Both Clathrin-Mediated and Membrane Microdomain-Associated Endocytosis Contribute to the Cellular Adaptation to Hyperosmotic Stress in Arabidopsis
Abstract
:1. Introduction
2. Results
2.1. Hyperosmotic Stress Promoted the Diffusion and Endocytosis of Clathrin at the Plasma Membrane
2.2. Clathrin-Mediated Endocytosis Is Involved in Responding to Hyperosmotic Stress
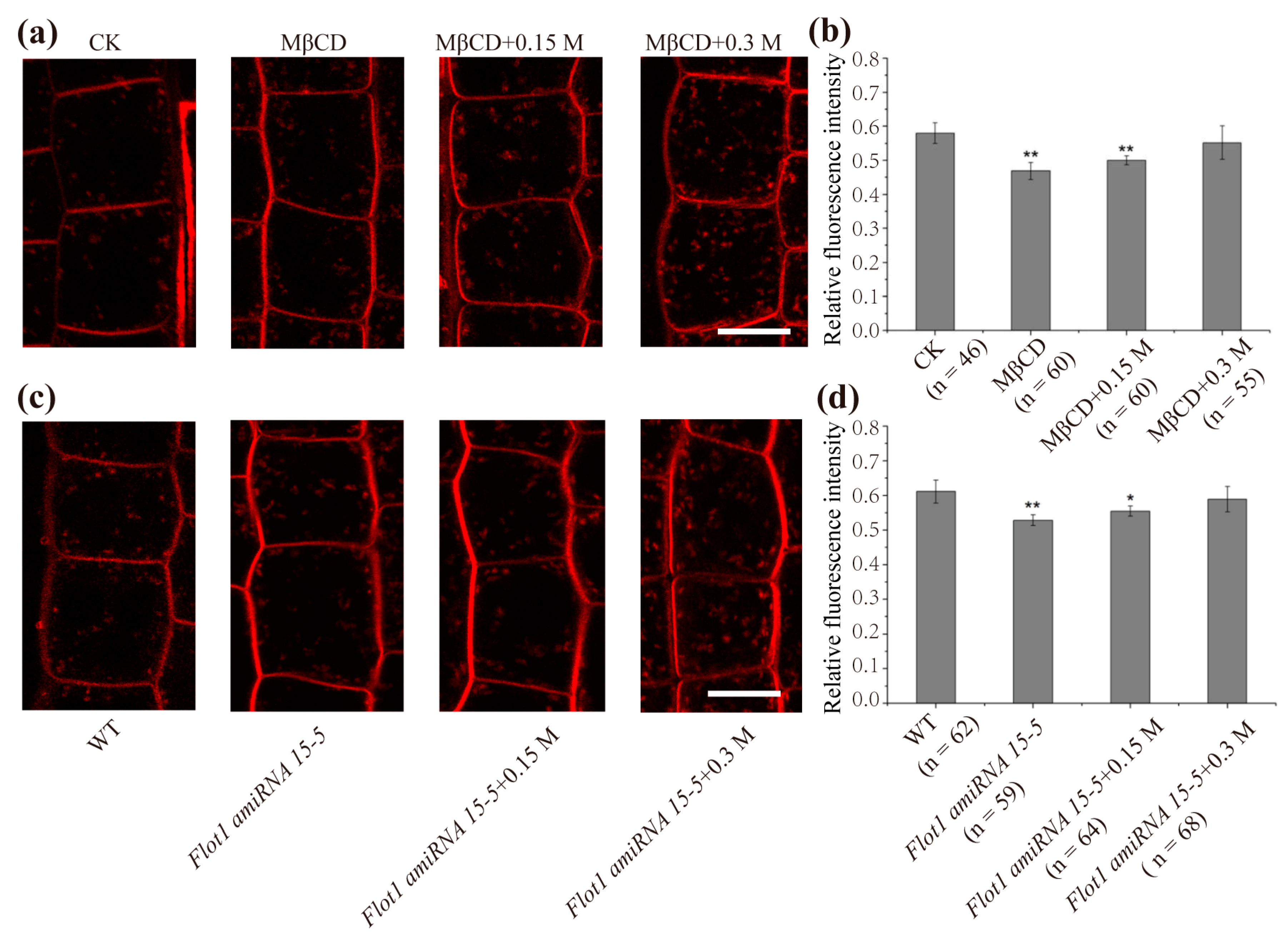
2.3. Membrane Microdomain-Associated Endocytosis Shows an Increase under Hyperosmotic Conditions
2.4. Hyperosmotic Treatments Resulted in a Change in Membrane Raft Microdomains
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Drug Treatments and FM4-64 Staining and Imaging
4.3. Quantitative Analysis of Endocytosis
4.4. Di-4-ANEPPDHQ Staining and Imaging
4.5. Membrane Order Determination by GP Processing
4.6. VA-TIRFM and Fluorescence Image Analysis
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfe, J.; Steponkus, P.L. Mechanical properties of the plasma membrane of isolated plant protoplasts: Mechanism of hyperosmotic and extracellular freezing injury. Plant Physiol. 1983, 71, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.S.; Nakashima, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Plant gene networks in osmotic stress response: From genes to regulatory networks. Methods Enzymol. 2007, 428, 109–128. [Google Scholar] [CrossRef]
- Rodas-Junco, B.A.; Racagni-Di-Palma, G.E.; Canul-Chan, M.; Usorach, J.; Hernández-Sotomayor, S.M.T. Link between lipid second messengers and osmotic stress in plants. Int. J. Mol. Sci. 2021, 22, 2658. [Google Scholar] [CrossRef]
- López-Hernández, T.; Haucke, V.; Maritzen, T. Endocytosis in the adaptation to cellular stress. Cell Stress 2020, 4, 230–247. [Google Scholar] [CrossRef]
- Fan, L.; Li, R.; Pan, J.; Ding, Z.; Lin, J. Endocytosis and its regulation in plants. Trends Plant Sci. 2015, 20, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.S.; Bandyopadhyay, A.; Holstein, S.E.; Peer, W.A. Endocytotic cycling of PM proteins. Annu. Rev. Plant Biol. 2005, 56, 221–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandmann, V.; Homann, U. Clathrin-independent endocytosis contributes to uptake of glucose into BY-2 protoplasts. Plant J. 2012, 70, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Bitsikas, V.; Correa, I.R., Jr.; Nichols, B.J. Clathrin-independent pathways do not contribute significantly to endocytic flux. Elife 2014, 3, e03970. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, Y.; Li, R.; He, Q.; Fang, X.; Luu, D.T.; Maurel, C.; Lin, J. Single-molecule analysis of PIP2; 1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 2011, 23, 3780–3797. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Liu, P.; Wan, Y.; Chen, T.; Wang, Q.; Mettbach, U.; Baluska, F.; Samaj, J.; Fang, X.; Lucas, W.J.; et al. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell 2012, 24, 2105–2122. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; He, Q.; Qi, Z.; Zhang, Y.; Lu, L.; Xue, J.; Li, J.; Li, R. Dynamics and Endocytosis of Flot1 in Arabidopsis Require CPI1 Function. Int. J. Mol. Sci. 2020, 21, 1552. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xing, J.; Lin, J. At the intersection of exocytosis and endocytosis in plants. New Phytol. 2019, 224, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yan, G. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuraoda, R.; Kato, M.; Tsuge, T.; Aoyama, T. Arabidopsis phosphatidylinositol 4-phosphate 5-kinase genes PIP5K7, PIP5K8, and PIP5K9 are redundantly involved in root growth adaptation to osmotic stress. Plant J. 2021, 106, 913–927. [Google Scholar] [CrossRef]
- Dai, J.; Sheetz, M.P. Regulation of endocytosis, exocytosis, and shape by membrane tension. Cold Spring Harb. Symp. Quant. Biol. 1995, 60, 567–571. [Google Scholar] [CrossRef]
- Zwiewka, M.; Nodzynski, T.; Robert, S.; Vanneste, S.; Friml, J. Osmotic stress modulates the balance between exocytosis and clathrin-mediated endocytosis in Arabidopsis thaliana. Mol. Plant. 2015, 8, 1175–1187. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, S.R.; Wu, J.Z.; Freeman, S.A. Solute transport controls membrane tension and organellar volume. Cell. Physiol. Biochem. 2021, 55, 1–24. [Google Scholar] [CrossRef]
- Ferguson, J.P.; Huber, S.D.; Willy, N.M.; Aygün, E.; Goker, S.; Atabey, T.; Kural, C. Mechanoregulation of clathrin-mediated endocytosis. J. Cell Sci. 2017, 130, 3631–3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucher, D.; Frey, F.; Sochacki, K.A.; Kummer, S.; Bergeest, J.P.; Godinez, W.J.; Kräusslich, H.G.; Rohr, K.; Taraska, J.W.; Schwarz, U.S.; et al. Clathrin-adaptor ratio and membrane tension regulate the flat-to-curved transition of the clathrin coat during endocytosis. Nat. Commun. 2018, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Mazheika, I.; Voronko, O.; Kamzolkina, O. Early endocytosis as a key to understanding mechanisms of plasma membrane tension regulation in filamentous fungi. Biol. Cell. 2020, 112, 409–426. [Google Scholar] [CrossRef]
- Konopka, C.A.; Backues, S.K.; Bednarek, S.Y. Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell. 2008, 20, 1363–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, E.; Fujimoto, M.; Ebine, K.; Uemura, T.; Ueda, T.; Nakano, A. Dynamic behavior of clathrin in Arabidopsis thaliana unveiled by live imaging. Plant J. 2012, 69, 204–216. [Google Scholar] [CrossRef]
- Nagy, E. 2-Molecular Diffusion; Elsevier: Amsterdam, The Netherlands, 2012; pp. 35–44. [Google Scholar] [CrossRef]
