GM3 Ganglioside Linked to Neurofibrillary Pathology in a Transgenic Rat Model for Tauopathy
Abstract
:1. Introduction
2. Results
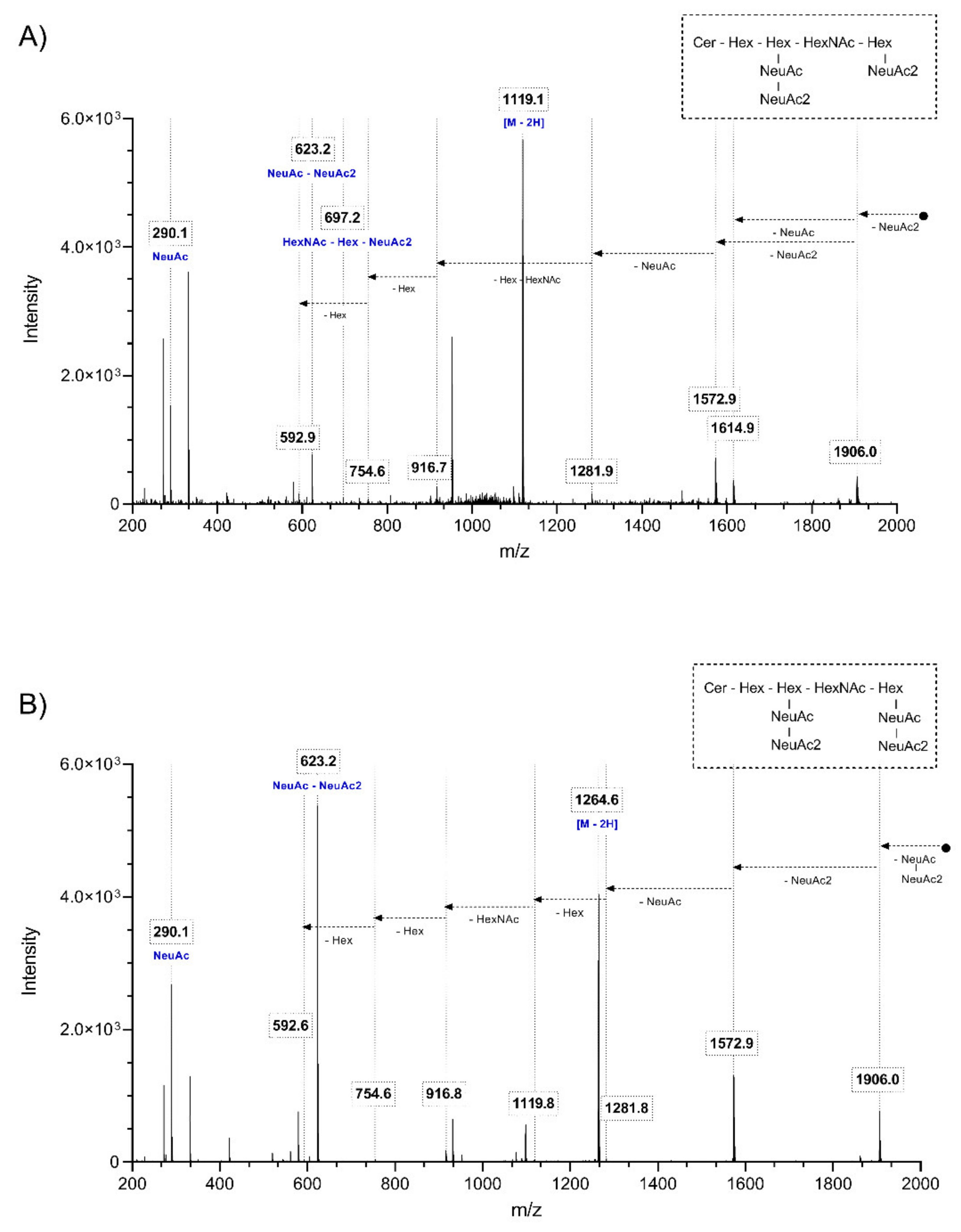
2.1. Characterization of the Glycosphingolipid Profile in Rat Brain Tissue
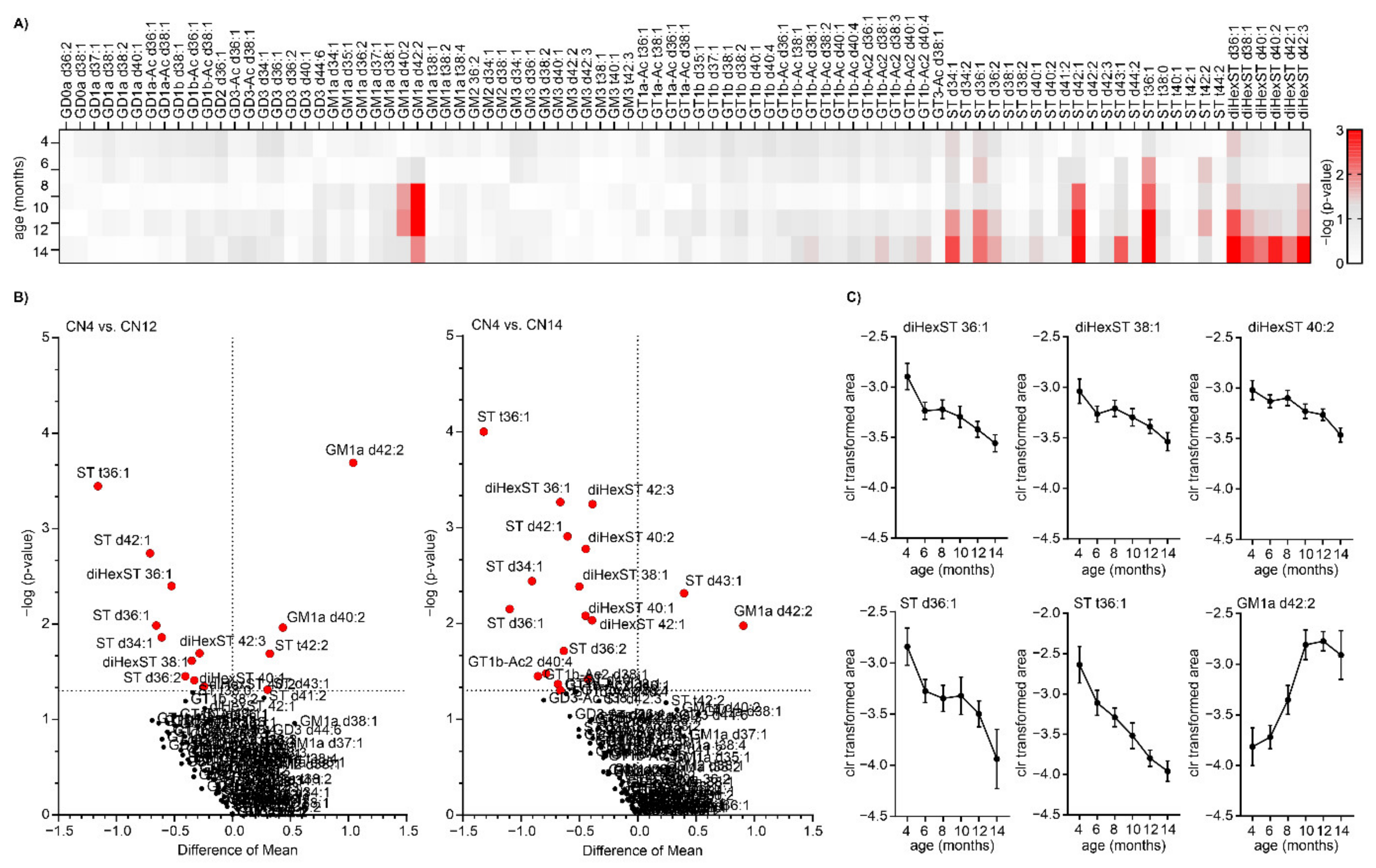
2.2. Glycosphingolipid Screening Revealed Changes in Ganglioside and Sulfatide Levels in Aging
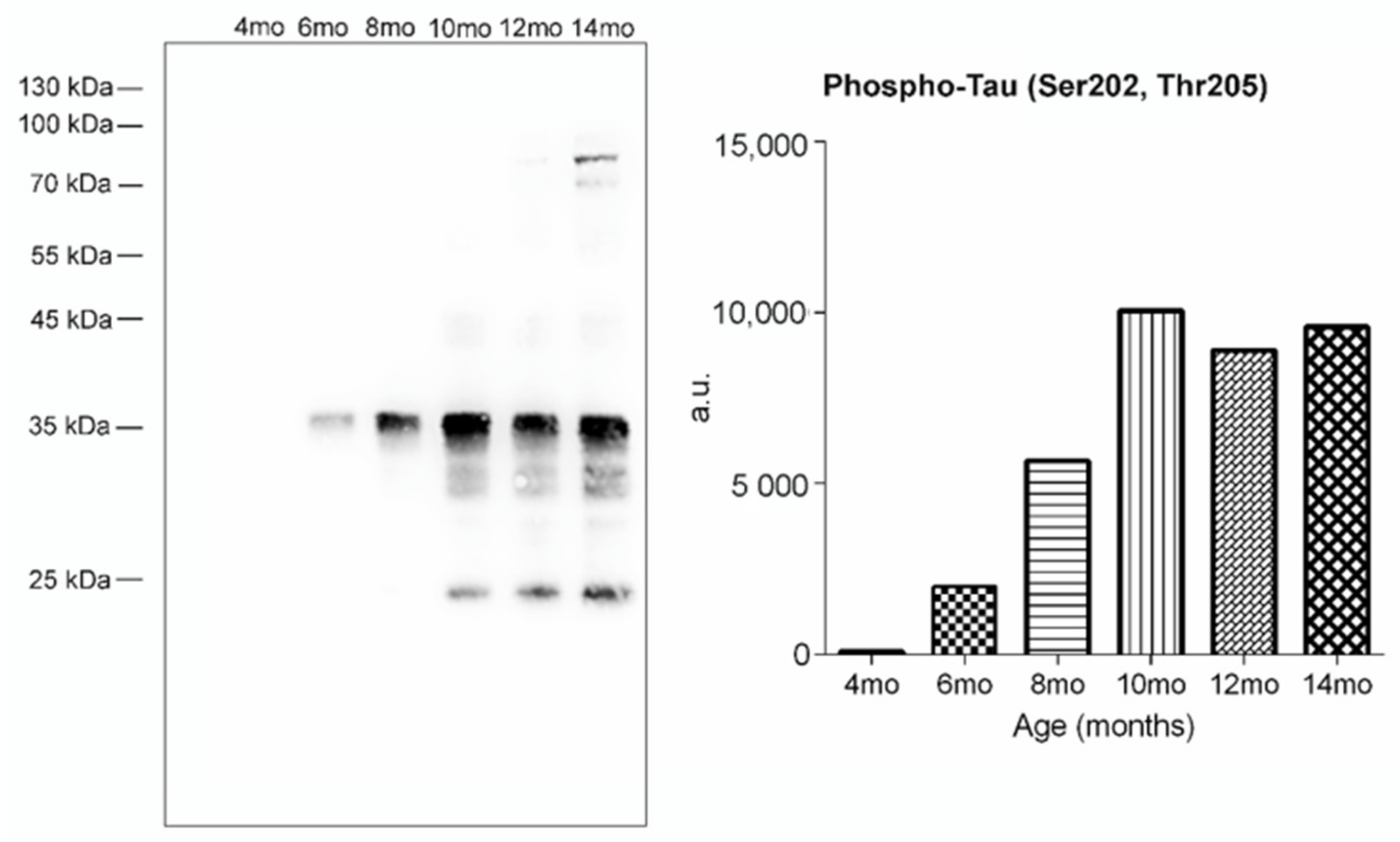
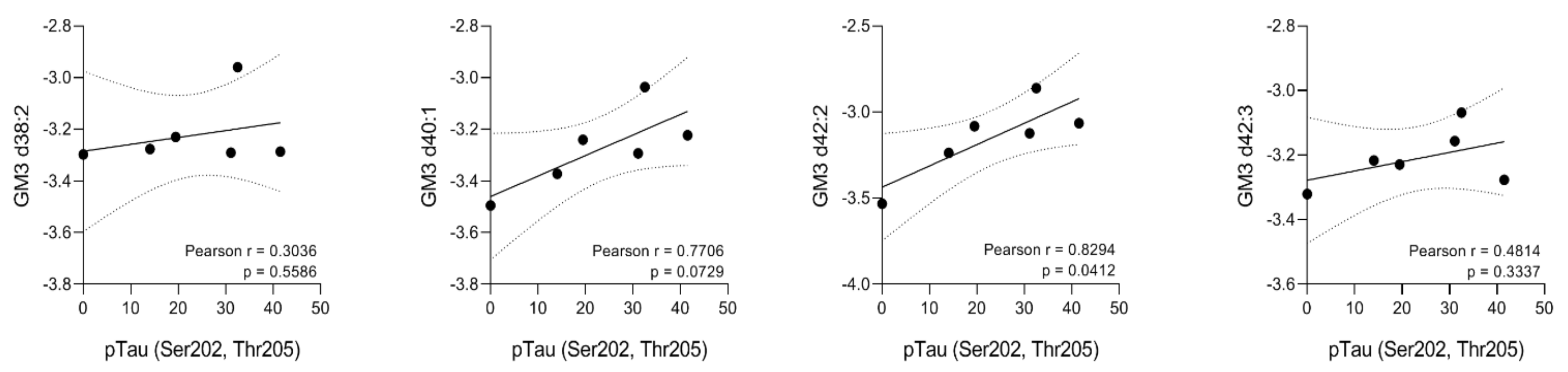
2.3. Glycosphingolipid Screening in an SHR24 Rat Model for Tauopathy
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Reagents
4.3. Sample Preparation
4.4. UHPLC-Q-TOF Analysis
4.5. Immunohistochemical Staining
4.6. Preparation of Sarkosyl-Insoluble PHF-tau from Transgenic Rat Brains
4.7. Biochemical Western Blot Analysis
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belarbi, K.; Cuvelier, E.; Bonte, M.-A.; Desplanque, M.; Gressier, B.; Devos, D.; Chartier-Harlin, M.-C. Glycosphingolipids and Neuroinflammation in Parkinson’s Disease. Mol. Neurodegener. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Bottai, D.; Adami, R.; Ghidoni, R. The Crosstalk between Glycosphingolipids and Neural Stem Cells. J. Neurochem. 2019, 148, 698–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, R.D. Stuck on Sugars—How Carbohydrates Regulate Cell Adhesion, Recognition, and Signaling. Glycoconj. J. 2019, 36, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Chakraborty, G.; Shah, R.; Mao, R.-F.; Kumar, A.; Yang, D.-S.; Dobrenis, K.; Saito, M. Elevation of GM2 Ganglioside during Ethanol-induced Apoptotic Neurodegeneration in the Developing Mouse Brain. J. Neurochem. 