Overlap and Specificity in the Substrate Spectra of Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3
Abstract
:1. Introduction
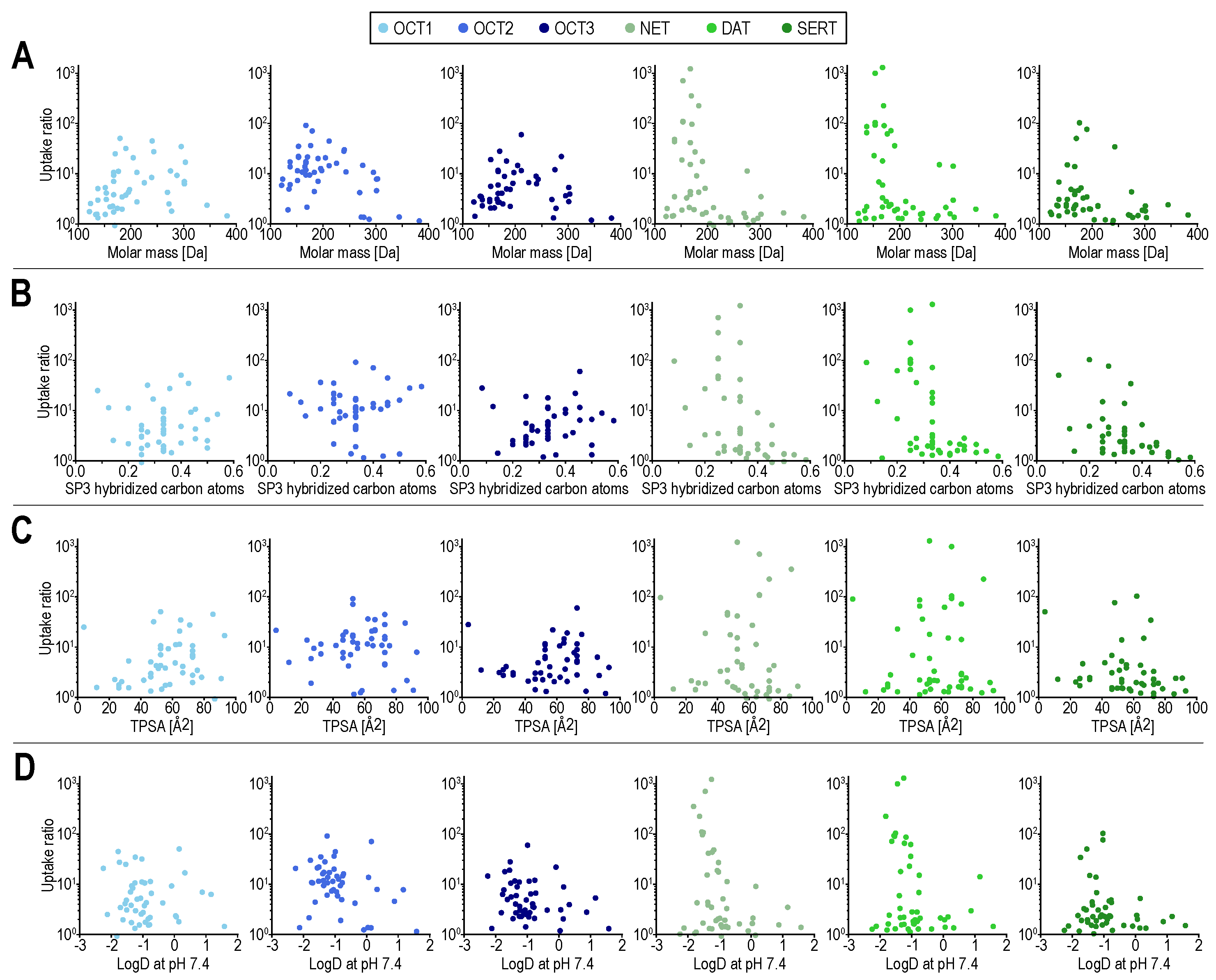
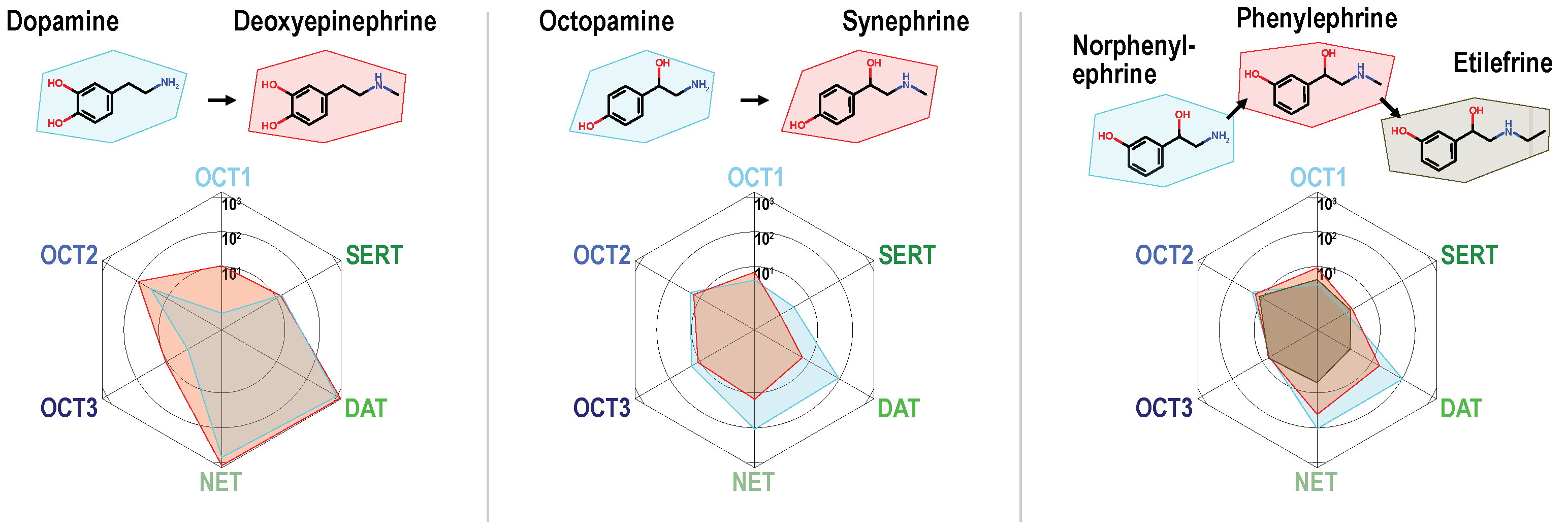
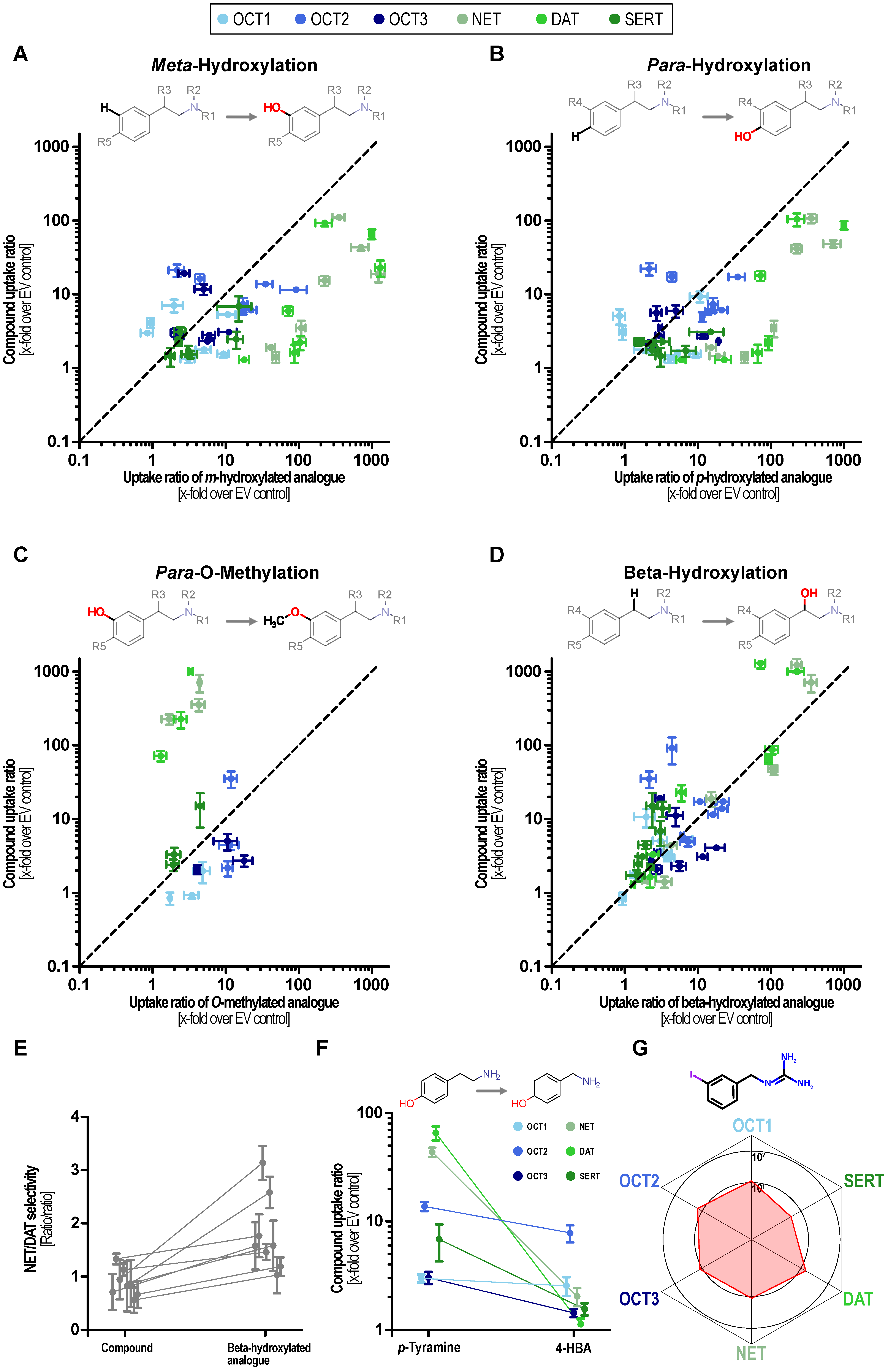
2. Results
3. Discussion
4. Materials and Methods
4.1. Test Compounds
4.2. In Vitro Transport Experiments
4.3. Concentration Analyses
4.4. Calculations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libersat, F.; Pflueger, H.-J. Monoamines and the Orchestration of Behavior. BioScience 2004, 54, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Flügge, G. Regulation of monoamine receptors in the brain: Dynamic changes during stress. Int. Rev. Cytol. 2000, 195, 145–213. [Google Scholar]
- Eisenhofer, G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacol. Ther. 2001, 91, 35–62. [Google Scholar] [CrossRef]
- Iversen, L.L. The uptake of catechol amines at high perfusion concentrations in the rat isolated heart: A novel catechol amine uptake process. Br. J. Pharmacol. Chemother. 1965, 25, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.S.; Andersen, J.; Jorgensen, T.N.; Sorensen, L.; Eriksen, J.; Loland, C.J.; Stromgaard, K.; Gether, U. SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef]
- Gu, H.; Wall, S.C.; Rudnick, G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J. Biol. Chem. 1994, 269, 7124–7130. [Google Scholar] [CrossRef]
- Daws, L.C. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol. Ther. 2009, 121, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Canales, J.J.; Björgvinsson, T.; Thomsen, M.; Qu, H.; Liu, Q.R.; Torres, G.E.; Caine, S.B. Monoamine transporters: Vulnerable and vital doorkeepers. Prog. Mol. Biol. Transl. Sci. 2011, 98, 1–46. [Google Scholar]
- Howell, L.L.; Kimmel, H.L. Monoamine transporters and psychostimulant addiction. Biochem. Pharmacol. 2008, 75, 196–217. [Google Scholar] [CrossRef]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef]
- Seitz, T.; Stalmann, R.; Dalila, N.; Chen, J.; Pojar, S.; Dos Santos Pereira, J.N.; Kratzner, R.; Brockmöller, J.; Tzvetkov, M.V. Global genetic analyses reveal strong inter-ethnic variability in the loss of activity of the organic cation transporter OCT1. Genome Med. 2015, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Busch, A.E.; Karbach, U.; Miska, D.; Gorboulev, V.; Akhoundova, A.; Volk, C.; Arndt, P.; Ulzheimer, J.C.; Sonders, M.S.; Baumann, C.; et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol. Pharmacol. 