Verification of the Impact of Blood Glucose Level on Liver Carcinogenesis and the Efficacy of a Dietary Intervention in a Spontaneous Metabolic Syndrome Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Blood Glucose Level Measurement
2.4. Urine Sugar Measurement
2.5. Glucose Tolerance Test
2.6. Morphological Assessment of the Liver
2.7. Morphological Assessment of the Pancreas
2.8. Analysis of mRNA Expression in Liver
2.9. Analysis of Plasma SCFAs
2.10. Statistical Analyses
3. Results
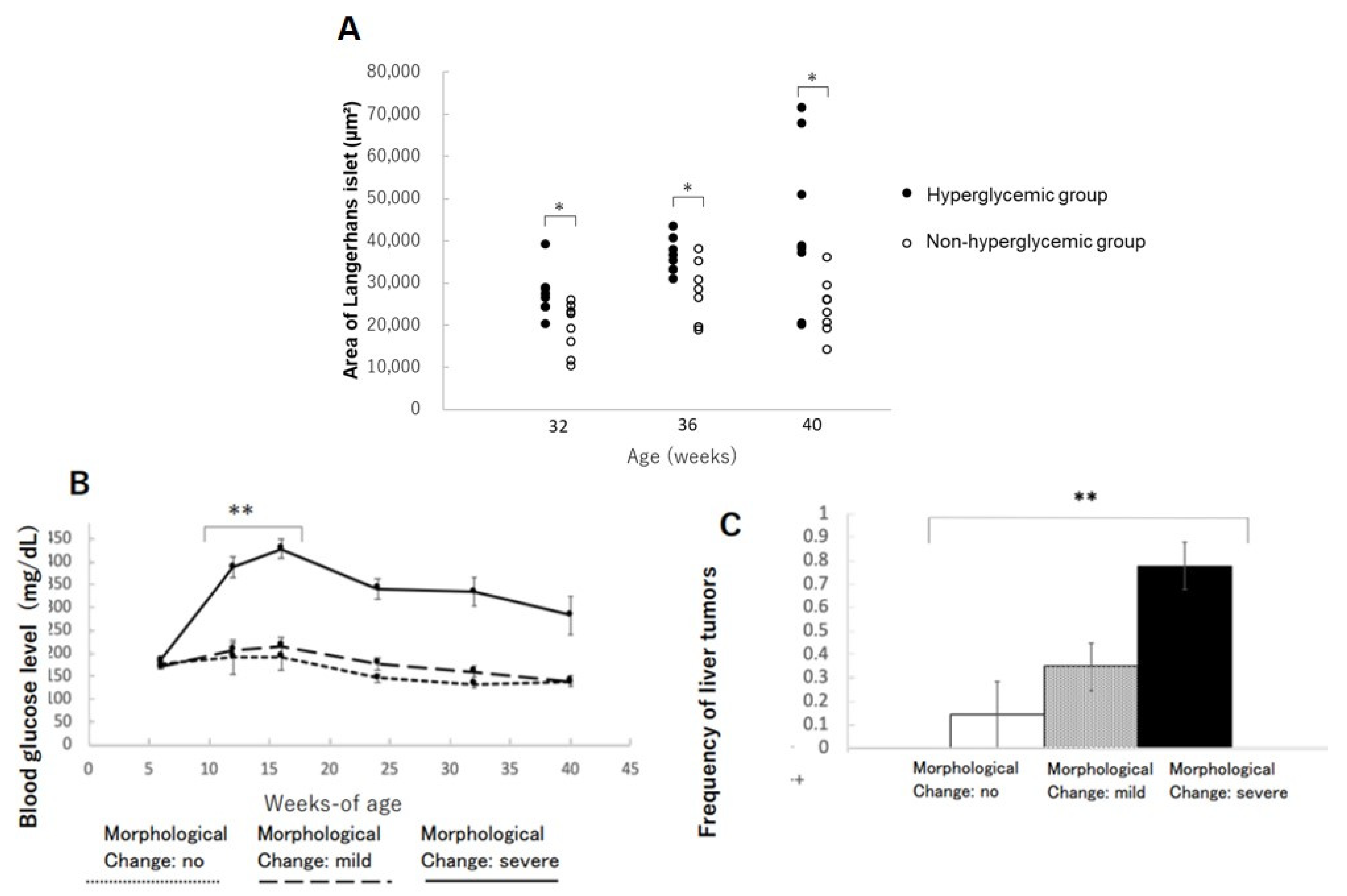
3.1. Pathological Characteristics of Islets of Langerhans
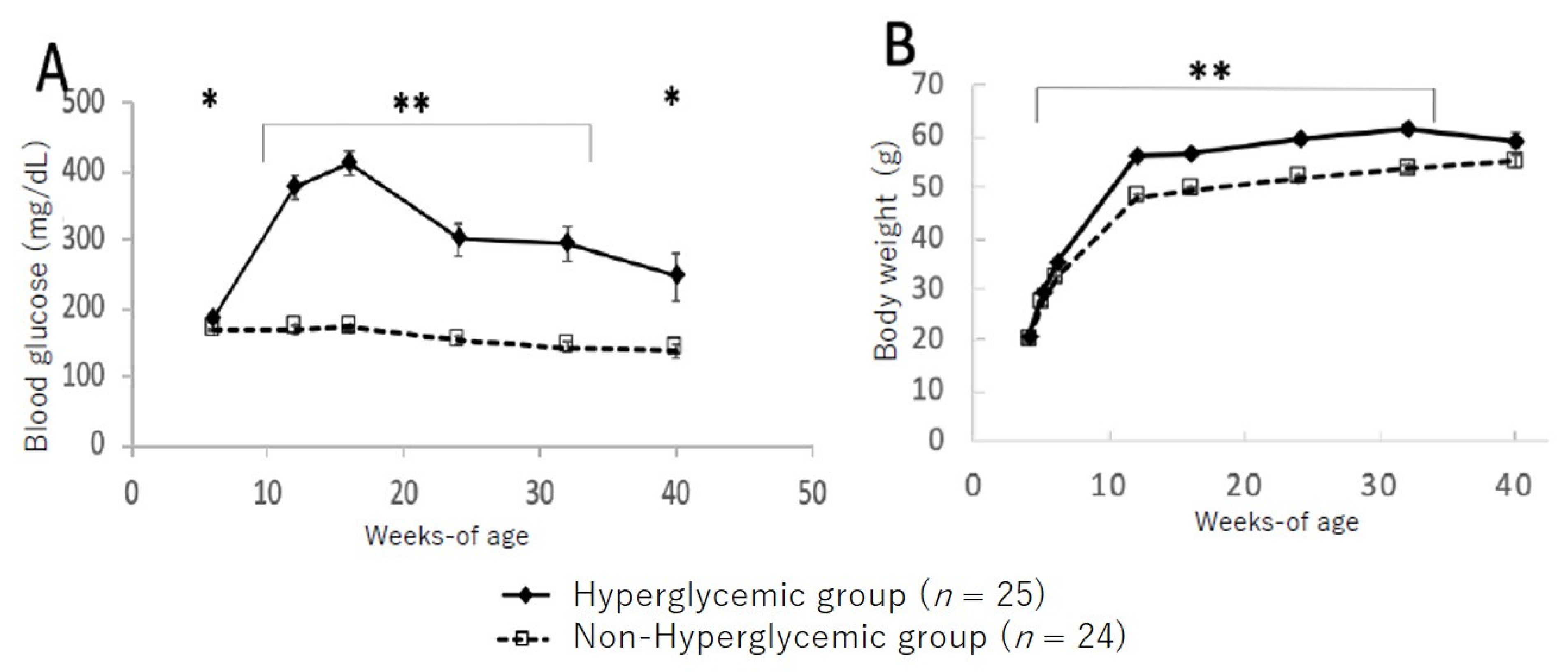
3.2. Changes in Blood Glucose Level and Body Weight between Hyperglycemic and Non-Hyperglycemic TSOD Mice
3.3. Frequency of Liver Tumors
3.4. Changes in Islets of Langerhans in the Pancreas
3.5. Changes in Bodyweight and Glucose Levels
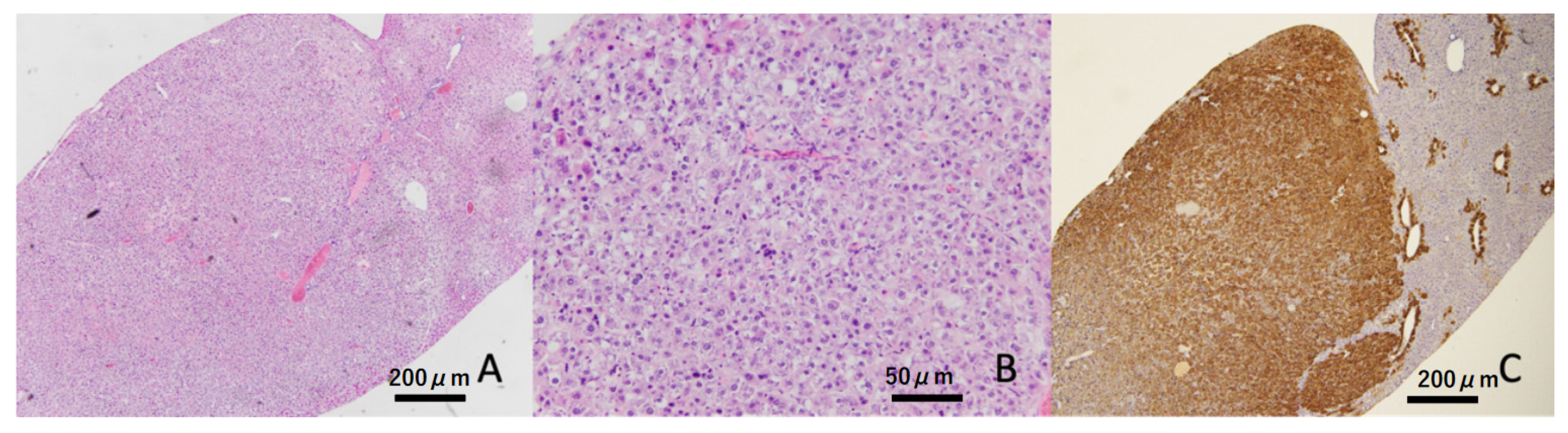
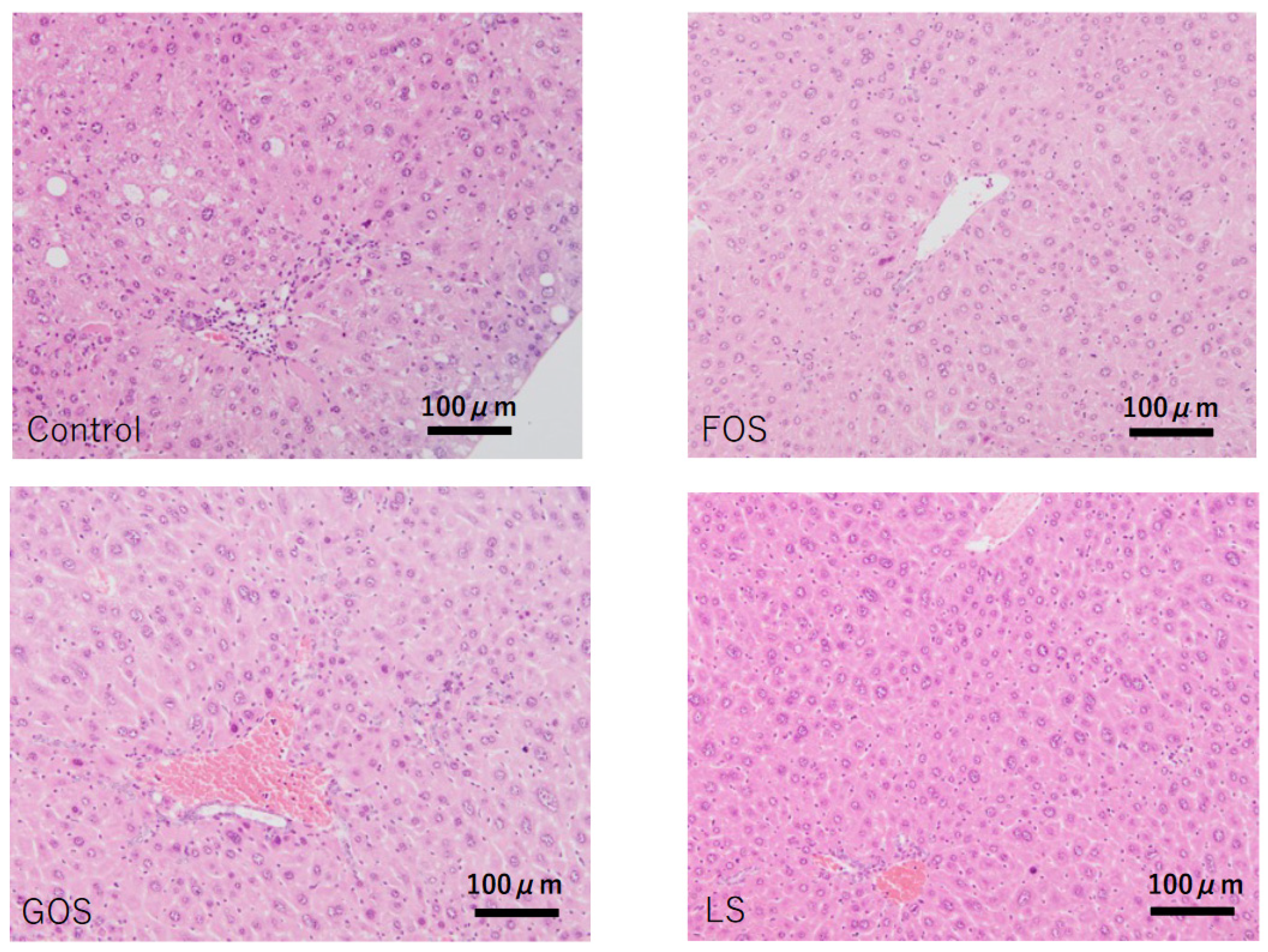
3.6. Histopathological Findings in the Liver
3.7. mRNA Expression of Lipid Metabolism-Related Genes in the Liver
3.8. Histopathological Findings in the Pancreas
3.9. Quantitation of Plasma SCFAs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TSOD | Tsumura Suzuki Obese Diabetes |
| MS | metabolic syndrome |
| NAFLD | non-alcoholic fatty liver disease |
| MAFLD | metabolic associated fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| FOS | fructooligosaccharide |
| GOS | galactooligosaccharide |
| LS | lactosucrose |
| HCC | hepatocellular carcinoma |
| HE | hematoxylin and eosin |
| SCFAs | short chain fatty acids |
References
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Pais, R.; Maurel, T. Natural History of NAFLD. J. Clin. Med. 2021, 10, 1161. [Google Scholar] [CrossRef]
