Fibrinogen and Antifibrinolytic Proteins: Interactions and Future Therapeutics
Abstract
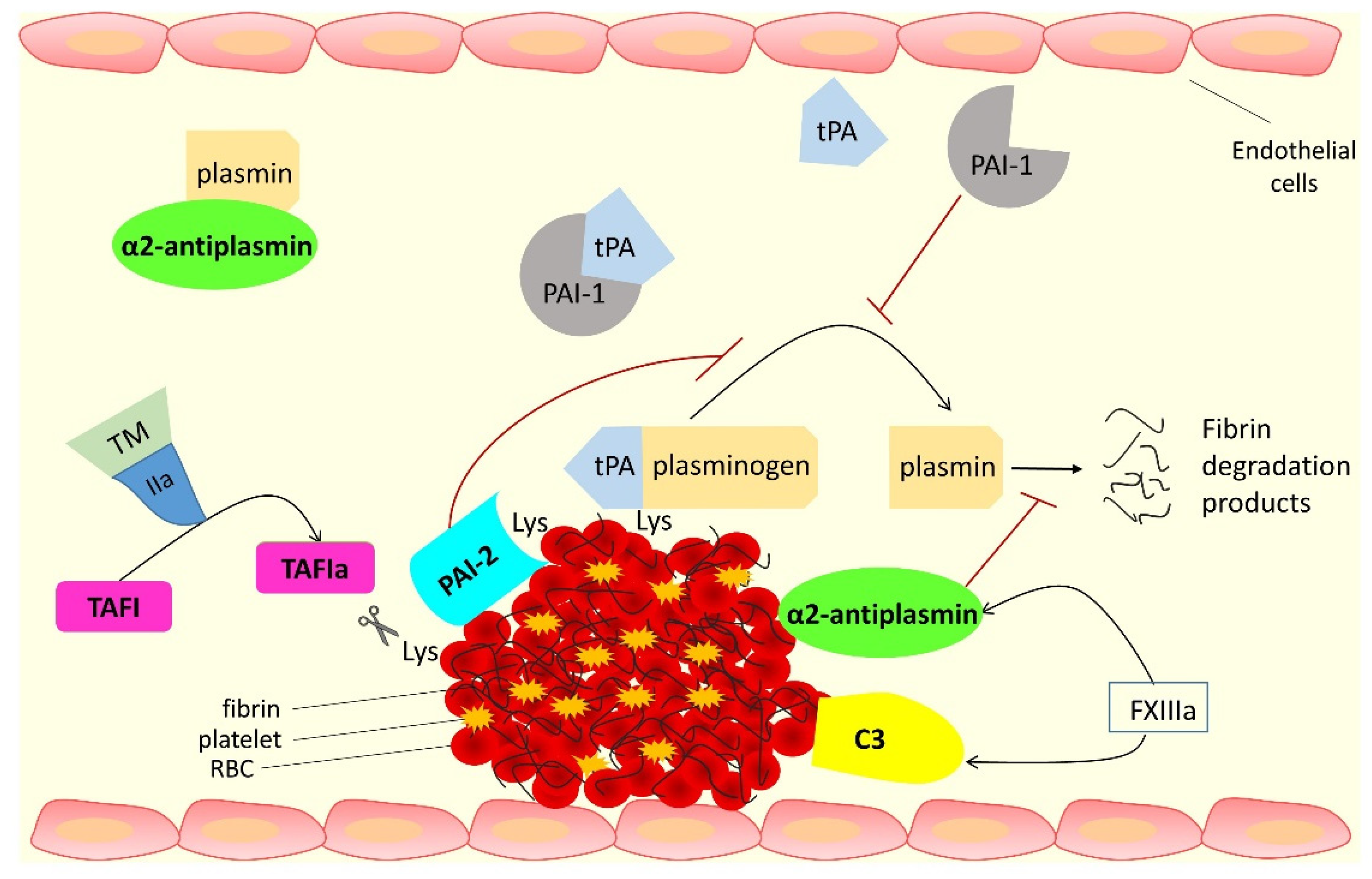
:1. Introduction
2. Interactions of Fibrinogen with Antifibrinolytic Proteins
2.1. Alpha-2 Antiplasmin (α2AP)
2.1.1. Role of α2AP Genetic and Post-Translational Variants
2.1.2. Effects of Congenital and Acquired Deficiency of α2AP
2.1.3. Role of α2AP in Thrombotic Disorders
2.2. Thrombin Activatable Fibrinolysis Inhibitor (TAFI)
2.2.1. TAFI Activation and Role of TAFI in Fibrinolysis
2.2.2. Role of TAFI Genetic and Post-Translational Variants
2.2.3. Effects of TAFI Deficiency
2.2.4. Role of TAFI in Thrombotic Disorders
2.3. Complement C3
2.3.1. Interaction of C3 with Fibrin(ogen) and Role in Fibrinolysis
2.3.2. Role of C3 Genetic and Post-Translational Variants
2.3.3. Effects of C3 Deficiency
2.3.4. Role of C3 in Thrombotic Disorders
2.4. PAI-2
2.4.1. Cross-Linking to Fibrin and Inhibition of Plasmin Generation by PAI-2
2.4.2. Role of PAI-2 Genetic and Post-Translational Variants
2.4.3. Effects of PAI-2 Deficiency
2.4.4. Role of PAI-2 in Thrombotic Disorders
3. Targeting the Antifibrinolytic Proteins for Developing Therapeutics
3.1. Therapeutics for Thrombotic Disorders
3.1.1. Targeting α2AP
3.1.2. Targeting TAFI
3.1.3. Targeting Complement C3
3.1.4. Targeting PAI-2
3.2. Therapeutics for Bleeding Disorders
4. Conclusions and the Future
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A. Thrombus Structural Composition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Eikelboom, J.W.; Anand, S.S.; Shestakovska, O.; Yusuf, S.; Fo, K.A.A. The COMPASS Trial: Net Clinical Benefit of Low-Dose Rivaroxaban Plus Aspirin as Compared With Aspirin in Patients With Chronic Vascular Disease. Circulation 2020, 142, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Sumaya, W.; Wallentin, L.; James, S.K.; Siegbahn, A.; Gabrysch, K.; Himmelmann, A.; Ajjan, R.A.; Storey, R.F. Impaired Fibrinolysis Predicts Adverse Outcome in Acute Coronary Syndrome Patients with Diabetes: A PLATO Sub-Study. Thromb. Haemost. 2020, 120, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumaya, W.; Wallentin, L.; James, S.K.; Siegbahn, A.; Gabrysch, K.; Bertilsson, M.; Himmelmann, A.; Ajjan, R.A.; Storey, R.F. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: A PLATO substudy. Eur. Heart J. 2018, 39, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Kietsiriroje, N.; Ariens, R.A.S.; Ajjan, R.A. Fibrinolysis in Acute and Chronic Cardiovascular Disease. Semin. Thromb. Hemost. 2021, 47, 490–505. [Google Scholar] [CrossRef]
- Kearney, K.; Tomlinson, D.; Smith, K.; Ajjan, R. Hypofibrinolysis in diabetes: A therapeutic target for the reduction of cardiovascular risk. Cardiovasc. Diabetol. 2017, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Kuruvilla, M.; Gurk-Turner, C. A review of warfarin dosing and monitoring. Bayl. Univ. Med Cent. Proc. 2001, 14, 305–306. [Google Scholar] [CrossRef]
- Mekaj, A.; Mekaj, Y.; Duci, S.; Miftari, E. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Gaule, T.G.; Ajjan, R.A. Fibrin(ogen) as a Therapeutic Target: Opportunities and Challenges. Int. J. Mol. Sci. 2021, 22, 6916. [Google Scholar] [CrossRef] [PubMed]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahni, A.; Francis, C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000, 96, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Lip, G.Y. Fibrinogen: Biochemistry, epidemiology and determinants. QJM 2003, 96, 711–729. [Google Scholar] [CrossRef] [Green Version]
- Sahni, A.; Odrljin, T.; Francis, C.W. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J. Biol. Chem. 1998, 273, 7554–7559. [Google Scholar] [CrossRef] [Green Version]
- Campbell, P.G.; Durham, S.K.; Hayes, J.D.; Suwanichkul, A.; Powell, D.R. Insulin-like growth factor-binding protein-3 binds fibrinogen and fibrin. J. Biol. Chem. 1999, 274, 30215–30221. [Google Scholar] [CrossRef] [Green Version]
- Sarker, H.; Hardy, E.; Haimour, A.; Maksymowych, W.P.; Botto, L.D.; Fernandez-Patron, C. Identification of fibrinogen as a natural inhibitor of MMP-2. Sci. Rep. 2019, 9, 4340. [Google Scholar] [CrossRef] [Green Version]
- Kawashita, E.; Kanno, Y.; Asayama, H.; Okada, K.; Ueshima, S.; Matsuo, O.; Matsuno, H. Involvement of α -antiplasmin in dendritic growth of hippocampal neurons. J. Neurochem. 2013, 126, 58–69. [Google Scholar] [CrossRef]
- Menoud, P.A.; Sappino, N.; Boudal-Khoshbeen, M.; Vassalli, J.D.; Sappino, A.P. The kidney is a major site of alpha(2)-antiplasmin production. J. Clin. Investig. 1996, 97, 2478–2484. [Google Scholar] [CrossRef] [Green Version]
- Collen, D. Identification and some properties of a new fast-reacting plasmin inhibitor in human plasma. Eur. J. Biochem. 1976, 69, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Mullertz, S.; Clemmensen, I. The primary inhibitor of plasmin in human plasma. Biochem. J. 1976, 159, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangert, K.; Johnsen, A.H.; Christensen, U.; Thorsen, S. Different N-terminal forms of alpha 2-plasmin inhibitor in human plasma. Biochem. J. 1993, 291 Pt 2, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Abdul, S.; Leebeek, F.W.G.; Rijken, D.C.; De Willige, S.U. Natural heterogeneity of α2-antiplasmin: Functional and clinical consequences. Blood 2016, 127, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Wiman, B.; Collen, D. Purification and characterization of human antiplasmin, the fast-acting plasmin inhibitor in plasma. Eur. J. Biochem. 1977, 78, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Moroi, M.; Aoki, N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J. Biol. Chem. 1976, 251, 5956–5965. [Google Scholar]
