Melatonin and Pathological Cell Interactions: Mitochondrial Glucose Processing in Cancer Cells
Abstract
:1. Introduction
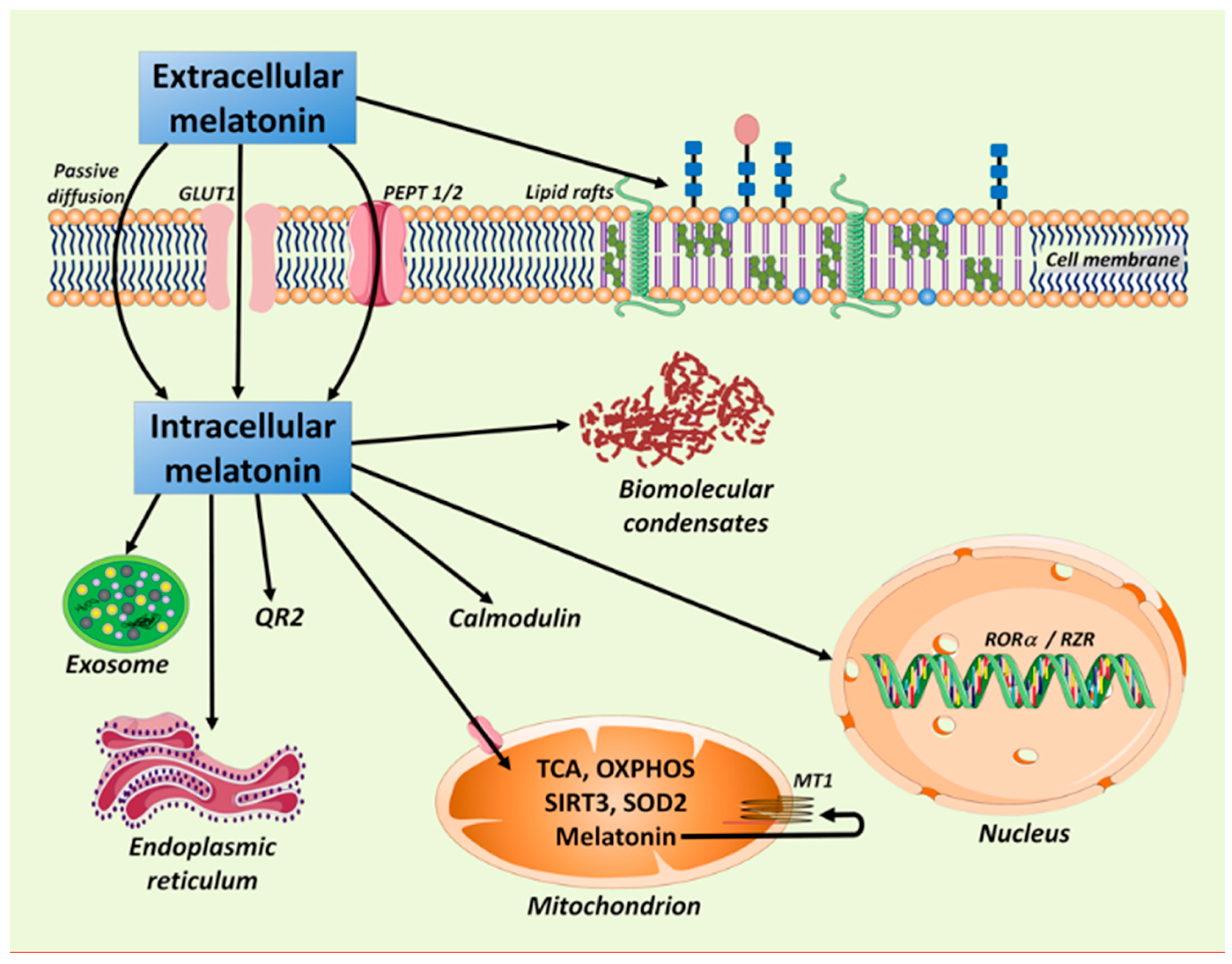
2. Melatonin: In the Right Place and at the Right Time in All Cells
3. Melatonin in Mitochondria: Some Assembly Required
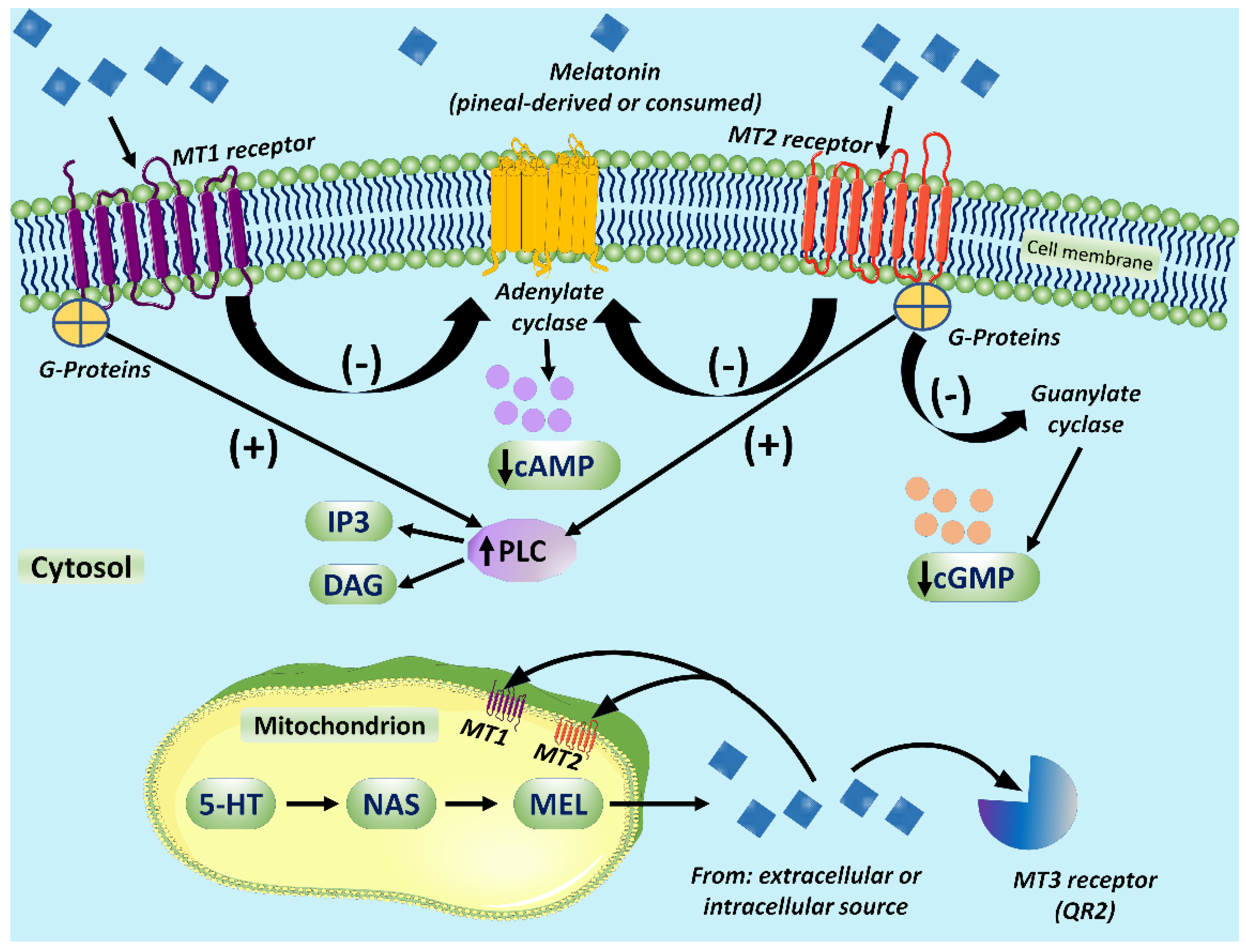
4. Melatonin Signaling via the Cellular Membrane Receptors
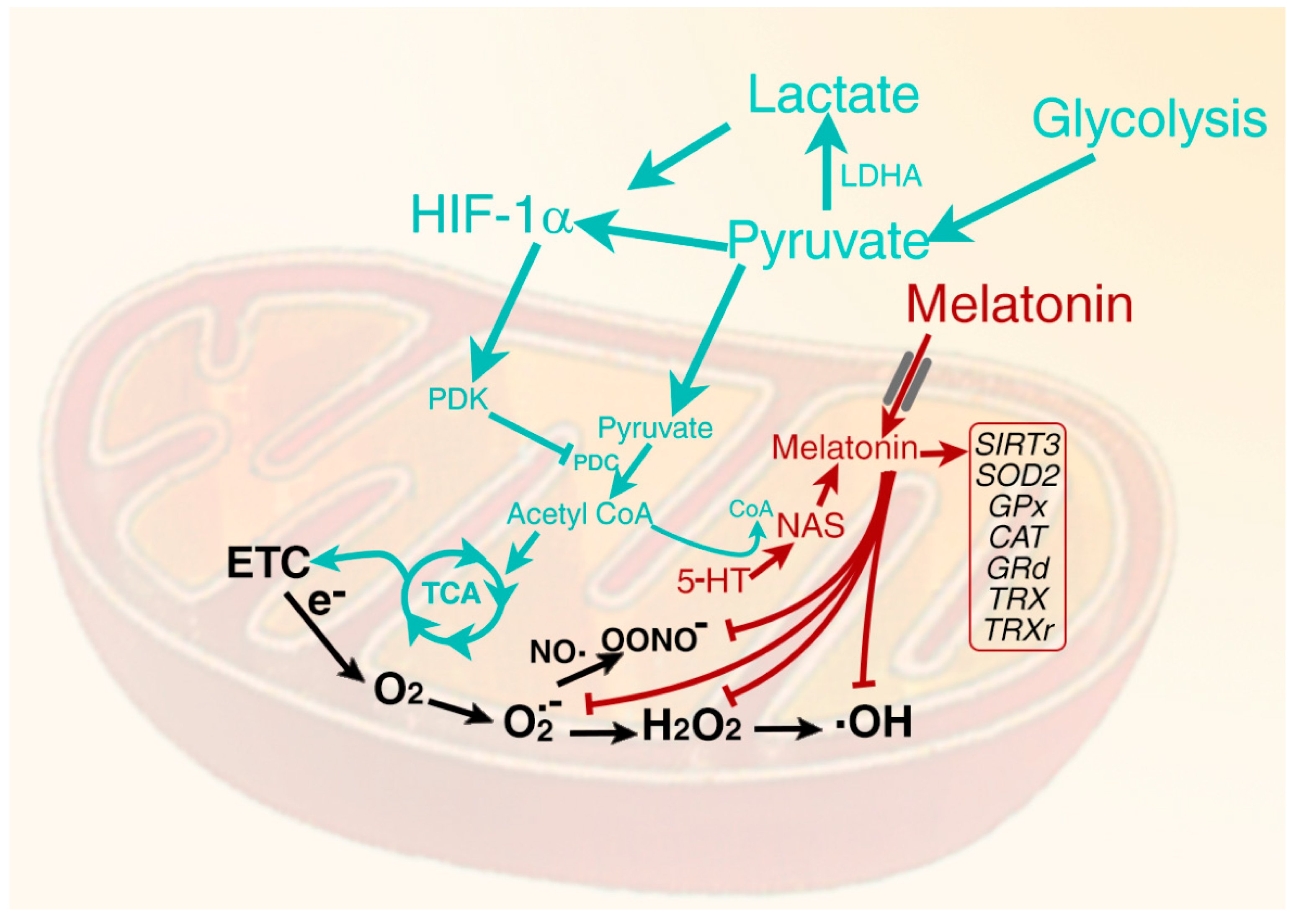
5. Melatonin in Mitochondria: Relation to Oxidative Stress and Glucose Metabolism
6. Role of Hypoxia Inducible Factor in Determining the Metabolic Phenotype
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dauchy, R.T.; Blask, D.E.; Dauchy, E.M.; Davidson, L.K.; Tirrell, P.C.; Greene, M.W.; Tirrell, R.P.; Hill, C.R.; Sauer, L.A. Antineoplastic effects of melatonin on a rare malignancy of mesenchymal origin: Melatonin receptor-mediated inhibition of signal transduction, linoleic, acid metabolism and growth in tissue-isolated human leiomyosarcoma xenografts. J. Pineal Res. 2009, 47, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An inhibitor of breast cancer. Endocr. Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samec, M.; Liskova, A.; Koklesova, L.; Zhai, K.; Varghese, E.; Samuel, S.M.; Sudomova, M.; Lucansky, V.; Kassayova, M.; Pec, M.; et al. Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects. Cancers 2021, 13, 3018. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martin, E.; Lopez-Munoz, F.; Reiter, R.J.; Romero, A. Understanding the oncostatic actions displayed by melatonin in colorectal cancer therapy. Future Med. Chem. 2020, 12, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Boutriq, S.; Plaza-Andrades, I.; Peralta-Linero, J.; Alba, E.; Gonzalez-Gonzalez, A.; Queipo-Ortuno, M.I. A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent. Cancers 2021, 13, 3141. [Google Scholar] [CrossRef]
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A. Putting cancer to sleep at night: The neuroendocrine/circadian mealtonin signal. Endocrine 2005, 27, 179–188. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; Yang, X.; Liu, L.; Hu, J.; Hou, Y.; Tao, H.; Sugimura, H.; Chen, Z.; Wang, L.; et al. Melatonin inhibits lipid accumulation to repress prostate cancer progression by mediating the epigenetic modification of CES1. Clin. Transl. Med. 2021, 11, e449. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Molecular targets for the management of gastrointestinal cancer using melatonin, a natural endogenous body hormone. Biomed. Pharmacother. 2021, 140, 111782. [Google Scholar] [CrossRef]
- Anderson, G. The effects of melatonin on signaling pathways and molecules involved in glioma: Melatonin and glioblastoma pathophysiology and treatment. Fundam. Clin. Pharmacol. 2020, 34, 189–191. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Kamali, M.; Mehrzadi, S.; Hosseinzadeh, A. Melatonin and neuroblastoma: A novel therapeutic approach. Mol. Biol. Rep. 2021, 48, 4659–4665. [Google Scholar] [CrossRef]
- Chuffa, L.G.A.; Reiter, R.J.; Lupi, L.A. Melatonin as a promising agent to treat ovarian cancer: Molecular mechanisms. Carcinogenesis 2017, 38, 945–952. [Google Scholar] [CrossRef]
- Yasin, H.K.; Taylor, A.H.; Ayakannu, T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers 2021, 13, 2149. [Google Scholar] [CrossRef]
- Mehrzadi, M.H.; Hosseinzedah, A.; Juybari, K.B.; Mehrzadi, S. Melatonin and urological cancers: A new therapeutic approach. Cancer Cell Int. 2020, 20, 444. [Google Scholar]
- Maleki, M.; Khelghati, N.; Alemi, F.; Younesi, S.; Asemi, Z.; Abolhasan, R.; Bazdar, M.; Samadi-Kafil, H.; Yousefi, B. Multiple interactions between melatonin and non-coding RNAs in cancer biology. Chem. Biol. Drug Des. 2021, 98, 323–340. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef] [Green Version]