2012, 121, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Boutry, M.; Branchu, J.; Lustremant, C.; Pujol, C.; Pernelle, J.; Matusiak, R.; Seyer, A.; Poirel, M.; Chu-Van, E.; Pierga, A.; et al. Inhibition of Lysosome Membrane Recycling Causes Accumulation of Gangliosides That Contribute to Neurodegeneration. Cell Rep. 2018, 23, 3813–3826. [Google Scholar] [CrossRef]
- Ohmi, Y.; Ohkawa, Y.; Tajima, O.; Sugiura, Y.; Furukawa, K.; Furukawa, K. Ganglioside Deficiency Causes Inflammation and Neurodegeneration via the Activation of Complement System in the Spinal Cord. J. Neuroinflamm. 2014, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Zhou, Y.; Holtzman, D.M.; Han, X. Apolipoprotein E Mediates Sulfatide Depletion in Animal Models of Alzheimer’s Disease. Neurobiol. Aging 2010, 31, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Marbois, B.N.; Faull, K.F.; Fluharty, A.L.; Raval-Fernandes, S.; Rome, L.H. Analysis of Sulfatide from Rat Cerebellum and Multiple Sclerosis White Matter by Negative Ion Electrospray Mass Spectrometry. Biochim. Biophys. Acta (BBA) Cell Biol. Lipids 2000, 1484, 59–70. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, M.; Li, J.-L.; Cairns, N.J.; Han, X. Specific Changes of Sulfatide Levels in Individuals with Pre-Clinical Alzheimer’s Disease: An Early Event in Disease Pathogenesis. J. Neurochem. 2013, 127, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Mansson, J.-E.; Vanier, M.-T.; Svennerholm, L. Changes in the Fatty Acid and Sphingosine Composition of the Major Gangliosides of Human Brain with Age. J. Neurochem. 1978, 30, 273–275. [Google Scholar] [CrossRef]
