Role of Defect Engineering and Surface Functionalization in the Design of Carbon Nanotube-Based Nitrogen Oxide Sensors
Abstract
1. Introduction
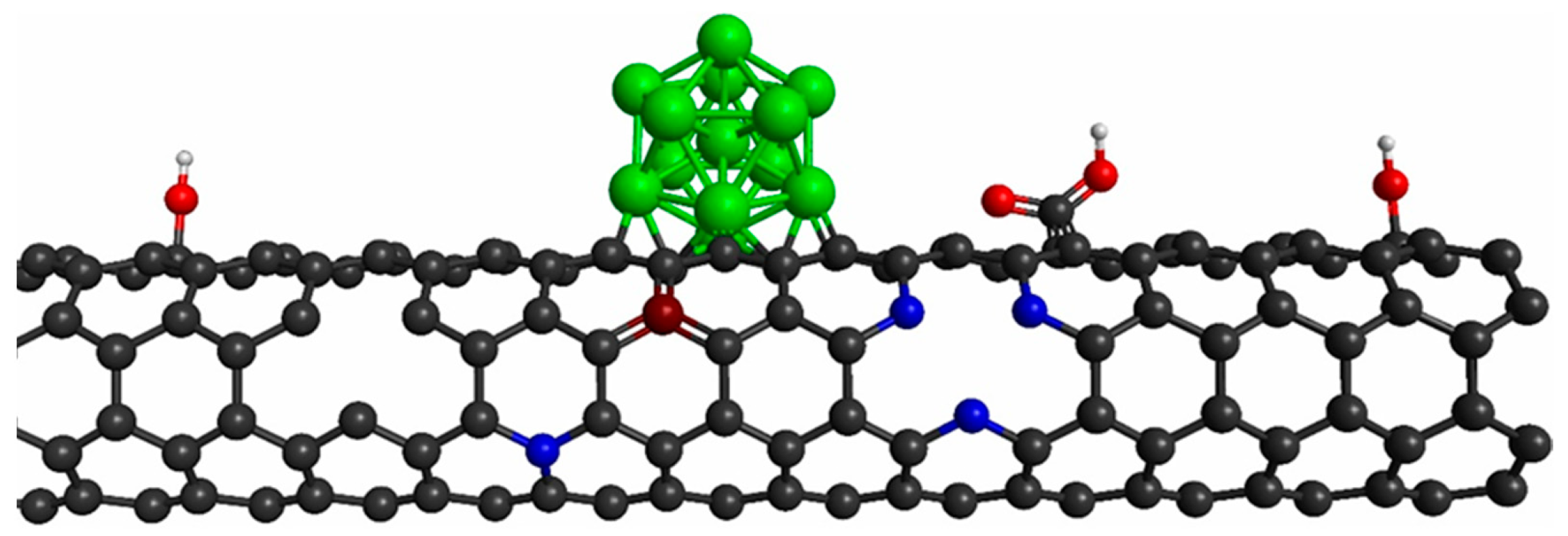
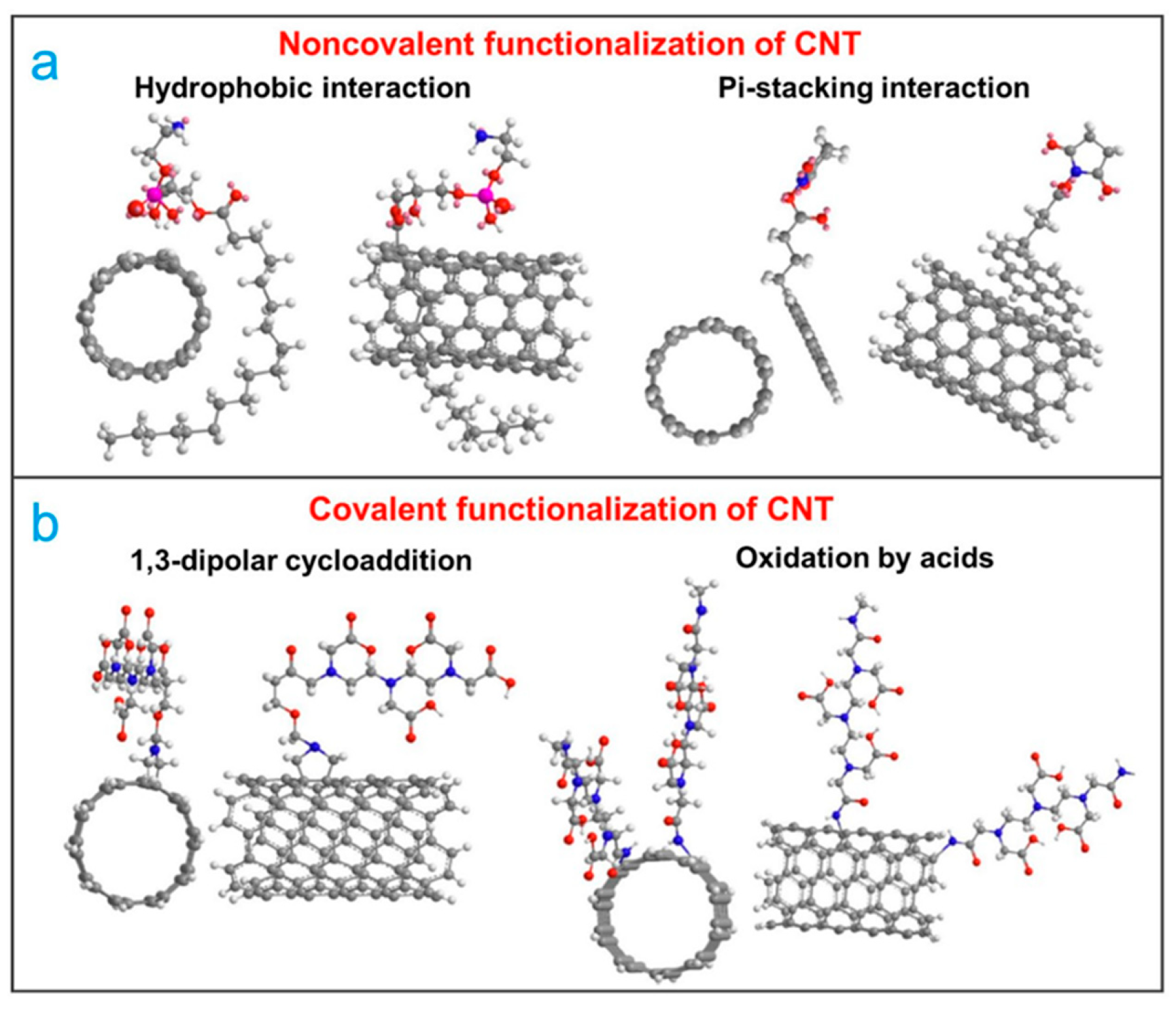
2. Surface Functionalization and Defect Engineering on CNTs
3. Experimental Studies
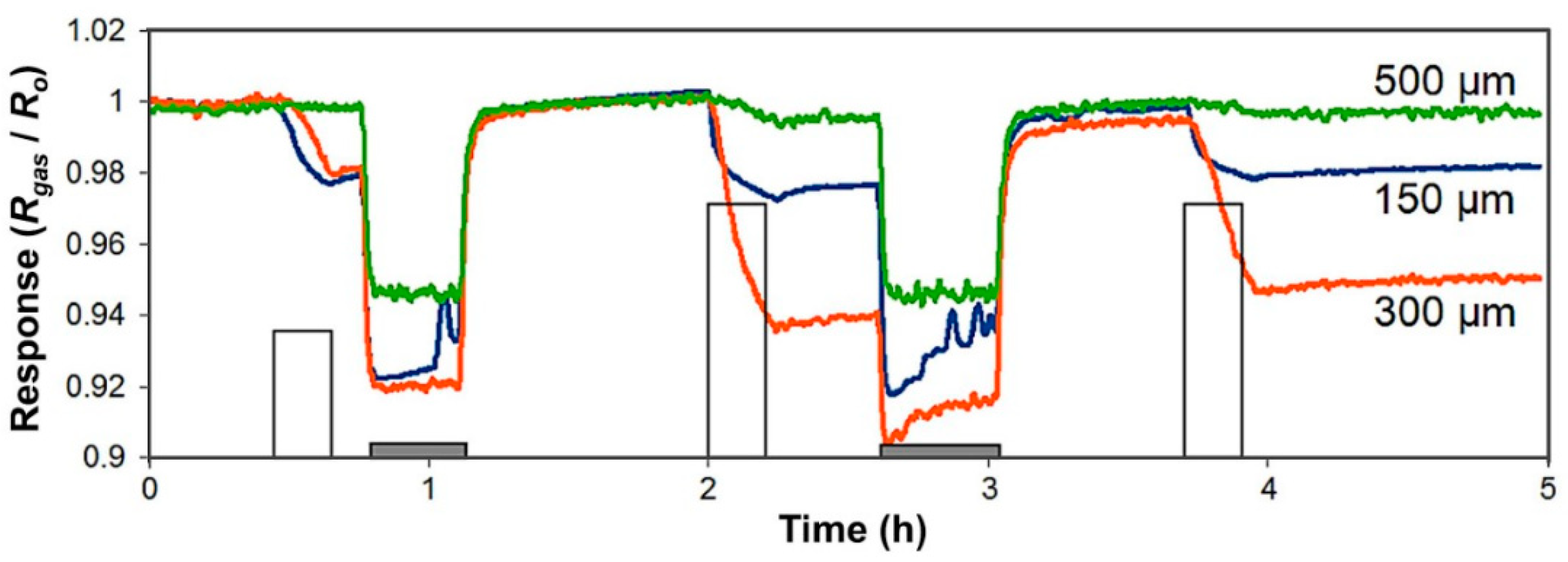
3.1. Functionalized CNTs
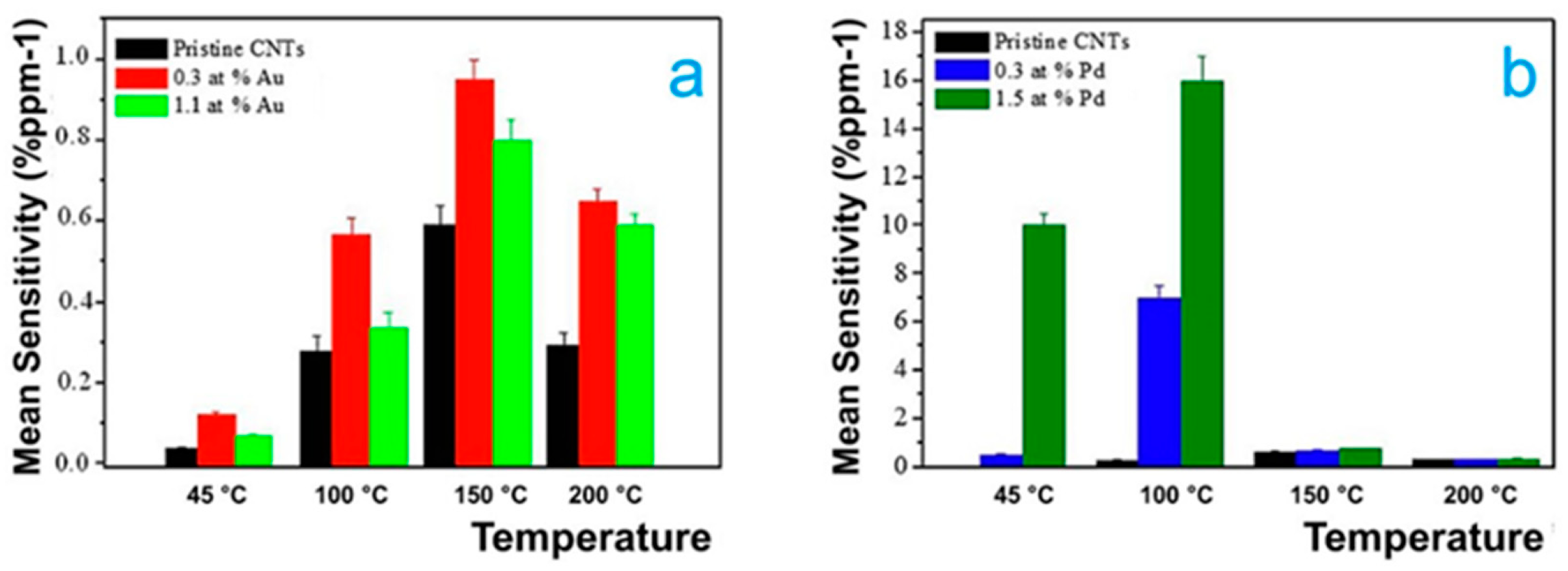
3.2. Decorated CNTs
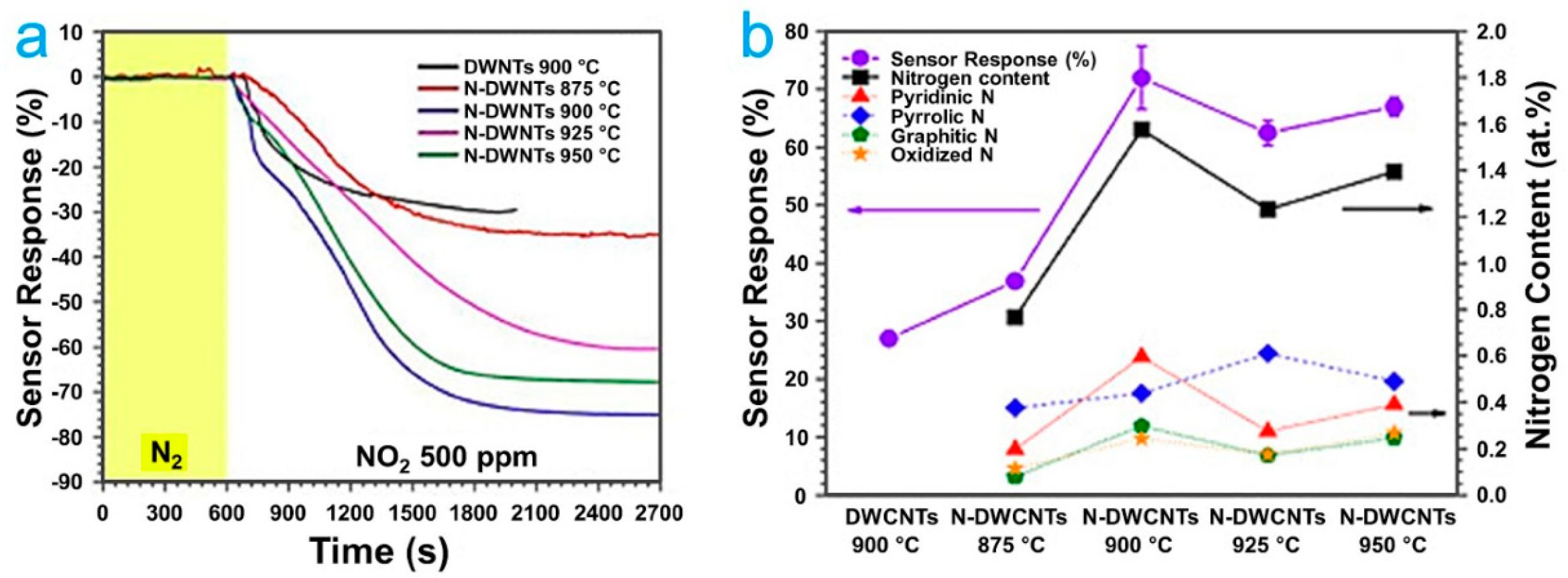
3.3. Doped CNTs
4. Theoretical Studies
4.1. Decorated CNTs
4.2. Doped CNTs
4.3. Vacancies
5. Combined Theoretical and Experimental Studies
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.D.; Yu, Y.; Dargusch, M.; Liu, Q.; Chen, Z.G. Carbon allotrope hybrids advance thermoelectric development and applications. Renew. Sustain. Energy Rev. 2021, 141, 110800. [Google Scholar] [CrossRef]
- Daniels, S.L. Products of incomplete combustion (Ox, COx, HOx, NOx, SOx ROx, MOx and POx). J. Hazard. Mater. 1989, 22, 161–173. [Google Scholar] [CrossRef]
- Usman, M.; Ma, Z.; Zafar, M.W.; Haseeb, A.; Ashraf, R.U. Are air pollution, economic and non-economic factors associated with per capita health expenditures? Evidence from emerging economies. Int. J. Environ. Res. Public Health 2019, 16, 1967. [Google Scholar] [CrossRef]
- Timilsina, G.R.; Csordás, S.; Mevel, S. When does a carbon tax on fossil fuels stimulate biofuels? Ecol. Econ. 2011, 70, 2400–2415. [Google Scholar] [CrossRef]
- Helm, D. The future of fossil fuels—Is it the end? Oxf. Rev. Econ. Policy 2016, 32, 191–205. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, Y.J.; Liu, Y.L.; Zhao, J.X. Boosting sensitivity of Boron Nitride Nanotube (BNNT) to nitrogen dioxide by Fe encapsulation. J. Mol. Graph. Model. 2014, 51, 1–6. [Google Scholar] [CrossRef]
- Kumar, U.; Yadav, B.C.; Haldar, T.; Dixit, C.K.; Yadawa, P.K. Synthesis of MWCNT/PPY nanocomposite using oxidation polymerization method and its employment in sensing such as CO2 and humidity. J. Taiwan Inst. Chem. Eng. 2020, 113, 419–427. [Google Scholar] [CrossRef]
- Nundy, S.; Eom, T.-Y.; Kang, J.-G.; Suh, J.; Cho, M.; Park, J.-S.; Lee, H.-J. Flower-shaped ZnO nanomaterials for low-temperature operations in NOX gas sensors. Ceram. Int. 2020, 46, 5706–5714. [Google Scholar] [CrossRef]
- Trung, D.D.; Van Toan, N.; Van Tong, P.; Van Duy, N.; Hoa, N.D.; Van Hieu, N. Synthesis of single-crystal SnO2 nanowires for NOx gas sensors application. Ceram. Int. 2012, 38, 6557–6563. [Google Scholar] [CrossRef]
- Cantalini, C.; Sun, H.T.; Faccio, M.; Ferri, G.; Pelino, M. Niobium-doped α-Fe2O3 semiconductor ceramic sensors for the measurement of nitric oxide gases. Sens. Actuators B Chem. 1995, 25, 673–677. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef]
- Falcao, E.H.; Wudl, F. Carbon allotropes: Beyond graphite and diamond. J. Chem. Technol. Biotechnol. 2007, 82, 524–531. [Google Scholar] [CrossRef]
- Rodríguez-Quintana, R.; Carbajal-Franco, G.; Rojas-Chávez, H. DFT study of the H2 molecules adsorption on pristine and Ni doped graphite surfaces. Mater. Lett. 2021, 293, 129660. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Niu, J.J.; Wang, J.N.; Jiang, Y.; Su, L.F.; Ma, J. An approach to carbon nanotubes with high surface area and large pore volume. Microporous Mesoporous Mater. 2007, 100, 1–5. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Yan, X.; Cao, S.; Li, M.; Guo, Y.; Huan, Y.; Wen, X.; Zhang, Y. A three-dimensional reticulate CNT-aerogel for a high mechanical flexibility fiber supercapacitor. Nanoscale 2018, 10, 9360–9368. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, W.; Li, F.; Cong, A.H.; Cheng, H.