1998, 54, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, H.; Wang, J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J. Pharmacol. Exp. Ther. 2010, 335, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzvetkov, M.V.; Matthaei, J.; Pojar, S.; Faltraco, F.; Vogler, S.; Prukop, T.; Seitz, T.; Brockmöller, J. Increased Systemic Exposure and Stronger Cardiovascular and Metabolic Adverse Reactions to Fenoterol in Individuals with Heritable OCT1 Deficiency. Clin. Pharmacol. Ther. 2018, 103, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, J.; Seitz, T.; Jensen, O.; Tann, A.; Prukop, T.; Tadjerpisheh, S.; Brockmöller, J.; Tzvetkov, M.V. OCT1 Deficiency Affects Hepatocellular Concentrations and Pharmacokinetics of Cycloguanil, the Active Metabolite of the Antimalarial Drug Proguanil. Clin. Pharmacol. Ther. 2019, 105, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Tzvetkov, M.V.; Saadatmand, A.R.; Lötsch, J.; Tegeder, I.; Stingl, J.C.; Brockmöller, J. Genetically polymorphic OCT1: Another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opioidergic drug tramadol. Clin. Pharmacol. Ther. 2011, 90, 143–150. [Google Scholar] [CrossRef]
- Bönisch, H. Extraneuronal transport of catecholamines. Pharmacology 1980, 21, 93–108. [Google Scholar]
- Gasser, P.J. Organic Cation Transporters in Brain Catecholamine Homeostasis. Handb. Exp. Pharmacol. 2021, 266, 187–197. [Google Scholar]
- Bacq, A.; Balasse, L.; Biala, G.; Guiard, B.; Gardier, A.M.; Schinkel, A.; Louis, F.; Vialou, V.; Martres, M.P.; Chevarin, C.; et al. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol. Psychiatry 2012, 17, 926–939. [Google Scholar] [CrossRef] [Green Version]
- Vialou, V.; Balasse, L.; Callebert, J.; Launay, J.M.; Giros, B.; Gautron, S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J. Neurochem. 2008, 106, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Inazu, M.; Takeda, H.; Matsumiya, T. Expression and functional characterization of the extraneuronal monoamine transporter in normal human astrocytes. J. Neurochem. 2003, 84, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kekuda, R.; Huang, W.; Fei, Y.J.; Leibach, F.H.; Chen, J.; Conway, S.J.; Ganapathy, V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J. Biol. Chem. 1998, 273, 32776–32786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, E.C.; Khuri, N.; Liang, X.; Stecula, A.; Chien, H.C.; Yee, S.W.; Huang, Y.; Sali, A.; Giacomini, K.M. Discovery of Competitive and Noncompetitive Ligands of the Organic Cation Transporter 1 (OCT1; SLC22A1). J. Med. Chem. 2017, 60, 2685–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlin, G.; Karlsson, J.; Pedersen, J.M.; Gustavsson, L.; Larsson, R.; Matsson, P.; Norinder, U.; Bergström, C.A.; Artursson, P. Structural requirements for drug inhibition of the liver specific human organic cation transport protein 1. J. Med. Chem. 2008, 51, 5932–5942. [Google Scholar] [CrossRef]