- Lacerda, J.Z.; Ferreira, L.C.; Lopes, B.C.; Aristizabal-Pachon, A.F.; Bajgelman, M.C.; Borin, T.F.; Zuccari, D.A.P.C. Therapeutic potential of melatonin in the regulation of MiR-148a-3p and angiogenic factors in breast cancer. Microna 2019, 8, 237–247. [Google Scholar] [CrossRef]
- Colombo, J.; Jardim-Perassi, B.V.; Ferreira, J.P.S.; Braga, C.Z.; Sonehara, N.M.; Junior, R.P.; Moschetta, M.G.; Girol, A.P.; Zuccari, D.A.P.C. Melatonin differentially modulates NF-B expression in breast and liver cancer cells. Anticancer Agents Med. Chem. 2018, 18, 1688–1694. [Google Scholar] [CrossRef]
- Shen, D.; Ju, L.; Zhou, F.; Yu, M.; Ma, H.; Zhang, Y.; Liu, T.; Xiao, Y.; Wang, X.; Qian, K. The inhibitory effect of melatonin on human prostate cancer. Cell Commun. Signal 2021, 19, 34. [Google Scholar] [CrossRef]
- Jardim-Perassi, B.V.; Alexandre, P.A.; Sonehara, N.M.; de Paula-Junior, R.; Reis Junior, O.; Fukumasu, H.; Chammas, R.; Coutinho, L.L.; Zuccari, D.A.P.S. RNA-Seq transcriptome analysis shows anti-tumor actions of melatonin in a breast cancer xenograft model. Sci. Rep. 2019, 9, 966. [Google Scholar] [CrossRef] [Green Version]
- Ezzati, M.; Velaei, K.; Kheirjou, R. Melatonin and its mechanism of action in the female reproductive system and related malignancies. Mol. Cell Biochem. 2021, 476, 3177–3190. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Rueda, N. Breast cancer therapy based on melatonin. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 108–116. [Google Scholar] [CrossRef]
- Razi Soofiyani, S.; Ahangari, H.; Soleimanian, A. The role of circadin genes in eh pathogenesis of colorectal cancer. Genes 2021, 804, 145894. [Google Scholar]
- Jin, Y.; Choi, Y.J.; Heo, K.; Park, S.J. Melatonin as an Oncostatic Molecule Based on Its Anti-Aromatase Role in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 438. [Google Scholar] [CrossRef]
- Chuffa, L.G.A.; Seiva, F.R.F.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Lupi, L.A. Mitochondrial functions and melatonin: A tour of the reproductive cancers. Cell Mol. Life Sci. 2019, 76, 837–863. [Google Scholar] [CrossRef]
- Chuffa, L.G.; Lupi Junior, L.A.; Seiva, F.R.; Martinez, M.; Domeniconi, R.F.; Pinheiro, P.F.; Dos Santos, L.D.; Martinez, F.E. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J. Proteome Res. 2016, 15, 3872–3882. [Google Scholar] [CrossRef]
- Sanchez-Hidalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Blask, D.E.; Dauchy, R.T.; Dauchy, E.M.; Mao, L.; Hill, S.M.; Greene, M.W.; Belancio, V.P.; Sauer, L.A.; Davidson, L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE 2014, 9, e102776. [Google Scholar]
- Dauchy, R.T.; Wren-Dail, M.A.; Dupepe, L.M.; Hill, S.M.; Xiang, S.; Anbalagan, M.; Belancio, V.P.; Dauchy, E.M.; Blask, D.E. Effect of daytime blue-enriched LED light on the nighttime circadian melatonin inhibition of hepatoma 7288CTC Warburg effect and progression. Comp. Med. 2018, 68, 269–279. [Google Scholar] [CrossRef]
- Mao, L.; Dauchy, R.T.; Blask, D.E.; Dauchy, E.M.; Slakey, L.M.; Brimer, S.; Yuan, L.; Xiang, S.; Hauch, A.; Smith, K.; et al. Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J. Pineal Res. 2016, 60, 167–177. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S. Anti-Warburg effect of melatonin: A proposed mechanism to explain its inhibition of multiple diseases. Int. J. Mol. Sci. 2021, 22, 764. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular mechanisms of melatonin-mediated cell protection and signaling in health and disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Puente-Moncada, N.; Reiter, R.J.; Sanchez-Sanchez, A.M.; Herrera, F.; Rodriguez-Blanco, J.; Duarte-Olivenza, C.; Turos-Cabal, M.; Antolin, I.; Martin, V.J. Regulation of cancer cell glucose metabolism is determinant for cancer cell fate after melatonin administration. J. Cell Physiol. 