-M. Synthesis and high thermal stability of double-walled carbon nanotubes using nickel formate dihydrate as catalyst precursor. J. Phys. Chem. C 2007, 111, 5006–5013. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Struzzi, C.; Aparicio Rebollo, F.J.; de Marco, P.; Mudimela, P.R.; Colomer, J.F.; Amati, M.; Gregoratti, L.; Petaccia, L.; Snyders, R.; et al. Tuning electronic properties of carbon nanotubes by nitrogen grafting: Chemistry and chemical stability. Carbon 2015, 83, 118–127. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Y.; Hu, P.; Yu, G.; Wang, X.; Zhu, D. High-mobility thin-film transistors based on aligned carbon nanotubes. Appl. Phys. Lett. 2003, 83, 150. [Google Scholar] [CrossRef]
- Sayago, I.; Santos, H.; Horrillo, M.C.; Aleixandre, M.; Fernández, M.J.; Terrado, E.; Tacchini, I.; Aroz, R.; Maser, W.K.; Benito, A.M.; et al. Carbon nanotube networks as gas sensors for NO2 detection. Talanta 2008, 77, 758–764. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P.; Chouksey, A.; Lal, M.; Rawat, J.S.B.S.; Tandon, R.P.; Chaudhury, P.K. Effect of single wall carbon nanotube networks on gas sensor response and detection limit. Sens. Actuators B Chem. 2017, 240, 1134–1140. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Harussani, M.M.; Zulaikha, N.D.S.; Norhana, A.H.; Syakir, M.I.; Norli, A. Composites based on conductive polymer with carbon nanotubes in DMMP gas sensors—An overview. Polimery 2021, 66, 85–97. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J. Hazard. Mater. 2017, 324, 762–772. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Non-covalently anchored multi-walled carbon nanotubes with hexa-decafluorinated zinc phthalocyanine as Ppb level chemiresistive chlorine sensor. Appl. Surf. Sci. 2018, 427, 202–209. [Google Scholar] [CrossRef]
- Kothari, R.; Kundalwal, S.I.; Sahu, S.K. Transversely isotropic thermal properties of carbon nanotubes containing vacancies. Acta Mech. 2018, 229, 2787–2800. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.Y. Geometry and electronic properties of single vacancies in achiral carbon nanotubes. Eur. Phys. J. B Condens. Matter Complex. Syst. 2006, 54, 243–247. [Google Scholar] [CrossRef]
- Kuzmany, H.; Kukovecz, A.; Simon, F.; Holzweber, M.; Kramberger, C.; Pichler, T. Functionalization of carbon nanotubes. Synth. Met. 2004, 141, 113–122. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Abdellah, A.; Scarpa, G.; Lugli, P. Metallic nanoparticles functionalizing carbon nanotube networks for gas sensing applications. Nanotechnology 2014, 25, 055208. [Google Scholar] [CrossRef]
- Aroutiounian, V.M. Gas sensors based on functionalized carbon nanotubes. J. Contemp. Phys. Arm. Acad. Sci. 2015, 50, 333–354. [Google Scholar] [CrossRef]
- Zhang, W.-S.; Liu, Y.-T.; Yao, T.-T.; Wu, G.-P.; Liu, Q. Oxygen defect engineering toward the length-selective tailoring of carbon nanotubes via a two-step electrochemical strategy. J. Phys. Chem. C 2020, 124, 27097–27106. [Google Scholar] [CrossRef]
- Hoefer, M.A.; Bandaru, P.R. Defect engineering of the electrochemical characteristics of carbon nanotube varieties. J. Appl. Phys. 2010, 108, 034308. [Google Scholar] [CrossRef]
- Dilonardo, E.; Penza, M.; Alvisi, M.; Rossi, R.; Cassano, G.; Di Franco, C.; Palmisano, F.; Torsi, L.; Cioffi, N. Gas sensing properties of MWCNT layers electrochemically decorated with Au and Pd nanoparticles. Beilstein J. Nanotechnol. 2017, 8, 592–603. [Google Scholar] [CrossRef]
