Stimuli-Responsive Polymeric Nanomaterials for the Delivery of Immunotherapy Moieties: Antigens, Adjuvants and Agonists
Abstract
:1. Introduction
2. Stimuli-Responsive Polymeric Nanomaterials
2.1. Endogenous Stimuli
2.2. Exogenous Stimuli
3. Stimuli-Responsive Polymers for Antigens, Adjuvants and Agonists
3.1. Antigens
3.1.1. Endogenous Stimuli
3.1.2. Exogenous Stimuli
3.2. Adjuvants
3.2.1. Endogenous Stimuli
3.2.2. Exogenous Stimuli
3.3. Agonists
3.3.1. Endogenous Stimuli
3.3.2. Exogeneous Stimuli
3.4. Codelivery of Antigens and Adjuvants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1. | APC | Antigen presenting cells |
| 2. | APNA | Activatable polymer nanoagonist |
| 3. | ATP | Adenosine triphosphate |
| 4. | CCPS | Chimeric cross-linked polymersomes |
| 5. | CD8+ | Cytotoxic T cells |
| 6. | CD4+ | Helper T cells |
| 7. | CD44 | Cluster of differentiation-44 |
| 8. | Ce6 | Chlorin e6 |
| 9. | cGAMP | Cyclic guanosine monophosphate |
| 10. | CHex-Dex | 2-carboxycyclohexane-1-carboxylated dextran |
| 11. | CHex-HA | 2-carboxycyclohexane-1-carboxylated hyaluronic acid |
| 12. | CO2 | Carbon-dioxide |
| 13. | CpG | 5′-C-phosphate-G-3′ (Oligonucleotide) |
| 14. | CRT | Calreticulin |
| 15. | CTL | Cytotoxic T lymphocytes |
| 16. | CTLA-4 | Cytotoxic T-lymphocyte associated antigen 4 |
| 17. | DCs | Dendritic cells |
| 18. | DNA | Deoxy-ribo nucleic acid |
| 19. | DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoetanolamine |
| 20. | DMAEMA | Dimethylaminoethyl methacrylate |
| 21. | DOX | Doxorubicin |
| 22. | DSPE-PEG | 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol) |
| 23. | EGFR | Epidermal growth factor receptor |
| 24. | FDA | Food and Drug Administration |
| 25. | GLUT 1 | Glucose transporter 1 |
| 26. | GSH | Glutathione |
| 27. | HA | Hyaluronic acid |
| 28. | HIF 1α | Hypoxia inducible factor-1 |
| 29. | HMGB1 | High mobility group box 1 |
| 30. | H2O2 | Hydrogen peroxide |
| 31. | HPAA | Hyperbranched polyamidoamine |
| 32. | HPAA-F7 | Fluorinated hyperbranched polyamidoamine |
| 33. | HPHH | 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a |
| 34. | ICD | Immunogenic cell death |
| 35. | iDC | Immature dendritic cells |
| 36. | IL-6 | Interleukin-6 |
| 37. | IL-12 | Interleukin-12 |
| 38. | IRF3 | Interferon regulatory factor 3 |
| 39. | NK cells | Natural killer cells |
| 40. | NPs | Nanoparticles |
| 41. | LASER | light amplification by stimulated emission of radiation |
| 42. | MAA | Methacrylic acid |
| 43. | mDC | Mature dendritic cells |
| 44. | MGlu-HAA | 3-methylglutarylated hyaluronic acid |
| 45. | MGlu-HPG | 3-methyl-glutarylated hyperbranched poly(glycidol) |
| 46. | MHC I | Major histocompatibility complex-I |
| 47. | NIR | Near infra red |
| 48. | OEGMA | Oligoethylene glycol methacrylate |
| 49. | OVA | Ovalbumin |
| 50. | PAA | Polyacrylic acid |
| 51. | PAM | Poly(D,L-lactic-coglycolic acid) |
| 52. | PAMAM | Polyamidoamine |
| 53. | PCL-PEG | Poly(ε-caprolactone)-poly(ethylene glycol) |
| 54. | PCL-PEI | poly-ε-caprolactone-polyethylene imine |
| 55. | PD-1 | Programmed cell death protein -1 |
| 56. | PD-L1 | Programmed death ligand -1 |
| 57. | PDSMA | Pyridyl disulfide ethyl methacrylate |
| 58. | PDT | Photodynamic therapy |
| 59. | PEG | Poly(D,L-lactide-co-glycolide) |
| 60. | PEI | Poly(ethylene glycol) |
| 61. | PEOz-PLA | Poly(2-ethyl-2-oxazoline)-poly(L-lactide) |
| 62. | PGA | Poly glycolic acid |
| 63. | pH | Potential of hydrogen |
| 64. | PiPOx | Pipecolic acid and sarcosine oxidase |
| 65. | PLA | Polylactic acid |
| 66. | PLG | Poly(D,L-lactide-co-glycolide) |
