Rare Glutamic Acid Methyl Ester Peptaibols from Sepedonium ampullosporum Damon KSH 534 Exhibit Promising Antifungal and Anticancer Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Compounds 1–7
2.2. In Situ Chemical Analysis
2.3. Solid Phase Synthesis and Absolute Configuration of Ampullosporin F (1) and G (2)
2.4. Evaluation of Antifungal Activities
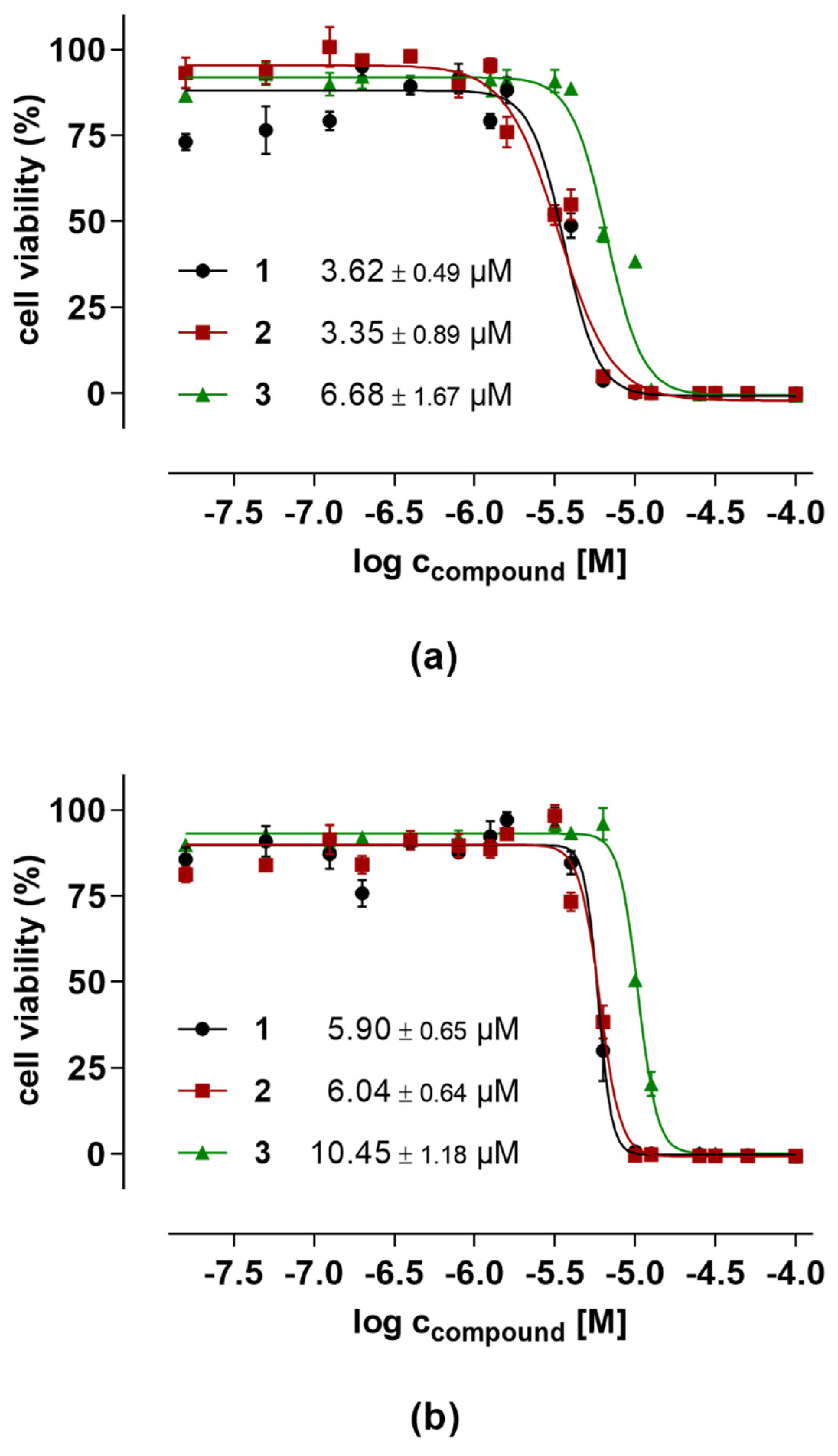
2.5. Evaluation of Anticancer Activities
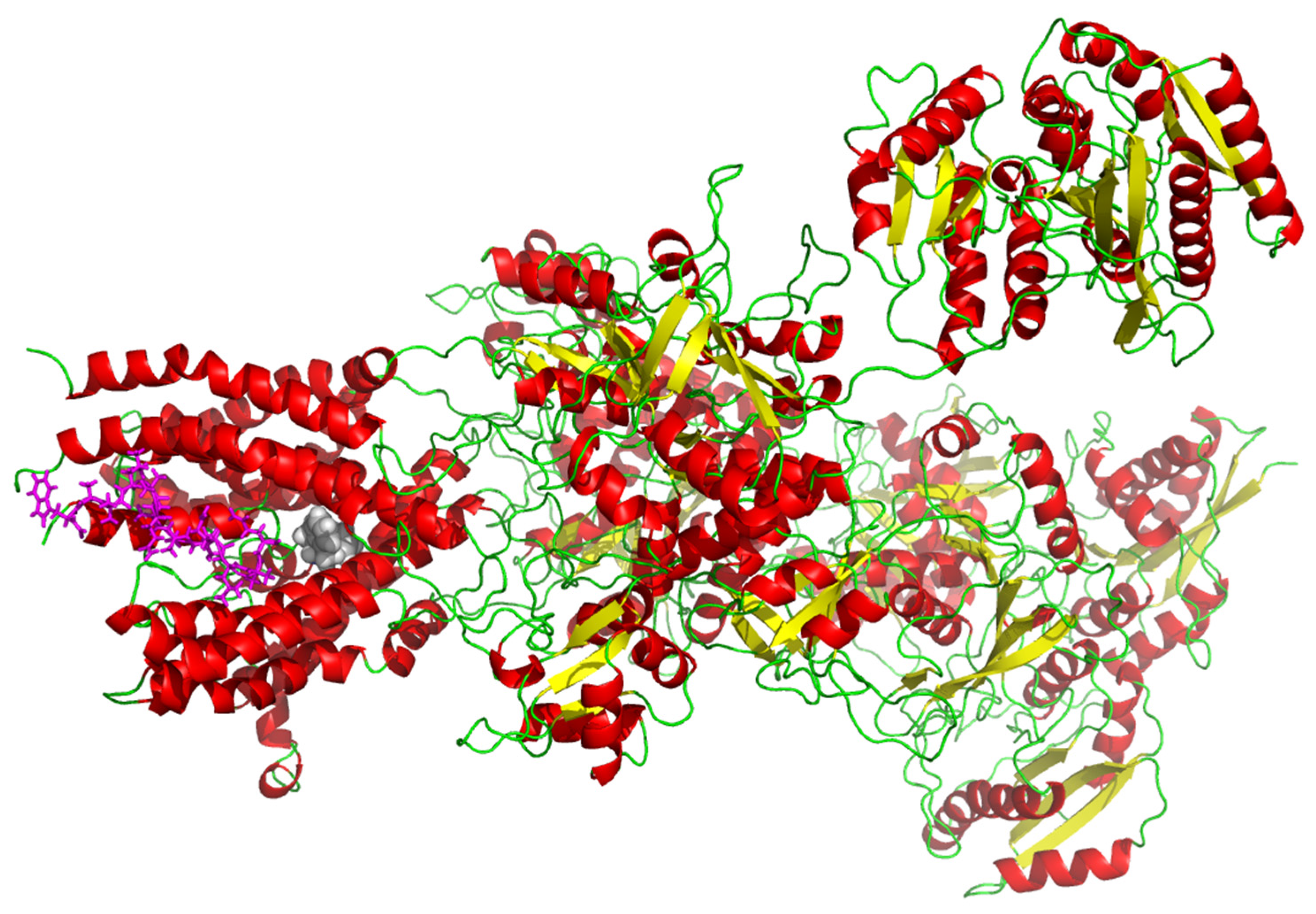
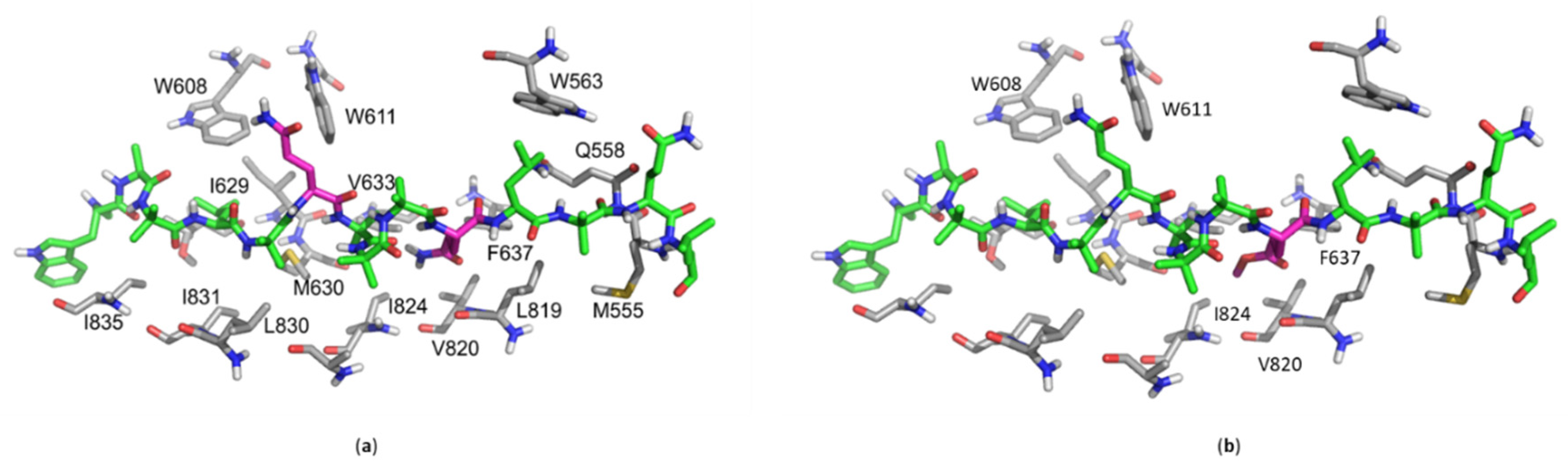
2.6. In Silico Molecular Docking
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Strain and Cultivation
3.3. Extraction and Isolation
3.4. Sample Preparation for LC-MS Screening
3.5. Solid-Phase Peptide Synthesis
3.6. Antifungal Assay
3.7. In Vitro Cell Proliferation Assay—Anticancer Activity
3.8. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degenkolb, T.; Brückner, H. Peptaibiomics: Towards a Myriad of Bioactive Peptides Containing Cα-Dialkylamino Acids? Chem. Biodivers. 2008, 5, 1817–1843. [Google Scholar] [CrossRef] [PubMed]
- Ayers, S.; Ehrmann, B.M.; Adcock, A.F.; Kroll, D.J.; Carcache de Blanco, E.J.; Shen, Q.; Swanson, S.M.; Falkinham, J.O., III; Wani, M.C.; Mitchell, S.M.; et al. Peptaibols from two unidentified fungi of the order Hypocreales with cytotoxic, antibiotic, and anthelmintic activities. J. Pept. Sci. 2012, 18, 500–510. [Google Scholar] [CrossRef]
- Carroux, A.; Van Bohemen, A.I.; Roullier, C.; Robiou du Pont, T.; Vansteelandt, M.; Bondon, A.; Zalouk-Vergnoux, A.; Pouchus, Y.F.; Ruiz, N. Unprecedented 17-residue peptaibiotics produced by marine-derived Trichoderma atroviride. Chem. Biodivers. 2013, 10, 772–786. [Google Scholar] [CrossRef]
- Liu, D.; Lin, H.; Proksch, P.; Tang, X.; Shao, Z.; Lin, W. Microbacterins A and B, new peptaibols from the deep sea actinomycete Microbacterium sediminis sp. nov. YLB-01(T). Org. Lett. 2015, 17, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Neumann, N.K.; Stoppacher, N.; Zeilinger, S.; Degenkolb, T.; Bruckner, H.; Schuhmacher, R. The peptaibiotics database—A comprehensive online resource. Chem. Biodivers. 2015, 12, 743–751. [Google Scholar] [CrossRef]
- Mohamed-Benkada, M.; Francois Pouchus, Y.; Verite, P.; Pagniez, F.; Caroff, N.; Ruiz, N. Identification and biological activities of long-chain peptaibols produced by a marine-derived strain of Trichoderma longibrachiatum. Chem. Biodivers. 2016, 13, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Risinger, A.L.; Mitchell, C.A.; You, J.; Stamps, B.W.; Pan, N.; King, J.B.; Bopassa, J.C.; Judge, S.I.V.; Yang, Z.; et al. Unique amalgamation of primary and secondary structural elements transform peptaibols into potent bioactive cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2017, 114, E8957–E8966. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chavez, J.; Raja, H.A.; Graf, T.N.; Gallagher, J.M.; Metri, P.; Xue, D.; Pearce, C.J.; Oberlies, N.H. Prealamethicin F50 and related peptaibols from Trichoderma arundinaceum: Validation of their authenticity via in situ chemical analysis. RSC Adv. 2017, 7, 45733–45751. [Google Scholar] [CrossRef] [PubMed]