2021, 236, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Duraj, T.; Garcia-Romero, N.; Carrion-Navarro, J.; Madurga, R.; de Mendivil, A.O.; Prat-Acin, R.; Garcia-Canamaque, L.; Ayuso-Sacido, A. Beyond the Warburg effect: Oxidative and glycolytic phenotypes coexist within the metabolic heterogeneity of glioblastoma. Cells 2021, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Ma, Q. Switching diseased cells from cytosolic aerobic glycolysis to mitochondrial oxidative phosphorylation: A metabolic rhythm regulated by melatonin? J. Pineal Res. 2021, 70, e12677. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rosales-Corral, S.; de Almeida Chuffa, L.G. Melatonin inhibits Warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: A mechanistic hypothesis. Cell Mol. Life Sci. 2020, 77, 2527–2542. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Rodriguez, C.; Martin, V.; Rosales-Corral, S.; de Campos Zuccari, D.A.P.; de Almeida Chuffa, L.G. Part-time cancers and role of melatonin in determining their metabolic phenotype. Life Sci. 2021, 8, 119597. [Google Scholar] [CrossRef]
- Ma, W.Q.; Sun, X.J.; Zhu, Y.; Liu, N.E. PDK4 promotes vascular calcification by interfering with autophagic activity and metabolic reprogramming. Cell Death Dis. 2020, 11, 991. [Google Scholar] [CrossRef]
- Kornberg, M.D. The immunologic Warburg effect: Evidence and therapeutic opportunities in autoimmunity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1486. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, Y.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Rosner, J.M. Retinal localization of the hydroxyindole-O-methyl transferase (HIOMT) in the rat. Endocrinology 1971, 89, 301–303. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Nagle, C.A.; Rosner, J.M. Periodic changes in rat retinal and pineal melatonin synthesis. Acta Physiol. Lat. Am. 1974, 24, 97–98. [Google Scholar]
- Cardinali, D.P.; Wurtman, R.J. Hydroxyindole-O-methyl transferases in rat pineal, retina and harderian gland. Endocrinology 1972, 91, 247–252. [Google Scholar] [CrossRef]
- Yu, H.S.; Pang, S.F.; Tang, P.L. Increase in the level of retinal melatonin and persistence of its diurnal rhythm in rats after pinealectomy. J. Endocrinol. 1981, 91, 477–481. [Google Scholar] [CrossRef]
- Reiter, R.J.; Richardson, B.A.; Matthews, S.A.; Ferguson, B.N. Rhythms in immunoreactive melatonin in the retina and Harderian gland of rats; persistence after pinealectomy. Life Sci. 1983, 32, 1229–1236. [Google Scholar] [CrossRef]
- Vivien-Roels, B.; Pevet, P.; Beck, O.; Fevre-Montange, M. Identification of melatonin in the compound eyes of an insect, the locust (Locusta migratoria), by radioimmunoassay and gas chromatography-mass spectrometry. Neurosci. Lett. 1984, 49, 153–157. [Google Scholar] [CrossRef]
- Blanc, A.; Vivien-Roels, B.; Pevet, P.; Attia, J.; Buisson, B. Melatonin and 5-methoxytryptophol (5-ML) in nervous and/or neurosensory structures of a gastropod mollusca (Helix aspersa maxima): Synthesis and diurnal rhythms. Gen. Comp. Endocrinol. 2003, 131, 168–175. [Google Scholar] [CrossRef]
- Poeggeler, B.; Hardeland, R. Detection and quantification of melatonin in a dinoflagellate, Gonyaulax polyedra: Solutions to the problem of methoxyindole destruction in non-vertebrate material. J. Pineal Res. 1994, 17, 1–10. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B.J. Non-vertebral melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar] [CrossRef]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, J.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X. Melatonin: An antioxidant in edible plants. Ann. N. Y. Acad. Sci. 2002, 957, 341–344. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin: Signaling mechanisms of a pleiotropic agent. Biofactors 2009, 35, 183–192. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in plants—Diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef]