| 67. | PLGA | Poly(lactic-co-glycolic acid) |
| 68. | PMAA | Poly(methacrylic acid) |
| 69. | PNIPAAM | Poly(g-glutamic acid |
| 70. | PTT | Photothermal therapy |
| 71. | PVA | Polyinyl alcohol |
| 72. | RGD | Arginine-glycine-aspartic acid motif |
| 73. | RIG-1 | Retinoic acid-inducible gene I |
| 74. | ROS | Reactive oxygen species |
| 75. | SO2 | Sulfur dioxide |
| 76. | STING | Stimulator of interferon genes |
| 77. | TAA | Tumour associated antigens |
| 78. | TBK1 | TANK binding kinase 1 |
| 79. | TCA | Tricarboxylic acid cycle |
| 80. | TCR | T cell receptor |
| 81. | TLR 2 | Toll like receptor 2 |
| 82. | TLR 4 | Toll like receptor 4 |
| 83. | TLR7/8 | Toll like receptor 7/8 |
| 84. | TME | Tumour micro-environment |
| 85. | TNF α | Tumour necrosis factor α |
| 86. | UV | Ultraviolet |
| 87. | VEGF | Vascular endothelial growth factor |
References
- Ye, Y.; Wang, J.; Hu, Q.; Hochu, G.M.; Xin, H.; Wang, C.; Gu, Z. Synergistic Transcutaneous Immunotherapy Enhances Antitumor Immune Responses through Delivery of Checkpoint Inhibitors. ACS Nano 2016, 10, 8956–8963. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Domchek, S.M.; Clark, A.S. Immunotherapy for Breast Cancer: What Are We Missing? Clin. Cancer Res. 2017, 23, 2640–2646. [Google Scholar] [CrossRef] [Green Version]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melero, I.; Berman, D.M.; Aznar, M.A.; Korman, A.J.; Perez-Gracia, J.L.; Haanen, J.B.A.G. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Cancer 2015, 15, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sommermeyer, D.; Cabanov, A.; Kosasih, P.; Hill, T.; Riddell, S.R. Inclusion of Strep-tag II in design of antigen receptors for T-cell immunotherapy. Nat. Biotechnol. 2016, 34, 430–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Wang, C.; Zhang, X.; Hu, Q.; Zhang, Y.; Liu, Q.; Wen, D.; Milligan, J.; Bellotti, A.; Huang, L.; et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol. 2017, 2, aan5692. [Google Scholar] [CrossRef] [Green Version]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Wang, Q.; Wang, X.; Ke, L.; Shi, K. Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy. Theranostics 2019, 9, 126–151. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Hou, Y.; Zhang, J.; Liang, X.-J. Nanomaterials in medicine and pharmaceuticals: Nanoscale materials developed with less toxicity and more efficacy. Eur. J. Nanomed. 2013, 5, 61–79. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Malachowski, T.; Hassel, A. Engineering nanoparticles to overcome immunological barriers for enhanced drug delivery. Eng. Regen. 2020, 1, 35–50. [Google Scholar] [CrossRef]
- Camacho, A.I.; Martins, R.D.C.; Tamayo, I.; de Souza, J.; Lasarte, J.J.; Mansilla, C.; Esparza, I.; Irache, J.M.; Gamazo, C. Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine 2011, 29, 7130–7135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Q.; Li, L.; Mu, Q.; Zhang, Q. Immunomodulation of Nanoparticles in Nanomedicine Applications. BioMed Res. Int. 2014, 2014, 426028. [Google Scholar] [CrossRef]
- Guo, S.; Fu, D.; Utupova, A.; Sun, D.; Zhou, M.; Jin, Z.; Zhao, K. Applications of polymer-based nanoparticles in vaccine field. Nanotechnol. Rev. 2019, 8, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, Z.; Chen, X. Recent Advances in Stimuli-Responsive Platforms for Cancer Immunotherapy. Acc. Chem. Res. 2020, 53, 2044–2054. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, M.; Nechansky, A.; Kircheis, R. Cancer immunotherapy. Biotechnol. J. 2006, 1, 138–147. [Google Scholar] [CrossRef]
