A Comparison of the Effect of Lead (Pb) on the Slow Vacuolar (SV) and Fast Vacuolar (FV) Channels in Red Beet (Beta vulgaris L.) Taproot Vacuoles
Abstract
:1. Introduction
2. Results
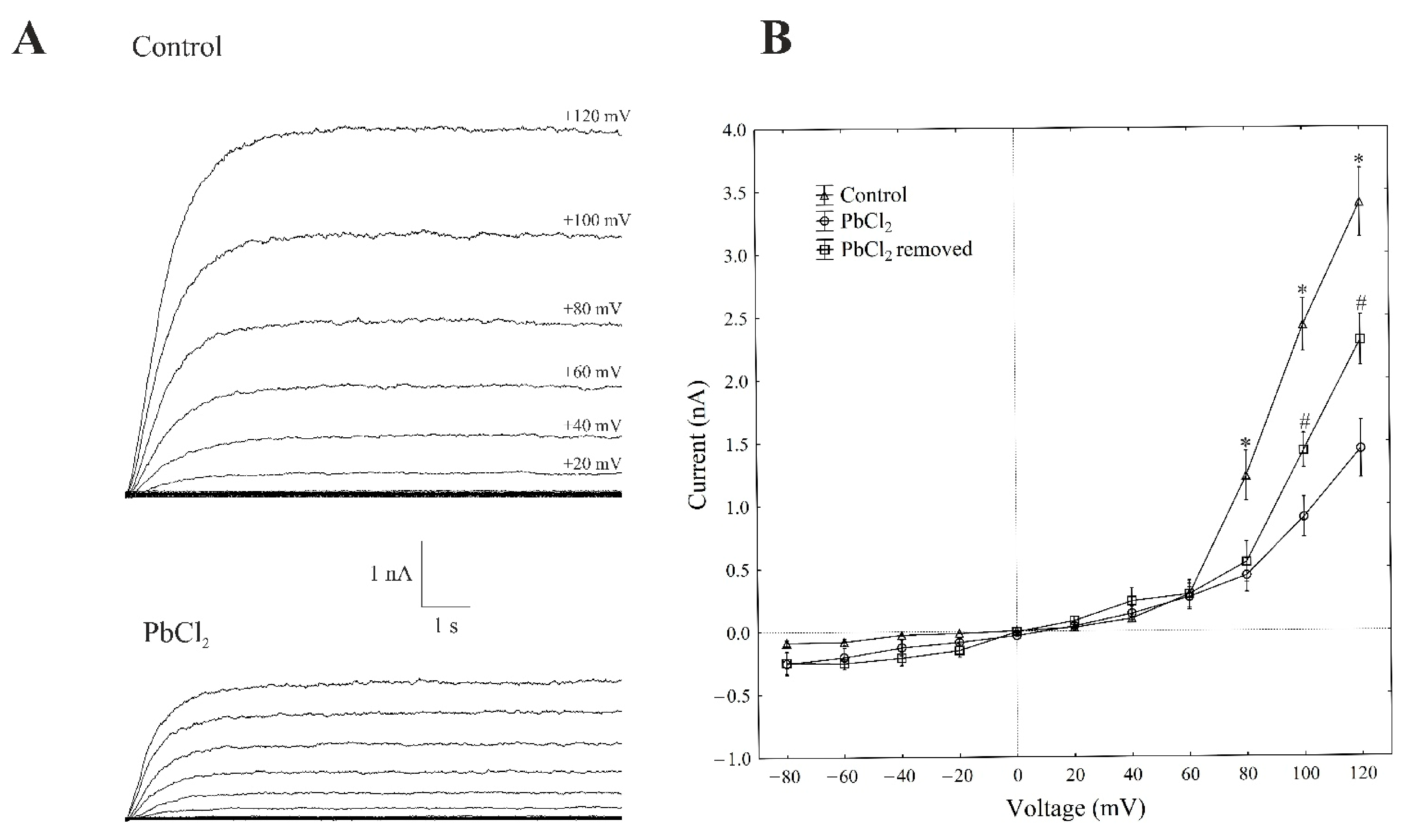
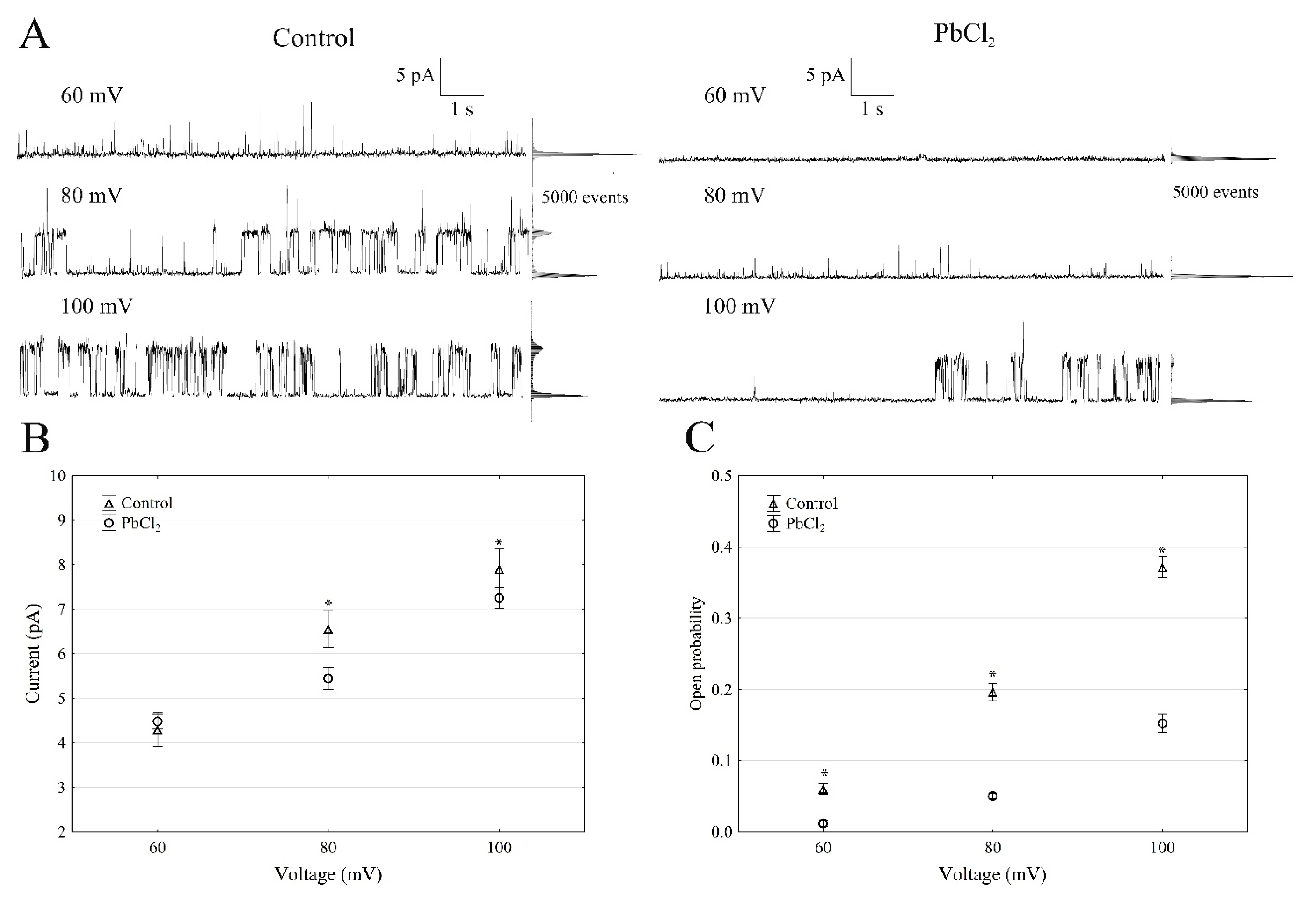
2.1. Cytosolic Lead Diminished Whole-Vacuole SV Currents and Single-Channel Activity
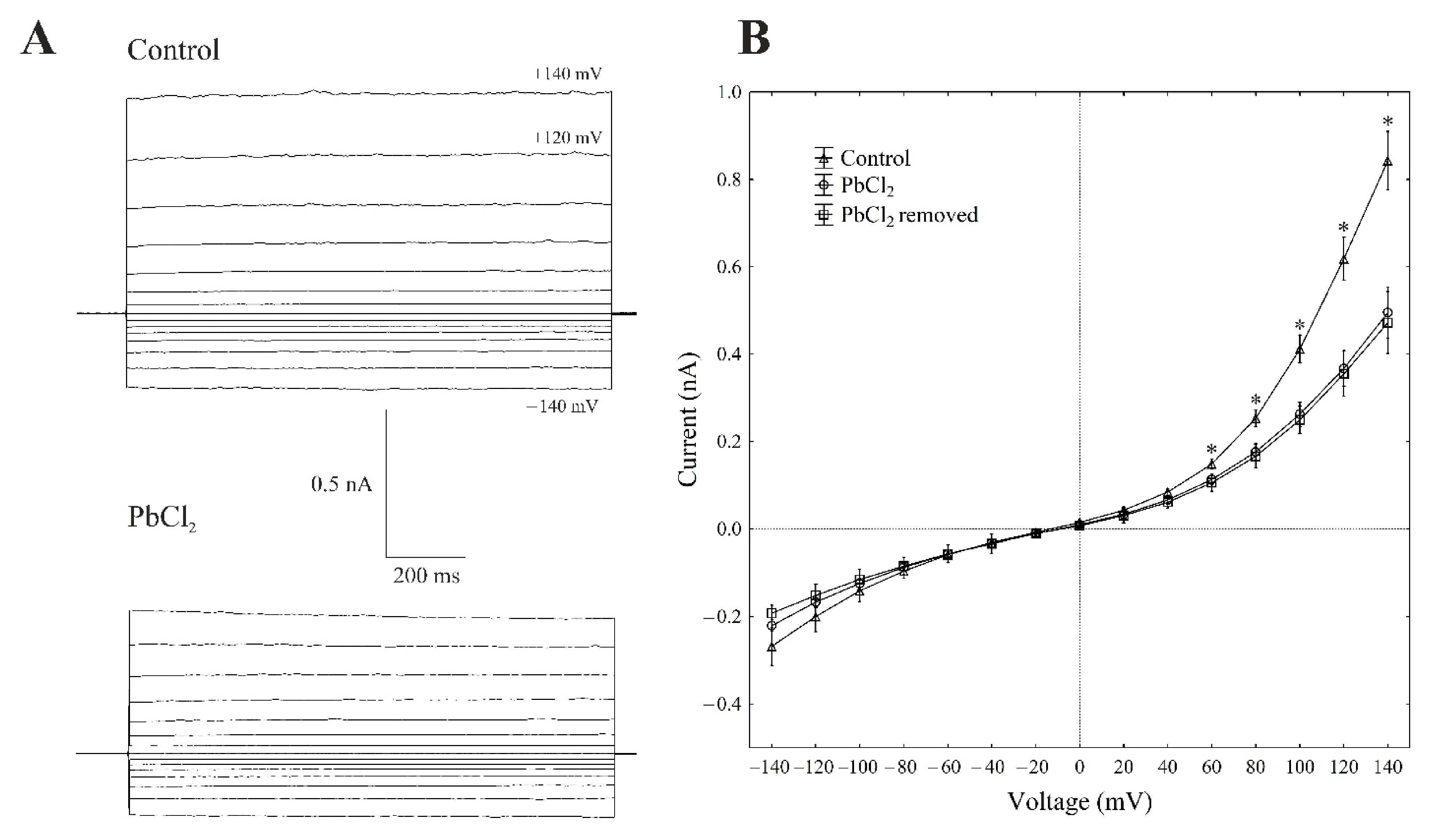
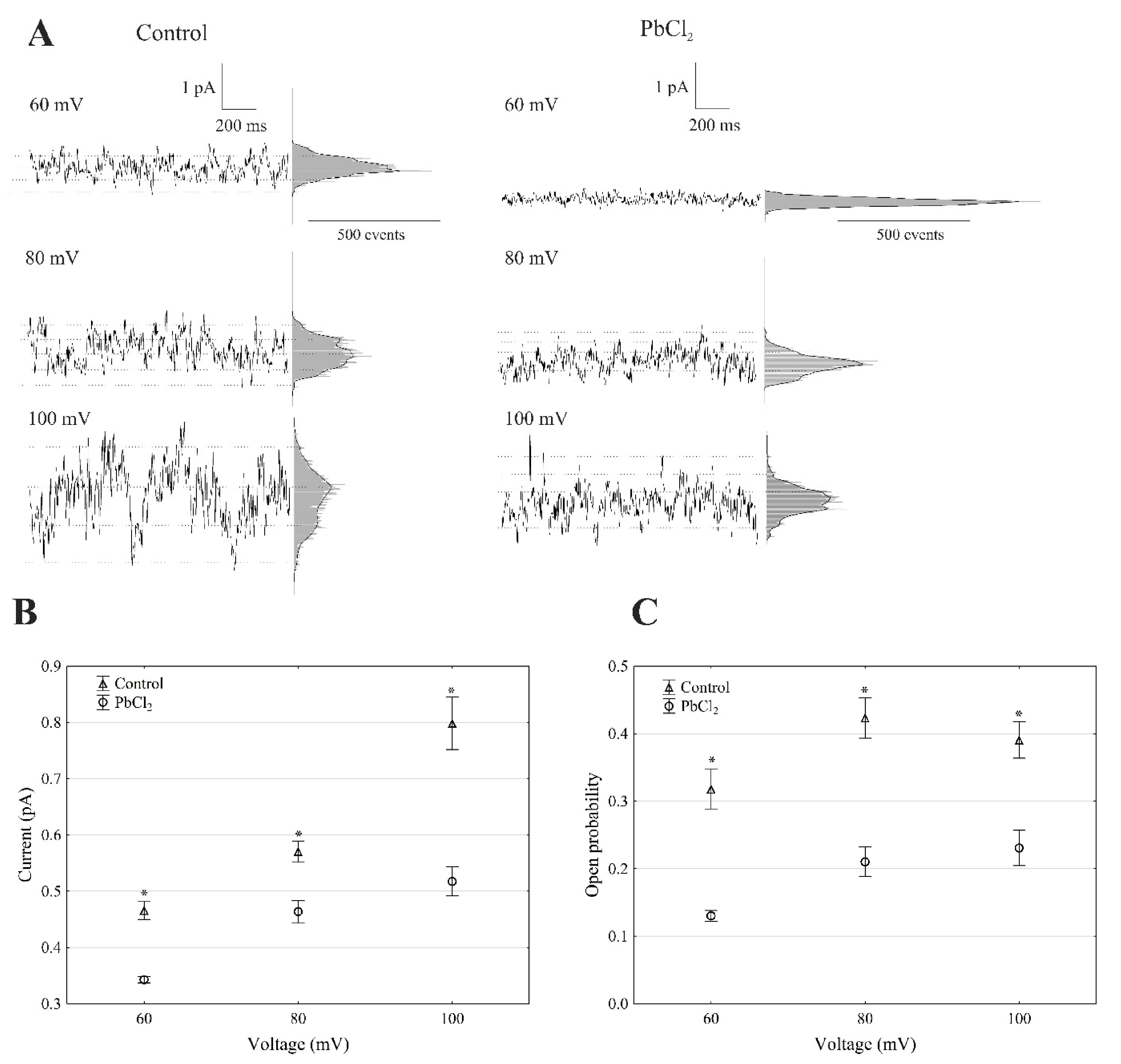
2.2. Cytosolic Lead Modulated the Whole-Vacuole FV Currents and Single-Channel Activity
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.-T.; Yang, L.-H.; Ferjani, A.; Lin, W.-H. Multiple functions of the vacuole in plant growth and fruit quality. Mol. Hortic. 2021, 1, 4. [Google Scholar] [CrossRef]
- Martinoia, E. Vacuolar Transporters—Companions on a Longtime Journey. Plant Physiol. 2018, 176, 1384–1407. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N.; Yazaki, K. Dynamism of vacuoles toward survival strategy in plants. Biochim. Biophys. Acta (BBA)—Biomembr. 2019, 1862, 183127. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, K.; Wang, Z.; Zhu, K.; Tan, X.; Cao, J. A Review of Plant Vacuoles: Formation, Located Proteins, and Functions. Plants 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedrich, R.; Mueller, T.D.; Becker, D.; Marten, I. Structure and Function of TPC1 Vacuole SV Channel Gains Shape. Mol. Plant 2018, 11, 764–775. [Google Scholar] [CrossRef] [Green Version]