- Kolar, J.; Machackova, I. Melatonin in higher plants: Occurrence and possible functions. J. Pineal Res. 2005, 39, 333–341. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Zhao, D.; Yao, Z.; Zhang, J.; Zhang, R.; Mou, Z.; Zhang, X.; Li, Z.; Feng, X.; Chen, S.; Reiter, R.J. Melatonin synthesis genes N-acetylserotonin methyltransferases evolved into caffeic acid O-methyltransferases and both assisted in plant terrestrialization. J. Pineal Res. 2021, 71, e12737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and Function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [Green Version]
- Treister-Goltzman, Y.; Peleg, R. Melatonin and the health of menopausal women: A systematic review. J. Pineal Res. 2021, 71, e12743. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin against environmental plant stressors: A review. Curr. Protein Pept. Sci. 2021. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef] [Green Version]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y.J. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondria regulation of cell cycle and proliferation. Antioxid. Redox. Signal 2012, 16, 1150–1180. [Google Scholar] [CrossRef] [Green Version]
- Idelchik, M.D.P.S.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Su, S.C.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017, 62, 12370. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Orso, F.; Dettori, D.; Lacerda, J.Z.; Borin, T.F.; Taverna, D.; Zuccari, D.A.P.C. The role of melatonin on miRNAs modulation in triple-negative breast cancer cells. PLoS ONE 2020, 15, e0228062. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Manchester, L.C. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev. Med. Chem. 2013, 13, 373–384. [Google Scholar]
- Messner, M.; Huether, G.; Lort, T.; Ramadori, G.; Schworer, H. Presence of melatonin in the human hepatobiliary-gastrointestinal tract. Life Sci. 2001, 69, 543–551. [Google Scholar] [CrossRef]
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Hacker, R.R.; Brown, G.M.; Bartos, L.J. Melatonin concentrations in the luminal fluid, mucosa, and muscularis of the bovine and porcine gastrointestinal tract. J. Pineal Res. 1999, 26, 56–63. [Google Scholar] [CrossRef]
- Pinato, L.; da Silveira Cruz-Machado, S.; Franco, D.G.; Campos, L.M.; Cecon, E.; Fernandes, P.A.; Bittencourt, J.C.; Markus, R.P. Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct. Funct. 2015, 220, 827–840. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Chamorro, I.; Alvarez-Sanchez, N.; Escalante-Andiocoechea, C.; Carrillo-Vico, A.; Rubio, A.; Guerrero, J.M.; Molinero, P.; Lardone, P.J. Temporal expression patterns of the melatoninergic system in the human thymus of children. Mol. Metab. 2019, 28, 83–90. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, M.A.; Guerrero, J.M.; Delgado, F. Presence of the pineal hormone melatonin in rat cochlea: Its variations with lighting conditions. Neurosci. Lett. 1997, 238, 81–83. [Google Scholar] [CrossRef]
- Martin, X.D.; Malina, H.Z.; Brennan, M.C.; Hendrickson, P.H.; Lichter, P.R. The ciliary body—The third organ found to synthesize indoleamines in humans. Eur. J. Ophthalmol. 1992, 2, 67–72. [Google Scholar] [CrossRef]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, J.M. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 2000, 28, 193–202. [Google Scholar] [CrossRef]
- Slominiski, A.T.; Kleszczynski, K.; Semak, I.; Janjetovic, Z.; Zmijewski, M.A.; Kim, T.K.; Slominiski, R.M.; Reiter, R.J.; Fischer, T.W. Local melatoninergic system as the protector of skin integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732. [Google Scholar] [CrossRef] [Green Version]