- Muangrat, W.; Wongwiriyapan, W.; Yordsri, V.; Chobsilp, T.; Inpaeng, S.; Issro, C.; Domanov, O.; Ayala, P.; Pichler, T.; Shi, L. Unravel the active site in nitrogen-doped double-walled carbon nanotubes for nitrogen dioxide gas sensor. Phys. Status Solidi Appl. Mater. Sci. 2018, 215, 1–6. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. Room temperature trace level detection of NO2 gas using SnO2 modified carbon nanotubes based sensor. J. Mater. Chem. 2012, 22, 23608–23616. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Adeel, M.; Rizwan, K.; Bilal, M.; Iqbal, H.M.N. Carbon nanotubes-based cues: A pathway to future sensing and detection of hazardous pollutants. J. Mol. Liq. 2019, 292, 111425. [Google Scholar] [CrossRef]
- Dai, H. Carbon nanotubes: Synthesis, integration, and properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef]
- Ibrahim, K.S. Carbon nanotubes-properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar] [CrossRef]
- Beitollahi, H.; Movahedifar, F.; Tajik, S.; Jahani, S. A review on the effects of introducing CNTs in the modification process of electrochemical sensors. Electroanalysis 2019, 31, 1195–1203. [Google Scholar] [CrossRef]
- Bassyouni, M.; Mansi, A.E.; ElGabry, A.; Ibrahim, B.A.; Kassem, O.A.; Alhebeshy, R. Utilization of carbon nanotubes in removal of heavy metals from wastewater: A review of the CNTs’ potential and current challenges. Appl. Phys. A 2020, 126, 1–33. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop. Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and water treatment application of carbon nanotubes (CNTs)-based composite membranes: A review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, H.; Hu, Y.; Peng, L.-M. Carbon nanotube-based flexible electronics. J. Mater. Chem. C 2018, 6, 7714–7727. [Google Scholar] [CrossRef]
- Elias, A.; Uddin, N.; Hossain, A.; Saha, J.K.; Siddiquey, I.A.; Sarker, D.R.; Diba, Z.R.; Uddin, J.; Choudhury, M.H.R.; Firoz, S.H. An experimental and theoretical study of the effect of Ce doping in ZnO/CNT composite thin film with enhanced visible light photo-catalysis. Int. J. Hydrogen Energy 2019, 44, 20068–20078. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Qian, D.; Rantell, T. Multiwall carbon nanotubes: Synthesis and application. Acc. Chem. Res. 2002, 35, 1008–1017. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Dilonardo, E.; Penza, M.; Alvisi, M.; Di Franco, C.; Rossi, R.; Palmisano, F.; Torsi, L.; Cioffi, N. Electrophoretic deposition of Au NPs on MWCNT-based gas sensor for tailored gas detection with enhanced sensing properties. Sens. Actuators B Chem. 2016, 223, 417–428. [Google Scholar] [CrossRef]
- Sinha, N.; Ma, J.; Yeow, J.T. Carbon nanotube-based sensors. J. Nanosci. Nanotechnol. 2006, 6, 573–590. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon nanotube-based chemical sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef]
- Hu, C.Y.; Xu, Y.J.; Duo, S.W.; Zhang, R.F.; Li, M.S. Non-covalent functionalization of carbon nanotubes with surfactants and polymers. J. Chin. Chem. Soc. 2009, 56, 234–239. [Google Scholar] [CrossRef]
- Kocharova, N.; Ääritalo, T.; Leiro, J.; Kankare, J.; Lukkari, J. Aqueous dispersion, surface thiolation, and direct self-assembly of carbon nanotubes on gold. Langmuir 2007, 23, 3363–3371. [Google Scholar] [CrossRef]
- Kotagiri, N.; Kim, J.W. Stealth nanotubes: Strategies of shielding carbon nanotubes to evade opsonization and improve biodistribution. Int. J. Nanomed. 2014, 9, 85–105. [Google Scholar] [CrossRef][Green Version]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon nanotube-based chemiresistive sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yee, M.J.; Yon, L.S.; Bing, C.H.; Khalid, M.; Abdullah, E.C. An overview of functionalised carbon nanomaterial for organic pollutant removal. J. Ind. Eng. Chem. 2018, 67, 175–186. [Google Scholar] [CrossRef]
- Pomoell, J.A.V.; Krasheninnikov, A.V.; Nordlund, K.; Keinonen, J. Ion ranges and irradiation-induced defects in multiwalled carbon nanotubes. J. Appl. Phys. 2004, 96, 2864–2871. [Google Scholar] [CrossRef]