- Pacifici, N.; Bolandparvaz, A.; Lewis, J.S. Stimuli-Responsive Biomaterials for Vaccines and Immunotherapeutic Applications. Adv. Ther. 2020, 3, 2000129. [Google Scholar] [CrossRef]
- Sever, R.; Brugge, J.S. Signal Transduction in Cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2019, 77, 1745–1770. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017, 4, 25–27. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, W.; Yu, J.; Li, Y.; Zhao, C. Recent Development of pH-Responsive Polymers for Cancer Nanomedicine. Molecules 2018, 24, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, S.; Tan, Z.; Wu, X.; Huang, X. Synthesis of Carboxy-Dimethylmaleic Amide Linked Polymer Conjugate Based Ultra-pH-sensitive Nanoparticles for Enhanced Antitumor Immunotherapy. ACS Macro Lett. 2020, 9, 1693–1699. [Google Scholar] [CrossRef]
- Kim, H.; Sehgal, D.; Kucaba, T.A.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Acidic pH-responsive polymer nanoparticles as a TLR7/8 agonist delivery platform for cancer immunotherapy. Nanoscale 2018, 10, 20851–20862. [Google Scholar] [CrossRef]
- Smith, A.A.A.; Gale, E.C.; Roth, G.A.; Maikawa, C.L.; Correa, S.; Yu, A.C.; Appel, E.A. Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment. Biomacromolecules 2020, 21, 3704–3712. [Google Scholar] [CrossRef]
- Huang, Z.; Gan, J.; Long, Z.; Guo, G.; Shi, X.; Wang, C.; Zang, Y.; Ding, Z.; Chen, J.; Zhang, J.; et al. Targeted delivery of let-7b to reprogramme tumor-associated macrophages and tumor infiltrating dendritic cells for tumor rejection. Biomaterials 2016, 90, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-responsive polymers for drug delivery: From molecular design to applications. Polym. Chem. 2013, 5, 1519–1528. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, G.; Wang, S.; Yu, G.; Yang, Z.; Lin, L.; Zhou, Z.; Liu, Y.; Dai, Y.; Zhang, F.; et al. In Situ Dendritic Cell Vaccine for Effective Cancer Immunotherapy. ACS Nano 2019, 13, 3083–3094. [Google Scholar] [CrossRef]
- Poole, K.M.; Nelson, C.E.; Joshi, R.V.; Martin, J.R.; Gupta, M.K.; Haws, S.C.; Kavanaugh, T.E.; Skala, M.C.; Duvall, C.L. ROS-responsive microspheres for on demand antioxidant therapy in a model of diabetic peripheral arterial disease. Biomaterials 2014, 41, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Jo, S.D.; Seah, G.L.; Kim, I.; Nam, Y.S. ROS-induced biodegradable polythioketal nanoparticles for intracellular delivery of anti-cancer therapeutics. J. Ind. Eng. Chem. 2015, 21, 1137–1142. [Google Scholar] [CrossRef]
- Lee, S.H.; Boire, T.C.; Lee, J.B.; Gupta, M.K.; Zachman, A.L.; Rath, R.; Sung, H.-J. ROS-cleavable proline oligomer crosslinking of polycaprolactone for pro-angiogenic host response. J. Mater. Chem. B 2014, 2, 7109–7113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible Polymeric Nanoparticles Degrade and Release Cargo in Response to Biologically Relevant Levels of Hydrogen Peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, X.; Chen, Y.; Wu, X.; Li, P.; Liu, Y. Enzyme-responsive sulfatocyclodextrin/prodrug supramolecular assembly for controlled release of anti-cancer drug chlorambucil. Chem. Commun. 2018, 55, 953–956. [Google Scholar] [CrossRef]