- Sica, V.P.; Rees, E.R.; Raja, H.A.; Rivera-Chavez, J.; Burdette, J.E.; Pearce, C.J.; Oberlies, N.H. In situ mass spectrometry monitoring of fungal cultures led to the identification of four peptaibols with a rare threonine residue. Phytochemistry 2017, 143, 45–53. [Google Scholar] [CrossRef] [PubMed]
- van Bohemen, A.I.; Ruiz, N.; Zalouk-Vergnoux, A.; Michaud, A.; Robiou du Pont, T.; Druzhinina, I.; Atanasova, L.; Prado, S.; Bodo, B.; Meslet-Cladiere, L.; et al. Pentadecaibins I-V: 15-Residue Peptaibols produced by a marine-derived Trichoderma sp. of the Harzianum clade. J. Nat. Prod. 2021, 84, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Dentinger, B.T.M.; Nielson, J.R.; Peterson, R.T.; Winter, J.M. Emerimicins V-X, 15-Residue Peptaibols discovered from an Acremonium sp. through integrated genomic and chemical approaches. J. Nat. Prod. 2021, 84, 1113–1126. [Google Scholar] [CrossRef]
- Kimonyo, A.; Bruckner, H. Sequences of metanicins, 20-residue peptaibols from the ascomycetous fungus CBS 597.80. Chem. Biodivers. 2013, 10, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Panizel, I.; Yarden, O.; Ilan, M.; Carmeli, S. Eight new peptaibols from sponge-associated Trichoderma atroviride. Mar. Drugs 2013, 11, 4937–4960. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Mine, K.; Akiyama, K.; Ohki, S.; Hayashi, H. Anti-plant viral activity of peptaibols, trichorzins HA II, HA V, and HA VI, isolated from Trichoderma harzianum HK-61. J. Pestic. Sci. 2018, 43, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.S.S.; Müller, H.; Henkel, T.; Lagojda, A.; Kleymann, G. New antiviral peptaibols from the mycoparasitic fungus Sepedonium microspermum. In Book of Abstracts, 13; Irseer Naturstofftage der DECHEMA: Irsee, Germany, 2001. [Google Scholar]
- Fragiadaki, I.; Katogiritis, A.; Calogeropoulou, T.; Bruckner, H.; Scoulica, E. Synergistic combination of alkylphosphocholines with peptaibols in targeting Leishmania infantum in vitro. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Laub, A.; Porzel, A.; Schmidt, J.; Wessjohann, L.; Westermann, B.; Arnold, N. Isolation and total synthesis of Albupeptins A–D: 11-Residue peptaibols from the fungus Gliocladium album. Eur. J. Org. Chem. 2015, 34, 7449–7459. [Google Scholar] [CrossRef]
- Otto, A.; Laub, A.; Haid, M.; Porzel, A.; Schmidt, J.; Wessjohann, L.; Arnold, N. Tulasporins A–D, 19-Residue peptaibols from the mycoparasitic fungus Sepedonium tulasneanum. Nat. Prod. Commun. 2016, 11, 1821–1824. [Google Scholar] [CrossRef]
- Otto, A.; Laub, A.; Wendt, L.; Porzel, A.; Schmidt, J.; Palfner, G.; Becerra, J.; Kruger, D.; Stadler, M.; Wessjohann, L.; et al. Chilenopeptins A and B, Peptaibols from the Chilean Sepedonium aff. chalcipori KSH 883. J. Nat. Prod. 2016, 79, 929–938. [Google Scholar] [CrossRef]
- Shi, W.L.; Chen, X.L.; Wang, L.X.; Gong, Z.T.; Li, S.; Li, C.L.; Xie, B.B.; Zhang, W.; Shi, M.; Li, C.; et al. Cellular and molecular insight into the inhibition of primary root growth of Arabidopsis induced by peptaibols, a class of linear peptide antibiotics mainly produced by Trichoderma spp. J. Exp. Bot. 2016, 67, 2191–2205. [Google Scholar] [CrossRef]