- Rosenberg, A.; Stern, N. Changes in Sphingosine and Fatty Acid Components of the Gangliosides in Developing Rat and Human Brain. J. Lipid Res. 1966, 7, 122–131. [Google Scholar] [CrossRef]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT Mutations, Tauopathy, and Mechanisms of Neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and Tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Sang, T.-K.; Chiang, A.-S. Untangling the Tauopathy for Alzheimer’s Disease and Parkinsonism. J. Biomed. Sci. 2018, 25, 54. [Google Scholar] [CrossRef] [Green Version]
- Hájek, R.; Jirásko, R.; Lísa, M.; Cífková, E.; Holčapek, M. Hydrophilic Interaction Liquid Chromatography—Mass Spectrometry Characterization of Gangliosides in Biological Samples. Anal. Chem. 2017, 89, 12425–12432. [Google Scholar] [CrossRef]
- Ovsepyan, L.M.; Kazaryan, G.S.; Akopdzhanyan, A.A.; Lvov, M.V. Age-Dependent Changes in Phospholipid Content and Neutral Lipid Contents during Aging. Adv. Gerontol. 2013, 3, 42–45. [Google Scholar] [CrossRef]
- Vos, J.P.; Lopes-Cardozo, M.; Gadella, B.M. Metabolic and Functional Aspects of Sulfogalactolipids. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1994, 1211, 125–149. [Google Scholar] [CrossRef]
- Svennerholm, L.; Boström, K.; Fredman, P.; Jungbjer, B.; Månsson, J.-E.; Rynmark, B.-M. Membrane Lipids of Human Peripheral Nerve and Spinal Cord. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1992, 1128, 1–7. [Google Scholar] [CrossRef]
- Marcus, J.; Honigbaum, S.; Shroff, S.; Honke, K.; Rosenbluth, J.; Dupree, J.L. Sulfatide Is Essential for the Maintenance of CNS Myelin and Axon Structure. Glia 2006, 53, 372–381. [Google Scholar] [CrossRef]