- Sandoval, P.J.; Zorn, K.M.; Clark, A.M.; Ekins, S.; Wright, S.H. Assessment of Substrate-Dependent Ligand Interactions at the Organic Cation Transporter OCT2 Using Six Model Substrates. Mol. Pharmacol. 2018, 94, 1057–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgen, A.S.; Iversen, L.L. The inhibition of noradrenaline uptake by sympathomimetic amines in the rat isolated heart. Br. J. Pharmacol. Chemother. 1965, 25, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Rothman, R.B.; Baumann, M.H. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003, 479, 23–40. [Google Scholar] [CrossRef]
- Eildal, J.N.N.; Andersen, J.; Kristensen, A.S.; Jørgensen, A.M.; Bang-Andersen, B.; Jørgensen, M.; Strømgaard, K. From the Selective Serotonin Transporter Inhibitor Citalopram to the Selective Norepinephrine Transporter Inhibitor Talopram: Synthesis and Structure−Activity Relationship Studies. J. Med. Chem. 2008, 51, 3045–3048. [Google Scholar] [CrossRef]
- Niello, M.; Cintulová, D.; Raithmayr, P.; Holy, M.; Jäntsch, K.; Colas, C.; Ecker, G.F.; Sitte, H.H.; Mihovilovic, M.D. Effects of Hydroxylated Mephedrone Metabolites on Monoamine Transporter Activity In Vitro. Front. Pharmacol. 2021, 12, 654061. [Google Scholar] [CrossRef]
- Dessalew, N. QSAR study on dual SET and NET reuptake inhibitors: An insight into the structural requirement for antidepressant activity. J. Enzym. Inhib. Med. Chem. 2009, 24, 262–271. [Google Scholar] [CrossRef]
- Wang, P.; Jing, C.; Yu, P.; Lu, M.; Xu, X.; Pei, Q.; Yan, F. Profiling the structural determinants of aminoketone derivatives as hNET and hDAT reuptake inhibitors by field-based QSAR based on molecular docking. Technol. Health Care: Off. J. Eur. Soc. Eng. Med. 2021, 29, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Reith, M.E.; Blough, B.E.; Hong, W.C.; Jones, K.T.; Schmitt, K.C.; Baumann, M.H.; Partilla, J.S.; Rothman, R.B.; Katz, J.L. Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug Alcohol. Depend. 2015, 147, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Seddik, A.; Holy, M.; Weissensteiner, R.; Zdrazil, B.; Sitte, H.H.; Ecker, G.F. Probing the Selectivity of Monoamine Transporter Substrates by Means of Molecular Modeling. Mol. Inform. 2013, 32, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, M.H.; Ayestas, M.A., Jr.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Burnette, W.B.; Bailey, M.D.; Kukoyi, S.; Blakely, R.D.; Trowbridge, C.G.; Justice, J.B., Jr. Human norepinephrine transporter kinetics using rotating disk electrode voltammetry. Anal. Chem. 1996, 68, 2932–2938. [Google Scholar] [CrossRef] [PubMed]