- Mendonça, F.M.; De Sousa, F.R.; Barbosa, A.L.; Martins, S.C.; Araújo, R.L.; Soares, R.; Abreu, C. Metabolic syndrome and risk of cancer: Which link? Metabolism 2015, 64, 182–189. [Google Scholar] [CrossRef]
- Mantovani, A.; Targher, G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: Spotlight on nonalcoholic fatty liver disease. Ann. Transl. Med. 2017, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, C.I.; Chen, H.J.; Lai, H.C.; Liu, C.S.; Lin, W.Y.; Li, T.C.; Lin, C.C. Hyperglycemia and chronic liver diseases on risk of hepatocellular carcinoma in Chinese patients with type 2 diabetes-National cohort of Taiwan Diabetes Study. Int. J. Cancer 2015, 136, 2668–2679. [Google Scholar] [CrossRef]
- Nishida, T.; Tsuneyama, K.; Fujimoto, M.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; Nakanishi, Y.; Sasaki, Y.; Suzuki, W.; et al. Spontaneous onset of nonalcoholic steatohepatitis and hepatocellular carcinoma in a mouse model of metabolic syndrome. Lab. Investig. 2013, 93, 230–241. [Google Scholar] [CrossRef]
- Takahashi, T.; Deuschle, U.; Taira, S.; Nishida, T.; Fujimoto, M.; Hijikata, T.; Tsuneyama, K. Tsumura-Suzuki obese diabetic mice-derived hepatic tumors closely resemble human hepatocellular carcinomas in metabolism-related genes expression and bile acid accumulation. Hepatol. Int. 2018, 12, 254–261. [Google Scholar] [CrossRef]
- Tsuneyama, K.; Nishitsuji, K.; Matsumoto, M.; Kobayashi, T.; Morimoto, Y.; Tsunematsu, T.; Ogawa, H. Animal models for analyzing metabolic syndrome-associated liver diseases. Pathol. Int. 2017, 67, 539–546. [Google Scholar] [CrossRef]