- Slominiski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef]
- Yu, H.S.; Yee, R.W.; Howes, K.A.; Reiter, R.J. Diurnal rhythms of immunoreactive melatonin in the aqueous humor and serum of male pigmented rabbits. Neurosci. Lett. 1990, 116, 309–314. [Google Scholar] [CrossRef]
- Brzezinski, A.; Seibel, M.M.; Lynch, H.J.; Deng, M.H.; Wurtman, R.J. Melatonin in human preovulatory follicular fluid. J. Clin. Endocrinol. Metab. 1987, 64, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H.C. Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochim. Biophys. Acta 1999, 1472, 206–214. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Manchester, L.C.; Liu, X.; Tan, D.X. Melatonin in the biliary tract and liver: Health implications. Curr. Pharm. Des. 2014, 20, 4788–4801. [Google Scholar] [CrossRef]
- Ostrycharz, E.; Wasik, U.; Kempinska-Podhorodecka, A.; Banales, J.M.; Milkiewicz, P.; Milkiewicz, M. Melatonin protects cholangiocytes from oxidative stress-induced proapoptotic and proinflammatory stimuli via miR-132 and miR-34. Int. J. Mol. Sci. 2020, 21, 9667. [Google Scholar] [CrossRef]
- Shiesh, S.C.; Chen, C.Y.; Lin, X.Z.; Liu, Z.A.; Tsao, H.C. Melatonin prevents pigment gallstone formation induced by bile duct ligation in guinea pigs. Hepatology 2000, 32, 455–460. [Google Scholar] [CrossRef]
- Laothrong, U.; Hiraku, Y.; Oikaw, S.; Intuyod, K.; Murata, M.; Pinlaor, S. Melatonin induces apoptosis in cholangiocarcinoma cell lines by activating the reactive oxygen species-mediated mitochondrial pathway. Oncol. Rept. 2015, 33, 1443–1449. [Google Scholar] [CrossRef] [Green Version]
- Koppisetti, S.; Jenigiri, B.; Terron, M.P.; Tengattini, S.; Tamura, H.; Flores, L.J.; Tan, D.X.; Reiter, R.J. Reactive oxygen species and the hypomotility of the gall bladder as targets for the treatment of gallstones with melatonin: A review. Dig. Dis. Sci. 2008, 53, 2592–2603. [Google Scholar] [CrossRef]
- Xia, D.; Yang, L.; Li, Y.; Chen, J.; Zhang, X.; Wang, H.; Zhai, S.; Jiang, X.; Meca, G.; Wang, S.; et al. Melatonin alleviates Ochratoxin A-induced liver inflammation involved intestinal microbiota homeostasis and microbiota-independent manner. J. Hazard Mater. 2021, 413, 125239. [Google Scholar] [CrossRef]
- Ebihara, S.; Hudson, D.J.; Marks, T.; Menaker, M. Pineal indole metabolism in the mouse. Brain Res. 1987, 416, 136–140. [Google Scholar] [CrossRef]
- Goto, M.; Oshima, I.; Tomita, T.; Ebihara, S. Melatonin content of the pineal gland in different mouse strains. J. Pineal Res. 1989, 7, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Corvera, A.; Cerrillo, I.; Molinero, P.; Naranjo, M.C.; Lardone, P.J.; Sanchez-Hidalgo, M.; Carrascosa-Salmoral, M.P.; Medrano-Campillo, P.; Guerrero, J.M.; Rubio, A. Evidence of immune system melatonin production by two pineal melatonin deficient mice, C57BL/6 and Swiss strains. J. Pineal Res. 2009, 47, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Peng, T.I.; Hsu, L.F.; Jou, S.B.; Reiter, R.J.; Yang, C.M.; Chiao, C.C.; Lin, Y.F.; Chen, C.C. Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca2+-mediated permeability transition and beyond in rat brain astrocytes. J. Pineal Res. 2010, 48, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Peng, T.I.; Reiter, R.J. Protective stabilization of mitochondrial permeability transition and mitochondrial oxidation during mitochondrial Ca2+ stress by melatonin’s cascade metabolites C3-OHM and AFMK in RBA1 astrocytes. J. Pineal Res. 2019, 66, e12538. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [Green Version]
- Acuna-Castroviejo, D.; Rahim, I.; Acuna-Fernandez, C.; Fernandez-Ortiz, M.; Solera-Marin, J.; Sayed, R.K.A.; Diaz-Casado, M.E.; Rusanova, I.; Lopez, L.C.; Escames, G. Melatonin, clock genes and mitochondria in sepsis. Cell Mol. Life Sci. 2017, 74, 3965–3987. [Google Scholar] [CrossRef]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Kerenyi, N.A.; Balogh, I.; Somogyi, E.; Sotonyi, P. Cytochemical investigation of acetyl-serotonin-transferase activity in the pineal gland. Cell. Mol. Biol. Incl. Cyto. Enzymol. 1979, 25, 259–262. [Google Scholar]