- Xie, A.; Hanif, S.; Ouyang, J.; Tang, Z.; Kong, N.; Kim, N.Y.; Qi, B.; Patel, D.; Shi, B.; Tao, W. Stimuli-responsive prodrug-based cancer nanomedicine. EBioMedicine 2020, 56, 102821. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Shen, W.; Liu, W.; Yang, H.; Zhang, P.; Xiao, C.; Chen, X. A glutathione-responsive sulfur dioxide polymer prodrug as a nanocarrier for combating drug-resistance in cancer chemotherapy. Biomaterials 2018, 178, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species (Apex) 2016, 1, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Shim, M.S.; Xia, Y. A Reactive Oxygen Species (ROS)-Responsive Polymer for Safe, Efficient, and Targeted Gene Delivery in Cancer Cells. Angew. Chem. Int. Ed. 2013, 52, 6926–6929. [Google Scholar] [CrossRef]
- Brégeon, D.; Doetsch, P.W. Transcriptional mutagenesis: Causes and involvement in tumour development. Nat. Rev. Cancer 2011, 11, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y.; Han, H.S.; Kim, S.-H.; Son, S.; Jo, D.-G.; Ahn, C.-H.; Suh, Y.D.; Kim, K.; et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2013, 35, 1735–1743. [Google Scholar] [CrossRef]
- Thambi, T.; Park, J.H.; Lee, D.S. Hypoxia-responsive nanocarriers for cancer imaging and therapy: Recent approaches and future perspectives. Chem. Commun. 2016, 52, 8492–8500. [Google Scholar] [CrossRef]
- Son, S.; Rao, N.V.; Ko, H.; Shin, S.; Jeon, J.; Han, H.S.; Nguyen, V.Q.; Thambi, T.; Suh, Y.D.; Park, J.H. Carboxymethyl dextran-based hypoxia-responsive nanoparticles for doxorubicin delivery. Int. J. Biol. Macromol. 2018, 110, 399–405. [Google Scholar] [CrossRef]
- Romano, A.; Roppolo, I.; Rossegger, E.; Schlögl, S.; Sangermano, M. Recent Trends in Applying Ortho-Nitrobenzyl Esters for the Design of Photo-Responsive Polymer Networks. Materials 2020, 13, 2777. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Gao, C. Shape Transformation of Light-Responsive Pyrene-Containing Micelles and Their Influence on Cytoviability. Biomacromolecules 2015, 16, 2276–2281. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2013, 43, 148–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wachtveitl, J.; Zumbusch, A. Azobenzene: An Optical Switch for in vivo Experiments. ChemBioChem 2011, 12, 1169–1170. [Google Scholar] [CrossRef] [PubMed]
- Alsuraifi, A.; Curtis, A.; Lamprou, D.A.; Hoskins, C. Stimuli Responsive Polymeric Systems for Cancer Therapy. Pharmaceutics 2018, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhang, Y.; She, J.; Zhou, X.; Xu, J.; Han, X.; Wang, C.; Zhu, M.; Liu, Z. Ultrasound-Mediated Remotely Controlled Nanovaccine Delivery for Tumor Vaccination and Individualized Cancer Immunotherapy. Nano Lett. 2021, 21, 1228–1237. [Google Scholar] [CrossRef]
- Wang, J.; Pelletier, M.; Zhang, H.; Xia, H.; Zhao, Y. High-Frequency Ultrasound-Responsive Block Copolymer Micelle. Langmuir 2009, 25, 13201–13205. [Google Scholar] [CrossRef]
- Cazares-Cortes, E.; Espinosa, A.; Guigner, J.-M.; Michel, A.; Griffete, N.; Wilhelm, C.; Ménager, C. Doxorubicin Intracellular Remote Release from Biocompatible Oligo(ethylene glycol) Methyl Ether Methacrylate-Based Magnetic Nanogels Triggered by Magnetic Hyperthermia. ACS Appl. Mater. Interfaces 2017, 9, 25775–25788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-B.; Yuan, C.-H.; Ke, A.-R.; Quan, Z.-L. Electrical response characterization of PVA–P(AA/AMPS) IPN hydrogels in aqueous Na2SO4 solution. Sens. Actuators B Chem. 2008, 134, 281–286. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-Responsive Polymer Micro- and Nano-Capsules. Polymers 2016, 9, 8. [Google Scholar] [CrossRef]
- Wang, Z.; Ju, Y.; Ali, Z.; Yin, H.; Sheng, F.; Lin, J.; Wang, B.; Hou, Y. Near-infrared light and tumor microenvironment dual responsive size-switchable nanocapsules for multimodal tumor theranostics. Nat. Commun. 2019, 10, 4418. [Google Scholar] [CrossRef] [Green Version]