- Ritzau, M.; Heinze, S.; Dornberger, K.; Berg, A.; Fleck, W.; Schlegel, B.; Hartl, A.; Grafe, U. Ampullosporin, a new peptaibol-type antibiotic from Sepedonium ampullosporum HKI-0053 with neuroleptic activity in mice. J. Antibiot. 1997, 50, 722–728. [Google Scholar] [CrossRef]
- Kronen, M.; Kleinwachter, P.; Schlegel, B.; Hartl, A.; Grafe, U. Ampullosporines B,C,D,E1,E2,E3 and E4 from Sepedonium ampullosporum HKI-0053: Structures and biological activities. J. Antibiot. 2001, 54, 175–178. [Google Scholar] [CrossRef]
- Berek, I.; Becker, A.; Schroder, H.; Hartl, A.; Hollt, V.; Grecksch, G. Ampullosporin A, a peptaibol from Sepedonium ampullosporum HKI-0053 with neuroleptic-like activity. Behav. Brain Res. 2009, 203, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Speckbacher, V.; Zeilinger, S. Secondary Metabolites of Mycoparasitic Fungi. In Secondary Metabolites-Sources and Applications, 1st ed.; Vijayakumar, R., Raja, S., Eds.; Intechopen: London, UK, 2018; pp. 37–55. [Google Scholar]
- Milov, A.D.; Tsvetkov, Y.D.; Raap, J.; De Zotti, M.; Formaggio, F.; Toniolo, C. Conformation, self-aggregation, and membrane interaction of peptaibols as studied by pulsed electron double resonance spectroscopy. Biopolymers 2016, 106, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Sahr, T.A.H.; Besl, H.; Fischer, M. Infrageneric classification of the boleticolous genus Sepedonium: Species delimitation and phylogenetic relationships. Mycologia 1999, 91, 935–943. [Google Scholar] [CrossRef]
- Divekar, P.V.; Vining, L.C. Reaction of anhydrosepedonin with alkali synthesis of a degradation product and some related dimethylhydroxybenzoic acids. Can. J. Chem. 1964, 42, 63–68. [Google Scholar] [CrossRef]
- Divekar, P.V.; Raistrick, H.; Dobson, T.A.; Vining, L.C. Studies in the biochemistry of microorganisms part 117. Sepedonin, a tropolone metabolite of Sepedonium chrysospermum Fries. Can. J. Chem. 1965, 43, 1835–1848. [Google Scholar] [CrossRef]
- Shibata, S.; Shoji, J.; Ohta, A.; Watanabe, M. Metabolic products of fungi. XI. Some observation on the occurrence of skyrin and rugulosin in mold metabolites, with a reference to structural relationship between penicilliopsin and skyrin. Pharm. Bull 1957, 5, 380–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quang, D.N.; Schmidt, J.; Porzel, A.; Wessjohann, L.; Haid, M.; Arnold, N. Ampullosine, a new isoquinoline alkaloid from Sepedonium ampullosporum (Ascomycetes). Nat. Prod. Commun. 2010, 5, 869–872. [Google Scholar] [CrossRef]
- Closse, A.; Hauser, D. Isolierung und Konstitutionsermittlung von Chrysodin. Helv. Chim. Acta. 1973, 56, 2694–2698. [Google Scholar] [CrossRef]
- Mitova, M.I.; Stuart, B.G.; Cao, G.H.; Blunt, J.W.; Cole, A.L.; Munro, M.H. Chrysosporide, a cyclic pentapeptide from a New Zealand sample of the fungus Sepedonium chrysospermum. J. Nat. Prod. 2006, 69, 1481–1484. [Google Scholar] [CrossRef]
- Laub, A.; Lam, Y.T.H.; Mendez, Y.; Vidal, A.V.; Porzel, A.; Schmidt, J.; Wessjohann, L.A.; Westermann, B.; Arnold, N. Identification and total synthesis of two new cyclic pentapeptides from Sepedonium microspermum Besl. Manuscript in preparation.
