Roles of OX40 and OX40 Ligand in Mycosis Fungoides and Sézary Syndrome
Abstract
:1. Introduction
2. Results
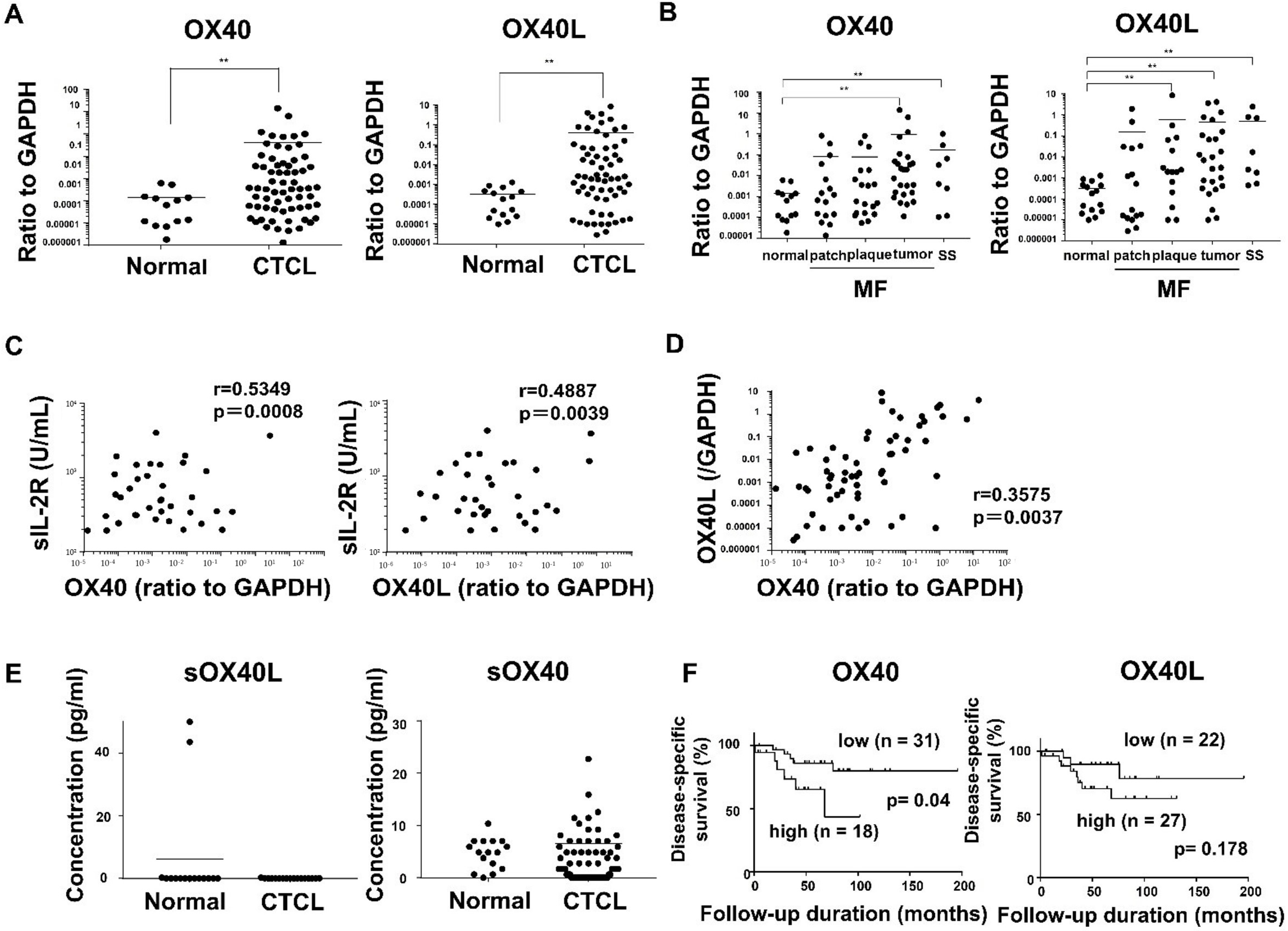
2.1. OX40 and OX40L Expression in MF/SS Patients
2.2. Immunohistochemical Staining of OX40 and OX40L in the Skin from MF/SS Patients
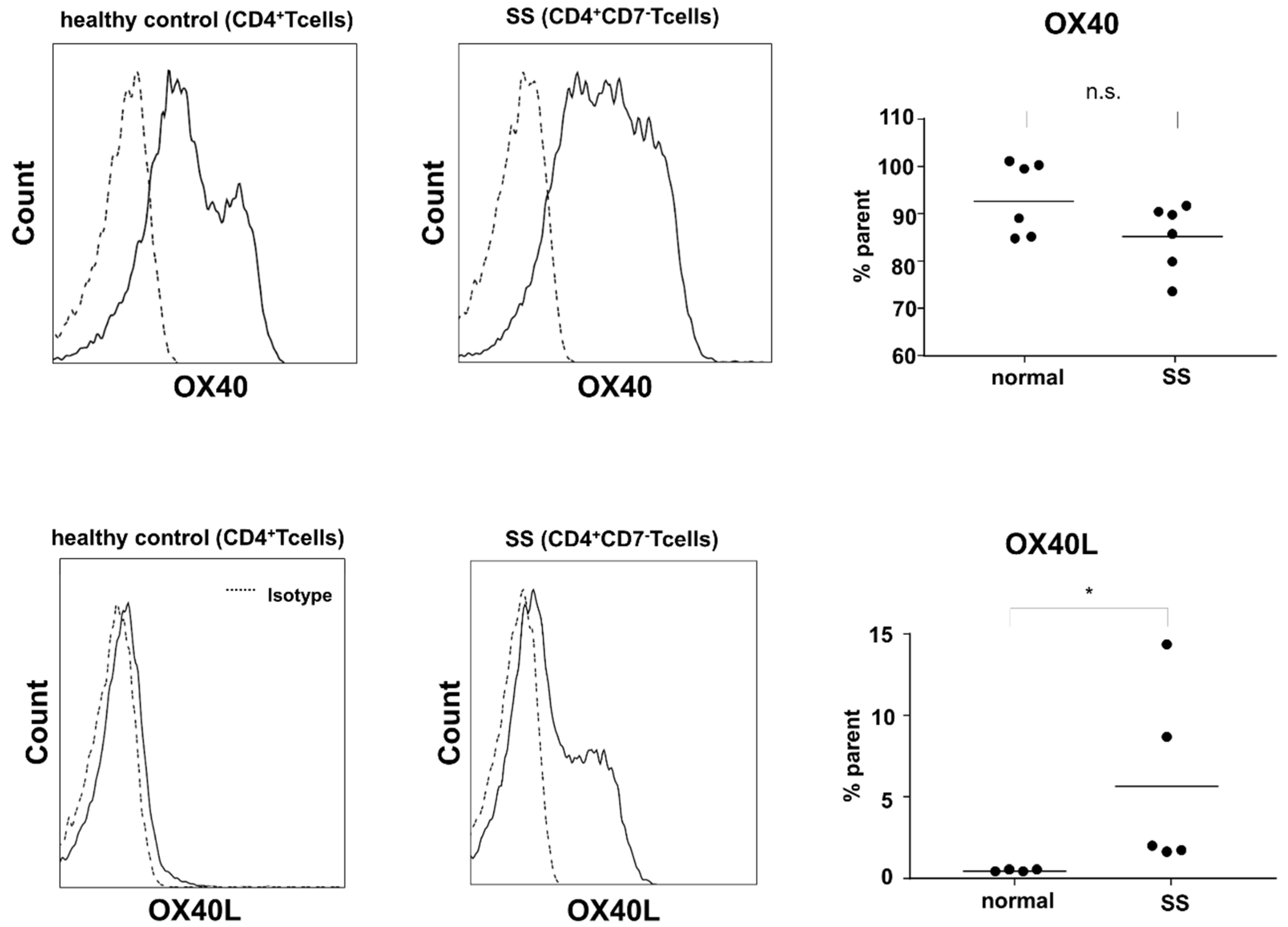
2.3. Expression of OX40 and OX40L in Sézary Cells
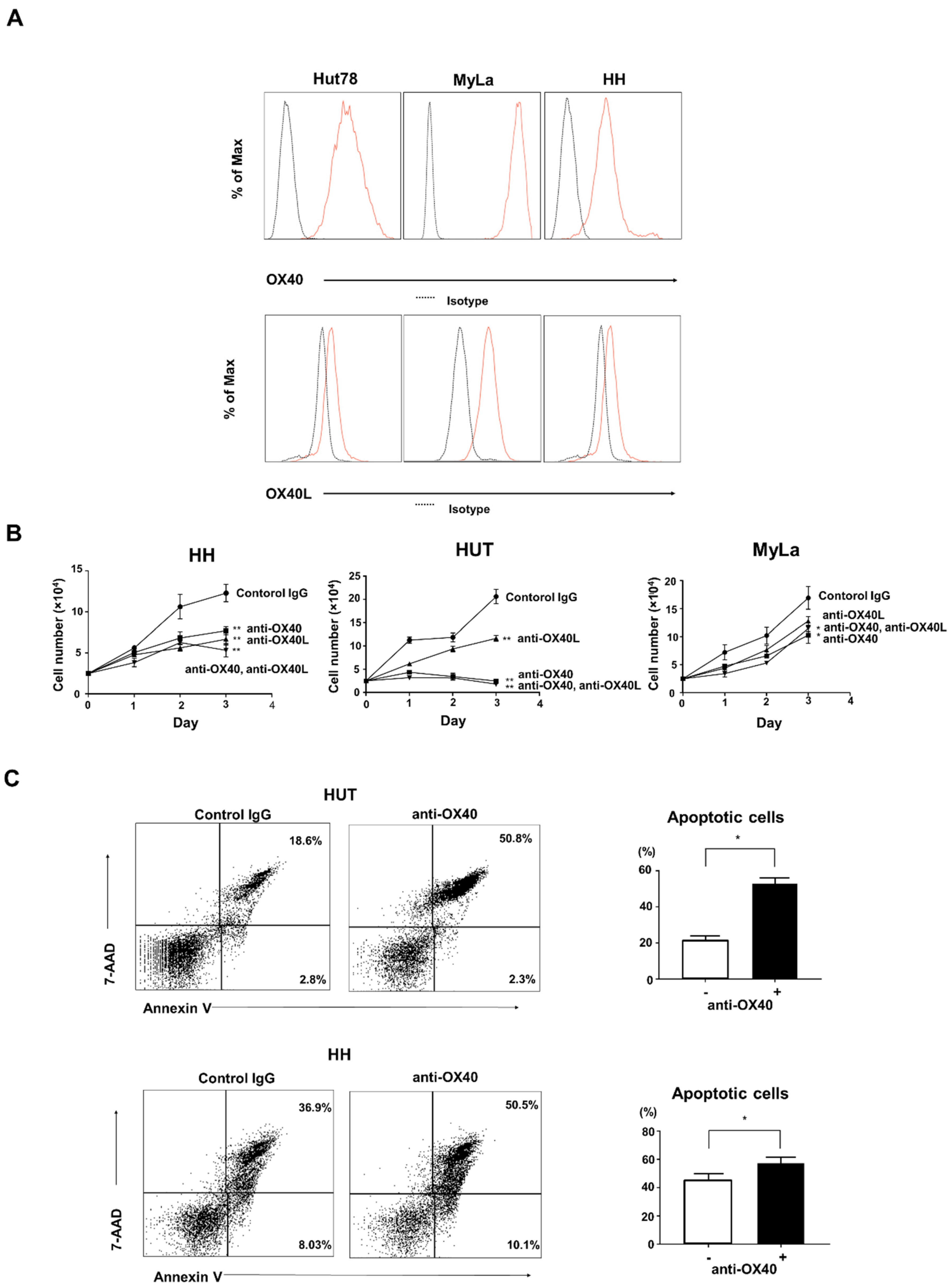
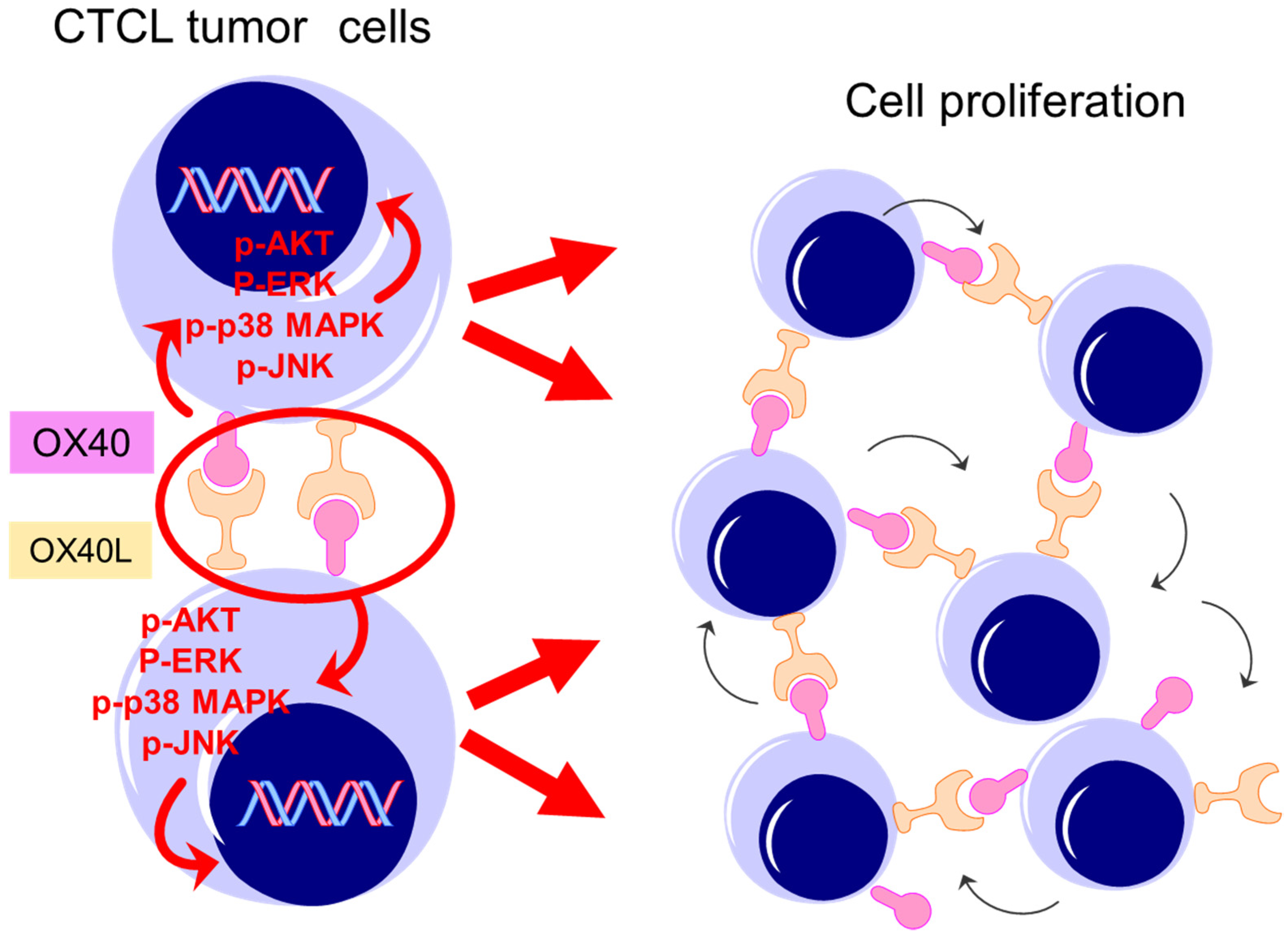
2.4. Effects of OX40 and OX40L on CTCL Cell Lines
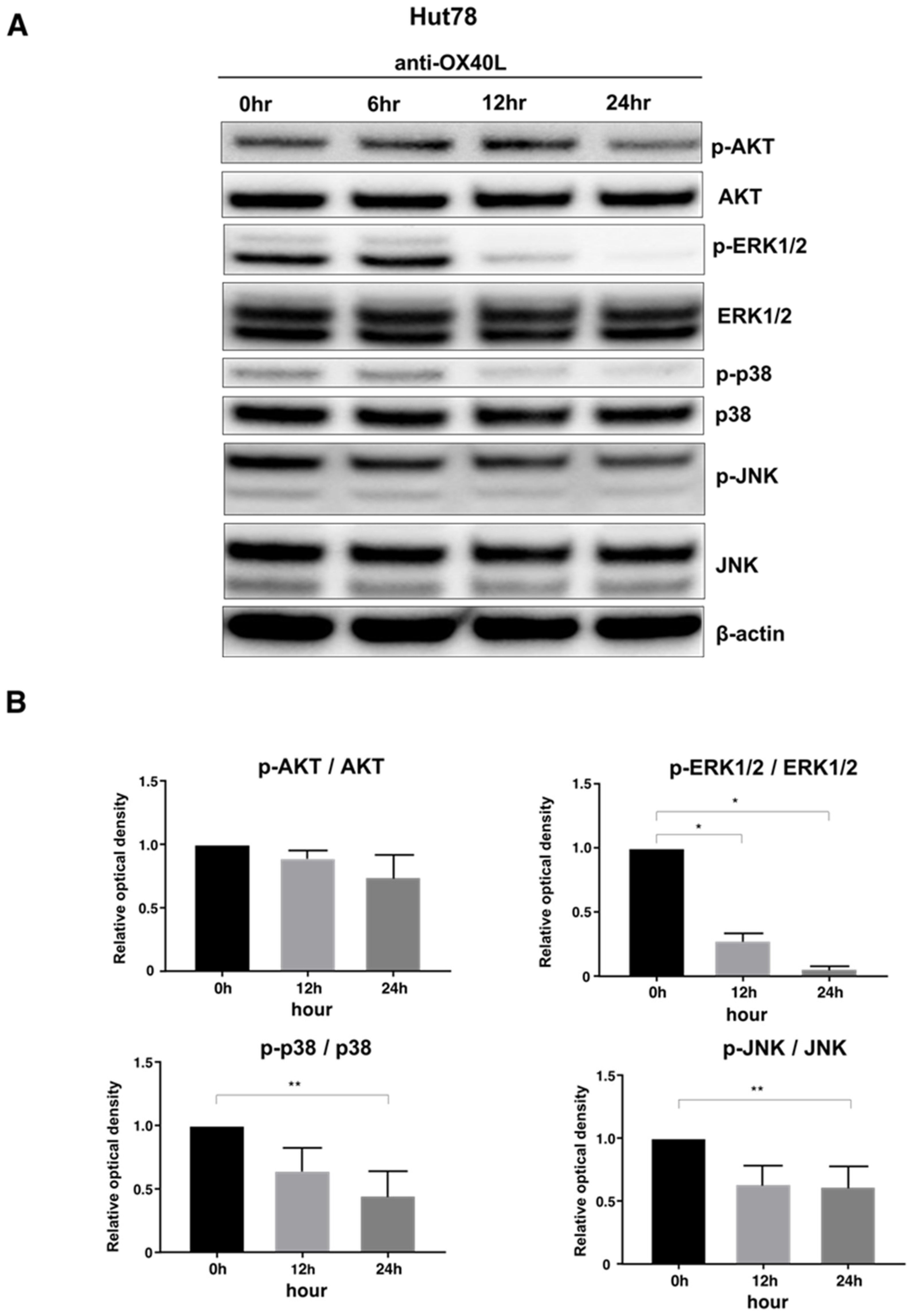
2.5. Examination of Intracellular Signal Changes Due to Inhibition of OX40–OX40L Interaction
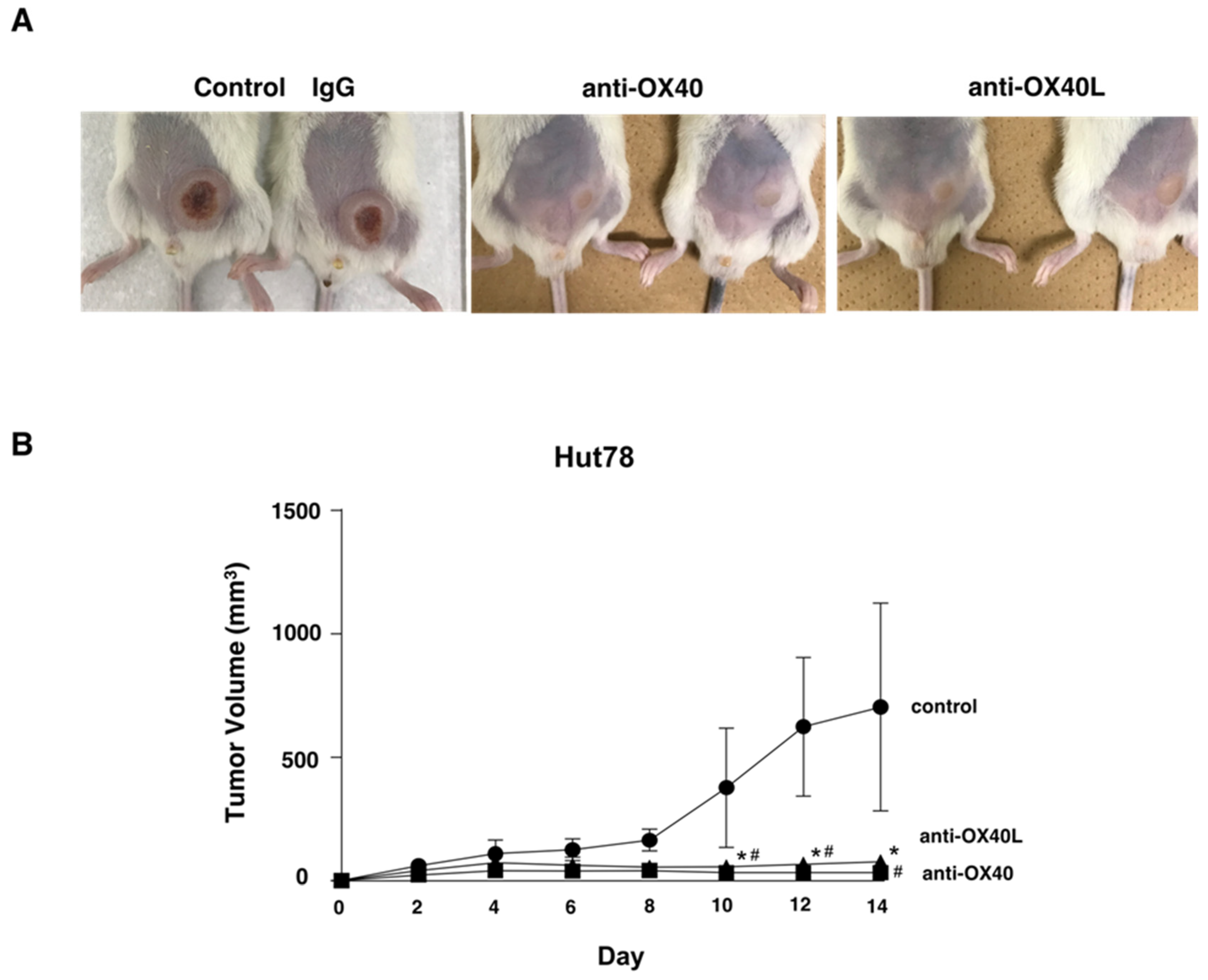
2.6. Examination in a Tumor Growth Model Using Mice
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. Cell Lines
4.3. Quantitative RT-PCR
4.4. Survival Analysis
4.5. Immunohistochemistry
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Flow Cytometric Analyses
4.8. Western Blotting
4.9. Proliferation Assays by Cell Count
4.10. Apoptosis Assays
4.11. In Vivo Animal Experiments