- Son, S.; Shin, E.; Kim, B.-S. Light-Responsive Micelles of Spiropyran Initiated Hyperbranched Polyglycerol for Smart Drug Delivery. Biomacromolecules 2014, 15, 628–634. [Google Scholar] [CrossRef]
- Sánchez-Moreno, P.; De Vicente, J.; Nardecchia, S.; Marchal, J.A.; Boulaiz, H. Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials 2018, 8, 935. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.E.; Yokoyama, M.; Yamato, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.G.; Husseini, G.A.; Staples, B.J. Ultrasonic drug delivery—A general review. Expert Opin. Drug Deliv. 2004, 1, 37–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Chen, Y.; Yu, T.; Guo, Y.; Liu, F.; Yao, Y.; Li, P.; Wang, D.; Wang, Z.; Chen, Y.; et al. Drug Release from Phase-Changeable Nanodroplets Triggered by Low-Intensity Focused Ultrasound. Theranostics 2018, 8, 1327–1339. [Google Scholar] [CrossRef]
- Thévenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wankhede, M.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Magnetic nanoparticles: An emerging technology for malignant brain tumor imaging and therapy. Expert Rev. Clin. Pharmacol. 2012, 5, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Neofytou, E.; Cahill, T.J., III; Beygui, R.E.; Zare, R.N. Drug Release from Electric-Field-Responsive Nanoparticles. ACS Nano 2011, 6, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Sela, M. Encyclopedia of Immunology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 201–207. ISBN 9780122267659. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, e1800917. [Google Scholar] [CrossRef] [Green Version]
- Knight, F.C.; Gilchuk, P.; Kumar, A.; Becker, K.W.; Sevimli, S.; Jacobson, M.E.; Suryadevara, N.; Wang-Bishop, L.; Boyd, K.L.; Crowe, J.E., Jr.; et al. Mucosal Immunization with a pH-Responsive Nanoparticle Vaccine Induces Protective CD8+ Lung-Resident Memory T Cells. ACS Nano 2019, 13, 10939–10960. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yuba, E.; Hayashi, H.; Harada, A.; Kono, K. Development of pH-Responsive Hyaluronic Acid-Based Antigen Carriers for Induction of Antigen-Specific Cellular Immune Responses. ACS Biomater. Sci. Eng. 2019, 5, 5790–5797. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Uesugi, S.; Miyazaki, M.; Kado, Y.; Harada, A.; Kono, K. Development of pH-sensitive Dextran Derivatives with Strong Adjuvant Function and Their Application to Antigen Delivery. Membranes 2017, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yu, T.; Zeng, Y.; Lian, K.; Zhou, X.; Ke, J.; Li, Y.; Yuan, H.; Hu, F. pH-Responsive Biomimetic Polymeric Micelles as Lymph Node-Targeting Vaccines for Enhanced Antitumor Immune Responses. Biomacromolecules 2020, 21, 2818–2828. [Google Scholar] [CrossRef]
- Yoshizaki, Y.; Yuba, E.; Sakaguchi, N.; Koiwai, K.; Harada, A.; Kono, K. pH-sensitive polymer-modified liposome-based immunity-inducing system: Effects of inclusion of cationic lipid and CpG-DNA. Biomaterials 2017, 141, 272–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Hou, B.; Wang, D.; Sun, F.; Song, R.; Shao, Q.; Wang, H.; Yu, H.; Li, Y. Engineering Polymeric Prodrug Nanoplatform for Vaccination Immunotherapy of Cancer. Nano Lett. 2020, 20, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Okubo, M.; Miyazaki, M.; Yuba, E.; Harada, A. Chondroitin Sulfate-Based pH-Sensitive Polymer-Modified Liposomes for Intracellular Antigen Delivery and Induction of Cancer Immunity. Bioconjugate Chem. 2019, 30, 1518–1529. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Z.; Wang, C.; Su, Q.; Song, H.; Zhang, C.; Huang, P.; Liang, X.