- Zhao, X.; Li, R.; Lu, C.; Baluska, F.; Wan, Y. Di-4-ANEPPDHQ, a fluorescent probe for the visualisation of membrane microdomains in living Arabidopsis thaliana cells. Plant Physiol. Biochem. 2015, 87, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Qu, Y.; Li, R.; Baluška, F.; Wan, Y. Mapping of membrane lipid order in root apex zones of Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1151. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, Y.; Sui, N. Transcriptional regulation of bHLH during plant response to stress. Biochem. Biophys. Res. Commun. 2018, 503, 397–401. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.; Mittler, R. Plant signaling in biotic and abiotic stress. J. Exp. Bot. 2020, 71, 1649–1651. [Google Scholar] [CrossRef] [Green Version]
- Ueda, M.; Tsutsumi, N.; Fujimoto, M. Salt stress induces internalization of plasma membrane aquaporin into the vacuole in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2016, 474, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Yuan, S.; Sun, L.; Wang, X.; Hong, Y. Cytidinediphosphate-diacylglycerol synthase 5 is required for phospholipid homeostasis and is negatively involved in hyperosmotic stress tolerance. Plant J. 2018, 94, 1038–1050. [Google Scholar] [CrossRef]
- Mettlen, M.; Chen, P.H.; Srinivasan, S.; Danuser, G.; Schmid, S.L. Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. [Google Scholar] [CrossRef]
- Kozera, L.; White, E.; Calaghan, S. Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS ONE 2009, 4, e8312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Singh, R.D.; Godin, L.; Pagano, R.E.; Hubmayr, R.D. Endocytic response of type I alveolar epithelial cells to hypertonic stress. Am. J. Physiol. Lung. Cell Mol. Physiol. 2011, 300, L560–L568. [Google Scholar] [CrossRef] [Green Version]
- Owen, D.M.; Lanigan, P.M.; Dunsby, C.; Munro, I.; Grant, D.; Neil, M.A.; French, P.M.; Magee, A.I. Fluorescence lifetime imaging provides enhanced contrast when imaging the phase-sensitive dye di-4-ANEPPDHQ in model membranes and live cells. Biophys. J. 2006, 90, L80–L82. [Google Scholar] [CrossRef] [Green Version]
- Obaid, A.L.; Loew, L.M.; Wuskell, J.P.; Salzberg, B.M. Novel naphthylstyryl-pyridinium potentiometric dyes offer advantages for neural network analysis. J. Neurosci. Methods 2004, 134, 179–190. [Google Scholar] [CrossRef]
- Owen, D.M.; Gaus, K. Optimized time-gated generalized polarization imaging of Laurdan and di-4-ANEPPDHQ for membrane order image contrast enhancement. Microsc. Res. Tech. 2010, 73, 618–622. [Google Scholar] [CrossRef]
- Grosjean, K.; Der, C.; Robert, F.; Thomas, D.; Mongrand, S.; Simon-Plas, F.; Gerbeau-Pissot, P. Interactions between lipids and proteins are critical for organization of plasma membrane-ordered domains in tobacco BY-2 cells. J. Exp. Bot. 2018, 69, 3545–3557. [Google Scholar] [CrossRef]
- Imran, M.; Sergent, O.; Tête, A.; Gallais, I.; Chevanne, M.; Lagadic-Gossmann, D.; Podechard, N. Membrane remodeling as a key player of the hepatotoxicity induced by co-exposure to benzo[a]pyrene and ethanol of obese zebrafish larvae. Biomolecules 2018, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Millard, A.C.; Wuskell, J.P.; Dong, X.; Wu, D.; Clark, H.A.; Loew, L.M. Characterization and application of a new optical probe for membrane lipid domains. Biophys. J. 2006, 90, 2563–2575. [Google Scholar] [CrossRef] [Green Version]