- D’Amore, A.; Gradogna, A.; Palombi, F.; Minicozzi, V.; Ceccarelli, M.; Carpaneto, A.; Filippini, A. The Discovery of Naringenin as Endolysosomal Two-Pore Channel Inhibitor and Its Emerging Role in SARS-CoV-2 Infection. Cells 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead Toxicity in Cereals: Mechanistic Insight into Toxicity, Mode of Action, and Management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef]
- Kurtyka, R.; Burdach, Z.; Siemieniuk, A.; Karcz, W. Single and combined effects of Cd and Pb on the growth, medium pH, membrane potential and metal contents in maize (Zea mays L.) coleoptile segments. Ecotoxicol. Environ. Saf. 2018, 161, 8–16. [Google Scholar] [CrossRef]
- Liao, J.; Cai, X.; Yang, Y.; Chen, Q.; Gao, S.; Liu, G.; Sun, L.; Luo, Z.; Lei, T.; Jiang, M. Dynamic study of the lead (Pb) tolerance and accumulation characteristics of new dwarf bamboo in Pb-contaminated soil. Chemosphere 2021, 282, 131089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shang, D.; Ning, J.; Zhai, Y.; Sheng, X.; Ding, H. Subcellular distribution and chemical forms of lead in the red algae, Porphyra yezoensis. Chemosphere 2019, 227, 172–178. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R.; Hanus-Fajerska, E. Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and kinetics of adsorption and removal of heavy metals from wastewater using nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Narayan, O.P. Metal transporters in organelles and their roles in heavy metal transportation and sequestration mechanisms in plants. Physiol. Plant. 2021, 173, 259–275. [Google Scholar] [CrossRef]