- Dornberger, K.; Ihn, W.; Ritzau, M.; Grafe, U.; Schlegel, B.; Fleck, W.F.; Metzger, J.W. Chrysospermins, new peptaibol antibiotics from Apiocrea chrysosperma Ap101. J. Antibiot. 1995, 48, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Hulsmann, H.; Heinze, S.; Ritzau, M.; Schlegel, B.; Grafe, U. Isolation and structure of peptaibolin, a new peptaibol from Sepedonium strains. J. Antibiot. 1998, 51, 1055–1058. [Google Scholar] [CrossRef][Green Version]
- Neuhof, T.; Berg, A.; Besl, H.; Schwecke, T.; Dieckmann, R.; von Dohren, H. Peptaibol production by Sepedonium strains parasitizing Boletales. Chem. Biodivers. 2007, 4, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Mitova, M.I.; Murphy, A.C.; Lang, G.; Blunt, J.W.; Cole, A.L.; Ellis, G.; Munro, M.H. Evolving trends in the dereplication of natural product extracts. 2. The isolation of chrysaibol, an antibiotic peptaibol from a New Zealand sample of the mycoparasitic fungus Sepedonium chrysospermum. J. Nat. Prod. 2008, 71, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Amemiya, M.; Sawa, R.; Kubota, Y.; Kunisada, T.; Momose, I.; Kawada, M.; Shibasaki, M. Acremopeptin, a new peptaibol from Acremonium sp. PF1450. J. Antibiot. 2017, 70, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; McGaw, L.J. Natural cyclic peptides as an attractive modality for therapeutics: A mini review. Molecules 2018, 23, 2080. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.H.; Khalil, Z.; Dewapriya, P.; Salim, A.A.; Lin, H.W.; Capon, R.J. Trichodermides A–E: New peptaibols isolated from the australian termite nest-derived fungus Trichoderma virens CMB-TN16. J. Nat. Prod. 2018, 81, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Marik, T.; Tyagi, C.; Racic, G.; Rakk, D.; Szekeres, A.; Vagvolgyi, C.; Kredics, L. New 19-residue peptaibols from Trichoderma Clade Viride. Microorganisms 2018, 6, 85. [Google Scholar] [CrossRef]
- Ojo, O.S.; Nardone, B.; Musolino, S.F.; Neal, A.R.; Wilson, L.; Lebl, T.; Slawin, A.M.Z.; Cordes, D.B.; Taylor, J.E.; Naismith, J.H.; et al. Synthesis of the natural product descurainolide and cyclic peptides from lignin-derived aromatics. Org. Biomol. Chem. 2018, 16, 266–273. [Google Scholar] [CrossRef]
- Singh, V.P.; Yedukondalu, N.; Sharma, V.; Kushwaha, M.; Sharma, R.; Chaubey, A.; Kumar, A.; Singh, D.; Vishwakarma, R.A. Lipovelutibols A–D: Cytotoxic lipopeptaibols from the himalayan cold habitat fungus Trichoderma velutinum. J. Nat. Prod. 2018, 81, 219–226. [Google Scholar] [CrossRef]
- Touati, I.; Ruiz, N.; Thomas, O.; Druzhinina, I.S.; Atanasova, L.; Tabbene, O.; Elkahoui, S.; Benzekri, R.; Bouslama, L.; Pouchus, Y.F.; et al. Hyporientalin A, an anti-Candida peptaibol from a marine Trichoderma orientale. World J. Microbiol. Biotechnol. 2018, 34, 98. [Google Scholar] [CrossRef] [PubMed]