- Solis, E., Jr. Electrophysiological Actions of Synthetic Cathinones on Monoamine Transporters. Curr. Top. Behav. Neurosci. 2017, 32, 73–92. [Google Scholar] [PubMed]
- Taubert, D.; Grimberg, G.; Stenzel, W.; Schömig, E. Identification of the endogenous key substrates of the human organic cation transporter OCT2 and their implication in function of dopaminergic neurons. PLoS ONE 2007, 2, e385. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Kudo, T.; Lapa, C.; Buck, A.; Higuchi, T. Recent advances in radiotracers targeting norepinephrine transporter: Structural development and radiolabeling improvements. J. Neural Transm. 2020, 127, 851–873. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, C.; Jordan, J. Norepinephrine transporter function and human cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1273–H1282. [Google Scholar] [CrossRef] [Green Version]
- Pandit-Taskar, N.; Modak, S. Norepinephrine Transporter as a Target for Imaging and Therapy. J. Nucl. Med.: Off. Publ. Soc. Nucl. Med. 2017, 58 (Suppl. 2), 39s–53s. [Google Scholar] [CrossRef] [Green Version]
- López Quiñones, A.J.; Wagner, D.J.; Wang, J. Characterization of Meta-Iodobenzylguanidine (mIBG) Transport by Polyspecific Organic Cation Transporters: Implication for mIBG Therapy. Mol. Pharmacol. 2020, 98, 109–119. [Google Scholar] [CrossRef]
- Jensen, O.; Brockmöller, J.; Dücker, C. Identification of Novel High-Affinity Substrates of OCT1 Using Machine Learning-Guided Virtual Screening and Experimental Validation. J. Med. Chem. 2021, 64, 2762–2776. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Rafehi, M.; Gebauer, L.; Brockmöller, J. Cellular Uptake of Psychostimulants—Are High- and Low-Affinity Organic Cation Transporters Drug Traffickers? Front. Pharmacol. 2021, 11, 609811. [Google Scholar] [CrossRef]
- Koepsell, H. General Overview of Organic Cation Transporters in Brain. Handb. Exp. Pharmacol. 2021, 266, 1–39. [Google Scholar] [PubMed]
- Jha, P.; Ragnarsson, L.; Lewis, R.J. Structure-Function of the High Affinity Substrate Binding Site (S1) of Human Norepinephrine Transporter. Front. Pharmacol. 2020, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Yang, D.; Zhao, Z.; Wen, P.C.; Yoshioka, C.; Tajkhorshid, E.; Gouaux, E. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 2019, 569, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiergerd, S.M.; Schenk, J.O. Striatal transporter for dopamine: Catechol structure-activity studies and susceptibility to chemical modification. J. Neurochem. 1994, 62, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of compounds in preworkout supplements (“boosters”). Eur. J. Pharmacol. 2019, 859, 172515. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011, 116, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Burchett, S.A.; Hicks, T.P. The mysterious trace amines: Protean neuromodulators of synaptic transmission in mammalian brain. Prog. Neurobiol. 2006, 79, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D.; Hart, S.; Pryor, A.R.; Hunter, S.; Gardiner, D. Pharmacological characterization of a high-affinity p-tyramine transporter in rat brain synaptosomes. Sci. Rep. 2016, 6, 38006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Vallender, E.J.; Yu, N.; Kirstein, S.L.; Yang, H.; Bahn, M.E.; Westmoreland, S.V.; Miller, G.M. Cloning, expression, and functional analysis of rhesus monkey trace amine-associated receptor 6: Evidence for lack of monoaminergic association. J. Neurosci. Res. 2008, 86, 3435–3446. [Google Scholar] [CrossRef] [Green Version]
- Cichero, E.; Espinoza, S.; Gainetdinov, R.R.; Brasili, L.; Fossa, P. Insights into the structure and pharmacology of the human trace amine-associated receptor 1 (hTAAR1): Homology modelling and docking studies. Chem. Biol. Drug Des. 2013, 81, 509–516. [Google Scholar] [CrossRef]
- Cichero, E.; Espinoza, S.; Franchini, S.; Guariento, S.; Brasili, L.; Gainetdinov, R.R.; Fossa, P. Further insights into the pharmacology of the human trace amine-associated receptors: Discovery of novel ligands for TAAR1 by a virtual screening approach. Chem. Biol. Drug Des. 2014, 84, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Wanpen, S.; Govitrapong, P.; Shavali, S.; Sangchot, P.; Ebadi, M. Salsolinol, a dopamine-derived tetrahydroisoquinoline, induces cell death by causing oxidative stress in dopaminergic SH-SY5Y cells, and the said effect is attenuated by metallothionein. Brain Res. 2004, 1005, 67–76. [Google Scholar] [CrossRef]
- Bruhn, J.G.; Lundström, J. Alkaloids of Carnegiea gigantea. Arizonine, a new tetrahydroisoquinoline alkaloid. Lloydia 1976, 39, 197–203. [Google Scholar]
- Inwang, E.E.; Mosnaim, A.D.; Sabelli, H.C. Isolation and characterization of phenylethylamine and phenylethanolamine from human brain. J. Neurochem. 1973, 20, 1469–1473. [Google Scholar] [CrossRef]
- Clement, B.A.; Goff, C.M.; Forbes, T.D.A. Toxic amines and alkaloids from acacia rigidula. Phytochemistry 1998, 49, 1377–1380. [Google Scholar] [CrossRef]
- Bush, L.P.; Jeffreys, J.A. Isolation and separation of tall fescue and ryegrass alkaloids. J. Chromatogr. 1975, 111, 165–170. [Google Scholar] [CrossRef]