- Wiman, B.; Collen, D. On the mechanism of the reaction between human alpha 2-antiplasmin and plasmin. J. Biol. Chem. 1979, 254, 9291–9297. [Google Scholar] [CrossRef]
- Collen, D.; Wiman, B. Turnover of antiplasmin, the fast-acting plasmin inhibitor of plasma. Blood 1979, 53, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Sakata, Y.; Aoki, N. Significance of cross-linking of alpha 2-plasmin inhibitor to fibrin in inhibition of fibrinolysis and in hemostasis. J. Clin. Investig. 1982, 69, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Aoki, N. Cross-linking site in fibrinogen for alpha 2-plasmin inhibitor. J. Biol. Chem. 1986, 261, 15591–15595. [Google Scholar] [CrossRef]
- Duval, C.; Ariens, R.A.S. Fibrinogen splice variation and cross-linking: Effects on fibrin structure/function and role of fibrinogen γ′ as thrombomobulin II. Matrix Biol. 2017, 60–61, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.N.; Jackson, K.W.; Christiansen, V.J.; Chung, K.H.; McKee, P.A. A novel plasma proteinase potentiates alpha2-antiplasmin inhibition of fibrin digestion. Blood 2004, 103, 3783–3788. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.N.; Jackson, K.W.; Christiansen, V.J.; Lee, C.S.; Chun, J.G.; McKee, P.A. Why alpha-antiplasmin must be converted to a derivative form for optimal function. J. Thromb. Haemost. 2007, 5, 2095–2104. [Google Scholar] [CrossRef]
- Lee, K.N.; Jackson, K.W.; Christiansen, V.J.; Dolence, E.K.; McKee, P.A. Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor alpha2-antiplasmin. J. Thromb. Haemost. 2011, 9, 987–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, V.J.; Jackson, K.W.; Lee, K.N.; McKee, P.A. The effect of a single nucleotide polymorphism on human alpha 2-antiplasmin activity. Blood 2007, 109, 5286–5292. [Google Scholar] [CrossRef]
- Bronić, A.; Ferenčak, G.; Bernat, R.; Krleza, J.L.; Dumić, J.; Dabelić, S. Association of fibrinogen and plasmin inhibitor, but not coagulation factor XIII gene polymorphisms with coronary artery disease. J. Med. Biochem. 2021, 40, 138–149. [Google Scholar] [CrossRef]
- Wiman, B.; Lijnen, H.R.; Collen, D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim. Biophys. Acta BBA Protein Struct. 1979, 579, 142–154. [Google Scholar] [CrossRef]
- Wiman, B. Affinity-chromatographic purification of human alpha 2-antiplasmin. Biochem. J. 1980, 191, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Wiman, B.; Nilsson, T.; Cedergren, B. Studies on a form of alpha 2-antiplasmin in plasma which does not interact with the lysine-binding sites in plasminogen. Thromb. Res. 1982, 28, 193–199. [Google Scholar] [CrossRef]
- Kluft, C.; Los, P.; Jie, A.F.; van Hinsbergh, V.W.; Vellenga, E.; Jespersen, J.; Henny, C.P. The mutual relationship between the two molecular forms of the major fibrinolysis inhibitor alpha-2-antiplasmin in blood. Blood 1986, 67, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.G.; Sofian, T.; Law, R.; Coughlin, P.B.; Horvath, A. Contribution of conserved lysine residues in the alpha2-antiplasmin C terminus to plasmin binding and inhibition. J. Biol. Chem. 2011, 286, 24544–24552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Udvardy, M.; Schwartzott, D.; Jackson, K.; McKee, P.A. Hybrid peptide containing RGDF (Arg-Gly-Asp-Phe) coupled with the carboxy terminal part of alpha 2-antiplasmin capable of inhibiting platelet aggregation and promoting fibrinolysis. Blood Coagul. Fibrinolysis 1995, 6, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.A.; Plow, E.F.; Donnelly, K.J.; Hougie, C.; Griffin, J.H. A bleeding disorder due to deficiency of alpha 2-antiplasmin. Blood 1982, 59, 1246–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, S.L.; Mathew, P. Alpha2-antiplasmin and its deficiency: Fibrinolysis out of balance. Haemophilia 2008, 14, 1250–1254. [Google Scholar] [CrossRef]
- Singh, S.; Saleem, S.; Reed, G.L. Alpha2-Antiplasmin: The Devil You Don’t Know in Cerebrovascular and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 608899. [Google Scholar] [CrossRef]
- Favier, R.; Aoki, N.; de Moerloose, P. Congenital alpha(2)-plasmin inhibitor deficiencies: A review. Br. J. Haematol. 2001, 114, 4–10. [Google Scholar] [CrossRef]
- Aoki, N.; Yamanaka, T. The alpha2-plasmin inhibitor levels in liver diseases. Clin. Chim. Acta 1978, 84, 99–105. [Google Scholar]
- Avvisati, G.; Cate, J.W.T.; Sturk, A.; Lamping, R.; Petti, M.C.; Mandelli, F. Acquired alpha-2-antiplasmin deficiency in acute promyelocytic leukaemia. Br. J. Haematol. 1988, 70, 43–48. [Google Scholar] [CrossRef]
- Avvisati, G.; Cate, J.W.T.; Sturk, A.; Lamping, R.; Petti, M.C.; Mandelli, F. Effective therapy with tranexamic acid in a case of chronic disseminated intravascular coagulation with acquired alpha2-antiplasmin deficiency associated with AL amyloidosis. Thromb. Haemost. 2009, 102, 1285–1287. [Google Scholar]
- Leebeek, F.W.; Kluft, C.; Knot, E.A.; Los, P.; Cohen, A.F.; Six, A.J. Plasmin inhibitors in the prevention of systemic effects during thrombolytic therapy: Specific role of the plasminogen-binding form of alpha 2-antiplasmin. J. Am. Coll. Cardiol. 1990, 15, 1212–1220. [Google Scholar] [CrossRef] [Green Version]