-J.; Dong, A.; Kong, D.; et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials 2019, 230, 119649. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, Y.; Xue, W.; Liu, Z. Fluorinated Redox-Responsive Poly(amidoamine) as a Vaccine Delivery System for Antitumor Immunotherapy. ACS Biomater. Sci. Eng. 2018, 5, 644–653. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.-J.; Luo, Y.-L.; Chen, Y.-F.; Fan, Y.-N.; Du, J.-Z.; Wang, J. Programmable Delivery of Immune Adjuvant to Tumor-Infiltrating Dendritic Cells for Cancer Immunotherapy. Nano Lett. 2020, 20, 4882–4889. [Google Scholar] [CrossRef]
- Im, S.; Lee, J.; Park, D.; Park, A.; Kim, Y.-M.; Kim, W.J. Hypoxia-Triggered Transforming Immunomodulator for Cancer Immunotherapy via Photodynamically Enhanced Antigen Presentation of Dendritic Cell. ACS Nano 2018, 13, 476–488. [Google Scholar] [CrossRef]
- Deng, S.; Iscaro, A.; Zambito, G.; Mijiti, Y.; Minicucci, M.; Essand, M.; Lowik, C.; Muthana, M.; Censi, R.; Mezzanotte, L.; et al. Development of a New Hyaluronic Acid Based Redox-Responsive Nanohydrogel for the Encapsulation of Oncolytic Viruses for Cancer Immunotherapy. Nanomaterials 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Shi, G.; Song, H.; Shi, S.; Zhang, X.; Huang, P.; Wang, Z.; Wang, W.; Wang, C.; et al. A Light Responsive Nanoparticle-Based Delivery System Using Pheophorbide A Graft Polyethylenimine for Dendritic Cell-Based Cancer Immunotherapy. Mol. Pharm. 2017, 14, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Kool, M.; Soullié, T.; van Nimwegen, M.; Willart, M.A.M.; Muskens, F.; Jung, S.; Hoogsteden, H.C.; Hammad, H.; Lambrecht, B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008, 205, 869–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKee, A.S.; Munks, M.W.; MacLeod, M.K.L.; Fleenor, C.J.; Van Rooijen, N.; Kappler, J.W.; Marrack, P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 2009, 183, 4403–4414. [Google Scholar] [CrossRef] [Green Version]
- Kawai, M.; Nakamura, T.; Miura, N.; Maeta, M.; Tanaka, H.; Ueda, K.; Higashi, K.; Moribe, K.; Tange, K.; Nakai, Y.; et al. DNA-loaded nano-adjuvant formed with a vitamin E-scaffold intracellular environmentally-responsive lipid-like material for cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2587–2597. [Google Scholar] [CrossRef]
- Van Herck, S.; Van Hoecke, L.; Louage, B.; Lybaert, L.; De Coen, R.; Kasmi, S.; Esser-Kahn, A.P.; David, S.A.; Nuhn, L.; Schepens, B.; et al. Transiently Thermoresponsive Acetal Polymers for Safe and Effective Administration of Amphotericin B as a Vaccine Adjuvant. Bioconjugate Chem. 2017, 29, 748–760. [Google Scholar] [CrossRef]
- Hu, L.; Cao, Z.; Ma, L.; Liu, Z.; Liao, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. The potentiated checkpoint blockade immunotherapy by ROS-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials 2019, 223, 119469. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wang, X.; Hou, Y.; Hong, X.; Gong, T.; Zhang, Z.; Sun, X. Rational design of Polymeric Hybrid Micelles to Overcome Lymphatic and Intracellular Delivery Barriers in Cancer Immunotherapy. Theranostics 2017, 7, 4383–4398. [Google Scholar] [CrossRef]
- Choi, Y.; Shi, Y.; Haymaker, C.L.; Naing, A.; Ciliberto, G.; Hajjar, J. T-cell agonists in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000966. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Khanna, V.; Kucaba, T.A.; Zhang, W.; Sehgal, D.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. TLR7/8 Agonist-Loaded Nanoparticles Augment NK Cell-Mediated Antibody-Based Cancer Immunotherapy. Mol. Pharm. 2020, 17, 2109–2124. [Google Scholar] [CrossRef]