- Han, X. Potential Mechanisms Contributing to Sulfatide Depletion at the Earliest Clinically Recognizable Stage of Alzheimer’s Disease: A Tale of Shotgun Lipidomics. J. Neurochem. 2007, 103, 171–179. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W.; Kelley, J.; Morris, J.C. Substantial Sulfatide Deficiency and Ceramide Elevation in Very Early Alzheimer’s Disease: Potential Role in Disease Pathogenesis. J. Neurochem. 2002, 82, 809–818. [Google Scholar] [CrossRef]
- Couttas, T.A.; Kain, N.; Suchowerska, A.K.; Quek, L.-E.; Turner, N.; Fath, T.; Garner, B.; Don, A.S. Loss of Ceramide Synthase 2 Activity, Necessary for Myelin Biosynthesis, Precedes Tau Pathology in the Cortical Pathogenesis of Alzheimer’s Disease. Neurobiol. Aging 2016, 43, 89–100. [Google Scholar] [CrossRef]
- Strnad, Š.; Pražienková, V.; Sýkora, D.; Cvačka, J.; Maletínská, L.; Popelová, A.; Vrkoslav, V. The Use of 1,5-Diaminonaphthalene for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging of Brain in Neurodegenerative Disorders. Talanta 2019, 201, 364–372. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Milani, D.; Leon, A. Monosialoganglioside GM1 Protects against Anoxia-Induced Neuronal Death in Vitro. Exp. Neurol. 1989, 106, 297–305. [Google Scholar] [CrossRef]
- Favaron, M.; Manev, H.; Alho, H.; Bertolino, M.; Ferret, B.; Guidotti, A.; Costa, E. Gangliosides Prevent Glutamate and Kainate Neurotoxicity in Primary Neuronal Cultures of Neonatal Rat Cerebellum and Cortex. Proc. Natl. Acad. Sci. USA 1988, 85, 7351–7355. [Google Scholar] [CrossRef] [Green Version]
- Park, D.H.; Wang, L.; Pittock, P.; Lajoie, G.; Whitehead, S.N. Increased Expression of GM1 Detected by Electrospray Mass Spectrometry in Rat Primary Embryonic Cortical Neurons Exposed to Glutamate Toxicity. Anal. Chem. 2016, 88, 7844–7852. [Google Scholar] [CrossRef]