- Margulis, L.; Bermudes, D. Symbiosis as a mechanism of evolution: Status of cell symbiosis theory. Symbiosis 1985, 1, 101–124. [Google Scholar]
- Lang, B.F.; Gray, M.W.; Burger, G. Mitochondrial genome evolution and the origin of eukaryotes. Annu. Rev. Genet. 1999, 33, 351–397. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocytes’ quality under in vitro conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Z.; Cui, M.; Zhang, L.; Li, Q. Melatonin restores the developmental competence of heat stressed porcine oocytes and alters the expression of genes related to oocyte maturation. Animals 2021, 11, 1086. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, J.; Bu, S.; Li, B.; Zhang, Q.; Wang, Q.; Lai, D. Melatonin protects against chronic stress-induced oxidative meiotic defects in mice MII oocytes by regulating SIRT1. Cell Cycle 2020, 19, 1677–1695. [Google Scholar] [CrossRef]
- Zhang, Z.; Mu, Y.; Ding, D.; Zou, W.; Li, X.; Chen, B.; Leung, P.C.; Chang, H.M.; Zhu, Q.; Wang, K.; et al. Melatonin improves the effect of cryopreservation on human oocytes by suppressing oxidative stress and maintaining the permeability of the oolemma. J. Pineal Res. 2021, 70, e12707. [Google Scholar] [CrossRef]
- Pang, Y.W.; Sun, Y.Q.; Sun, W.J.; Du, W.H.; Hao, H.S.; Zhao, S.J.; Zhu, H.B. Melatonin inhibits paraquat-induced cell death in bovine preimplantation embryos. J. Pineal Res. 2016, 60, 155–166. [Google Scholar] [CrossRef]
- Gelaleti, G.B.; Borin, T.F.; Maschio-Signorini, L.B.; Moschetta, M.G.; Hellmén, E.; Viloria-Petit, A.M.; Zuccari, D.A.P.C. Melatonin and IL-25 modulate apoptosis and angiogenesis mediators in metastatic (CF-41) and non-metastatic (CMT-U229) canine mammary tumour cells. Vet. Comp. Oncol. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Brzozowska, I.M.; Hoa, N.; Jones, M.L.; Tarnawski, A.S. Melatonin signaling in mitochondria extends beyond neurons and neuroprotection: Implications for angiogenesis and cardio/gastroprotection. Proc. Natl. Acad. Sci. USA 2018, 115, E1942–E1943. [Google Scholar] [CrossRef] [Green Version]
- Boutin, J.A.; Ferry, G.J. Is there sufficient evidence that the melatonin binding site MT3 is quinone reductase 2? J. Pharmacol. Exp. Ther. 2019, 368, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: What’s new? Br. J. Pharmacol. 2008, 154, 1182–1195. [Google Scholar] [CrossRef] [Green Version]
- Hevia, D.; Gonzalez-Menendez, P.; Quiros-Gonzalez, I.; Miar, A.; Rodriguez-Garcia, A.; Tan, D.X.; Reiter, R.J.; Mayo, J.C.; Sainz, R.M. Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 2015, 58, 234–250. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; Gonzalez-Menendez, P.; Hevia, D.; Cernuda-Cernuda, R. Melatonin transport into mitochondria. Cell Mol. Life Sci. 2017, 74, 3927–3940. [Google Scholar] [CrossRef]
- Huo, X.; Wang, C.; Yu, Z.; Peng, Y.; Wang, S.; Feng, S.; Zhang, S.; Tian, X.; Sun, C.; Liu, K.; et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef]
- Acuna-Castroviejo, D.; Noguiera-Navarro, M.T.; Reiter, R.J.; Escames, G. Melatonin actions in the heart; more than a hormone. Melatonin Res. 2018, 1, 21–26. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Recent findings in melatonin research and their relevance to the CNS. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 102–114. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Govitrapong, P. Melatonin receptor as a drug target for neuroprotection. Curr. Mol. Pharmacol. 2021, 14, 150–164. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Kang, M.H.; Kim, J.H. Role and therapeutic potential of melatonin in various type of cancers. Onco. Targets Ther. 2021, 14, 2019–2052. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin receptors: Molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Labani, N.; Cecon, E.; Jockers, R. Melatonin target proteins: Too many or not enough? Front. Endocrinol. 2019, 10, 791. [Google Scholar] [CrossRef] [Green Version]