- Wang, B.; Van Herck, S.; Chen, Y.; Bai, X.; Zhong, Z.; De Swarte, K.; Lambrecht, B.N.; Sanders, N.N.; Lienenklaus, S.; Scheeren, H.W.; et al. Potent and Prolonged Innate Immune Activation by Enzyme-Responsive Imidazoquinoline TLR7/8 Agonist Prodrug Vesicles. J. Am. Chem. Soc. 2020, 142, 12133–12139. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.E.; Becker, K.W.; Palmer, C.R.; Pastora, L.E.; Fletcher, R.B.; Collins, K.A.; Fedorova, O.; Duvall, C.L.; Pyle, A.M.; Wilson, J.T. Structural Optimization of Polymeric Carriers to Enhance the Immunostimulatory Activity of Molecularly Defined RIG-I Agonists. ACS Cent. Sci. 2020, 6, 2008–2022. [Google Scholar] [CrossRef]
- Jacobson, M.E.; Wang-Bishop, L.; Becker, K.W.; Wilson, J.T. Delivery of 5′-triphosphate RNA with endosomolytic nanoparticles potently activates RIG-I to improve cancer immunotherapy. Biomater. Sci. 2018, 7, 547–559. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Liu, Y.-H.; Fang, Z.-S.; Lin, C.-L.; Lin, J.-C.; Yao, B.-Y.; Hu, C.-M.J. Synthetic Immunogenic Cell Death Mediated by Intracellular Delivery of STING Agonist Nanoshells Enhances Anticancer Chemo-immunotherapy. Nano Lett. 2020, 20, 2246–2256. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-Containing Nanoparticles Combine the NK Cells Mediated Immunotherapy with Radiotherapy and Chemotherapy. Adv. Mater. 2020, 32, e1907568. [Google Scholar] [CrossRef] [PubMed]
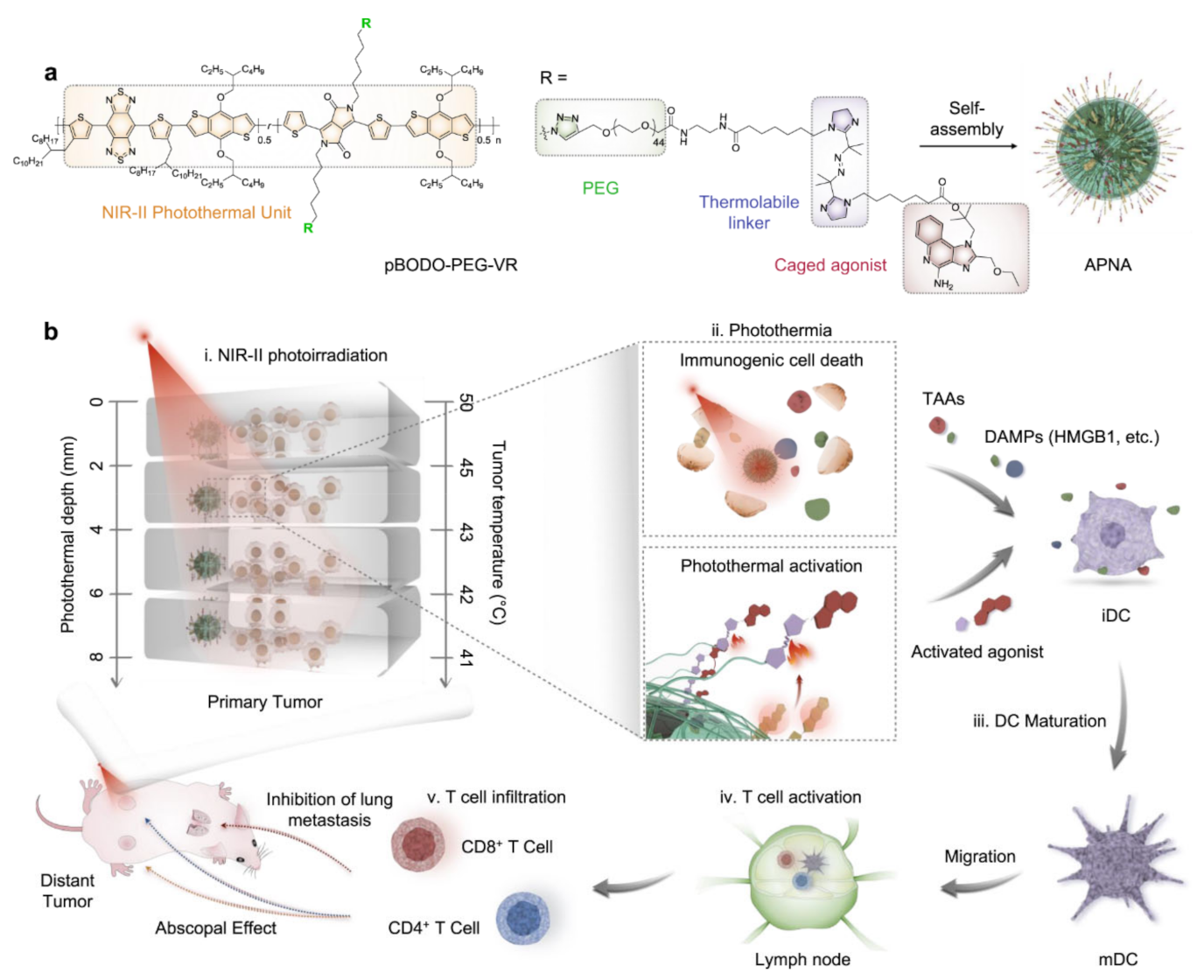
- Jiang, Y.; Huang, J.; Xu, C.; Pu, K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat. Commun. 2021, 12, 742. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Chen, Q.; Zhang, J.; Li, W.; Hu, H.; Zhao, X.; Qiao, M.; Chen, D. Synthetic Polymeric Mixed Micelles Targeting Lymph Nodes Trigger Enhanced Cellular and Humoral Immune Responses. ACS Appl. Mater. Interfaces 2018, 10, 2874–2889. [Google Scholar] [CrossRef] [PubMed]
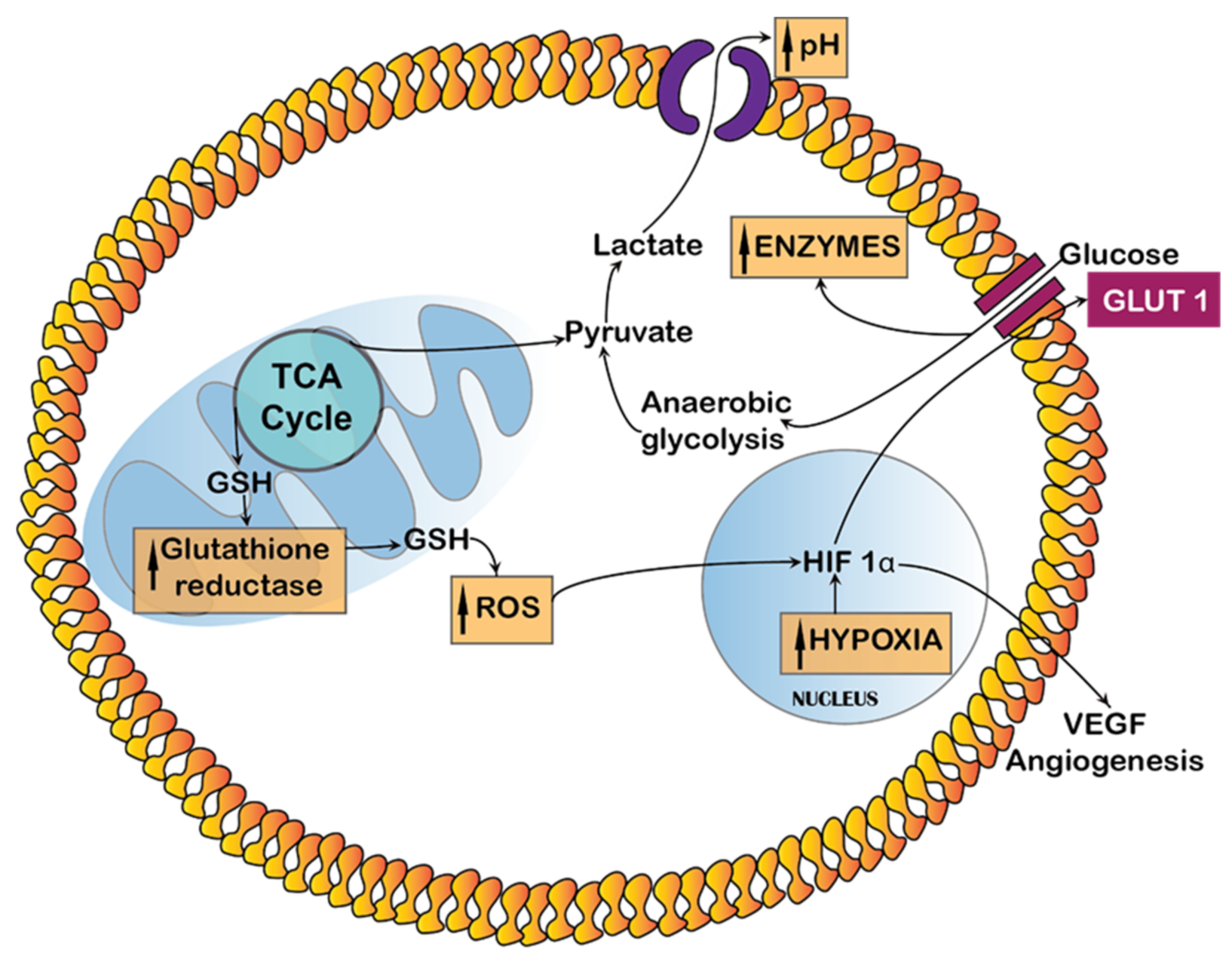
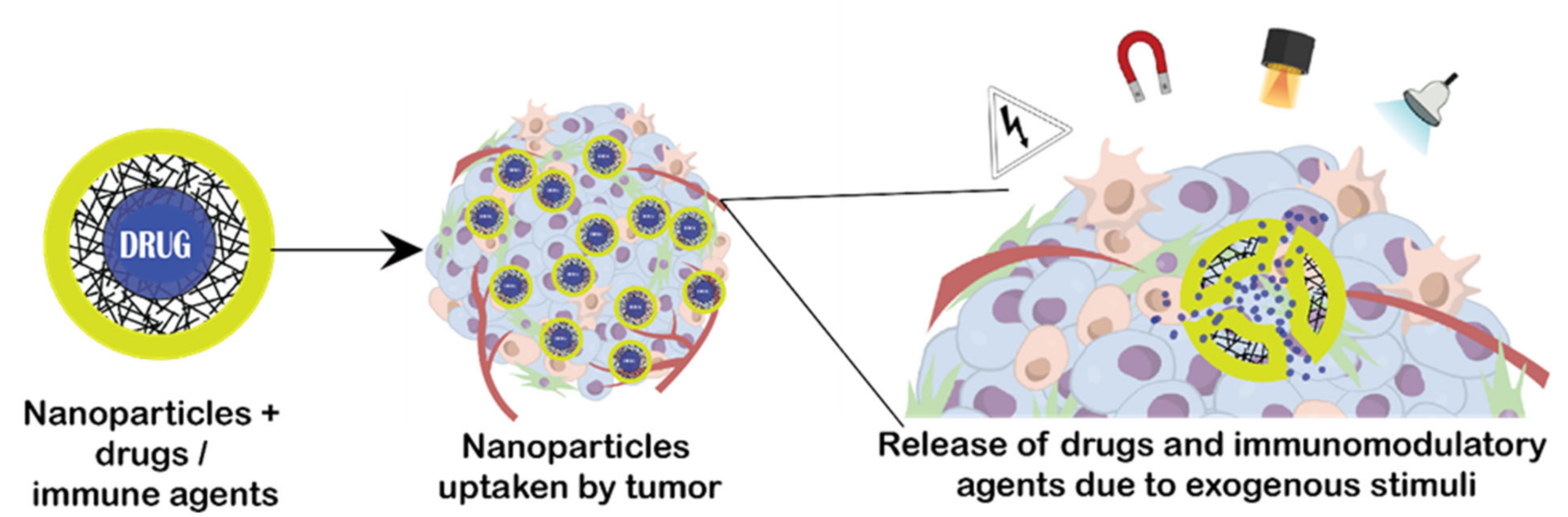
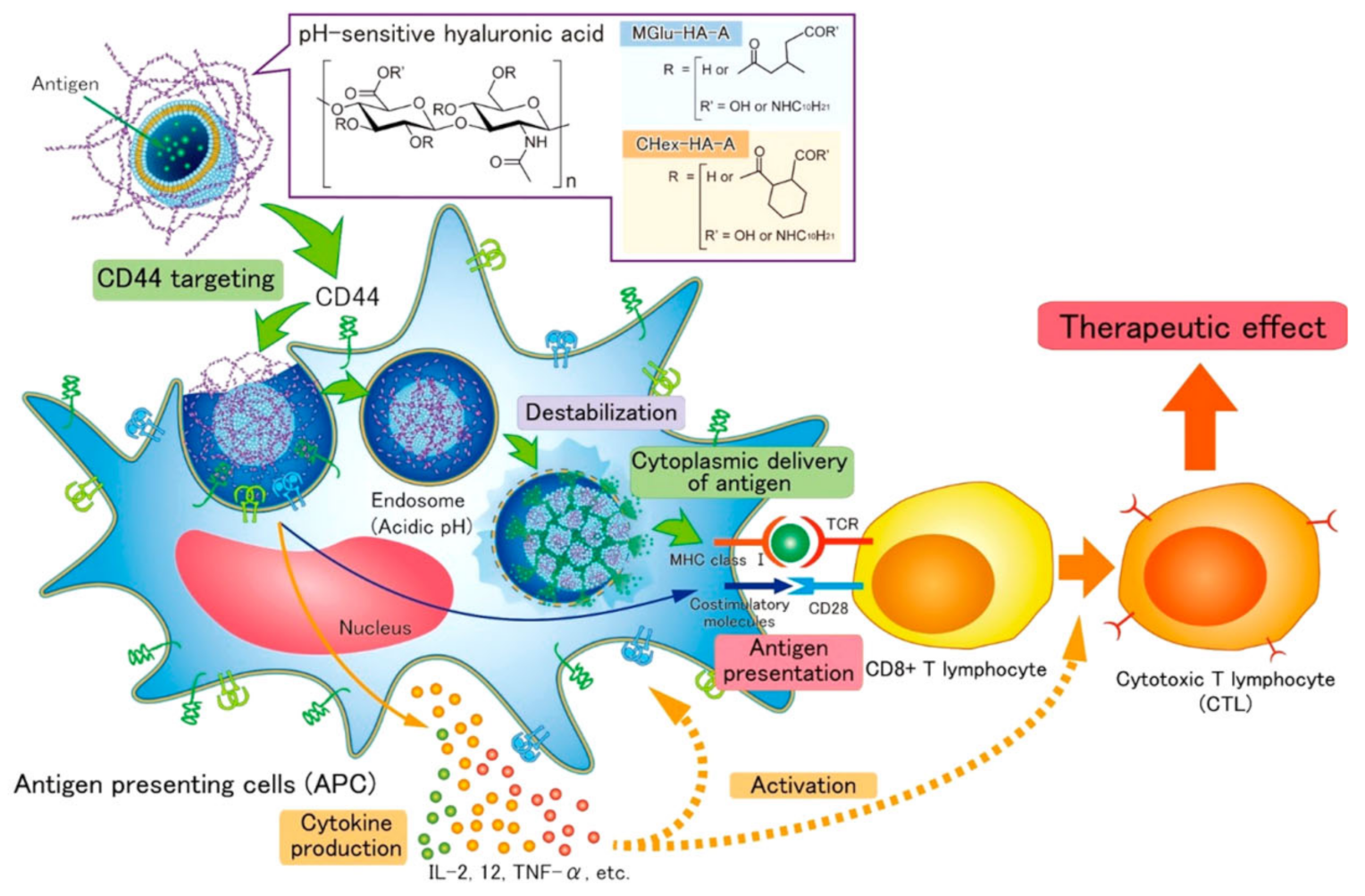
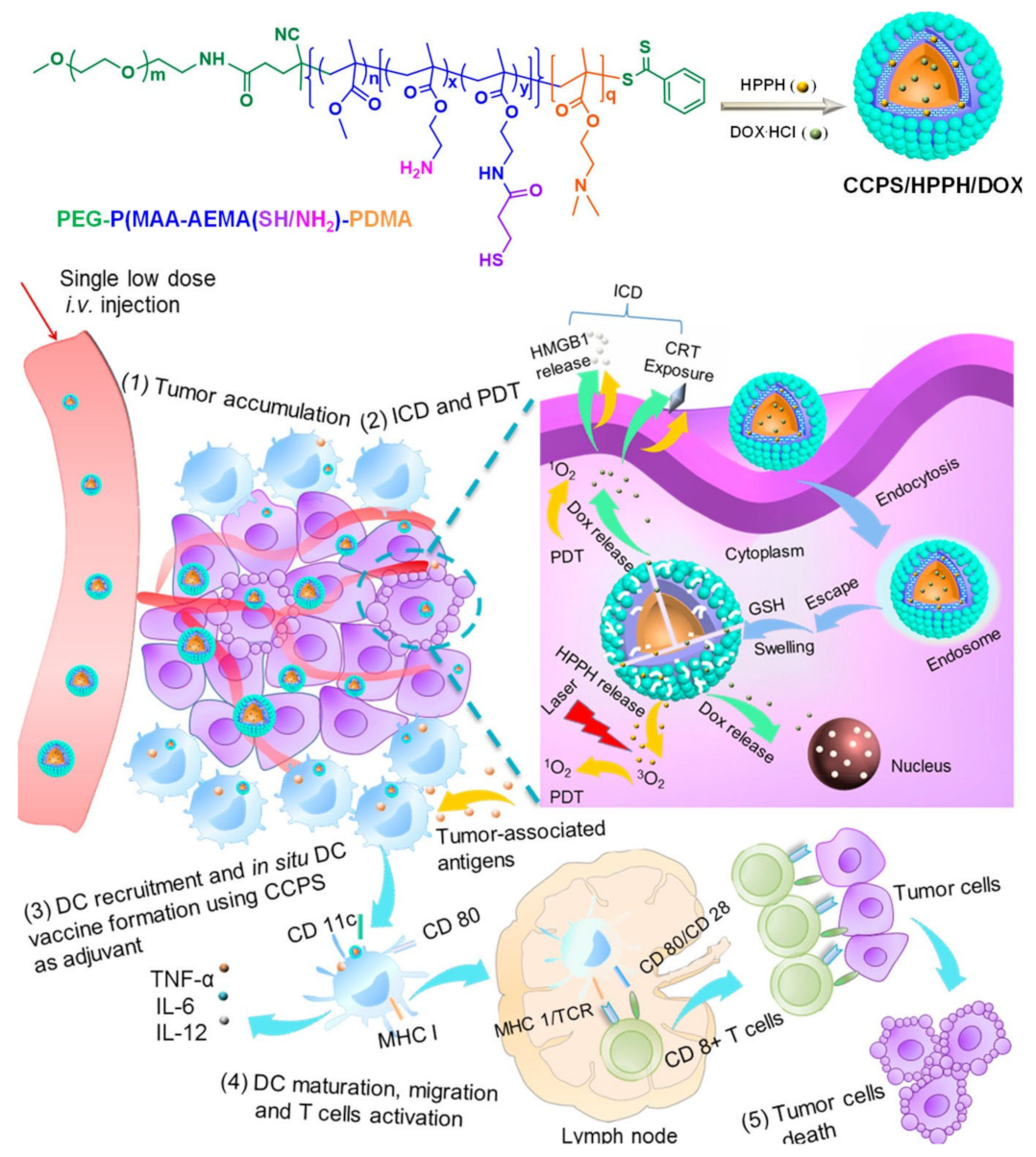
| Stimuli | Nanomaterials | References |
|---|---|---|
| pH | PAA, PMAA, PEI, PNIPAAM, PAM, PLGA, PEG histamine modified Alanine, PLA | [27,28,29] |
| GSH | Polymers with disulphide linkage PEG-P (MMA-co-AEMA (SH/NH2)-PDMA | [30,31] |
| ROS | Poly (propylene sulphide), poly(thioketal), phenyl boronic acid, poly- L-(methionine), poly- L-(proline) | [32,33,34,35] |
| Enzymes | Sulfato-b-cyclodextrin | [36] |
| Hypoxia | Nitrobenzoyl alcohols, Nitroimidazoles, Azo linkers | [37] |
| Stimuli | Nanomaterials | References |
|---|---|---|
| Light | O-nitro benzyl, pyrene, spiropyran, and azobenzene | [48,49,50,51] |
| Thermo | HPMA, PNIPAAM, PiPOx, Modified Poly acrylamides | [52] |
| Ultrasound | PLGA, tetrahydropyranyl groups | [53,54] |
| Magnetic | OEGMA and MAA loaded with superparamagnetic iron oxide | [55] |
| Electric | PVA, poly (acrylic acid-co-2-acrylsmido-2-methyl propyl sulfonic acid) | [56] |
| Stimuli | Nanomaterials | Cancer | Antigen and Mechanism of Action | References |
|---|---|---|---|---|
| pH | HA liposomes | Lymphoma | Ovalbumin Targeting DC | [72] |
| Chitosan micelles | Melanoma | Ovalbumin Targeting DC | [74] | |
| MGlu-HPG-modified liposomes | Lymphoma | CPG Targeting DC | [75] | |
| 5,6-dimethylxanthenone-4-acetic acid-based micelles | Melanoma and breast cancer | Ovalbumin Activating STING pathway | [76] | |
| Chondroitin sulphate derived liposomes | Melanoma | Ovalbumin Targeting DC | [77] | |
| Caprolactone based hydrogel | Breast cancer | CPG Targeting DC | [78] | |