- Katoch, M.; Singh, D.; Kapoor, K.K.; Vishwakarma, R.A. Trichoderma lixii (IIIM-B4), an endophyte of Bacopa monnieri L. producing peptaibols. BMC Microbiol. 2019, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Momose, I.; Onodera, T.; Doi, H.; Adachi, H.; Iijima, M.; Yamazaki, Y.; Sawa, R.; Kubota, Y.; Igarashi, M.; Kawada, M. Leucinostatin Y: A Peptaibiotic produced by the entomoparasitic fungus Purpureocillium lilacinum 40-H-28. J. Nat. Prod. 2019, 82, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Krumpe, L.R.H.; Smith, E.; Henrich, C.J.; Brownell, I.; Wendt, K.L.; Cichewicz, R.H.; O’Keefe, B.R.; Gustafson, K.R. Roseabol A, a new peptaibol from the fungus Clonostachys rosea. Molecules 2021, 26, 3594. [Google Scholar] [CrossRef]
- Rawa, M.S.A.; Nogawa, T.; Okano, A.; Futamura, Y.; Nakamura, T.; Wahab, H.A.; Osada, H. A new peptaibol, RK-026A, from the soil fungus Trichoderma sp. RK10-F026 by culture condition-dependent screening. Biosci. Biotechnol. Biochem. 2021, 85, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Rawa, M.S.A.; Nogawa, T.; Okano, A.; Futamura, Y.; Wahab, H.A.; Osada, H. Zealpeptaibolin, an 11-mer cytotoxic peptaibol group with 3 Aib-Pro motifs isolated from Trichoderma sp. RK10-F026. J. Antibiot. 2021, 74, 485–495. [Google Scholar] [CrossRef]
- Zhang, S.H.; Yang, J.; Ma, H.; Yang, Y.; Zhou, G.F.; Zhao, X.; Xu, R.; Nie, D.; Zhang, G.G.; Shan, J.J.; et al. Longibramides A-E, Peptaibols isolated from a mushroom derived fungus Trichoderma longibrachiatum Rifai DMG-3-1-1. Chem. Biodivers. 2021, 18, 2100128. [Google Scholar] [CrossRef]
- Tullberg, M.; Grøtli, M.; Luthman, K. Efficient synthesis of 2,5-diketopiperazines using microwave assisted heating. Tetrahedron 2006, 62, 7484–7491. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic metabolites from the antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef]
- Wang, F.Z.; Huang, Z.; Shi, X.F.; Chen, Y.C.; Zhang, W.M.; Tian, X.P.; Li, J.; Zhang, S. Cytotoxic indole diketopiperazines from the deep sea-derived fungus Acrostalagmus luteoalbus SCSIO F457. Bioorg. Med. Chem. Lett. 2012, 22, 7265–7267. [Google Scholar] [CrossRef]
- Sun, S.; Dai, X.; Sun, J.; Bu, X.; Weng, C.; Li, H.; Zhu, H. A diketopiperazine factor from Rheinheimera aquimaris QSI02 exhibits anti-quorum sensing activity. Sci. Rep. 2016, 6, 39637. [Google Scholar] [CrossRef] [PubMed]
- Caballero, E.; Avendaño, C.; Menéndez, J.C. Stereochemical issues related to the synthesis and reactivity of pyrazino[2′,1′-5,1]pyrrolo[2,3-b]indole-1,4-diones. Tetrahedron Asymmetry 1998, 9, 967–981. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, F.; Guo, X.-J.; Zhang, Y.-R.; Kang, Z.; Zhu, H.-J. Antibacterial Indole alkaloids and anthraquinones from a sewage-derived fungus Eurotium sp. Chem. Nat. Compd. 2018, 54, 399–401. [Google Scholar] [CrossRef]
- Bovio, E.; Garzoli, L.; Poli, A.; Luganini, A.; Villa, P.; Musumeci, R.; McCormack, G.P.; Cocuzza, C.E.; Gribaudo, G.; Mehiri, M.; et al. Marine fungi from the sponge Grantia compressa: Biodiversity, chemodiversity, and biotechnological potential. Mar. Drugs 2019, 17, 220. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Du, J.; Goehring, A.; Gouaux, E. Cryo-EM structures of the triheteromeric NMDA receptor and its allosteric modulation. Science 2017, 355, 6331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Chang, S.; Xu, P.; Miao, M.; Wu, H.; Zhang, Y.; Zhang, T.; Wang, H.; Zhang, J.; Xie, C.; et al. Structural basis of the proton sensitivity of human GluN1-GluN2A NMDA receptors. Cell Rep. 2018, 25, 3582–3590. [Google Scholar] [CrossRef]