- Hirayama, I.; Yi, Z.; Izumi, S.; Arai, I.; Suzuki, W.; Nagamachi, Y. Genetic analysis of obese diabetes in the TSOD mouse. Diabetes 1999, 48, 1183–1191. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial medicine: Prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Xiao, J.; Takahashi, S.S.; Saarela, M.; Maukonen, J.; von Wright, A.; Vilpponen-Salmela, T.; Patterson, A.J.; Scott, K.P.; Hämynen, H.; Mättö, J.; et al. Antibiotic susceptibility of Bifidobacterial strains distributed in the Japanese market. Biosci. Biotechnol. Biochem. 2017, 74, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Takai, A.; Kikuchi, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Matsumoto, K.; Tsunashima, H.; Onda, T.; Kuniyoshi, N.; Nariyama, T.; et al. Fructo-oligosaccharides ameliorate steatohepatitis, visceral adiposity, and associated chronic inflammation via increased production of short-chain fatty acids in a mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ichimura, M.; Tsuneyama, K.; Moritoki, Y.; Tsunashima, H.; Omagari, K.; Hara, M.; Yasuda, I.; Miyakawa, H.; Kikuchi, K. Fructo-oligosaccharides and intestinal barrier function in a methionine–choline-deficient mouse model of nonalcoholic steatohepatitis. PLoS ONE 2017, 12, e0175406. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Takahashi, T.; Ogawa, H.; Uehara, H.; Tsunematsu, T.; Baba, H.; Morimoto, Y.; Tsuneyama, K. Daily Coffee Intake Inhibits Pancreatic Beta Cell Damage and Nonalcoholic Steatohepatitis in a Mouse Model of Spontaneous Metabolic Syndrome, Tsumura-Suzuki Obese Diabetic Mice. Metab. Syndr. Relat. Disord. 2017, 15, 170–177. [Google Scholar] [CrossRef]
- Nishida, T.; Tsuneyama, K.; Fujimoto, M.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; Nakanishi, Y.; Hatta, H.; Imura, J. Aberrant iron metabolism might have an impact on progression of diseases in Tsumura Suzuki obese diabetes mice, a model of spontaneous metabolic syndrome. Pathol. Int. 2016, 66, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, L.; Vigneri, R.; Tumminia, A.; Frasca, F.; Squatrito, S.; Frittitta, L.; Vigneri, P. Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 808–815. [Google Scholar] [CrossRef]