- Suri, M.F.K.; Yamagishi, K.; Aleksic, N.; Hannan, P.J.; Folsom, A.R. Novel hemostatic factor levels and risk of ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc. Dis. 2010, 29, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Houng, A.K.; Reed, G.L. Venous stasis-induced fibrinolysis prevents thrombosis in mice: Role of α2-antiplasmin. Blood 2019, 134, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Butte, A.N.; Houng, A.K.; Jang, I.K.; Reed, G.L. Alpha 2-antiplasmin causes thrombi to resist fibrinolysis induced by tissue plasminogen activator in experimental pulmonary embolism. Circulation 1997, 95, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Okada, K.; Ueshima, S.; Matsuo, O.; Kozawa, O. Alpha2-antiplasmin plays a significant role in acute pulmonary embolism. J. Thromb. Haemost. 2003, 1, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Kozawa, O.; Okada, K.; Ueshima, S.; Matsuo, O.; Uematsu, T.; Matsuno, H. Plasmin generation plays different roles in the formation and removal of arterial and venous thrombus in mice. Thromb. Haemost. 2002, 87, 98–104. [Google Scholar] [CrossRef]
- Tersteeg, C.; Joly, B.S.; Gils, A.; Lijnen, R.; Deckmyn, H.; Declerck, P.J.; Plaimauer, B.; Coppo, P.; Veyradier, A.; Maas, C.; et al. Amplified endogenous plasmin activity resolves acute thrombotic thrombocytopenic purpura in mice. J. Thromb. Haemost. 2017, 15, 2432–2442. [Google Scholar] [CrossRef]
- Reed, G.L.; Houng, A.K.; Wang, D. Microvascular thrombosis, fibrinolysis, ischemic injury, and death after cerebral thromboembolism are affected by levels of circulating α2-antiplasmin. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2586–2593. [Google Scholar] [CrossRef] [Green Version]
- Foley, J.; Kim, P.Y.; Mutch, N.; Gils, A. Insights into thrombin activatable fibrinolysis inhibitor function and regulation. J. Thromb. Haemost. 2013, 11 (Suppl. 1), 306–315. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. Thrombin Activatable Fibrinolysis Inhibitor (TAFI): An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 3670. [Google Scholar] [CrossRef]
- Eaton, D.; Malloy, B.; Tsai, S.; Henzel, W.; Drayna, D. Isolation, molecular cloning, and partial characterization of a novel carboxypeptidase B from human plasma. J. Biol. Chem. 1991, 266, 21833–21838. [Google Scholar] [CrossRef]
- Vanhoof, G.; Wauters, J.; Schatteman, K.; Hendriks, D.; Goossens, F.; Bossuyt, P.; Scharpe, S. The gene for human carboxypeptidase U (CPU)—A proposed novel regulator of plasminogen activation—Maps to 13q14.11. Genomics 1996, 38, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.P.; Drayna, D. The gene encoding human plasma carboxypeptidase B (CPB2) resides on chromosome 13. Genomics 1992, 14, 549–550. [Google Scholar] [CrossRef]
- Bajzar, L.; Manuel, R.; Nesheim, M.E. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J. Biol. Chem. 1995, 270, 14477–14484. [Google Scholar] [CrossRef] [Green Version]
- Mosnier, L.O.; von dem Borne, P.A.; Meijers, J.C.; Bouma, B.N. Plasma TAFI levels influence the clot lysis time in healthy individuals in the presence of an intact intrinsic pathway of coagulation. Thromb. Haemost. 1998, 80, 829–835. [Google Scholar]
- Marx, P.F.; Brondijk, T.H.C.; Plug, T.; Romijn, R.A.; Hemrika, W.; Meijers, J.C.M.; Huizinga, E.G. Crystal structures of TAFI elucidate the inactivation mechanism of activated TAFI: A novel mechanism for enzyme autoregulation. Blood 2008, 112, 2803–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, S.-S.; Cooper, C.M.; Wood, T.; Shafer, J.A.; Gardell, S.J. Characterization of plasmin-mediated activation of plasma procarboxypeptidase B. Modulation by glycosaminoglycans. J. Biol. Chem. 1999, 274, 35046–35052. [Google Scholar] [CrossRef] [Green Version]
- Bajzar, L.; Morser, J.; Nesheim, M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J. Biol. Chem. 1996, 271, 16603–16608. [Google Scholar] [CrossRef] [Green Version]
- Bajzar, L. Thrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathway. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2511–2518. [Google Scholar] [CrossRef] [Green Version]
- Valnickova, Z.; Enghild, J.J. Human procarboxypeptidase U, or thrombin-activable fibrinolysis inhibitor, is a substrate for transglutaminases. Evidence for transglutaminase-catalyzed cross-linking to fibrin. J. Biol. Chem. 1998, 273, 27220–27224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouma, B.N.; Mosnier, L.O. Thrombin activatable fibrinolysis inhibitor (TAFI)—How does thrombin regulate fibrinolysis? Ann. Med. 2006, 38, 378–388. [Google Scholar] [CrossRef]
- Sakharov, D.V.; Plow, E.F.; Rijken, D.C. On the mechanism of the antifibrinolytic activity of plasma carboxypeptidase B. J. Biol. Chem. 1997, 272, 14477–14482. [Google Scholar] [CrossRef] [Green Version]
- Longstaff, C.; Kolev, K. Basic mechanisms and regulation of fibrinolysis. J. Thromb. Haemost. 2015, 13 (Suppl. S1), S98–S105. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Nesheim, M. A study of the protection of plasmin from antiplasmin inhibition within an intact fibrin clot during the course of clot lysis. J. Biol. Chem. 2004, 279, 13333–13339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miljić, P.; Heylen, E.; Willemse, J.; Djordjevic, V.; Radojkovic, D.; Colovic, M.; Elezovic, I.; Hendriks, D. Thrombin activatable fibrinolysis inhibitor (TAFI): A molecular link between coagulation and fibrinolysis. Srp. Arh. Celok. Lek. 2010, 138 (Suppl. S1), 74–78. [Google Scholar] [CrossRef] [PubMed]