- Nguyen, H.-H.; Imhof, D.; Kronen, M.; Schlegel, B.; Ha, A.; Gera, L.; Reissmann, S. Synthesis and biological evaluation of analogues of the peptaibol ampullosporin A. J. Med. Chem. 2002, 45, 2781–2787. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Chemical Computing Group Inc. Molecular Operating Environment v2019.0101; Chemical Computing Group Inc.: Montreal, QC, Canada, 2019. [Google Scholar]
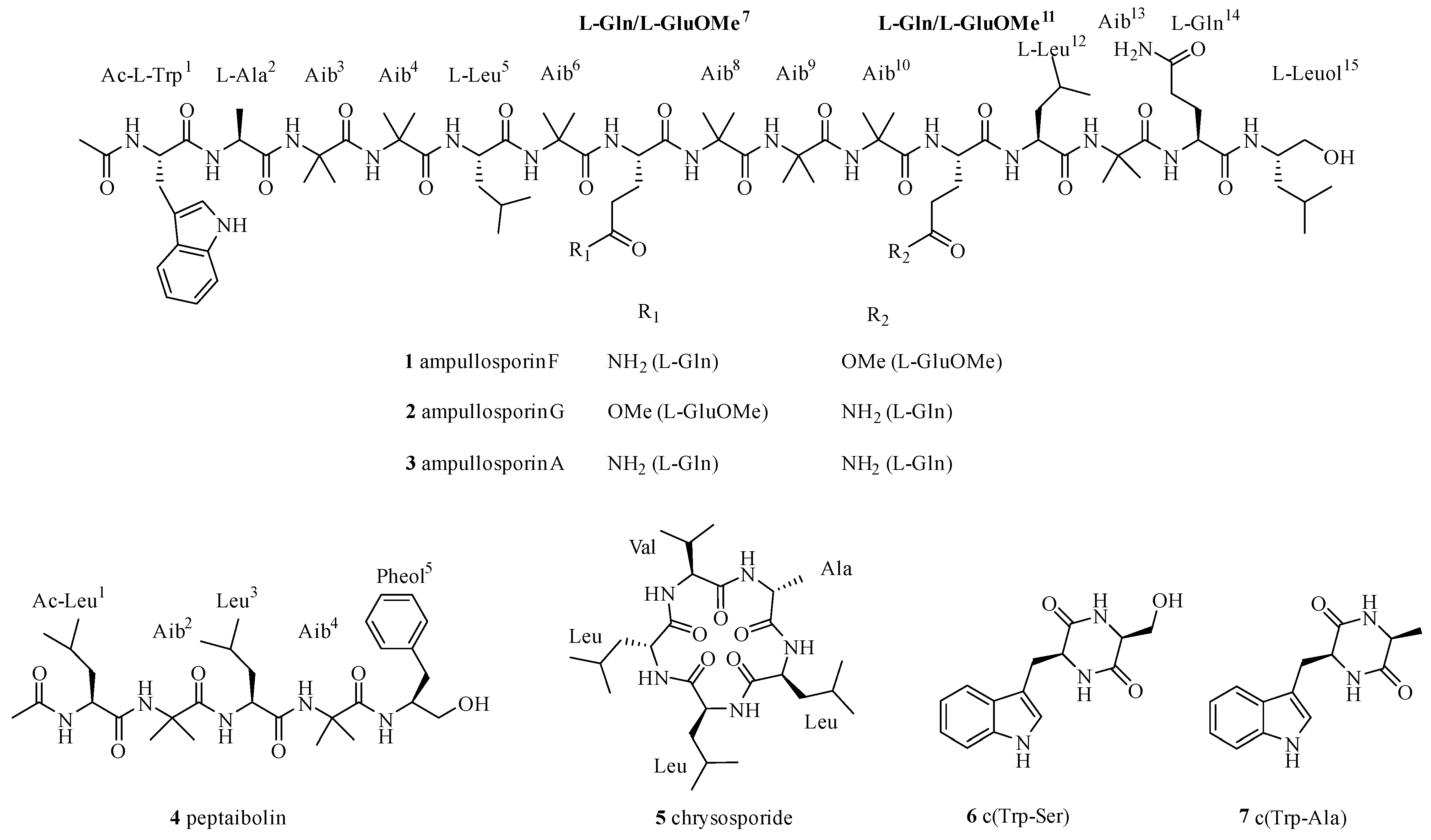
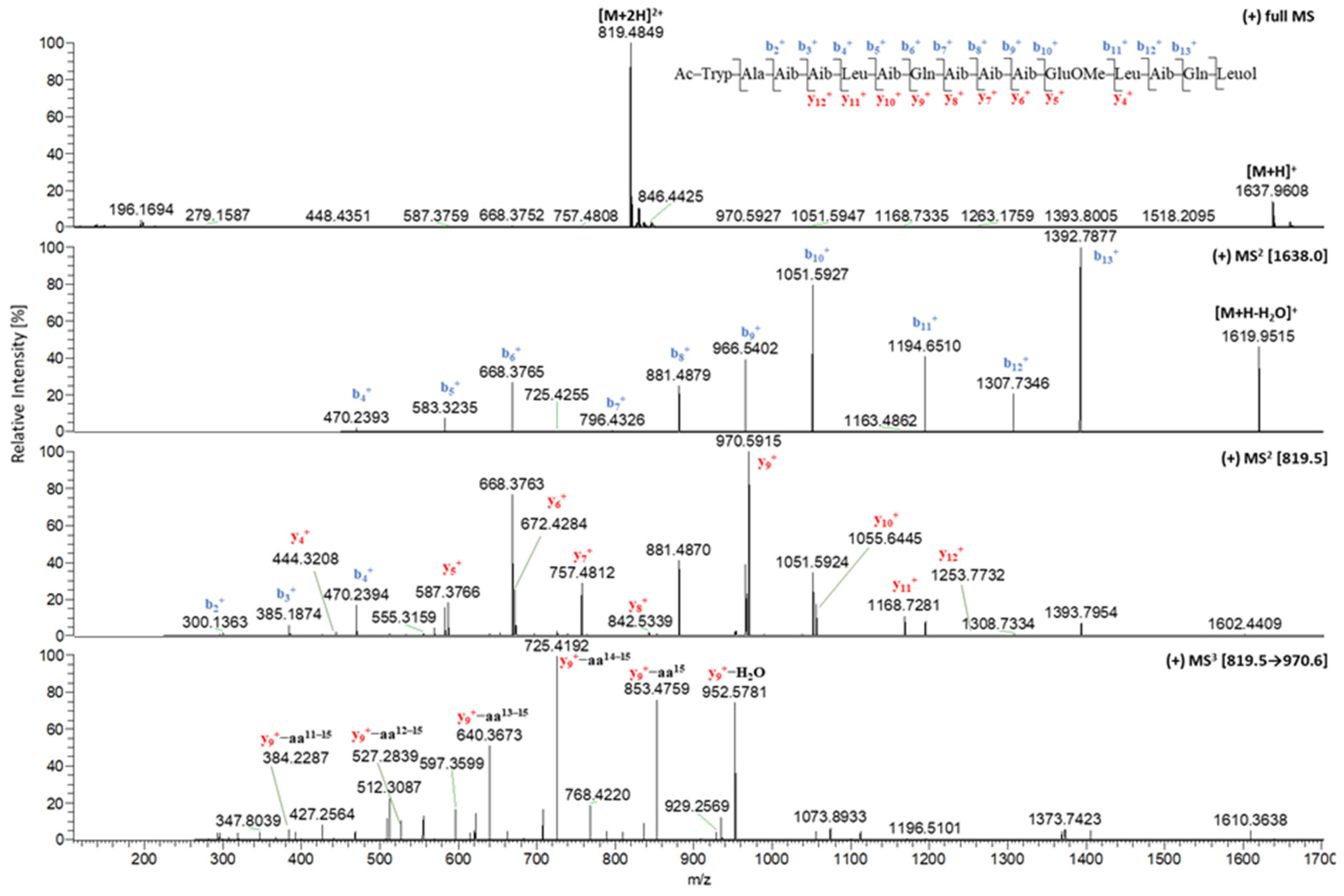

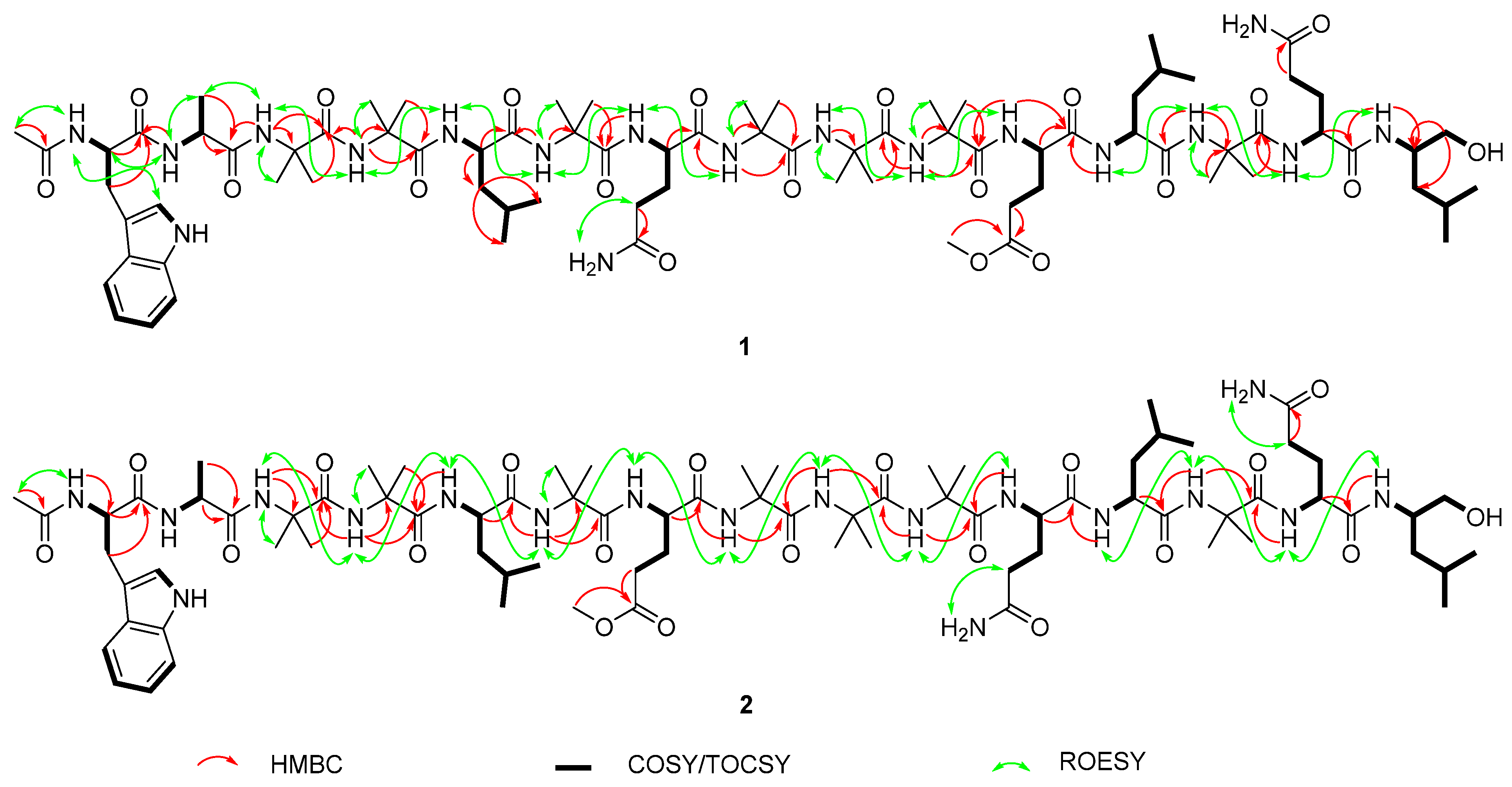
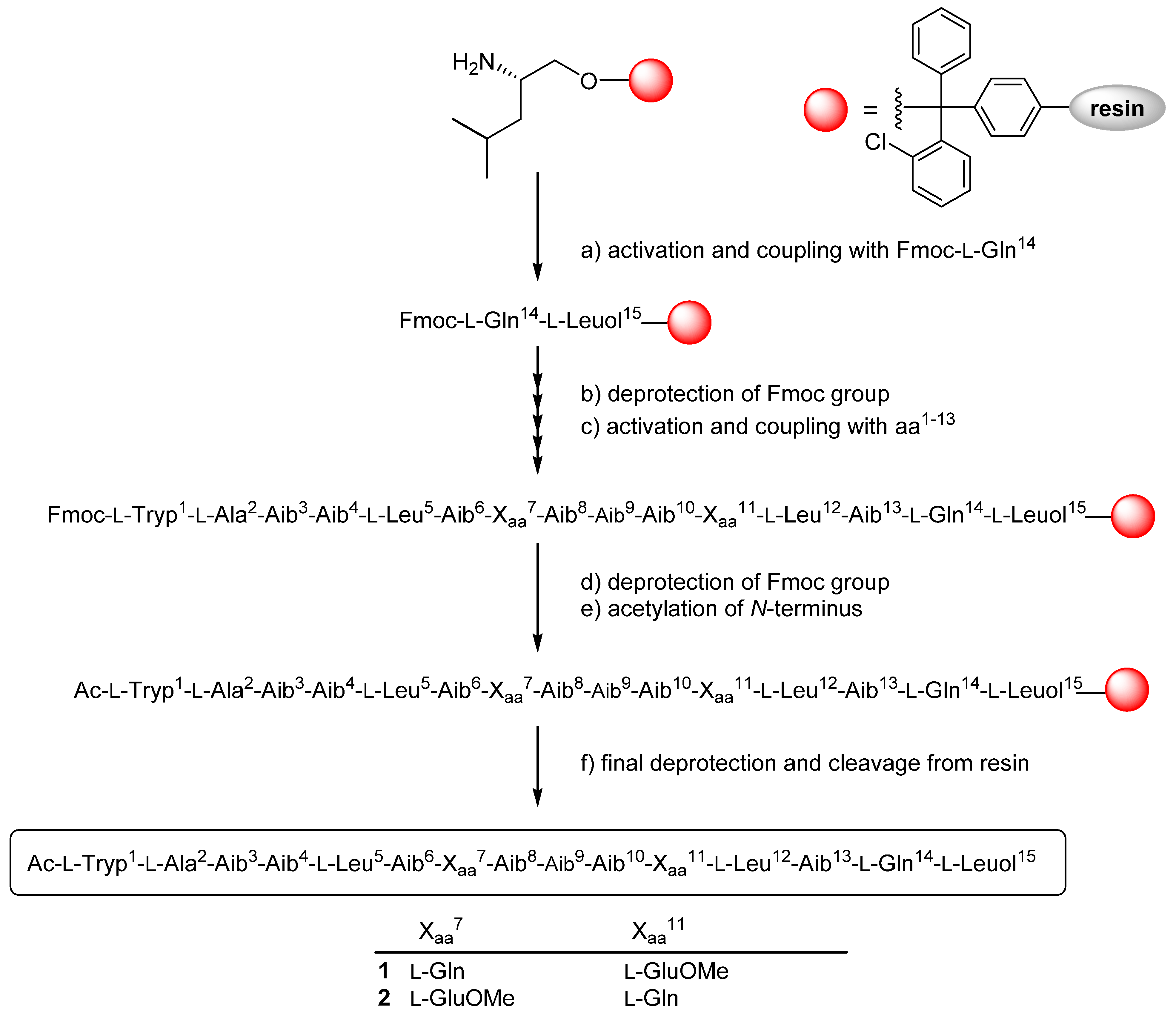

| 1 | 2 | 1 | 2 | ||
|---|---|---|---|---|---|
| tR (min) | 11.62 | 11.54 | y11+ | 1168.7280 | 1168.7294 |
| [M + 2H]2+ | 819.4849 | 819.4864 | y12+ | n.d. | n.d. |
| [M + H]+ | 1637.9608 | 1637.9646 | y13+ | n.d. | n.d. |
| b1+ | 229.0977 | 229.0978 | y14+ | n.d. | n.d. |
| b2+ | 300.1361 | 300.1345 | y9+ | 970.5923 | 970.5915 |
| b3+ | 385.1870 | 385.1874 | y9+-aa15 | 853.4770 | 853.4781 |
| b4+ | 470.2393 | 470.2400 | y9+-aa14–15 | 725.4192 | 725.4180 |
| b5+ | 583.3235 | 583.3235 | y9+-aa13–15 | 640.3662 | 640.3654 |
| b6+ | 668.3765 | 668.3763 | y9+-aa12–15 | 527.2811 | 527.2847 |
| b7+ | 796.4326 | 811.4340 | y9+-aa11–15 | n.d. | 399.2281 |
| b8+ | 881.4879 | 896.4871 | y9+-aa10–15 | n.d. | n.d. |
| b9+ | 966.5402 | 981.5396 | y9+-aa9–15 | n.d. | n.d. |
| b10+ | 1051.5927 | 1066.5923 | y9+-aa8–15 | n.d. | n.d. |
| b11+ | 1194.6510 | 1194.6440 | [M − H]− | 1635.9458 | 1635.9453 |
| b12+ | 1307.7346 | 1307.7357 | y2− | n.d. | n.d. |
| b13+ | 1392.7877 | 1392.7875 | y3− | n.d. | n.d. |
| b14+ | n.d. | n.d. | y4− | n.d. | n.d. |
| y1+ | n.d. | n.d. | y5− | n.d. | 570.3615 |
| y2+ | n.d. | 246.1820 | y6− | n.d. | 655.4136 |
| y3+ | n.d. | n.d. | y7− | n.d. | 740.4655 |
| y4+ | 444.3214 | 444.3169 | y8− | n.d. | 825.5215 |
| y5+ | 587.3766 | 572.3767 | y9− | 968.5731 | 968.5756 |
| y6+ | 672.4281 | 657.4289 | y10− | 1053.6372 | 1053.6267 |
| y7+ | 757.4816 | 742.4813 | y11− | 1166.7102 | 1166.7143 |
| y8+ | 842.5358 | 827.5331 | y12− | 1251.7668 | 1251.7572 |
| y9+ | 970.5923 | 970.5915 | y13− | 1336.8179 | 1336.8088 |
| y10+ | 1055.6453 | 1055.6426 | y14− | 1407.8525 | 1407.8543 |
| Pos. | δH, Mult. J (Hz) | δC/δN | Pos. | δH, Mult. J (Hz) | δC/δN | Pos. | δH, Mult. J (Hz) | δC/δN |
|---|---|---|---|---|---|---|---|---|
| Ac | β | 1.77 a; 1.58 a | 38.4 | C=O | 173.0 | |||
| CH3 | 1.86 s | 22.2 | γ | 1.72 a | 23.8 | α | 3.95 m | 54.4 |
| C=O | 170.2 | δ1 | 0.84 d 6.6 | 20.8 | β | 2.07 a; 2.00 a | 25.3 | |
| Trp1 | δ2 | 0.93 d 6.6 | 22.0 | γ | 2.60 m; 2.45 m | 29.6 | ||
| NH | 8.38 d 6.5 | 125.3 | Aib6 | C=O | 172.5 | |||
| C=O | 173.0 | NH | 7.87 s | 127.9 | O-CH3 | 3.55 s | 50.8 | |
| α | 4.40 | 54.4 | C=O | 175.5 | Leu12 | |||
| β | 3.12 dd 14.6/3.6 | 26.8 | α | 1.48 | 55.4 | NH | 7.42 d 7.3 | 115.4 |
| 2.98 dd 14.6/9.4 | β | 1.36 s | 22.6 | C=O | 172.8 | |||