- Pottosin, I.I.; Schonknecht, G. Vacuolar calcium channels. J. Exp. Bot. 2007, 58, 1559–1569. [Google Scholar] [CrossRef] [Green Version]
- Miśkiewicz, J.; Trela, Z.; Przestalski, S.; Karcz, W. Superstatistics analysis of the ion current distribution function: Met3PbCl influence study. Eur. Biophys. J. 2010, 39, 1397–1406. [Google Scholar] [CrossRef]
- Miśkiewicz, J.; Trela, Z.; Burdach, Z.; Karcz, W.; Balińska-Miśkiewicz, W. Long range correlations of the ion current in SV channels Met3PbCl influence study. PLoS ONE 2020, 15, e0229433. [Google Scholar] [CrossRef] [Green Version]
- Trela, Z.; Burdach, Z.; Przestalski, S.; Karcz, W. Effect of trimethyllead chloride on slowly activating (SV) channels in red beet (Beta vulgaris L.) taproots. Comptes Rendus Biol. 2012, 335, 722–730. [Google Scholar] [CrossRef]
- Tikhonova, L.I.; Pottosin, I.I.; And, K.D.; Schoenknecht, G. Fast-activating cation channel in barley mesophyll vacuoles Inhibition by calcium. Plant J. 1997, 11, 1059–1070. [Google Scholar] [CrossRef]
- Burdach, Z.; Siemieniuk, A.; Trela, Z.; Kurtyka, R.; Karcz, W. Role of auxin (IAA) in the regulation of slow vacuolar (SV) channels and the volume of red beet taproot vacuoles. BMC Plant Biol. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdach, Z.; Siemieniuk, A.; Karcz, W. Effect of Auxin (IAA) on the Fast Vacuolar (FV) Channels in Red Beet (Beta vulgaris L.) Taproot Vacuoles. Int. J. Mol. Sci. 2020, 21, 4876. [Google Scholar] [CrossRef] [PubMed]