- Owen, D.M.; Rentero, C.; Magenau, A.; Abu-Siniyeh, A.; Gaus, K. Quantitative imaging of membrane lipid order in cells and organisms. Nat. Protoc. 2012, 7, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Aron, M.; Browning, R.; Carugo, D.; Sezgin, E.; Bernardino de la Serna, J.; Eggeling, C.; Stride, E. Spectral imaging toolbox: Segmentation, hyperstack reconstruction, and batch processing of spectral images for the determination of cell and model membrane lipid order. BMC Bioinform. 2017, 18, 254. [Google Scholar] [CrossRef] [Green Version]
- Pike, L.J. Lipid rafts: Heterogeneity on the high seas. J. Biochem. 2004, 378, 281–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, L.J. Growth factor receptors, lipid rafts and caveolae: An evolving story. Biochim. Biophys. Acta. 2005, 1746, 260–273. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cui, Y.; Yu, M.; Su, B.; Gong, W.; Baluška, F.; Komis, G.; Šamaj, J.; Shan, X.; Lin, J. Phosphorylation-mediated dynamics of nitrate transceptor NRT1.1 regulate auxin flux and nitrate signaling in lateral root growth. Plant Physiol. 2019, 181, 480–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Jing, Y.; Xiao, J.; Zhang, Y.; Zhu, Y.; Julian, R.; Lin, J. Membrane microdomains and the cytoskeleton constrain AtHIR1 dynamics and facilitate the formation of an AtHIR1-associated immune complex. Plant J. 2017, 90, 3–16. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Fan, C.; Man, Y.; Zhang, Y.; Li, R.; Li, X.; Jing, Y. Both Clathrin-Mediated and Membrane Microdomain-Associated Endocytosis Contribute to the Cellular Adaptation to Hyperosmotic Stress in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 12534. https://doi.org/10.3390/ijms222212534
Wu Z, Fan C, Man Y, Zhang Y, Li R, Li X, Jing Y. Both Clathrin-Mediated and Membrane Microdomain-Associated Endocytosis Contribute to the Cellular Adaptation to Hyperosmotic Stress in Arabidopsis. International Journal of Molecular Sciences. 2021; 22(22):12534. https://doi.org/10.3390/ijms222212534
Chicago/Turabian StyleWu, Zheng, Chengyu Fan, Yi Man, Yue Zhang, Ruili Li, Xiaojuan Li, and Yanping Jing. 2021. "Both Clathrin-Mediated and Membrane Microdomain-Associated Endocytosis Contribute to the Cellular Adaptation to Hyperosmotic Stress in Arabidopsis" International Journal of Molecular Sciences 22, no. 22: 12534. https://doi.org/10.3390/ijms222212534
APA StyleWu, Z., Fan, C., Man, Y., Zhang, Y., Li, R., Li, X., & Jing, Y. (2021). Both Clathrin-Mediated and Membrane Microdomain-Associated Endocytosis Contribute to the Cellular Adaptation to Hyperosmotic Stress in Arabidopsis. International Journal of Molecular Sciences, 22(22), 12534. https://doi.org/10.3390/ijms222212534