- Benady, A.; Freidin, D.; Pick, C.G.; Rubovitch, V. GM1 Ganglioside Prevents Axonal Regeneration Inhibition and Cognitive Deficits in a Mouse Model of Traumatic Brain Injury. Sci. Rep. 2018, 8, 13340. [Google Scholar] [CrossRef] [Green Version]
- Caughlin, S.; Maheshwari, S.; Weishaupt, N.; Yeung, K.K.C.; Cechetto, D.F.; Whitehead, S.N. Age-Dependent and Regional Heterogeneity in the Long-Chain Base of A-Series Gangliosides Observed in the Rat Brain Using MALDI Imaging. Sci. Rep. 2017, 7, 16135. [Google Scholar] [CrossRef] [Green Version]
- Palestini, P.; Masserini, M.; Fiorilli, A.; Calappi, E.; Tettamanti, G. Age-Related Changes in the Ceramide Composition of the Major Gangliosides Present in Rat Brain Subcellular Fractions Enriched in Plasma Membranes of Neuronal and Myelin Origin. J. Neurochem. 1993, 61, 955–960. [Google Scholar] [CrossRef]
- Shin, M.-K.; Choi, M.-S.; Chae, H.-J.; Kim, J.-W.; Kim, H.-G.; Kim, K.-L. Ganglioside GQ1b Ameliorates Cognitive Impairments in an Alzheimer’s Disease Mouse Model, and Causes Reduction of Amyloid Precursor Protein. Sci. Rep. 2019, 9, 8512. [Google Scholar] [CrossRef] [Green Version]
- Caughlin, S.; Maheshwari, S.; Agca, Y.; Agca, C.; Harris, A.J.; Jurcic, K.; Yeung, K.K.-C.; Cechetto, D.F.; Whitehead, S.N. Membrane-Lipid Homeostasis in a Prodromal Rat Model of Alzheimer’s Disease: Characteristic Profiles in Ganglioside Distributions during Aging Detected Using MALDI Imaging Mass Spectrometry. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Kaya, I.; Brinet, D.; Michno, W.; Syvänen, S.; Sehlin, D.; Zetterberg, H.; Blennow, K.; Hanrieder, J. Delineating Amyloid Plaque Associated Neuronal Sphingolipids in Transgenic Alzheimer’s Disease Mice (TgArcSwe) Using MALDI Imaging Mass Spectrometry. ACS Chem. Neurosci. 