- Boffa, M.; Wang, W.; Bajzar, L.; Nesheim, M.E. Plasma and recombinant thrombin-activable fibrinolysis inhibitor (TAFI) and activated TAFI compared with respect to glycosylation, thrombin/thrombomodulin-dependent activation, thermal stability, and enzymatic properties. J. Biol. Chem. 1998, 273, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, G.J.; Vos, H.L.; Leebeek, F.W.; Bulk, S.; Schneider, M.; Boffa, M.; Koschinsky, M.; van Tilburg, N.H.; Nesheim, M.E.; Bertina, R.M.; et al. A novel, possibly functional, single nucleotide polymorphism in the coding region of the thrombin-activatable fibrinolysis inhibitor (TAFI) gene is also associated with TAFI levels. Blood 2001, 98, 1992–1993. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Boffa, M.; Stewart, R.; Rahman, M.; Koschinsky, M.; Nesheim, M. Two naturally occurring variants of TAFI (Thr-325 and Ile-325) differ substantially with respect to thermal stability and antifibrinolytic activity of the enzyme. J. Biol. Chem. 2002, 277, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.F.; Fagundes, M.G.; Meijers, J.C.; Reitsma, P.H.; Lourenço, D.; Morelli, V.; Maffei, F.H.; Ferrari, I.C.; Piccinato, C.E.; Silva, W.A.; et al. Identification of polymorphisms in the 5′-untranslated region of the TAFI gene: Relationship with plasma TAFI levels and risk of venous thrombosis. Haematologica 2001, 86, 510–517. [Google Scholar]
- Zidane, M.; de Visser, M.C.H.; Wolde, M.T.; Vos, H.L.; de Monyé, W.; Bertina, R.M.; Huisman, M.V.; The Antelope study group. Frequency of the TAFI -438 G/A and factor XIIIA Val34Leu polymorphisms in patients with objectively proven pulmonary embolism. Thromb. Haemost. 2003, 90, 439–445. [Google Scholar] [CrossRef]
- Martini, C.; Brandts, A.; de Bruijne, E.; Vlieg, A.V.H.; Leebeek, F.; Lisman, T.; Rosendaal, F. The effect of genetic variants in the thrombin activatable fibrinolysis inhibitor (TAFI) gene on TAFI-antigen levels, clot lysis time and the risk of venous thrombosis. Br. J. Haematol. 2006, 134, 92–94. [Google Scholar] [CrossRef] [Green Version]
- Valnickova, Z.; Christensen, T.; Skottrup, P.; Thøgersen, I.B.; Højrup, P.; Enghild, J.J. Post-translational modifications of human thrombin-activatable fibrinolysis inhibitor (TAFI): Evidence for a large shift in the isoelectric point and reduced solubility upon activation. Biochemistry 2006, 45, 1525–1535. [Google Scholar] [CrossRef]
- Colucci, M.; Binetti, B.M.; Branca, M.G.; Clerici, C.; Morelli, A.; Semeraro, N.; Gresele, P. Deficiency of thrombin activatable fibrinolysis inhibitor in cirrhosis is associated with increased plasma fibrinolysis. Hepatology 2003, 38, 230–237. [Google Scholar] [CrossRef]
- Nagashima, M.; Yin, Z.F.; Zhao, L.; White, K.; Zhu, Y.; Lasky, N.; Halks-Miller, M.; Broze, G.J., Jr.; Fay, W.P.; Morser, J. Thrombin-activatable fibrinolysis inhibitor (TAFI) deficiency is compatible with murine life. J. Clin. Investig. 2002, 109, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.-S.; Holahan, M.A.; Bailey, C.; Wu, G.; Colussi, D.; Carroll, S.S.; Cook, J.J. Demonstration of enhanced endogenous fibrinolysis in thrombin activatable fibrinolysis inhibitor-deficient mice. Blood Coagul. Fibrinolysis 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Wang, X.; Smith, P.L.; Hsu, M.-Y.; Tamasi, J.A.; Bird, E.; Schumacher, W.A. Deficiency in thrombin-activatable fibrinolysis inhibitor (TAFI) protected mice from ferric chloride-induced vena cava thrombosis. J. Thromb. Thrombolysis 2007, 23, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, E.; Peeters, M.; Hoylaerts, M.F.; Lijnen, H.R.; Meijers, J.C.M.; Declerck, P.J.; Gils, A. The hyperfibrinolytic state of mice with combined thrombin-activatable fibrinolysis inhibitor (TAFI) and plasminogen activator inhibitor-1 gene deficiency is critically dependent on TAFI deficiency. J. Thromb. Haemost. 2012, 10, 2555–2562. [Google Scholar] [CrossRef]
- Wyseure, T.; Yang, T.; Zhou, J.Y.; Cooke, E.J.; Wanko, B.; Olmer, M.; Agashe, R.; Morodomi, Y.; Behrendt, N.; Lotz, M.; et al. TAFI deficiency causes maladaptive vascular remodeling after hemophilic joint bleeding. JCI Insight 2019, 4, e128379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tilburg, N.H.; Rosendaal, F.R.; Bertina, R.M. Thrombin activatable fibrinolysis inhibitor and the risk for deep vein thrombosis. Blood 2000, 95, 2855–2859. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Schönauer, V.; Weltermann, A.; Minar, E.; Bialonczyk, C.; Hirschl, M.; Schneider, B.; Quehenberger, P.; Kyrle, P.A. Thrombin-activatable fibrinolysis inhibitor and the risk for recurrent venous thromboembolism. Blood 2004, 103, 3773–3776. [Google Scholar] [CrossRef] [PubMed]
- Leebeek, F.W.G.; Goor, M.P.J.; Guimarães, A.H.C.; Brouwers, G.-J.; Maat, M.P.M.; Dippel, D.W.J.; Rijken, D.C. High functional levels of thrombin-activatable fibrinolysis inhibitor are associated with an increased risk of first ischemic stroke. J. Thromb. Haemost. 2005, 3, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Kamphuisen, P.W.; Ten Wolde, M.; Jacobs, E.M.G.; Ullmann, E.F.; Büller, H.R. Screening of high factor VIII levels is not recommended in patients with recently diagnosed pulmonary embolism. J. Thromb. Haemost. 2003, 1, 2239–2240. [Google Scholar] [CrossRef] [Green Version]
- Schielen, W.J.; Voskuilen, M.; Tesser, G.I.; Nieuwenhuizen, W. The sequence A alpha-(148-160) in fibrin, but not in fibrinogen, is accessible to monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1989, 86, 8951–8954. [Google Scholar] [CrossRef] [Green Version]