- Micucci, C.; Orciari, S.; Catalano, A. Hyperglycemia promotes K-ras-induced lung tumorigenesis through BASCs amplification. PLoS ONE 2014, 9, e105550. [Google Scholar] [CrossRef]
- Liao, Y.F.; Yin, S.; Chen, Z.Q.; Li, F.; Zhao, B. High glucose promotes tumor cell proliferation and migration in lung adenocarcinoma via the RAGE-NOXs pathway. Mol. Med. Rep. 2018, 17, 8536–8541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Xiao, J.-Z.; Takahashi, S.; Odamaki, T.; Yaeshima, T.; Iwatsuki, K. Antibiotic susceptibility of bifidobacterial strains distributed in the Japanese market. Biosci. Biotechnol. Biochem. 2010, 74, 336–342. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Kim, T.Y.; Kang, H.; Oh, J.; Park, J.W.; Kim, S.C.; Kim, M.; Apostolidis, E.; Kim, Y.C.; Kwon, Y.I. Anti-Obesity and Anti-Adipogenic Effects of Chitosan Oligosaccharide (GO2KA1) in SD Rats and in 3T3-L1 Preadipocytes Models. Molecules 2021, 26, 331. [Google Scholar] [CrossRef] [PubMed]
- Ichimura-Shimizu, M.; Omagari, K.; Yamashita, M.; Tsuneyama, K. Development of a novel mouse model of diet-induced nonalcoholic steatohepatitis-related progressive bridging fibrosis. Biosci. Biotechnol. Biochem. 2021, 85, 941–947. [Google Scholar] [CrossRef]
- Mateus, V.A.; Monteiro, L.C.S.; Oliveira, R.B.; Maschio, D.A.; Collares-Buzato, C.B. Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp. Biol. Med. 2017, 242, 1214–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Weitkunat, K.; Schumann, S.; Petzke, K.J.; Blaut, M.; Loh, G.; Klaus, S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J. Nutr. Biochem. 