- Lovett, J.V.; Hoult, A.H.; Christen, O. Biologically active secondary metabolites of barley. IV. Hordenine production by different barley lines. J. Chem. Ecol. 1994, 20, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Camp, B.J.; Lyman, C.M. The isolation of N-methyl beta-phenylethylamine from Acacia berlandieri. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1956, 45, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.L.; Gödecke, T.; Nikolic, D.; Chen, S.N.; Ahn, S.; Dietz, B.; Farnsworth, N.R.; van Breemen, R.B.; Lankin, D.C.; Pauli, G.F.; et al. In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent. J. Agric. Food Chem. 2008, 56, 11718–11726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servillo, L.; Giovane, A.; Balestrieri, M.L.; Casale, R.; Cautela, D.; Castaldo, D. Citrus genus plants contain N-methylated tryptamine derivatives and their 5-hydroxylated forms. J. Agric. Food Chem. 2013, 61, 5156–5162. [Google Scholar] [CrossRef]
- Erspamer, V. Identification of octopamine as l-p-hydroxyphenylethanolamine. Nature 1952, 169, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Tao, L.; Luo, X.; Ding, L.; Guo, M.; Nie, L.; Yao, S. Determination of octopamine, synephrine and tyramine in Citrus herbs by ionic liquid improved ‘green’ chromatography. J. Chromatogr. A 2006, 1125, 182–188. [Google Scholar] [CrossRef]
- Linares, D.M.; Martín, M.C.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic amines in dairy products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef]
- Melzig, M.F.; Putscher, I.; Henklein, P.; Haber, H. In vitro pharmacological activity of the tetrahydroisoquinoline salsolinol present in products from Theobroma cacao L. like cocoa and chocolate. J. Ethnopharmacol. 2000, 73, 153–159. [Google Scholar] [CrossRef]
- Stewart, I.; Newhall, W.; Edwards, G. The Isolation and Identification of l-Synephrine in the Leaves and Fruit of Citrus. J. Biol. Chem. 1964, 239, 930–932. [Google Scholar] [CrossRef]
- Tittarelli, R.; Mannocchi, G.; Pantano, F.; Romolo, F.S. Recreational use, analysis and toxicity of tryptamines. Curr. Neuropharmacol. 2015, 13, 26–46. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785.e5. [Google Scholar] [CrossRef] [Green Version]
- Dyck, L.E.; Juorio, A.V.; Boulton, A.A. The in vitro release of endogenous m-tyramine, p-tyramine and dopamine from rat striatum. Neurochem. Res. 1982, 7, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Ladero, V.; Fernández, M.; Alvarez, M.A. Isolation and identification of tyramine-producing enterococci from human fecal samples. Can. J. Microbiol. 2009, 55, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, M.; Faltraco, F.; Matthaei, J.; Prukop, T.; Jensen, O.; Grytzmann, A.; Blome, F.G.; Berger, R.G.; Krings, U.; Vormfelde, S.V.; et al. Highly Variable Pharmacokinetics of Tyramine in Humans and Polymorphisms in OCT1, CYP2D6, and MAO-A. Front. Pharmacol. 2019, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Quach, A.; Ji, L.; Mishra, V.; Sznewajs, A.; Veatch, J.; Huberty, J.; Franc, B.; Sposto, R.; Groshen, S.; Wei, D.; et al. Thyroid and hepatic function after high-dose 131 I-metaiodobenzylguanidine (131 I-MIBG) therapy for neuroblastoma. Pediatric Blood Cancer 2011, 56, 191–201. [Google Scholar] [CrossRef] [Green Version]