| 1-NH | 10.86 br s | 131.2 | γ | 1.48 s | 26.1 | α | 4.08 m | 52.5 |
| 2 | 7.22 d 2.4 | 123.4 | Gln7 | β | 1.67 a; 1.59 a | 38.4 | ||
| 3 | 109.8 | NH | 7.41 d 6.2 | 112.9 | γ | 1.73 a | 23.7 | |
| 3a | 127.1 | C=O | 173.1 | δ1 | 0.86 d 6.6 | 22.2 | ||
| 4 | 7.56 d 8.1 | 117.9 | α | 3.82 m | 55.7 | δ2 | 0.83 d 6.6 | 20.6 |
| 5 | 6.96 t 7.5 | 117.7 | β | 2.00 a; 1.91 a | 25.8 | Aib13 | ||
| 6 | 7.05 a | 120.5 | γ | 2.22 a; 2.07 a | 31.0 | NH | 7.45 s | 126.5 |
| 7 | 7.33 d 8.1 | 111.0 | C=O | 172.9 | C=O | 173.4 | ||
| 7a | 136.0 | N-H2 | 7.12 br s | 107.7 | α | 56.0 | ||
| Ala2 | 6.75 br s | β | 1.38 s | 23.7 | ||||
| NH | 8.52 a | 121.9 | Aib8 | γ | 1.41 s | 25.4 | ||
| C=O | 174.0 | NH | 7.99 s | 128.5 | Gln14 | |||
| α | 4.04 a | 50.1 | C=O | 175.0 | NH | 7.19 d 7.7 | 109.2 | |
| β | 1.27 d 7.4 | 15.5 | α | 55.1 | C=O | 173.1 | ||
| Aib3 | 132.1 | β | 1.31 s | 22.0 | α | 3.98 m | 52.9 | |
| NH | 8.46 s | 174.6 | γ | 1.43 s | 25.3 | β | 2.05 a; 1.81 a | 26.7 |
| C=O | 55.4 | Aib9 | γ | 2.18 a; 2.08 a | 31.2 | |||
| α | 25.3 | NH | 7.96 s | 125.2 | C=O | 173.5 | ||
| β | 1.38 s | 22.6 | C=O | 175.0 | N-H2 | 7.13 br s | 107.9 | |
| γ | 1.35 s | 22.6 | α | 55.3 | 6.73 br s | |||
| Aib4 | β | 1.39 s | 26.0 | Leuol15 | ||||
| NH | 8.06 s | 124.3 | γ | 1.32 s | 22.0 | NH | 7.06 a | 118.8 |
| C=O | 176.3 | Aib10 | α | 3.78 m | 48.2 | |||
| α | 55.3 | NH | 7.57 s | 125.1 | β | 1.35 a | 39.4 | |
| β | 1.38 s | 26.2 | C=O | 175.8 | γ | 1.66 a | 23.5 | |
| γ | 1.36 s | 22.0 | α | 55.4 | δ1 | 0.86 d 6.6 | 23.2 | |
| Leu5 | β | 1.39 s | 22.3 | δ2 | 0.81 d 6.6 | 21.2 | ||
| NH | 7.71 d 4.1 | 114.8 | γ | 1.49 s | 26.1 | β’ | 3.30 a; 3.18 m | 63.5 |
| C=O | 173.7 | GluOMe11 | O-H | 4.50 t 6.0 | ||||
| α | 3.90 m | 54.1 | NH | 7.76 d 6.6 | 111.2 |
| Pos. | δH, Mult. J (Hz) | δC/δN | Pos. | δH, Mult. J (Hz) | δC/δN | Pos. | δH, Mult. J (Hz) | δC/δN |
|---|---|---|---|---|---|---|---|---|
| Ac | β | 1.55 a; 1.76 a | 38.7 | α | 3.93 m | 55.0 | ||
| CH3 | 1.85 br s | 22.3 | γ | 1.70 a | 24.3 | β | 1.94 a; 2.00 a | 26.3 |
| C=O | 170.4 | δ1 | 0.833 d 6.6 | 21.1 | γ | 2.13 m; 2.34 m | 31.5 | |
| Trp1 | δ2 | 0.914 d 6.6 | 22.1 | C=O | 173.2 | |||
| NH | 8.30 a | 125.2 | Aib6 | N-H2 | 7.12 br s | 107.6 | ||
| C=O | 173.0 | NH | 7.85 s | 127.8 | 6.70 br s | |||
| α | 4.39 m | 54.4 | C=O | 176.0 | Leu12 | |||
| β | 3.11 dd 14.4/4.2 | α | 55.6 | NH | 7.77 d 6.6 | 115.2 | ||
| 2.98 dd 14.4/9.6 | 26.8 | β | 1.35 s | 22.2 | C=O | 173.2 | ||
| 1-NH | 10.84 br s | 131.1 | γ | 1.45 s | 26.2 | α | 4.07 m | 52.6 |
| 2 | 7.21 d 2.4 | 123.5 | GluOMe7 | β | 1.58 a & 1.67 a | 38.5 | ||
| 3 | 109.6 | NH | 7.47 d 6.0 | 111.8 | γ | 1.70 a | 23.9 | |
| 3a | 126.9 | C=O | 172.8 | δ1 | 0.85 d 6.6 | 22.3 | ||
| 4 | 7.55 d 7.8 | 118.0 | α | 3.87 m | 55.2 | δ2 | 0.82 d 6.6 | 20.7 |
| 5 | 6.95 t-like 7.2 | 117.9 | β | 1.96 a; 2.03 a | 25.4 | Aib13 | ||
| 6 | 7.06 t-like 7.8 | 120.7 | γ | 2.49 a; 2.39 m | 29.7 | NH | 7.48 s | 126.7 |
| 7 | 7.33 d 8.1 | 111.1 | C=O | 172.2 | C=O | 173.7 | ||
| 7a | 135.9 | O-CH3 | 3.54 s | α | 56.0 | |||
| Ala2 | Aib8 | β | 1.37 s | 23.8 | ||||