- Bertl, A.; Slayman, C.L. Cation-selective channels in the vacuolar membrane of Saccharomyces: Dependence on calcium, redox state, and voltage. Proc. Natl. Acad. Sci. USA 1990, 87, 7824–7828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressler, J.; Kim, K.-A.; Chakraborti, T.; Goldstein, G. Molecular Mechanisms of Lead Neurotoxicity. Neurochem. Res. 1999, 24, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Sticht, H.; Meyerhoff, P.; Dietrich, P. Differential contribution of EF-hands to the Ca2+-dependent activation in the plant two-pore channel TPC1. Plant J. 2011, 68, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Kintzer, A.F.; Stroud, R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Zeng, W.; Chen, Q.; Lee, C.; Chen, L.; Yang, Y.; Cang, C.; Ren, D.; Jiang, Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 2015, 531, 196–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, D.; Hedrich, R. Channelling auxin action: Modulation of ion transport by indole-3-acetic acid. Plant Mol. Biol. 2002, 49, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G.; Brüdern, A.; Gradmann, D. Small Inward Rectifying K+ Channels in Coleoptiles: Inhibition by External Ca2+ and Function in Cell Elongation. J. Membr. Biol. 1996, 149, 9–20. [Google Scholar] [CrossRef]
- Thiel, G.; Macrobbie, E.; Blatt, M. Membrane transport in stomatal guard cells: The importance of voltage control. J. Membr. Biol. 1992, 126, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kotch, F.W.; Fettinger, J.; Davis, J.T. A Lead-Filled G-Quadruplex: Insight into the G-Quartet’s Selectivity for Pb2+ over K+. Org. Lett. 2000, 2, 3277–3280. [Google Scholar] [CrossRef] [PubMed]