- DuBois, S.G.; Messina, J.; Maris, J.M.; Huberty, J.; Glidden, D.V.; Veatch, J.; Charron, M.; Hawkins, R.; Matthay, K.K. Hematologic Toxicity of High-Dose Iodine-131–Metaiodobenzylguanidine Therapy for Advanced Neuroblastoma. J. Clin. Oncol. 2004, 22, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Ameling, S.; Noutsias, M.; Köck, K.; Triebel, I.; Bonitz, K.; Meissner, K.; Jedlitschky, G.; Herda, L.R.; Reinthaler, M.; et al. Selective regulation of cardiac organic cation transporter novel type 2 (OCTN2) in dilated cardiomyopathy. Am. J. Pathol. 2011, 178, 2547–2559. [Google Scholar] [CrossRef] [Green Version]
- Zwart, R.; Verhaagh, S.; Buitelaar, M.; Popp-Snijders, C.; Barlow, D.P. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol. Cell. Biol. 2001, 21, 4188–4196. [Google Scholar] [CrossRef] [Green Version]
- Bayer, M.; Kuçi, Z.; Schömig, E.; Gründemann, D.; Dittmann, H.; Handgretinger, R.; Bruchelt, G. Uptake of mIBG and catecholamines in noradrenaline- and organic cation transporter-expressing cells: Potential use of corticosterone for a preferred uptake in neuroblastoma- and pheochromocytoma cells. Nucl. Med. Biol. 2009, 36, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, J.; Kuron, D.; Faltraco, F.; Knoch, T.; Dos Santos Pereira, J.N.; Abu Abed, M.; Prukop, T.; Brockmöller, J.; Tzvetkov, M.V. OCT1 mediates hepatic uptake of sumatriptan and loss-of-function OCT1 polymorphisms affect sumatriptan pharmacokinetics. Clin. Pharmacol. Ther. 2016, 99, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, A.R.; Tadjerpisheh, S.; Brockmöller, J.; Tzvetkov, M.V. The prototypic pharmacogenetic drug debrisoquine is a substrate of the genetically polymorphic organic cation transporter OCT1. Biochem. Pharmacol. 2012, 83, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Brockmöller, J.; Seitz, T.; König, J.; Tzvetkov, M.V.; Chen, X. Tropane alkaloids as substrates and inhibitors of human organic cation transporters of the SLC22 (OCT) and the SLC47 (MATE) families. Biol. Chem. 2017, 398, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]

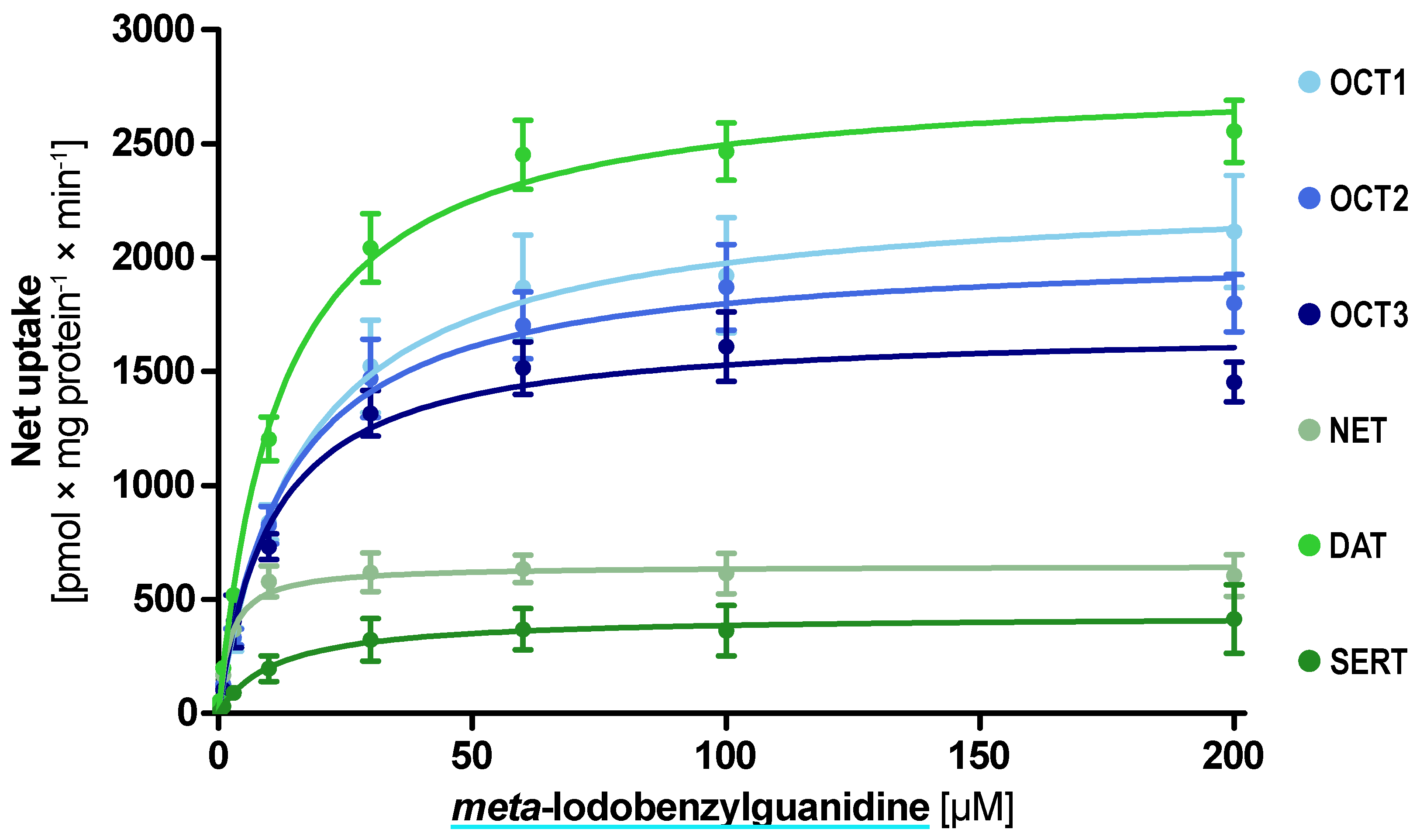
| Transporter | Uptake Ratio [x-Fold over EV Control] | Km ± SEM [µM] | Vmax ± SEM [pmol × mg Protein−1 × min−1] | Clint ± SEM [mL × g Protein−1 × min−1] |
|---|---|---|---|---|
| OCT1 | 11.4 ± 2.2 | 16.6 ± 3.7 | 2301 ± 129 | 138 ± 38.5 |