- Lehtinen, O.; Nikitin, T.; Krasheninnikov, A.V.; Sun, L.; Banhart, F.; Khriachtchev, L.; Keinonen, J. Characterization of ion-irradiation-induced defects in multi-walled carbon nanotubes. New J. Phys. 2011, 13, 073004. [Google Scholar] [CrossRef]
- Da Silva, L.B.; Fagan, S.B.; Mota, R. Ab initio study of deformed carbon nanotube sensors for carbon monoxide molecules. Nano Lett. 2004, 4, 65–67. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K. Ab initio study of doped carbon nanotube sensors. Nano Lett. 2003, 3, 513–517. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Zhang, Z.; Jia, X.; An, L. CO adsorption on Fe-doped vacancy-defected CNTs–A DFT study. Chem. Phys. Lett. 2019, 730, 316–320. [Google Scholar] [CrossRef]
- Tabtimsai, C.; Rakrai, W.; Phalinyot, S.; Wanno, B. Interaction investigation of single and multiple carbon monoxide molecules with Fe-, Ru-, and Os-doped single-walled carbon nanotubes by DFT study: Applications to gas adsorption and detection nanomaterials. J. Mol. Model. 2020, 26, 1–13. [Google Scholar] [CrossRef]
- Yeow, J.T.W.; Wang, Y. A review of carbon nanotubes-based gas sensors. J. Sens. 2009, 2009, 493904. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Mukhopadhyay, S.C.; Xu, Y. Carbon nanotubes and its gas-sensing applications: A review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Valentini, L.; Cantalini, C.; Armentano, I.; Kenny, J.M.; Lozzi, L.; Santucci, S. Highly sensitive and selective sensors based on carbon nanotubes thin films for molecular detection. Diam. Relat. Mater. 2004, 13, 1301–1305. [Google Scholar] [CrossRef]
- Piloto, C.; Mirri, F.; Bengio, E.A.; Notarianni, M.; Gupta, B.; Shafiei, M.; Pasquali, M.; Motta, N. Room temperature gas sensing properties of ultrathin carbon nanotube films by surfactant-free dip coating. Sens. Actuators B 2016, 227, 128–134. [Google Scholar] [CrossRef]
- Ray, A.; Sadhukhan, P.; Naskar, K.; Lal, G.; Bhar, R.; Sinha, C.; Das, S. Polyaniline-multiwalled carbon nanotube (PANI-MWCNT): Room temperature resistive carbon monoxide (CO) sensor. Synth. Met. 2018, 245, 182–189. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Wan, M. Nanostructures of polyaniline doped with inorganic acids. Macromolecules 2002, 35, 5937–5942. [Google Scholar] [CrossRef]
- Fei, J.; Cui, Y.; Yan, X.; Yang, Y.; Wang, K.; Li, J. Controlled fabrication of polyaniline spherical and cubic shells with hierarchical nanostructures. ACS Nano 2009, 3, 3714–3718. [Google Scholar] [CrossRef]
- Cho, S.; Kwon, O.S.; You, S.A.; Jang, J. Shape-controlled polyaniline chemiresistors for high-performance DMMP sensors: Effect of morphologies and charge-transport properties. J. Mater. Chem. A 2013, 1, 5679–5688. [Google Scholar] [CrossRef]
- Yun, J.; Jeon, S.; Kim, H.I. Improvement of NO gas sensing properties of polyaniline/MWCNT composite by photocatalytic effect of TiO2. J. Nanomater. 2013, 2013, 184345. [Google Scholar] [CrossRef]
- Mangu, R.; Rajaputra, S.; Singh, V.P. MWCNT-polymer composites as highly sensitive and selective room temperature gas sensors. Nanotechnology 2011, 22, 215502. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, J.J.; Chen, D.; Tuller, H.L.; Rutledge, G.C. Electrospun polyaniline fibers as highly sensitive room temperature chemiresistive sensors for ammonia and nitrogen dioxide gases. Adv. Funct. Mater. 2014, 24, 4005–4014. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, S.; Wu, Z.; Zhang, M.; Cao, Y.; Guo, J.; Zhong, F.; Duan, H.; Jia, D. High-performance gas sensor of polyaniline/carbon nanotube composites promoted by interface engineering. Sensors 2019, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- Pisal, S.H.; Harale, N.S.; Bhat, T.S.; Deshmukh, H.P.; Patil, P.S. Functionalized multi-walled carbon nanotubes for nitrogen sensor. IOSR J. Appl. Chem. 2014, 7, 49–52. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Kang, B.C.; Byun, Y.T.; Ha, T.J. High-performance gas sensors based on single-wall carbon nanotube random networks for the detection of nitric oxide down to the ppb-level. Nanoscale 2019, 11, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Ionete, E.I.; Spiridon, S.I.; Monea, B.F.; Stratulat, E. A room temperature gas sensor based on sulfonated SWCNTs for the detection of NO and NO2. Sensors 2019, 19, 1116. [Google Scholar] [CrossRef]
- Popescu, M.; Simandan, I.D.; Sava, F.; Velea, A.; Fagadar-Cosma, E. Sensor of nitrogen dioxide based on single wall carbon nanotubes and manganese-porphyrin. Dig. J. Nanomater. Biostructures 2011, 6, 1253–1256. [Google Scholar]
- Penza, M.; Rossi, R.; Alvisi, M.; Cassano, G.; Signore, M.A.; Serra, E.; Giorgi, R. Pt- and Pd-nanoclusters functionalized carbon nanotubes networked films for sub-ppm gas sensors. Sens. Actuators B Chem. 2008, 135, 289–297. [Google Scholar] [CrossRef]
- Leghrib, R.; Dufour, T.; Demoisson, F.; Claessens, N.; Reniers, F.; Llobet, E. Gas sensing properties of multiwall carbon nanotubes decorated with rhodium nanoparticles. Sens. Actuators B Chem. 2011, 160, 974–980. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, Y.J.; Ra, E.J.; Kim, K.K.; An, K.H.; Lee, Y.H.; Choi, J.Y.; Park, C.H.; Doo, S.K.; Park, M.H.; et al. Defect-induced loading of Pt nanoparticles on carbon nanotubes. Appl. Phys. Lett. 2007, 90, 94–97. [Google Scholar] [CrossRef]
- Mudimela, P.R.; Scardamaglia, M.; González-León, O.; Reckinger, N.; Snyders, R.; Llobet, E.; Bittencourt, C.; Colomer, J.F. Gas sensing with gold-decorated vertically aligned carbon nanotubes. Beilstein J. Nanotechnol. 2014, 5, 910–918. [Google Scholar] [CrossRef]
- Na, P.S.; Kim, H.; So, H.M.; Kong, K.J.; Chang, H.; Ryu, B.H.; Choi, Y.; Lee, J.O.; Kim, B.K.; Kim, J.J.; et al. Investigation of the humidity effect on the electrical properties of single-walled carbon nanotube transistors. Appl. Phys. Lett. 2005, 87, 10–13. [Google Scholar] [CrossRef]
- Loghin, F.C.; Falco, A.; Albrecht, A.; Salmerón, J.F.; Becherer, M.; Lugli, P.; Rivandeneyra, A. A handwriting method for low-cost gas sensors. ACS Appl. Mater. Interfaces 2018, 10, 34683–34689. [Google Scholar] [CrossRef]
- Fort, A.; Panzardi, E.; Al-Hamry, A.; Vignoli, V.; Mugnaini, M.; Addabbo, T.; Kanoun, O. Highly sensitive detection of NO2 by au and TiO2 nanoparticles decorated SWCNTs sensors. Sensors 2020, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, J.; Byun, Y.T. Highly sensitive and selective NO2 detection by Pt nanoparticles-decorated single-walled carbon nanotubes and the underlying sensing mechanism. Sens. Actuators B Chem. 2017, 238, 1032–1042. [Google Scholar] [CrossRef]
- Mahmood, W.K.; Naje, A.N. Fabrication of room temperature NO2 gas sensor based on silver nanoparticles-decorated carbon nanotubes. J. Nano- Electron. Phys. 2018, 10, 1–6. [Google Scholar] [CrossRef]
- Panzardi, E.; Lo Grasso, A.; Vignoli, V.; Mugnaini, M.; Lupetti, P.; Fort, A. NO2 Sensing with SWCNT decorated by nanoparticles in temperature pulsed mode: Modeling and characterization. Sensors 2020, 20, 4729. [Google Scholar] [CrossRef]
- Su, P.G.; Pan, T.T. Fabrication of a room-temperature NO2 gas sensor based on WO3 films and WO3/MWCNT nanocomposite films by combining polyol process with metal organic decomposition method. Mater. Chem. Phys. 2011, 125, 351–357. [Google Scholar] [CrossRef]
- Park, S.; Byoun, Y.; Kang, H.; Song, Y.-J.; Choi, S.-W. ZnO nanocluster-functionalized single-walled carbon nanotubes synthesized by microwave irradiation for highly sensitive NO2 detection at room temperature. ACS Omega 2019, 4, 10677–10686. [Google Scholar] [CrossRef]