- Mosa, A.; El-Ghamry, A.; Trüby, P.; Omar, M.; Gao, B.; Elnaggar, A.; Li, Y. Chemo-mechanical modification of cottonwood for Pb2+ removal from aqueous solutions: Sorption mechanisms and potential application as biofilter in drip-irrigation. Chemosphere 2016, 161, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, W.; Ma, G.; Li, Y.; Fan, L.; Li, X.; Lu, Y. Insights into the Competition between K+ and Pb2+ Binding to a G-Quadruplex and Discovery of a Novel K+–Pb2+–Quadruplex Intermediate. J. Phys. Chem. B 2018, 122, 9382–9388. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.J.; Sanders, D. Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. Plant J. 1996, 10, 1055–1069. [Google Scholar] [CrossRef]
- Hedrich, R.; Neher, E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 1987, 329, 833–836. [Google Scholar] [CrossRef]
- Pottosin, I.; Dobrovinskaya, O.; Muñiz, J. Conduction of Monovalent and Divalent Cations in the Slow Vacuolar Channel. J. Membr. Biol. 2001, 181, 55–65. [Google Scholar] [CrossRef]
- Ward, J.M.; Schroeder, J. Calcium-Activated K+ Channels and Calcium-Induced Calcium Release by Slow Vacuolar Ion Channels in Guard Cell Vacuoles Implicated in the Control of Stomatal Closure. Plant Cell 1994, 6, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, L.I.; Pottosin, I.I.; Schönknecht, G. Cytoplasmic magnesium regulates the fast activating vacuolar cation channel. J. Exp. Bot. 1999, 50, 1547–1552. [Google Scholar] [CrossRef]