- Janssen, B.; Huizinga, E.G.; Raaijmakers, H.C.A.; Roos, A.; Daha, M.R.; Nilsson-Ekdahl, K.; Nilsson, B.; Gros, P. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 2005, 437, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.S.; Solomon, E.; Chambers, S.; Bodmer, W.; Povey, S.; Fey, G. Assignment of the structural gene for the third component of human complement to chromosome 19. Proc. Natl. Acad. Sci. USA 1982, 79, 5021–5025. [Google Scholar] [CrossRef] [Green Version]
- Vik, D.P.; Amiguet, P.; Moffat, G.J.; Fey, M.; Amiguet-Barras, F.; Wetsel, R.A.; Tack, B.F. Structural features of the human C3 gene: Intron/exon organization, transcriptional start site, and promoter region sequence. Biochemistry 1991, 30, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Reis, E.S.; Mastellos, D.C.; Gros, P.; Lambris, J.D. Complement component C3—The “Swiss Army Knife” of innate immunity and host defense. Immunol. Rev. 2016, 274, 33–58. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.P.; Gasque, P. Extrahepatic complement biosynthesis: Where, when and why? Clin. Exp. Immunol. 1997, 107, 1–7. [Google Scholar] [CrossRef]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Brückner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010, 185, 5628–5636. [Google Scholar] [CrossRef] [Green Version]
- Distelmaier, K.; Adlbrecht, C.; Jakowitsch, J.; Winkler, S.; Dunkler, D.; Gerner, C.; Wagner, O.; Lang, I.M.; Kubicek, M. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thromb. Haemost. 2009, 102, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Howes, J.M.; Richardson, V.R.; Smith, K.A.; Schroeder, V.; Somani, R.; Shore, A.; Hess, K.; Ajjan, R.; Pease, R.J.; Keen, J.N.; et al. Complement C3 is a novel plasma clot component with anti-fibrinolytic properties. Diabetes Vasc. Dis. Res. 2012, 9, 216–225. [Google Scholar] [CrossRef] [PubMed]
- King, R.J.; Schuett, K.; Tiede, C.; Jankowski, V.; John, V.; Trehan, A.; Simmons, K.; Ponnambalam, S.; Storey, R.F.; Fishwick, C.W.G.; et al. Fibrinogen interaction with complement C3: A potential therapeutic target to reduce thrombosis risk. Haematologica 2021, 106, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Richardson, V.R.; Schroeder, V.; Grant, P.J.; Standeven, K.F.; Carter, A.M. Complement C3 is a substrate for activated factor XIII that is cross-linked to fibrin during clot formation. Br. J. Haematol. 2013, 160, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Nikolajsen, C.L.; Scavenius, C.; Enghild, J.J. Human complement C3 is a substrate for transglutaminases. A functional link between non-protease-based members of the coagulation and complement cascades. Biochemistry 2012, 51, 4735–4742. [Google Scholar] [CrossRef]
- Ajjan, R.; Futers, T.S.; Brown, J.M.; Cymbalista, C.M.; Boothby, M.; Carter, A.M.; Grant, P.J. Complement C3 and C-reactive protein levels in patients with stable coronary artery disease. Thromb. Haemost. 2005, 94, 1048–1053. [Google Scholar] [CrossRef]
- Onat, A.; Uzunlar, B.; Hergenç, G.; Yazici, M.; Sari, I.; Uyarel, H.; Can, G.; Sansoy, V. Cross-sectional study of complement C3 as a coronary risk factor among men and women. Clin. Sci. 2005, 108, 129–135. [Google Scholar] [CrossRef]
- Széplaki, G.; Prohaszka, Z.; Duba, J.; Rugonfalvi-Kiss, S.; Karádi, I.; Kókai, M.; Kramer, J.; Füst, G.; Kleiber, M.; Romics, L.; et al. Association of high serum concentration of the third component of complement (C3) with pre-existing severe coronary artery disease and new vascular events in women. Atherosclerosis 2004, 177, 383–389. [Google Scholar] [CrossRef]
- Carter, A.M.; Prasad, U.K.; Grant, P.J. Complement C3 and C-reactive protein in male survivors of myocardial infarction. Atherosclerosis 2009, 203, 538–543. [Google Scholar] [CrossRef]
- Hess, K.; Alzahrani, S.H.; Mathai, M.; Schroeder, V.; Carter, A.M.; Howell, G.J.; Koko, T.; Strachan, M.W.J.; Price, J.; Smith, K.; et al. A novel mechanism for hypofibrinolysis in diabetes: The role of complement C3. Diabetologia 2012, 55, 1103–1113. [Google Scholar] [CrossRef]
- Hess, K.; Alzahrani, S.H.; Price, J.F.; Strachan, M.W.; Oxley, N.; King, R.; Gamlen, T.; Schroeder, V.; Baxter, P.D.; Ajjan, R.A. Hypofibrinolysis in type 2 diabetes: The role of the inflammatory pathway and complement C3. Diabetologia 2014, 57, 1737–1741. [Google Scholar] [CrossRef]
- Lokki, A.I.; Kaartokallio, T.; Holmberg, V.; Onkamo, P.; Koskinen, L.L.E.; Saavalainen, P.; Heinonen, S.; Kajantie, E.; Kere, J.; Kivinen, K.; et al. Analysis of Complement C3 Gene Reveals Susceptibility to Severe Preeclampsia. Front. Immunol. 2017, 8, 589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, S.; Hu, S.; Yu, J.; Xiang, Y. Association between genetic variation of complement C3 and the susceptibility to advanced age-related macular degeneration: A meta-analysis. BMC Ophthalmol. 2018, 18, 274. [Google Scholar] [CrossRef]
- Yates, J.R.; Sepp, T.; Matharu, B.K.; Khan, J.C.; Thurlby, D.A.; Shahid, H.; Clayton, D.G.; Hayward, C.; Morgan, J.; Wright, A.F.; et al. Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 2007, 357, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, V.; Quan, M. SLE and Serum Complement: Causative, Concomitant or Coincidental? Open Rheumatol. J. 2017, 11, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajjan, R.A.; Grant, P.J.; Futers, T.S.; Brown, J.M.; Carter, A.M. The association of complement C3 genotype with coronary artery disease, markers of the metabolic syndrome and C3 plasma levels. Thromb. Haemost. 2006, 95, 393–394. [Google Scholar] [CrossRef]