2015, 26, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, S.; Roelofsen, H.; Rezaee, F.; Weening, D.; Hoek, A.; Vonk, R.; Venema, K. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur. J. Clin. Investig. 2012, 42, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yingyi, G.; Chen, L.; Lijuan, G.; Ou, S.; Peng, X. Lean rats gained more body weight from a high-fructooligosaccharide diet. Food Funct. 2015, 6, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]




| Genes | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| β-actin | CATCCGTAAAGACCTCTATGCCAAC | ATGGAGCCACCGATCCACA |
| Cd36 | TCCCAGAATTCTCAGCTGCTCC | CACATTTCAGAAGGCAGCAACTTC |
| Chrebp | CGACACTCACCCACCTCTTC | TTGTTCAGCCGGATCTTGTC |
| Cpt-1a | TCCTGAAGGAGGTACTGTCTG | CATAGCCGTCATCAGCAACC |
| Fasn | GACTCGGCTACTGACACGAC | CGAGTTGAGCTGGGTTAGGG |
| Fatp5 | GGGTGTGAGGGTAAGGTTGG | CCAGGGAATCCTGGATACGG |
| G6pase | CCAACGTATGGATTCCGGTGT | GCAAGGTAGATCCGGGACAG |
| Mttp | TGCTGTCCATTGGGGAACTC | CGACGGATCATTTTGCTTGC |
| Pepck1 | TGGAAGGTCGAATGTGTGGG | TAGCCCTTAAGTTGCCTTGGG |
| Pepck2 | GTGGTAACTCCTTGCTGGGC | TTGGTGATGCCCAAAATCAGCAT |
| 32-Weeks | 36-Weeks | 40-Weeks | |
|---|---|---|---|
| Hyperglycemic group | 4/8 (50%) | 8/8 (100%) | 6/8 (75%) |
| Non-hyperglycemic group | 0/8 (0%) | 1/8 (12.5%) | 4/8 (50%) |
| No | Mild | Severe | |
|---|---|---|---|
| Hyperglycemic group | 1/24 (4%) | 5/24 (20.8%) | 18/24 (75%) |
| Non-hyperglycemic group | 6/24 (25%) | 18/24 (75%) | 0/24 (0%) |
| Organ | Liver | Liver | Liver | Liver |
|---|---|---|---|---|
| Assessment Item | Steatosis | Lobular Inflammation | Ballooning | Fibrosis |
| Control-M1 | 1 | 2 | 0 | 1 |
| Control-M2 | 1 | 2 | 0 | 1 |
| Control-M3 | 0 | 2 | 0 | 0 |
| Control-M4 | 1 | 2 | 0 | 0 |
| Control-M5 | 1 | 2 | 0 | 0 |