| Redox | Hyperbranched poly-(amidoamine) based nanocomposite | Lymphoma | Ovalbumin Cytoplasmic delivery of antigens | [79] |
| pH and redox | PAMAM clusters | Pancreatic cancer | CPG Targeting draining lymph node | [80] |
| Hypoxia | Glycol chitosan-PEG mesoporous silica nanoparticles | Melanoma | CPG Targeting DC | [81] |
| Stimuli | Polymeric Nanoparticle | Cancer Type | Antigen and Mechanism of Action | Reference |
|---|---|---|---|---|
| Combination PDT and hypoxia | Glycol chitosan-PEG mesoporous silica nanoparticles | Melanoma | CPG and targeting DC | [81] |
| Laser and ROS | Polyethyleneimine based nanoparticles | Lymphoma | OVA and targeting DC | [83] |
| Stimuli | Polymeric Nanoparticle | Cancer Type | Mechanism of Action | References |
|---|---|---|---|---|
| pH sensitive | Cholesterol-DOPE-PEG based lipid nanoparticles | Lymphoma and melanoma | Activation of macrophages and plasmid DNA | [87] |
| ROS sensitive | poly (thioketal phosphoester) lecithin-PEG based nanoparticles | Breast cancer | Release of antigens due to LASER | [89] |
| Stimuli | Polymeric Nanoparticle | Cancer Type | Agonist and Mechanism of Action | Reference |
|---|---|---|---|---|
| pH responsive | polymer p(DMAEMA)-b-(DMAEMA-co-BMA-co- PAA) based nanoparticle | Colon cancer | 3pRNA and enhancing Anti PD L1 therapy | [96] |
| mPEG-block-[DMAEMA-co-AnMA] nanocarriers | Pancreatic cancer | 3pRNA and Endosomolytic carriers | [95] | |
| carboxyl terminated PLGA nanoshells | Colon cancer | cGMP and inducing immunogenic cell death | [97] | |
| PLGA nanoparticles | Lung adenocarcinoma | Small molecule 522 and antigen release due to CO2 production | [93] | |
| Enzyme responsive | PEG vesicular nanoparticles | Imidazoquinoline and bringing Dendritic cells to Lymph nodes | [94] |
| Stimuli | Polymeric Nanoparticle | Cancer Type | Agonist and Mechanism of Action | Reference |
|---|---|---|---|---|
| NIR II- PTT | DSPE-PEG nanoagonists | Breast cancer | Resiquimod and targeting DC | [99] |
| Radiation | PEG nanoparticles | Breast cancer | RGD peptide and targeting NK cells | [98] |
| Antigens | Adjuvants/Agonists | Polymer | Cancer Type | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Ovalbumin | Imiquimod | PLGA | Melanoma | Gel−sol−gel transformation for DC activation | [53] |
| TRP-2 peptide | CPG | PCL-PEG, PCL-PEI | Melanoma | Activating DC | [90] |
| Ovalbumin | CL264 | PEOz-PLA | Lymphoma | Activating DC | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagareddy, R.; Thomas, R.G.; Jeong, Y.Y. Stimuli-Responsive Polymeric Nanomaterials for the Delivery of Immunotherapy Moieties: Antigens, Adjuvants and Agonists. Int. J. Mol. Sci. 2021, 22, 12510. https://doi.org/10.3390/ijms222212510
Nagareddy R, Thomas RG, Jeong YY. Stimuli-Responsive Polymeric Nanomaterials for the Delivery of Immunotherapy Moieties: Antigens, Adjuvants and Agonists. International Journal of Molecular Sciences. 2021; 22(22):12510. https://doi.org/10.3390/ijms222212510
Chicago/Turabian StyleNagareddy, Raveena, Reju George Thomas, and Yong Yeon Jeong. 2021. "Stimuli-Responsive Polymeric Nanomaterials for the Delivery of Immunotherapy Moieties: Antigens, Adjuvants and Agonists" International Journal of Molecular Sciences 22, no. 22: 12510. https://doi.org/10.3390/ijms222212510
APA StyleNagareddy, R., Thomas, R. G., & Jeong, Y. Y. (2021). Stimuli-Responsive Polymeric Nanomaterials for the Delivery of Immunotherapy Moieties: Antigens, Adjuvants and Agonists. International Journal of Molecular Sciences, 22(22), 12510. https://doi.org/10.3390/ijms222212510