- Hair, P.S.; Echague, C.G.; Rohn, R.D.; Krishna, N.K.; Nyalwidhe, J.O.; Cunnion, K.M. Hyperglycemic conditions inhibit C3-mediated immunologic control of Staphylococcus aureus. J. Transl. Med. 2012, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Ghannam, A.; Pernollet, M.; Fauquert, J.-L.; Monnier, N.; Ponard, D.; Villiers, M.-B.; Péguet-Navarro, J.; Tridon, A.; Lunardi, J.; Gerlier, D.; et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J. Immunol. 2008, 181, 5158–5166. [Google Scholar] [CrossRef]
- Gotz, P.; Braumandl, A.; Kübler, M.; Kumaraswami, K.; Ishikawa-Ankerhold, H.; Lasch, M.; Deindl, E. C3 Deficiency Leads to Increased Angiogenesis and Elevated Pro-Angiogenic Leukocyte Recruitment in Ischemic Muscle Tissue. Int. J. Mol. Sci. 2021, 22, 5800. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, L.; Cui, J.; Chen, Y.; Yang, L.; Wan, J. Complement C3 deficiency ameliorates aging related changes in the kidney. Life Sci. 2020, 260, 118370. [Google Scholar] [CrossRef] [PubMed]
- Dahm, A.E.A.; Jacobsen, E.M.; Wik, H.S.; Jacobsen, A.F.; Mollnes, T.E.; Kanse, S.M.; Sandset, P.M. Elevated Complement C3 and C4 Levels are Associated with Postnatal Pregnancy-Related Venous Thrombosis. Thromb. Haemost. 2019, 119, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.A.; Conklin, J.; O’Malley, T.; Dervieux, T. Platelet-bound C4d, low C3 and lupus anticoagulant associate with thrombosis in SLE. Lupus Sci. Med. 2019, 6, e000318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekdahl, K.N.; Rönnblom, L.; Nilsson, B.; Sturfelt, G. Increased phosphate content in complement component C3, fibrinogen, vitronectin, and other plasma proteins in systemic lupus erythematosus: Covariation with platelet activation and possible association with thrombosis. Arthritis Rheum. 1997, 40, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Genton, C.; Kruithof, E.K.; Schleuning, W.D. Phorbol ester induces the biosynthesis of glycosylated and nonglycosylated plasminogen activator inhibitor 2 in high excess over urokinase-type plasminogen activator in human U-937 lymphoma cells. J. Cell Biol. 1987, 104, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Medcalf, R.L.; Stasinopoulos, S.J. The undecided serpin. The ins and outs of plasminogen activator inhibitor type 2. FEBS J. 2005, 272, 4858–4867. [Google Scholar] [CrossRef]
- Kruithof, E.K.; Baker, M.S.; Bunn, C.L. Biological and clinical aspects of plasminogen activator inhibitor type 2. Blood 1995, 86, 4007–4024. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.D.; Ahern, S.M.; Le Beau, M.M.; Lebo, R.V.; Sadler, J.E. Structure of the gene for human plasminogen activator inhibitor-2. The nearest mammalian homologue of chicken ovalbumin. J. Biol. Chem. 1989, 264, 5495–5502. [Google Scholar] [CrossRef]
- Kawano, T.; Morimoto, K.; Uemura, Y. Urokinase inhibitor in human placenta. Nature 1968, 217, 253–254. [Google Scholar] [CrossRef]
- Wun, T.C.; Reich, E. An inhibitor of plasminogen activation from human placenta. Purification and characterization. J. Biol. Chem. 1987, 262, 3646–3653. [Google Scholar]
- Kruithof, E.K.; Tran-Thang, C.; Gudinchet, A.; Hauert, J.; Nicoloso, G.; Genton, C.; Welti, H.; Bachmann, F. Fibrinolysis in pregnancy: A study of plasminogen activator inhibitors. Blood 1987, 69, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Robbie, L.A.; Dummer, S.; Booth, N.A.; Adey, G.D.; Bennett, B. Plasminogen activator inhibitor 2 and urokinase-type plasminogen activator in plasma and leucocytes in patients with severe sepsis. Br. J. Haematol. 2000, 109, 342–348. [Google Scholar] [CrossRef]
- Scherrer, A.; Kruithof, E.K.; Grob, J.P. Plasminogen activator inhibitor-2 in patients with monocytic leukemia. Leukemia 1991, 5, 479–486. [Google Scholar]
- Jensen, P.H.; Schüler, E.; Woodrow, G.; Richardson, M.; Goss, N.; Højrup, P.; Petersen, T.; Rasmussen, L. A unique interhelical insertion in plasminogen activator inhibitor-2 contains three glutamines, Gln83, Gln84, Gln86, essential for transglutaminase-mediated cross-linking. J. Biol. Chem. 1994, 269, 15394–15398. [Google Scholar] [CrossRef]
- Jensen, P.H.; Lorand, L.; Ebbesen, P.; Gliemann, J. Type-2 plasminogen-activator inhibitor is a substrate for trophoblast transglutaminase and factor XIIIa. Transglutaminase-catalyzed cross-linking to cellular and extracellular structures. Eur. J. Biochem. 1993, 214, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Robbie, L.A.; Kinghorn, S.; Exley, R.; Booth, N.A.; Ritchie, H. Monocyte plasminogen activator inhibitor 2 (PAI-2) inhibits u-PA-mediated fibrin clot lysis and is cross-linked to fibrin. Thromb. Haemost. 1999, 81, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, H.; Lawrie, L.C.; Mosesson, M.W.; Booth, N.A. Characterization of crosslinking sites in fibrinogen for plasminogen activator inhibitor 2 (PAI-2). Ann. N. Y. Acad. Sci. USA 2001, 936, 215–218. [Google Scholar] [CrossRef]
- Nieuwenhuizen, W. Sites in fibrin involved in the acceleration of plasminogen activation by t-PA. Possible role of fibrin polymerisation. Thromb. Res. 1994, 75, 343–347. [Google Scholar] [CrossRef]
- Ritchie, H.; Lawrie, L.C.; Crombie, P.W.; Mosesson, M.W.; Booth, N.A. Cross-linking of plasminogen activator inhibitor 2 and alpha 2-antiplasmin to fibrin(ogen). J. Biol. Chem. 2000, 275, 24915–24920. [Google Scholar] [CrossRef] [Green Version]
- Foy, C.A.; Grant, P.J. PCR-RFLP detection of PAI-2 gene variants: Prevalence in ethnic groups and disease relationship in patients undergoing coronary angiography. Thromb. Haemost. 1997, 77, 955–958. [Google Scholar] [CrossRef]