- Hedrich, R. Ion Channels in Plants. Physiol. Rev. 2012, 92, 1777–1811. [Google Scholar] [CrossRef] [PubMed]
- Coyaud, L.; Kurkdjian, A.; Kado, R.; Hedrich, R. Ion channels and ATP-driven pumps involved in ion transport across the tonoplast of sugarbeet vacuoles. Biochim. Biophys. Acta (BBA)—Biomembr. 1987, 902, 263–268. [Google Scholar] [CrossRef]
- Hedrich, R.; Marten, I. TPC1—SV channels gain shape. Mol. Plant 2011, 4, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Rucińska-Sobkowiak, R.; Nowaczyk, G.; Krzesłowska, M.; Rabęda, I.; Jurga, S. Water status and water diffusion transport in lupine roots exposed to lead. Environ. Exp. Bot. 2013, 87, 100–109. [Google Scholar] [CrossRef]
- Bonales-Alatorre, E.; Pottosin, I.; Shabala, L.; Chen, Z.-H.; Zeng, F.; Jacobsen, S.-E.; Shabala, S. Differential Activity of Plasma and Vacuolar Membrane Transporters Contributes to Genotypic Differences in Salinity Tolerance in a Halophyte Species, Chenopodium quinoa. Int. J. Mol. Sci. 2013, 14, 9267–9285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonales-Alatorre, E.; Shabala, S.; Chen, Z.-H.; Pottosin, I. Reduced Tonoplast Fast-Activating and Slow-Activating Channel Activity Is Essential for Conferring Salinity Tolerance in a Facultative Halophyte, Quinoa. Plant Physiol. 2013, 162, 940–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertl, A.; Blumwald, E.; Coronado, R.; Eisenberg, R.; Findlay, G.; Gradmann, D.; Hille, B.; Köhler, K.; Kolb, H.A.; MacRobbie, E.; et al. Electrical measurements on endomembranes. Science 1992, 258, 873–874. [Google Scholar] [CrossRef] [PubMed]