| NH | 8.30 a | 121.6 | NH | 7.97 s | 128.7 | γ | 1.41 s | 25.6 |
| C=O | 174.0 | C=O | 175.0 | Gln14 | ||||
| α | 4.01 m | 50.3 | α | 55.4 | NH | 7.22 d 8.4 | 109.3 | |
| β | 1.25 d 7.2 | 15.7 | β | 1.34 s | 22.4 | C=O | 170.6 | |
| Aib3 | γ | 1.42 s | 25.4 | α | 3.98 m | 53.0 | ||
| NH | 8.33 s | 131.9 | Aib9 | β | 1.80 a & 2.05 a | 26.6 | ||
| C=O | 174.7 | NH | 7.87 s | 125.0 | γ | 2.09 a & 2.18 a | 31.3 | |
| α | 55.4 | C=O | 175.0 | C=O | 173.8 | |||
| β | 1.33 s | 23.0 | α | 55.4 | N-H2 | 7.17 br s | 108.2 | |
| γ | 1.37 s | 25.4 | β | 1.31 s | 22.0 | 6.73 br s | ||
| Aib4 | γ | 1.37 s | 26.1 | Leuol15 | ||||
| NH | 8.06 s | 124.4 | Aib10 | NH | 7.07 d 8.4 | 119.1 | ||
| C=O | 176.4 | NH | 7.56 s | 125.1 | α | 3.76 m | 48.4 | |
| α | 55.4 | C=O | 175.9 | β | 1.34 a | 39.5 | ||
| β | 1.32 s | 22.0 | α | 55.5 | γ | 1.64 a | 23.7 | |
| γ | 1.37 s | 26.5 | β | 1.38 s | 22.6 | δ1 | 0.85 d 6.6 | 23.2 |
| Leu5 | γ | 1.48 s | 26.2 | δ2 | 0.81 d 6.6 | 21.3 | ||
| NH | 7.65 d 5.4 | 114.8 | Gln11 | β’ | 3.18 m, 3.30 m | 63.6 | ||
| C=O | 173.8 | NH | 7.74 d 5.4 | 111.9 | O-H | 4.54 t-like | ||
| α | 3.88 a | 54.2 | C=O | 173.5 |
| Compound | Toxicity a | Antifungal Activity b | |||
|---|---|---|---|---|---|
| HT29 | PC3 | B. cinerea | S. tritici | P. infestans | |
| 1 | 5.90 ± 0.65 | 3.62 ± 0.49 | 7.27 ± 0.95 | >125 | 14.75 ± 1.89 |
| 2 | 6.04 ± 0.64 | 3.35 ± 0.89 | 4.59 ± 0.34 | >125 | 14.79 ± 2.84 |
| 3 | 10.45 ± 1.18 | 6.68 ± 1.67 | 11.41 ± 1.48 | >125 | 19.49 ± 4.29 |
| Epoxiconazole c | <1.5 | <1.5 | |||
| Terbinafine c | 48.30 ± 0.2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, Y.T.H.; Ricardo, M.G.; Rennert, R.; Frolov, A.; Porzel, A.; Brandt, W.; Stark, P.; Westermann, B.; Arnold, N. Rare Glutamic Acid Methyl Ester Peptaibols from Sepedonium ampullosporum Damon KSH 534 Exhibit Promising Antifungal and Anticancer Activity. Int. J. Mol. Sci. 2021, 22, 12718. https://doi.org/10.3390/ijms222312718
Lam YTH, Ricardo MG, Rennert R, Frolov A, Porzel A, Brandt W, Stark P, Westermann B, Arnold N. Rare Glutamic Acid Methyl Ester Peptaibols from Sepedonium ampullosporum Damon KSH 534 Exhibit Promising Antifungal and Anticancer Activity. International Journal of Molecular Sciences. 2021; 22(23):12718. https://doi.org/10.3390/ijms222312718
Chicago/Turabian StyleLam, Yen T. H., Manuel G. Ricardo, Robert Rennert, Andrej Frolov, Andrea Porzel, Wolfgang Brandt, Pauline Stark, Bernhard Westermann, and Norbert Arnold. 2021. "Rare Glutamic Acid Methyl Ester Peptaibols from Sepedonium ampullosporum Damon KSH 534 Exhibit Promising Antifungal and Anticancer Activity" International Journal of Molecular Sciences 22, no. 23: 12718. https://doi.org/10.3390/ijms222312718
APA StyleLam, Y. T. H., Ricardo, M. G., Rennert, R., Frolov, A., Porzel, A., Brandt, W., Stark, P., Westermann, B., & Arnold, N. (2021). Rare Glutamic Acid Methyl Ester Peptaibols from Sepedonium ampullosporum Damon KSH 534 Exhibit Promising Antifungal and Anticancer Activity. International Journal of Molecular Sciences, 22(23), 12718. https://doi.org/10.3390/ijms222312718