- Baccar, H.; Thamri, A.; Clément, P.; Llobet, E.; Abdelghani, A. Pt- and Pd-decorated MWCNTs for vapour and gas detection at room temperature. Beilstein J. Nanotechnol. 2015, 6, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Zanolli, Z.; Leghrib, R.; Felten, A.; Pireaux, J.J.; Llobet, E.; Charlier, J.C. Gas sensing with au-decorated carbon nanotubes. ACS Nano 2011, 5, 4592–4599. [Google Scholar] [CrossRef]
- Choi, K.Y.; Park, J.S.; Park, K.B.; Kim, H.J.; Park, H.D.; Kim, S.D. Low power micro-gas sensors using mixed SnO2 nanoparticles and MWCNTs to detect NO2, NH3, and xylene gases for ubiquitous sensor network applications. Sens. Actuators B Chem. 2010, 150, 65–72. [Google Scholar] [CrossRef]
- Kim, D.; Park, K.M.; Shanmugam, R.; Yoo, B. Electrochemically decorated ZnTe nanodots on single-walled carbon nanotubes for room-temperature NO2 sensor application. J. Nanosci. Nanotechnol. 2014, 14, 8248–8252. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.; Lee, J.S.; Byun, Y.T. Negatively-doped single-walled carbon nanotubes decorated with carbon dots for highly selective NO2 detection. Nanomaterials 2020, 10, 2509. [Google Scholar] [CrossRef]
- Albiss, B.A.; Sakhaneh, W.A.; Jumah, I.; Obaidat, I.M. NO2 gas sensing properties of ZnO/single-wall carbon nanotube composites. IEEE Sens. J. 2010, 10, 1807–1812. [Google Scholar] [CrossRef]
- Evans, G.P.; Buckley, D.J.; Skipper, N.T.; Parkin, I.P. Single-walled carbon nanotube composite inks for printed gas sensors: Enhanced detection of NO2, NH3, EtOH and acetone. RSC Adv. 2014, 4, 51395–51403. [Google Scholar] [CrossRef]
- Yaqoob, U.; Phan, D.T.; Uddin, A.S.M.I.; Chung, G.S. Highly flexible room temperature NO2 sensor based on MWCNTs-WO3 nanoparticles hybrid on a PET substrate. Sens. Actuators B Chem. 2015, 221, 760–768. [Google Scholar] [CrossRef]
- Jones, R.O. Density functional theory: Its origins, rise to prominence, and future. Rev. Mod. Phys. 2015, 87, 897. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Tellez-Cruz, M.M.; Solorza-Feria, O.; Calaminici, P.; Medina, D.I. Catalytic activity trends from pure Pd nanoclusters to M@PdPt (M = Co, Ni, and Cu) core-shell nanoclusters for the oxygen reduction reaction: A first-principles analysis. Int. J. Hydrogen Energy 2020, 45, 13738–13745. [Google Scholar] [CrossRef]
- Zeng, Y.; Lin, S.; Gu, D.; Li, X. Two-dimensional nanomaterials for gas sensing applications: The role of theoretical calculations. Nanomaterials 2018, 8, 851. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Ehlert, C.; Gryn’Ova, G. Sensing and sensitivity: Computational chemistry of graphene-based sensors. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1526. [Google Scholar] [CrossRef]
- Bakar, A.; Afaq, A.; Ahmed, M.; Bashir, A.; Asif, M. Optoelectronic properties of CuCoMnZ (Z = Si, Sn, Sb): A DFT study. J. Electron. Mater. 2021, 50, 4006–4015. [Google Scholar] [CrossRef]
- Li, K.; Wang, W.; Cao, D. Metal (Pd, Pt)-decorated carbon nanotubes for CO and NO sensing. Sens. Actuators B Chem. 2011, 159, 171–177. [Google Scholar] [CrossRef]
- Dutta, A.; Pradhan, A.K.; Qi, F.; Mondal, P. Computation-led design of pollutant gas sensors with bare and carbon nanotube supported rhodium alloys. Monatsh. Chem. 2020, 151, 159–171. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Montejo-Alvaro, F.; Peña-Castañeda, Y.A.; Matadamas-Ortiz, P.T.; Medina, D.I. Recent developments in graphene-based toxic gas sensors: A theoretical overview. Sensors 2021, 21, 1992. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Janebi, H. B-, N-doped and BN codoped C60 heterofullerenes for environmental monitoring of NO and NO2: A DFT study. Mol. Phys. 2020, 118, e1631495. [Google Scholar] [CrossRef]