| FOS-M6 | 0 | 2 | 0 | 0 |
| FOS-M7 | 0 | 2 | 0 | 0 |
| FOS-M8 | 0 | 2 | 0 | 1 |
| FOS-M9 | 1 | 2 | 0 | 0 |
| GOS-M10 | 0 | 2 | 0 | 1 |
| GOS-M11 | 0 | 1 | 0 | 0 |
| GOS-M12 | 0 | 0 | 0 | 0 |
| GOS-M13 | 0 | 2 | 0 | 1 |
| LS-M14 | 0 | 0 | 0 | 0 |
| LS-M15 | 0 | 2 | 0 | 1 |
| LS-M16 | 0 | 1 | 0 | 1 |
| LS-M17 | 0 | 2 | 0 | 1 |
| Group | n | No | Mild | Severe |
|---|---|---|---|---|
| Control | 5 | 0 | 4 | 1 |
| FOS | 4 | 1 | 0 | 3 |
| GOS | 4 | 4 | 0 | 0 |
| LS | 4 | 4 | 0 | 0 |
| Acetate | Propionate | Butyrate | Isobutyrate | 2-Methyl Butyrate | Capronate | |
|---|---|---|---|---|---|---|
| (μM) | (μM) | (μM) | (μM) | (μM) | (μM) | |
| Control-M1 | 475.5 | 2.7 | 0.9 | 3.4 | 2.3 | 1.7 |
| Control-M2 | 283.9 | 3.5 | 1.4 | 1.9 | N.D. | 1.2 |
| Control-M3 | 945.3 | 5.5 | 2.4 | 3.7 | N.D. | 3.0 |
| Control-M4 | 589.9 | 5.6 | 2.6 | 2.7 | 1.7 | 2.4 |
| Control-M5 | 889.8 | N.D. | 0.9 | N.D. | N.D. | 3.5 |
| Ave (SD) | 636.9 (279.3) | 4.3 (1.4) | 1.6 (0.8) | 2.9 (0.8) | 2.0 (0.4) | 2.4 (0.9) |
| FOS-M6 | 363.6 | 2.7 | 0.9 | 1.4 | N.D. | 1.6 |
| FOS-M7 | 467.2 | N.D. | 1.5 | 2.0 | N.D. | 2.3 |
| FOS-M8 | 203.3 | 2.6 | 1.1 | 1.7 | 1.2 | 1.3 |
| FOS-M9 | 275.8 | 3.8 | N.D. | 2.3 | N.D. | 1.4 |
| Ave (SD) | 327.5 (113.9) | 3.0 (0.7) | 1.2 (0.3) | 1.9 (0.4) | 1.2 | 1.7 (0.5) |
| GOS-M10 | 286.8 | 4.7 | 1.3 | 1.7 | 1.1 | 1.6 |
| GOS-M11 | 358.7 | 3.1 | 1.3 | 1.1 | 0.6 | 1.8 |
| GOS-M12 | 431.5 | 3.6 | 0.9 | 1.2 | N.D. | 1.0 |
| GOS-M13 | 360.9 | 3.6 | 0.7 | 1.9 | 0.9 | 1.9 |
| Ave (SD) | 359.5 (59.1) | 3.8 (0.7) | 1.0 (0.3) | 1.5 (0.4) | 0.9 (0.3) | 1.6 (0.4) |
| LS-M14 | 417.6 | 4.7 | 0.7 | 2.1 | 1.2 | 1.5 |
| LS-M15 | 655.1 | 6.7 | 2.7 | N.D. | N.D. | 2.5 |
| LS-M16 | 579.0 | 5.6 | 2.3 | 2.3 | N.D. | 2.5 |
| LS-M17 | 425.8 | 4.2 | 1.9 | 2.4 | N.D. | 2.5 |