- Buyru, N.; Altinisik, J.; Gurel, C.B.; Ulutin, T. PCR-RFLP detection of PAI-2 variants in myocardial infarction. Clin. Appl. Thromb. Hemost. 2003, 9, 333–336. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.-Y.; Zhang, L.; Yang, Y.-N.; Xie, X.; Liu, F.; Chen, B.-D.; Ma, Y.-T. Variant of PAI-2 gene is associated with coronary artery disease and recurrent coronary event risk in Chinese Han population. Lipids Health Dis. 2015, 14, 148. [Google Scholar] [CrossRef] [Green Version]
- Saffari, B.; Jooyan, N.; Bahari, M.; Senemar, S.; Yavarian, M. Lack of association between Ser(413)/Cys polymorphism of plasminogen activator inhibitor type 2 (PAI-2) and premature coronary atherosclerotic disease. EXCLI J. 2012, 11, 407–415. [Google Scholar] [PubMed]
- Dougherty, K.M.; Pearson, J.M.; Yang, A.Y.; Westrick, R.J.; Baker, M.S.; Ginsburg, D. The plasminogen activator inhibitor-2 gene is not required for normal murine development or survival. Proc. Natl. Acad. Sci. USA 1999, 96, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Westrick, R.J.; Røjkjær, L.P.; Yang, A.Y.; Roh, M.H.; Siebert, A.E.; Ginsburg, D. Deficiency of plasminogen activator inhibitor-2 results in accelerated tumor growth. J. Thromb. Haemost. 2020, 18, 2968–2975. [Google Scholar] [CrossRef] [PubMed]
- Siefert, S.A.; Chabasse, C.; Mukhopadhyay, S.; Hoofnagle, M.; Strickland, D.K.; Sarkar, R.; Antalis, T.M. Enhanced venous thrombus resolution in plasminogen activator inhibitor type-2 deficient mice. J. Thromb. Haemost. 2014, 12, 1706–1716. [Google Scholar] [CrossRef] [Green Version]
- Reed, G.L., 3rd; Matsueda, G.R.; Haber, E. Inhibition of clot-bound alpha 2-antiplasmin enhances in vivo thrombolysis. Circulation 1990, 82, 164–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, G.L., 3rd; Matsueda, G.R.; Haber, E. Synergistic fibrinolysis: Combined effects of plasminogen activators and an antibody that inhibits alpha 2-antiplasmin. Proc. Natl. Acad. Sci. USA 1990, 87, 1114–1118. [Google Scholar] [CrossRef] [Green Version]
- Reed, G.L., 3rd; Matsueda, G.R.; Haber, E. Acceleration of plasma clot lysis by an antibody to alpha 2-antiplasmin. Trans. Assoc. Am. Physicians 1988, 101, 250–256. [Google Scholar] [PubMed]
- Sakata, Y.; Eguchi, Y.; Mimuro, J.; Matsuda, M.; Sumi, Y. Clot lysis induced by a monoclonal antibody against alpha 2-plasmin inhibitor. Blood 1989, 74, 2692–2697. [Google Scholar] [CrossRef]
- Houng, A.K.; Wang, D.; Reed, G.L. Reversing the deleterious effects of alpha2-antiplasmin on tissue plasminogen activator therapy improves outcomes in experimental ischemic stroke. Exp. Neurol. 2014, 255, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Houng, A.; Reed, G.L. Releasing the Brakes on the Fibrinolytic System in Pulmonary Emboli: Unique Effects of Plasminogen Activation and α2-Antiplasmin Inactivation. Circulation 2017, 135, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Ichinose, A.; Tamaki, T.; Aoki, N. Factor XIII-mediated cross-linking of NH2-terminal peptide of alpha 2-plasmin inhibitor to fibrin. FEBS Lett. 1983, 153, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Tamaki, T.; Aoki, N. Acceleration of fibrinolysis by the N-terminal peptide of alpha 2-plasmin inhibitor. Blood 1985, 66, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, W.P.; Eltringham-Smith, L.J.; Gataiance, S.; Bhakta, V. Addition of a sequence from alpha2-antiplasmin transforms human serum albumin into a blood clot component that speeds clot lysis. BMC Biotechnol. 2009, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakola, S.; Cahillane, G.; Stassen, J.-M.; Lijnen, H.R.; Verhamme, P. Neutralization of alpha(2)-antiplasmin by microplasmin: A randomized, double-blind, placebo-controlled, ascending-dose study in healthy male volunteers. Clin. Ther. 2009, 31, 1688–1706. [Google Scholar] [CrossRef]
- Sansilvestri-Morel, P.; Rupin, A.; Schaffner, A.-P.; Bertin, F.; Mennecier, P.; Lapret, I.; Declerck, P.J.; Baumy, P.; Vallez, M.-O.; Petit-Dop, F.; et al. S62798, a potent TAFIa inhibitor, accelerates endogenous fibrinolysis in a murine model of pulmonary thromboembolism. Thromb. Res. 2021, 204, 81–87. [Google Scholar] [CrossRef]
- Klement, P.; Liao, P.; Bajzar, L. A novel approach to arterial thrombolysis. Blood 1999, 94, 2735–2743. [Google Scholar] [CrossRef]
- da Cunha, V.; Vincelette, J.; Zhao, L.; Nagashima, M.; Kawai, K.; Yuan, S.; Emayan, K.; Islam, I.; Hosoya, J.; Sullivan, M.E.; et al. A novel inhibitor of activated thrombin activatable fibrinolysis inhibitor (TAFIa)—Part II: Enhancement of both exogenous and endogenous fibrinolysis in animal models of thrombosis. Thromb. Haemost. 2007, 97, 54–61. [Google Scholar] [CrossRef]
- Noguchi, K.; Edo, N.; Miyoshi, N.; Isobe, A.; Watanabe, A.; Ito, Y.; Morishima, Y.; Yamaguchi, K. Fibrinolytic potential of DS-1040, a novel orally available inhibitor of activated thrombin-activatable fibrinolysis inhibitor (TAFIa). Thromb. Res. 2018, 168, 96–101. [Google Scholar] [CrossRef]