2017, 8, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, K. Aβ–Ganglioside Interactions in the Pathogenesis of Alzheimer’s Disease. Biochim. Biophys. Acta (BBA) Biomembr. 2020, 1862, 183233. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Ihara, Y. GM1 Ganglioside-Bound Amyloid β-Protein in Alzheimer’s Disease Brain. Neurobiol. Aging 1998, 19, S65–S67. [Google Scholar] [CrossRef]
- Tooyama, I.; Yamada, T.; Kim, S.U.; McGeer, P.L. Immunohistochemical Study of A2B5-Positive Ganglioside in Postmortem Human Brain Tissue of Alzheimer Disease, Amyotrophic Lateral Sclerosis, Progressive Supranuclear Palsy and Control Cases. Neurosci. Lett. 1992, 136, 91–94. [Google Scholar] [CrossRef]
- Yasuhara, O.; Matsuo, A.; Tooyama, I.; Kimura, H.; McGeer, E.G.; McGeer, P.L. Pick’s Disease Immunohistochemistry: New Alterations and Alzheimer’s Disease Comparisons. Acta Neuropathol. 1995, 89, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.B.; Oliveira, T.G.; Cortes, E.P.; Honig, L.S.; Duff, K.E.; Small, S.A.; Wenk, M.R.; Shui, G.; Di Paolo, G. Comparative Lipidomic Analysis of Mouse and Human Brain with Alzheimer Disease. J. Biol. Chem. 2012, 287, 2678–2688. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.; Kim, Y.-S.; Kim, H.-T.; Kim, C.-H.; Cho, E.-W.; Kang, H.-Y.; Kim, N.-S.; Kim, C.-H.; Ryu, S.E.; Lee, J.-H.; et al. Ganglioside GM3 Is Involved in Neuronal Cell Death. FASEB J. 2006, 20, 1248–1250. [Google Scholar] [CrossRef]
- Adams, K.J.; Pratt, B.; Bose, N.; Dubois, L.G.; St. John-Williams, L.; Perrott, K.M.; Ky, K.; Kapahi, P.; Sharma, V.; MacCoss, M.J.; et al. Skyline for Small Molecules: A Unifying Software Package for Quantitative Metabolomics. J. Proteome Res. 2020, 19, 1447–1458. [Google Scholar] [CrossRef]