- Stauch, B.; Johansson, L.C.; Cherezov, V. Structural insights into melatonin receptors. FEBS J. 2020, 287, 1496–1510. [Google Scholar] [CrossRef] [Green Version]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A review of melatonin, its receptors and drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef]
- Wortzel, I.; Seger, R. The ERK cascade: Distinct functions within various subcellular organelles. Genes Cancer 2011, 2, 195–209. [Google Scholar] [CrossRef]
- Martin, V.; Herrera, F.; Garcia-Santos, G.; Antolin, I.; Rodriguez-Blanco, J.; Medina, M.; Rodriguez, C. Involvement of protein kinase C in melatonin’s oncostatic effect in C6 glioma cells. J. Pineal Res. 2007, 43, 239–244. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [Green Version]
- Andrews, C.D.; Foster, R.G.; Alexander, I.; Vasudevan, S.; Downes, S.M.; Heneghan, C.; Pluddemann, A. Sleep-wake disturbance related to ocular disease: A systematic review of phase-shifting pharmaceutical therapies. Transl. Vis. Sci. Technol. 2019, 8, 49. [Google Scholar] [CrossRef]
- Pevet, P. Melatonin receptors as therapeutic targets in the suprachiasmatic nucleus. Expert Opin. Ther. Targets 2016, 20, 1209–1218. [Google Scholar] [CrossRef]
- Madhu, L.N.; Kodali, M.; Attaluri, S.; Shuai, B.; Melissari, L.; Rao, X.; Shetty, A.K. Melatonin improves brain function in a model of chronic Gulf War illness with modulation of oxidative stress, NLRP3 inflammasomes, and BDNF-ERK-CREB pathway in the hippocampus. Redox Biol. 2021, 43, 101973. [Google Scholar] [CrossRef]
- Oishi, A.; Cecon, E.; Jockers, R. Melatonin receptor signaling: Impact of receptor oligomerization on receptor function. Int. Rev. Cell Mol. Biol. 2018, 338, 59–77. [Google Scholar]
- Bonmati-Carrion, M.A.; Tomas-Loba, A. Melatonin and cancer: A polyhedral network where the source matters. Antioxidants 2021, 10, 210. [Google Scholar] [CrossRef]
- Reiter, R.J.; Ma, Q.; Sharma, R. Melatonin in mitochondria: Mitigating clear and present dangers. Physiology 2020, 35, 86–95. [Google Scholar] [CrossRef]
- Sack, R.L.; Lewy, A.J.; Erb, D.L.; Vollmer, W.M.; Singer, C.M. Human melatonin production decreases with age. J. Pineal Res. 1986, 3, 379–388. [Google Scholar] [CrossRef]
- Scholtens, R.M.; van Munster, B.C.; van Kempen, M.F.; de Rooij, S.E. Physiological melatonin levels in healthy older people: A systematic review. J. Psychosom. Res. 2016, 86, 20–27. [Google Scholar] [CrossRef]
- Jauhari, A.; Baranov, S.V.; Suofu, Y.; Kim, J.; Singh, T.; Yablonska, S.; Li, F.; Wang, X.; Oberly, P.; Minnigh, M.B.; et al. Melatonin inhibits cytosolic mitochondria DNA-induced neuroinflammatory signaling in accelerated aging and neurodegeneration. J. Clin. Investig. 2020, 130, 3124–3136. [Google Scholar] [CrossRef] [Green Version]
- Blask, D.E.; Brainard, G.C.; Dauchy, R.T.; Hanifin, J.P.; Davidson, L.K.; Krause, J.A.; Sauer, L.A.; Rivera-Bermudez, M.A.; Dubocovich, M.L.; Jasser, S.A.; et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005, 65, 11174–11184. [Google Scholar] [CrossRef] [Green Version]
- Blask, D.E.; Dauchy, R.T.; Brainard, G.C.; Hanifin, J.P. Circadian stage-dependent inhibition of human breast cancer metabolism and growth by the nocturnal melatonin signal: Consequences of its disruption by light at night in rats and women. Integr. Cancer Ther. 2009, 8, 347–353. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Vinogradova, I.A.; Panchenko, A.V.; Popovich, I.G.; Zabezhinski, M.A. Light-at-night-induced circadian disruption, cancer and aging. Curr. Aging Sci. 2012, 5, 170–177. [Google Scholar] [CrossRef]