- Zhou, J.; Limsakun, T.; Yin, O.; Warren, V.; Zamora, C.; Atiee, G.; Kochan, J.; Pav, J.; Kobayashi, F.; Vashi, V.; et al. First-in-Human Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of an Oral Formulation of DS-1040, an Inhibitor of the Activated Form of Thrombin-Activatable Fibrinolysis Inhibitor, in Healthy Subjects. J. Clin. Pharmacol. 2019, 59, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Gils, A.; Ceresa, E.; Macovei, A.M.; Marx, P.F.; Peeters, M.; Compernolle, G.; Declerck, P.J. Modulation of TAFI function through different pathways--implications for the development of TAFI inhibitors. J. Thromb. Haemost. 2005, 3, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Hillmayer, K.; Vancraenenbroeck, R.; DE Maeyer, M.; Compernolle, G.; Declerck, P.J.; Gils, A. Discovery of novel mechanisms and molecular targets for the inhibition of activated thrombin activatable fibrinolysis inhibitor. J. Thromb. Haemost. 2008, 6, 1892–1899. [Google Scholar] [CrossRef]
- Vercauteren, E.; Emmerechts, J.; Peeters, M.; Hoylaerts, M.F.; Declerck, P.J.; Gils, A. Evaluation of the profibrinolytic properties of an anti-TAFI monoclonal antibody in a mouse thromboembolism model. Blood 2011, 117, 4615–4622. [Google Scholar] [CrossRef] [Green Version]
- Hendrickx, M.L.; Zatloukalova, M.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S.; Gils, A.; Declerck, P.J. Identification of a novel, nanobody-induced, mechanism of TAFI inactivation and its in vivo application. J. Thromb. Haemost. 2014, 12, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wyseure, T.; Rubio, M.; Denorme, F.; De Lizarrondo, S.M.; Peeters, M.; Gils, A.; De Meyer, S.; Vivien, D.; Declerck, P.J. Innovative thrombolytic strategy using a heterodimer diabody against TAFI and PAI-1 in mouse models of thrombosis and stroke. Blood 2015, 125, 1325–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.; Tiede, C.; Simmons, K.; Fishwick, C.; Tomlinson, D.; Ajjan, R. Inhibition of complement C3 and fibrinogen interaction: A potential novel therapeutic target to reduce cardiovascular disease in diabetes. Lancet 2015, 385 (Suppl. S1), S57. [Google Scholar] [CrossRef]
- Mastellos, D.C.; Pires da Silva, B.G.P.; Fonseca, B.A.L.; Fonseca, N.P.; Auxiliadora-Martins, M.; Mastaglio, S.; Ruggeri, A.; Sironi, M.; Radermacher, P.; Chrysanthopoulou, A.; et al. Complement C3 vs C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020, 220, 108598. [Google Scholar] [CrossRef]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef] [Green Version]
- Mastellos, D.C.; Ricklin, D.; Lambris, J.D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug Discov. 2019, 18, 707–729. [Google Scholar] [CrossRef]
- de Castro, C.; Grossi, F.; Weitz, I.C.; Maciejewski, J.; Sharma, V.; Roman, E.; Brodsky, R.A.; Tan, L.; Di Casoli, C.; El Mehdi, D.; et al. C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab. Am. J. Hematol. 2020, 95, 1334–1343. [Google Scholar] [CrossRef]
- Peacock-Young, B.; Macrae, F.L.; Newton, D.J.; Hill, A.; Ariëns, R.A. The prothrombotic state in paroxysmal nocturnal hemoglobinuria: A multifaceted source. Haematologica 2018, 103, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyand, A.C.; Pipe, S.W. New therapies for hemophilia. Blood 2019, 133, 389–398. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Lisman, T.; Berg, M.V.D.H.; Nieuwenhuis, K.H.; Meijers, J.C.; Bouma, B.N. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb. Haemost. 2001, 86, 1035–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, J.; Petersen, K.-U.; Rea, C.J.; Harpell, L.; Powell, S.; Lillicrap, D.; Nesheim, M.E.; Sørensen, B. Solulin increases clot stability in whole blood from humans and dogs with hemophilia. Blood 2012, 119, 3622–3628. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.W.; Kim, C.H.; Wang, X.; Pun, S.H.; White, N.J.; Kim, T.H. PolySTAT-modified chitosan gauzes for improved hemostasis in external hemorrhage. Acta Biomater. 2016, 31, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Kearney, K.; Pechlivani, N.; King, R.; Tiede, C.; Phoenix, F.; Cheah, R.; Macrae, F.; Simmons, K.; Manfield, I.W.; Smith, K.A.; et al. Affimer proteins as a tool to modulate fibrinolysis, stabilize the blood clot, and reduce bleeding complications. Blood 2019, 133, 1233–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| α2AP | TAFI | C3 | PAI-2 | |
|---|---|---|---|---|
| Mass (kDa) | ~70 | 56 | 187 | 47 |
| Human gene | SERPINF2 | CPB2 | C3 | SERPINB2 |
| Synthesis/expression | Liver, kidney, and brain | Liver and megakaryocytes | Liver and immune cells | Monocytes, macrophages, keratinocytes, fibroblasts, and placenta |
| Circulating plasma concentration | 70 µg/mL | 4–15 µg/mL | 1.2 mg/mL | Below detection limit |
| Antifibrinolytic function | Direct binding to, and inhibition of, plasmin and cross-linking into the clot making it more resistant to lysis | Protects the clot from lysis by cleaving off C-terminal lysine residues from fibrin, which reduces plasminogen and tPA binding and subsequent plasmin generation | Incorporation into the fibrin clot causes prolongation of fibrinolysis | Cross-linking into fibrin at a site close to tPA binding site affects fibrin clot lysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pechlivani, N.; Kearney, K.J.; Ajjan, R.A. Fibrinogen and Antifibrinolytic Proteins: Interactions and Future Therapeutics. Int. J. Mol. Sci. 2021, 22, 12537. https://doi.org/10.3390/ijms222212537
Pechlivani N, Kearney KJ, Ajjan RA. Fibrinogen and Antifibrinolytic Proteins: Interactions and Future Therapeutics. International Journal of Molecular Sciences. 2021; 22(22):12537. https://doi.org/10.3390/ijms222212537
Chicago/Turabian StylePechlivani, Nikoletta, Katherine J. Kearney, and Ramzi A. Ajjan. 2021. "Fibrinogen and Antifibrinolytic Proteins: Interactions and Future Therapeutics" International Journal of Molecular Sciences 22, no. 22: 12537. https://doi.org/10.3390/ijms222212537