| Ave (SD) | 519.4 (117) | 5.3 (1.1) | 1.9 (0.9) | 2.3 (0.1) | 1.2 | 2.3 (0.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichimura-Shimizu, M.; Kageyama, T.; Oya, T.; Ogawa, H.; Matsumoto, M.; Sumida, S.; Kakimoto, T.; Miyakami, Y.; Nagatomo, R.; Inoue, K.; et al. Verification of the Impact of Blood Glucose Level on Liver Carcinogenesis and the Efficacy of a Dietary Intervention in a Spontaneous Metabolic Syndrome Model. Int. J. Mol. Sci. 2021, 22, 12844. https://doi.org/10.3390/ijms222312844
Ichimura-Shimizu M, Kageyama T, Oya T, Ogawa H, Matsumoto M, Sumida S, Kakimoto T, Miyakami Y, Nagatomo R, Inoue K, et al. Verification of the Impact of Blood Glucose Level on Liver Carcinogenesis and the Efficacy of a Dietary Intervention in a Spontaneous Metabolic Syndrome Model. International Journal of Molecular Sciences. 2021; 22(23):12844. https://doi.org/10.3390/ijms222312844
Chicago/Turabian StyleIchimura-Shimizu, Mayuko, Takeshi Kageyama, Takeshi Oya, Hirohisa Ogawa, Minoru Matsumoto, Satoshi Sumida, Takumi Kakimoto, Yuko Miyakami, Ryosuke Nagatomo, Koichi Inoue, and et al. 2021. "Verification of the Impact of Blood Glucose Level on Liver Carcinogenesis and the Efficacy of a Dietary Intervention in a Spontaneous Metabolic Syndrome Model" International Journal of Molecular Sciences 22, no. 23: 12844. https://doi.org/10.3390/ijms222312844
APA StyleIchimura-Shimizu, M., Kageyama, T., Oya, T., Ogawa, H., Matsumoto, M., Sumida, S., Kakimoto, T., Miyakami, Y., Nagatomo, R., Inoue, K., Cheng, C., & Tsuneyama, K. (2021). Verification of the Impact of Blood Glucose Level on Liver Carcinogenesis and the Efficacy of a Dietary Intervention in a Spontaneous Metabolic Syndrome Model. International Journal of Molecular Sciences, 22(23), 12844. https://doi.org/10.3390/ijms222312844