| OCT2 | 14.7 ± 1.6 | 13.3 ± 2.4 | 2039 ± 86.9 | 152 ± 34.1 |
| OCT3 | 12.0 ± 1.0 | 10.5 ± 1.9 | 1689 ± 68.0 | 161 ± 35.7 |
| NET | 11.3 ± 2.1 | 2.27 ± 0.57 | 648 ± 28.2 | 284.6 ± 84.2 |
| DAT | 15.1 ± 3.0 | 12.2 ± 1.46 | 2800 ± 77.2 | 229 ± 33.8 |
| SERT | 4.3 ± 1.0 | 11.2 ± 6.2 | 429 ± 53.4 | 38.2 ± 25.9 |
| Name | Endogenous | Microbiom | Nutrition | Plants | Example and References |
|---|---|---|---|---|---|
| 3-Methoxy p-tyramine | x | x | Dopamine metabolite Cactus [58] | ||
| Bisnorephedrine | x | Trace amine neuromodulator [59] | |||
| Deoxyepinephrine | x | Acacia species [60] | |||
| Halostachine | x | Perennial ryegrass, tall fescue [61] | |||
| Hordenine | x | x | Barley, beer [62] Acacia species [60] | ||
| N-Methyl phenylethylamine | x | x | Trace amine neuromodulator Acacia species [63] | ||
| N-Methyl serotonin | x | Black cohosh [64] | |||
| N-Methyl tryptamine | x | x | Citrus plants [65] | ||
| N-Methyl p-tyramine | x | x | Acacica speciest [60] | ||
| Octopamine | x | x | x | Trace amine neuromodulator [66] Citrus herbs [67] | |
| Phenylethylamine | x | x | Cheese [68] | ||
| Salsolinol | x | x | x | Dopamine intermediate [57] Cocao, chochlate [69] | |
| Synephrine | x | x | Various Citrus trees [70] | ||
| Tryptamine | x | x | x | Trace amine neuromodulator [71] Commensual bacteria [72], Cheese [68] | |
| m-Tyramine | x | Trace amine neuromodulator [73] | |||
| p-Tyramine | x | x | x | Enterococcus species [74], cheese [68] Citrus herbs [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebauer, L.; Jensen, O.; Neif, M.; Brockmöller, J.; Dücker, C. Overlap and Specificity in the Substrate Spectra of Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3. Int. J. Mol. Sci. 2021, 22, 12816. https://doi.org/10.3390/ijms222312816
Gebauer L, Jensen O, Neif M, Brockmöller J, Dücker C. Overlap and Specificity in the Substrate Spectra of Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3. International Journal of Molecular Sciences. 2021; 22(23):12816. https://doi.org/10.3390/ijms222312816
Chicago/Turabian StyleGebauer, Lukas, Ole Jensen, Maria Neif, Jürgen Brockmöller, and Christof Dücker. 2021. "Overlap and Specificity in the Substrate Spectra of Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3" International Journal of Molecular Sciences 22, no. 23: 12816. https://doi.org/10.3390/ijms222312816
APA StyleGebauer, L., Jensen, O., Neif, M., Brockmöller, J., & Dücker, C. (2021). Overlap and Specificity in the Substrate Spectra of Human Monoamine Transporters and Organic Cation Transporters 1, 2, and 3. International Journal of Molecular Sciences, 22(23), 12816. https://doi.org/10.3390/ijms222312816