- Ramirez-de-Arellano, J.M.; Canales, M.; Magaña, L.F. Carbon nanostructures doped with transition metals for pollutant gas adsorption systems. Molecules 2021, 26, 5346. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; An, L.; Chen, T. Adsorption of nitrogen oxides on Al-doped carbon nanotubes: The first principles study. Adsorption 2020, 26, 587–595. [Google Scholar] [CrossRef]
- Demir, S.; Fellah, M.F. Carbon nanotubes doped with Ni, Pd and Pt: A density functional theory study of adsorption and sensing NO. Surf. Sci. 2020, 701, 121689. [Google Scholar] [CrossRef]
- Azizi, K.; Karimpanah, M. Computational study of Al-or P-doped single-walled carbon nanotubes as NH3 and NO2 sensors. Appl. Surf. Sci. 2013, 285, 102–109. [Google Scholar] [CrossRef]
- Tabtimsai, C.; Wanno, B.; Utairueng, A.; Promchamorn, P.; Kumsuwan, U. First principles investigation of NH3 and NO2 adsorption on transition metal-doped single-walled carbon nanotubes. J. Electron. Mater. 2019, 48, 7226–7238. [Google Scholar] [CrossRef]
- Tabtimsai, C.; Wanno, B.; Ruangpornvisuti, V. Theoretical investigation of CO2 and NO2 adsorption onto Co-, Rh-and Ir-doped (5, 5) single-walled carbon nanotubes. Mater. Chem. Phys. 2013, 138, 709–715. [Google Scholar] [CrossRef]
- Tabtimsai, C.; Keawwangchai, S.; Wanno, B.; Ruangpornvisuti, V. Gas adsorption on the Zn–, Pd–and Os–doped armchair (5, 5) single–walled carbon nanotubes. J. Mol. Model. 2012, 18, 351–358. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Wang, Z.; Ma, S. Small gas adsorption on Co–N4 porphyrin-like CNT for sensor exploitation: A first-principles study. Carbon Lett. 2020, 30, 177–187. [Google Scholar] [CrossRef]
- Luo, M.; Liang, Z.; Peera, S.G.; Chen, M.; Liu, C.; Yang, H.; Liu, J.; Kumar, U.P.; Liang, T. Theoretical study on the adsorption and predictive catalysis of MnN4 embedded in carbon substrate for gas molecules. Appl. Surf. Sci. 2020, 525, 146480. [Google Scholar] [CrossRef]
- Hankins, A.; Willard, T.C.; Liu, A.Y.; Paranjape, M. Role of defects in the sensing mechanism of CNTFET gas sensors. J. Appl. Phys. 2020, 128, 084501. [Google Scholar] [CrossRef]
- Zanolli, Z.; Charlier, J.C. Defective carbon nanotubes for single-molecule sensing. Phys. Rev. B 2009, 80, 155447. [Google Scholar] [CrossRef]
- Vasylenko, A.I.; Tokarchuk, M.V.; Jurga, S. Effect of a vacancy in single-walled carbon nanotubes on He and NO adsorption. J. Phys. Chem. C 2015, 119, 5113–5116. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.T.; Ortiz-Herrera, J.C.; López-Chávez, E.; Solorza-Feria, O.; Medina, D.I. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Guerra-Cabrera, W.; Flores-Rojas, E.; Ruiz-Villalobos, D.; Rojas-Chávez, H.; Peña-Castañeda, Y.A.; Medina, D.I. Pt-free metal nanocatalysts for the oxygen reduction reaction combining experiment and theory: An overview. Molecules 2021, 26, 6689. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Herrera-Rivera, M.R.; Rojas-Chávez, H.; Cruz-Martínez, H.; Medina, D.I. Recent advances in ZnO-based carbon monoxide sensors: Role of doping. Sensors 2021, 21, 4425. [Google Scholar] [CrossRef]
- Chikate, P.R.; Sharma, A.; Rondiya, S.R.; Cross, R.W.; Dzade, N.Y.; Shirage, P.M.; Devan, R.S. Hierarchically interconnected ZnO nanowires for low-temperature-operated reducing gas sensors: Experimental and DFT studies. New J. Chem. 2021, 3, 1404–1414. [Google Scholar] [CrossRef]
- Santucci, S.; Picozzi, S.; Di Gregorio, F.; Lozzi, L.; Cantalini, C.; Valentini, L.; Kenny, J.M.; Delley, B. NO2 and CO gas adsorption on carbon nanotubes: Experiment and theory. J. Chem. Phys. 2003, 119, 10904–10910. [Google Scholar] [CrossRef]