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood J. Am. Soc. Hematol. 2005, 105, 3768–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterry, W.; Mielke, V. CD4+ cutaneous T-cell lymphomas show the phenotype of helper/inducer T cells (CD45RA−, CDw29+). J. Investig. Dermatol. 2020, 93, 413–416. [Google Scholar] [CrossRef]
- Claudy, A.L.; Rouchouse, B.; Boucheron, S.; Lepetit, J.C. Treatment of cutaneous lymphoma with etretinate. Br. J. Dermatol. 1983, 109, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, M.J.; Lazarus, H.M.; Carreras, J.; Laport, G.G.; Cutler, C.S.; Wiernik, P.H.; Hale, G.A.; Maharaj, D.; Gale, R.P.; Rowlings, P.A.; et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014, 49, 1360–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, T.; Sugaya, M.; Cury-Martins, J.; Vasconcelos-Berg, R.; Suga, H.; Miyagaki, T.; Fujita, H.; Izutsu, K.; Sato, S.; Jose, A. Hematopoietic stem cell transplantation for cutaneous T-cell lymphoma: Summary of 11 cases from two facilities in Japan and Brazil. J. Dermatol. 2016, 43, 638–642. [Google Scholar] [CrossRef]
- de Masson, A.; Beylot-Barry, M.; Bouaziz, J.D.; de Peffault Latour, R.; Aubin, F.; Garciaz, S.; Michel, I.; Dereure, O.; Dalle, S.; Dompmartin, A.; et al. Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: A study from the French Society of Bone Marrow Transplantation and French Study Group on Cutaneous Lymphomas. Haematologica 2014, 99, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Delioukina, M.; Zain, J.; Palmer, J.M.; Tsai, N.; Thomas, S.; Forman, S. Reduced-intensity allogeneic hematopoietic cell transplantation using fludarabine-melphalan conditioning for treatment of mature T-cell lymphomas. Bone Marrow Transplant. 2012, 47, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Whittaker, S.; Hoppe, R.; Prince, H.M. How I treat mycosis fungoides and Sézary syndrome. Blood J. Am. Soc. Hematol. 2016, 127, 3142–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiroaki, K.; Tomomitsu, M.; Naomi, T.-S.; Rina, N.; Tomonori, O.; Hiraku, S.; Makoto, S.; Shinichi, S. Thrombospondin-1 promotes tumor progression in cutaneous T-cell lymphoma via CD47. Leukemia 2020, 34, 845–856. [Google Scholar]
- Paterson, D.J.; Jefferies, W.A.; Green, J.R.; Brandon, M.R.; Corthesy, P.; Puklavec, M.; Williams, A.F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol. Immunol. 1987, 24, 1281–1290. [Google Scholar] [CrossRef]
- Walker, J.; Gonzalez, I.; Meeuwsen, T.; Fox, B.A.; Moudgil, T.; Miller, W.; Haley, D.; Coffey, T.; Fisher, B.; Delanty-Miller, L.; et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013, 73, 7189–7198. [Google Scholar]
- Vetto, J.T.; Lum, S.; Morris, A.; Sicotte, M.; Davis, J.; Lemon, M.; Weinberg, A. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am. J. Surg. 1997, 174, 258–265. [Google Scholar] [CrossRef]
- Voo, K.S.; Bover, L.; Harline, M.L.; Vien, L.T.; Facchinetti, V.; Arima, K.; Kwak, L.W.; Liu, Y.J. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J. Immunol. 2013, 191, 3641–3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, A.D.; Rivera, M.M.; Prell, R.; Morris, A.; Ramstad, T.; Vetto, J.T.; Urba, W.J.; Alvord, G.; Bunce, C.; Shields, J. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 2000, 164, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.R.; Weinberg, A.; Prell, R.A.; Vella, A.T. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J. Immunol. 2000, 164, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Inoi, T.; Tozawa, H.; Yamamoto, N.; Hinuma, Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I (HTLV-I). Int. J. Cancer 1985, 36, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.I.; McAdam, A.J.; Buhlmann, J.E.; Scott, S.; Lupher, M.L., Jr.; Greenfield, E.A.; Baum, P.R.; Fanslow, W.C.; Calderhead, D.M.; Freeman, G.J.; et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity 1999, 11, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Ishii, N.; Takano, H.; Miura, S.; Ndhlovu, L.C.; Nose, M.; Noda, T.; Sugamura, K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 2000, 191, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, Y.; Tanaka, Y.; Tozawa, H.; Takahashi, Y.; Maliszewski, C.; Delespesse, G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997, 159, 3838–3848. [Google Scholar]
- Takasawa, N.; Ishii, N.; Higashimura, N.; Murata, K.; Tanaka, Y.; Nakamura, M.; Sasaki, T.; Sugamura, K. Expression of gp34 (OX40 ligand) and OX40 on human T cell clones. Jpn. J. Cancer Res. 2001, 92, 377–382. [Google Scholar] [CrossRef]
- Vieyra-Garcia, P.; Crouch, J.D.; O’Malley, J.T.; Seger, E.W.; Yang, C.H.; Teague, J.E.; Vromans, A.M.; Gehad, A.; Win, T.S.; Yu, Z.; et al. Benign T cells drive clinical skin inflammation in cutaneous T cell lymphoma. JCI Insight 2019, 4, e124233. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.; Binder, E.; Riedl, M.; Wechselberger, G.; Steichen, E.; Edelbauer, M. Enhanced activity of Akt in Teff cells from children with lupus nephritis is associated with reduced induction of tumor necrosis factor receptor-associated factor 6 and increased OX40 expression. Arthritis Rheum. 2013, 65, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, S.; Hori, T.; Imura, A.; Takaori-Kondo, A.; Uchiyama, T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J. Biol. Chem. 1998, 273, 5808–5814. [Google Scholar] [CrossRef] [Green Version]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef]
- Palma, C.; Binaschi, M.; Bigioni, M.; Maggi, C.A.; Goso, C. CD137 and CD137 ligand constitutively coexpressed on human T and B leukemia cells signal proliferation and survival. Int. J. Cancer 2004, 108, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Imura, A.; Hori, T.; Imada, K.; Kawamata, S.; Tanaka, Y.; Imamura, S.; Uchiyama, T. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: Implication for the possible involvement of OX40 in leukemic cell infiltration. Blood J. Am. Soc. Hematol. 1997, 89, 2951–2958. [Google Scholar] [CrossRef]
- Kamijo, H.; Miyagaki, T.; Shishido-Takahashi, N.; Nakajima, R.; Oka, T.; Suga, H.; Sugaya, M.; Sato, S. Aberrant CD137 ligand expression induced by GATA6 overexpression promotes tumor progression in cutaneous T-cell lymphoma. Blood J. Am. Soc. Hematol. 2018, 132, 1922–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonezawa, A.; Dutt, S.; Chester, C.; Kim, J.; Kohrt, H.E. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin. Cancer Res. 2015, 21, 3113–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.W.; Park, S.J.; Choi, B.K.; Kim, H.H.; Nam, K.O.; Kwon, B.S. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J. Immunol. 2002, 169, 4882–4888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollok, K.E.; Kim, Y.J.; Zhou, Z.; Hurtado, J.; Kim, K.K.; Pickard, R.T.; Kwon, B.S. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 1993, 150, 771–781. [Google Scholar] [PubMed]
- Infante, J.R.; Hansen, A.R.; Pishvaian, M.J.; Chow, L.Q.M.; McAuther, G.A.; Bauer, T.M.; Liu, S.V.; Sandhu, S.K.; Tsai, F.Y.; Kim, J.; et al. A phase Ib dose escalation study of the OX40 agonist MOXR9016 and the PD-L1 inhibitor atezolizumab in patient with advanced solid tumors. J. Clin. Oncol. 2016, 34, 101. [Google Scholar]