- Molleman, A. Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology; Wiley: Hoboken, NJ, USA, 2003; ISBN 047148685X. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siemieniuk, A.; Burdach, Z.; Karcz, W. A Comparison of the Effect of Lead (Pb) on the Slow Vacuolar (SV) and Fast Vacuolar (FV) Channels in Red Beet (Beta vulgaris L.) Taproot Vacuoles. Int. J. Mol. Sci. 2021, 22, 12621. https://doi.org/10.3390/ijms222312621
Siemieniuk A, Burdach Z, Karcz W. A Comparison of the Effect of Lead (Pb) on the Slow Vacuolar (SV) and Fast Vacuolar (FV) Channels in Red Beet (Beta vulgaris L.) Taproot Vacuoles. International Journal of Molecular Sciences. 2021; 22(23):12621. https://doi.org/10.3390/ijms222312621
Chicago/Turabian StyleSiemieniuk, Agnieszka, Zbigniew Burdach, and Waldemar Karcz. 2021. "A Comparison of the Effect of Lead (Pb) on the Slow Vacuolar (SV) and Fast Vacuolar (FV) Channels in Red Beet (Beta vulgaris L.) Taproot Vacuoles" International Journal of Molecular Sciences 22, no. 23: 12621. https://doi.org/10.3390/ijms222312621
APA StyleSiemieniuk, A., Burdach, Z., & Karcz, W. (2021). A Comparison of the Effect of Lead (Pb) on the Slow Vacuolar (SV) and Fast Vacuolar (FV) Channels in Red Beet (Beta vulgaris L.) Taproot Vacuoles. International Journal of Molecular Sciences, 22(23), 12621. https://doi.org/10.3390/ijms222312621