- Adjizian, J.-J.; Leghrib, R.; Koos, A.A.; Suarez-Martinez, I.; Crossley, A.; Wagner, P.; Grobert, N.; Llobet, E.; Ewels, C.P. Boron-and nitrogen-doped multi-wall carbon nanotubes for gas detection. Carbon 2014, 66, 662–673. [Google Scholar] [CrossRef]


| Sensor Type | Operating Temperature °C | Limit of Detection (ppm) | Response Time | Recovery Time | Reference |
|---|---|---|---|---|---|
| Pt-SWCNTs | 200 | 0.003 | <600 s | - | [87] |
| Pt-MWCNTs | 25 | 1.7 | - | - | [99] |
| Pt-SWCNTs | 25–150 | 2 | >180 s | 849–1411 s | [94] |
| Pd-SWCNTs | 200 | 0.009 | <600 s | - | [87] |
| Pd-MWCNTs | 25 | 1.7 | - | - | [99] |
| Pd-SWCNTs | 45–200 | 0.2 | <300 s | >1300 s | [38] |
| Au-MWCNTs | RT | 0.1 | >600 s | - | [100] |
| Au-MWCNTs | 45–200 | 0.2 | <300 s | >1300 s | [38] |
| Au-MWCNTs | 100–250 | 5 | >30 s | 7–4 min | [93] |
| SnO2-MWCNTs | 30–200 | 0.1 | <420 s | >8 min | [40] |
| SnO2-SWCNTs | 180–380 | 0.3 | <100 s | - | [101] |
| TiO2-SWCNTs | 100–250 | 5 | >60 s | 6–3 min | [93] |
| ZnTe-SWCNTs | RT | 0.5 | - | - | [102] |
| Rh-MWCNTs | RT | 0.05 | 20 min | - | [88] |
| Cdots-SWCNTs | RT | 0.1 | 381 s | 294 s | [103] |
| ZnO-SWCNTs | RT | 0.088 | <220 s | - | [98] |
| ZnO-SWCNTs | 25–300 | 1 | 300 s | 5–8 min | [104] |
| Ag-SWCNTs | RT | - | 8 s | 15 s | [95] |
| WO3-SWCNTs | 250–300 | 0.05 | 25 min | - | [105] |
| WO3-SWCNTs | RT | 0.1 | 10 min | 27 min | [106] |
| Doping Atom | Eads (in eV) | Methodology | Reference |
|---|---|---|---|
| Al | −2.20 | B3LYP | [120] |
| Al | −4.24 | BPE | [118] |
| P | −1.60 | B3LYP | [120] |
| Cr | −2.34 | B3LYP | [121] |
| Mn | −1.82 | B3LYP | [121] |
| Co | −2.36 | B3LYP | [122] |
| Zn | −2.02 | B3LYP | [123] |
| Mo | −3.17 | B3LYP | [121] |
| Tc | −2.06 | B3LYP | [121] |
| Rh | −2.08 | B3LYP | [122] |
| Pd | −2.09 | B3LYP | [123] |
| W | −3.90 | B3LYP | [121] |
| Re | −2.83 | B3LYP | [121] |
| Os | −2.50 | B3LYP | [123] |
| Ir | −2.62 | B3LYP | [122] |
| Sensor Type | Operating Temperature | NO2 | |||
|---|---|---|---|---|---|
| 0.05 ppm | 0.2 ppm | 0.5 ppm | 1.0 ppm | ||
| N-CNT | Ambient | −0.75 | −2.01 | −3.27 | −5.5 |
| 150 °C | −0.54 | −1.21 | −1.87 | −2.76 | |
| B-CNT | Ambient | 0.00 | −0.91 | −1.39 | −1.63 |
| 150 °C | −1.33 | −1.98 | −3.56 | −3.98 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés-Madrigal, M.A.; Montejo-Alvaro, F.; Cernas-Ruiz, A.S.; Rojas-Chávez, H.; Román-Doval, R.; Cruz-Martinez, H.; Medina, D.I. Role of Defect Engineering and Surface Functionalization in the Design of Carbon Nanotube-Based Nitrogen Oxide Sensors. Int. J. Mol. Sci. 2021, 22, 12968. https://doi.org/10.3390/ijms222312968
Valdés-Madrigal MA, Montejo-Alvaro F, Cernas-Ruiz AS, Rojas-Chávez H, Román-Doval R, Cruz-Martinez H, Medina DI. Role of Defect Engineering and Surface Functionalization in the Design of Carbon Nanotube-Based Nitrogen Oxide Sensors. International Journal of Molecular Sciences. 2021; 22(23):12968. https://doi.org/10.3390/ijms222312968
Chicago/Turabian StyleValdés-Madrigal, Manuel A., Fernando Montejo-Alvaro, Amelia S. Cernas-Ruiz, Hugo Rojas-Chávez, Ramon Román-Doval, Heriberto Cruz-Martinez, and Dora I. Medina. 2021. "Role of Defect Engineering and Surface Functionalization in the Design of Carbon Nanotube-Based Nitrogen Oxide Sensors" International Journal of Molecular Sciences 22, no. 23: 12968. https://doi.org/10.3390/ijms222312968
APA StyleValdés-Madrigal, M. A., Montejo-Alvaro, F., Cernas-Ruiz, A. S., Rojas-Chávez, H., Román-Doval, R., Cruz-Martinez, H., & Medina, D. I. (2021). Role of Defect Engineering and Surface Functionalization in the Design of Carbon Nanotube-Based Nitrogen Oxide Sensors. International Journal of Molecular Sciences, 22(23), 12968. https://doi.org/10.3390/ijms222312968