- Di, C.; Lin, X.; Zhang, Y.; Zhong, W.; Yuan, Y.; Zhou, T.; Liu, J.; Xia, Z. Basophil-associated OX40 Ligand Participates in the Initiation of Th2 Responses during Airway Inflammation. J. Biol. Chem. 2015, 290, 12523–12536. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawana, Y.; Suga, H.; Kamijo, H.; Miyagaki, T.; Sugaya, M.; Sato, S. Roles of OX40 and OX40 Ligand in Mycosis Fungoides and Sézary Syndrome. Int. J. Mol. Sci. 2021, 22, 12576. https://doi.org/10.3390/ijms222212576
Kawana Y, Suga H, Kamijo H, Miyagaki T, Sugaya M, Sato S. Roles of OX40 and OX40 Ligand in Mycosis Fungoides and Sézary Syndrome. International Journal of Molecular Sciences. 2021; 22(22):12576. https://doi.org/10.3390/ijms222212576
Chicago/Turabian StyleKawana, Yuki, Hiraku Suga, Hiroaki Kamijo, Tomomitsu Miyagaki, Makoto Sugaya, and Shinichi Sato. 2021. "Roles of OX40 and OX40 Ligand in Mycosis Fungoides and Sézary Syndrome" International Journal of Molecular Sciences 22, no. 22: 12576. https://doi.org/10.3390/ijms222212576
APA StyleKawana, Y., Suga, H., Kamijo, H., Miyagaki, T., Sugaya, M., & Sato, S. (2021). Roles of OX40 and OX40 Ligand in Mycosis Fungoides and Sézary Syndrome. International Journal of Molecular Sciences, 22(22), 12576. https://doi.org/10.3390/ijms222212576