- Gardlo, A.; Friedecký, D.; Hron, K.; Najdekr, L.; Karlíková, R.; Adam, T. Package ‘Metabol’; Palacky University: Olomouc, Czech Republic, 2019; Available online: https://github.com/AlzbetaG/Metabol/ (accessed on 15 August 2021).
- Team, R.C. R: A Language and Environment for Statistical Computing; R Project for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olešová, D.; Majerová, P.; Hájek, R.; Piešťanský, J.; Brumarová, R.; Michalicová, A.; Jurkanin, B.; Friedecký, D.; Kováč, A. GM3 Ganglioside Linked to Neurofibrillary Pathology in a Transgenic Rat Model for Tauopathy. Int. J. Mol. Sci. 2021, 22, 12581. https://doi.org/10.3390/ijms222212581
Olešová D, Majerová P, Hájek R, Piešťanský J, Brumarová R, Michalicová A, Jurkanin B, Friedecký D, Kováč A. GM3 Ganglioside Linked to Neurofibrillary Pathology in a Transgenic Rat Model for Tauopathy. International Journal of Molecular Sciences. 2021; 22(22):12581. https://doi.org/10.3390/ijms222212581
Chicago/Turabian StyleOlešová, Dominika, Petra Majerová, Roman Hájek, Juraj Piešťanský, Radana Brumarová, Alena Michalicová, Bernadeta Jurkanin, David Friedecký, and Andrej Kováč. 2021. "GM3 Ganglioside Linked to Neurofibrillary Pathology in a Transgenic Rat Model for Tauopathy" International Journal of Molecular Sciences 22, no. 22: 12581. https://doi.org/10.3390/ijms222212581
APA StyleOlešová, D., Majerová, P., Hájek, R., Piešťanský, J., Brumarová, R., Michalicová, A., Jurkanin, B., Friedecký, D., & Kováč, A. (2021). GM3 Ganglioside Linked to Neurofibrillary Pathology in a Transgenic Rat Model for Tauopathy. International Journal of Molecular Sciences, 22(22), 12581. https://doi.org/10.3390/ijms222212581