- Kneisley, L.W.; Moskowitz, M.A.; Lynch, H.G. Cervical spinal cord lesions disrupt the rhythm in human melatonin excretion. J. Neural Transm. Suppl. 1978, 13, 311–323. [Google Scholar]
- Reiter, R.J.; Hester, R.J. Interrelationships of the pineal gland, the superior cervical ganglia and the photoperiod in the regulation of the endocrine systems of hamsters. Endocrinology 1966, 79, 1168–1170. [Google Scholar] [CrossRef]
- Mul Fedele, M.L.; Galiana, M.D.; Golombeck, D.A.; Munoz, E.M.; Plano, S.A. Alterations n metabolism and diurnal rhythms following bilateral surgical removal of the superior cervical ganglia in rats. Front. Endocrinol. 2018, 8, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinker, G.X.; Oba-Shinjo, S.M.; Carvalho-Sousa, C.E.; Muxel, S.M.; Marie, S.K.; Markus, R.P.; Fernandes, P.A. Melatonergic system-based two-gene index is prognostic in human gliomas. J. Pineal Res. 2016, 60, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Paulose, J.K.; Cassone, C.V.; Cassone, V.M. Aging, melatonin biosynthesis, and circadian clockworks in the gastrointestinal system of the laboratory mouse. Physiol. Genom. 2019, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Benloucif, S.; Masana, M.I.; Dubocovich, M.L. Responsiveness to melatonin and its receptor expression in aging circadian clock of mice. Am. J. Physiol. 1997, 273, R1855–R1860. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sampath, H. Mitochondrial DNA integrity: Role in health and disease. Cells 2019, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- La Morgia, C.; Maresca, A.; Caporali, L.; Valentino, M.L.; Carelli, V. Mitochondrial diseases in adults. J. Intern. Med. 2020, 287, 592–608. [Google Scholar] [CrossRef]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Musatov, A.; Robinson, N.C. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on Cytochrome c oxidase. Free Radic. Res. 2012, 46, 1313–1326. [Google Scholar] [CrossRef]
- Javadov, S.; Jang, S.; Chapa-Dubocq, X.R.; Khuchua, Z.; Camara, A.K. Mitochondrial respiratory supercomplexes in mammalian cells: Structural versus functional role. J. Mol. Med. 2021, 99, 57–73. [Google Scholar] [CrossRef]
- Mailloux, R.J. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid. Med. Cell Longev. 2018, 2018, 7857251. [Google Scholar] [CrossRef]
- Hernansanz-Agustin, P.; Enriquez, J.A. Generation of reactive oxygen species by mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.X. Melatonin: An established antioxidant worthy of use in clinical trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, M.J.; Acuna-Castroviejo, D. Melatonin mitigates mitochondrial meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018, 19, 2439. [Google Scholar] [CrossRef] [Green Version]
- Baburina, Y.; Lomovsky, A.; Krestinina, O. Melatonin as a potential multitherapeutic agent. J. Pers. Med. 2021, 11, 274. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Q.; Gao, W.; Li, B.; Xia, Z.; Zhao, B. Melatonin protects against cerebral ischemia-perfusion injury in diabetic mice by ameliorating mitochondrial impairments: Involvement of the Akt-SIRT3-SOD2 signaling pathway. Aging 2021, in press. [Google Scholar]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Furumoto, T. Pyruvate transport systems in organelles: Future directions in C4 biology research. Curr. Opin. Plant Biol. 2016, 31, 143–148. [Google Scholar] [CrossRef]
- Saed, C.T.; Tabatabaei-Dakhili, S.A.; Ussher, J.R. Pyruvate dehydrogenase as a therapeutic target for nonalcoholic fatty liver disease. ACS Pharmacol. Transl. Sci. 2021, 4, 582–588. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Mohammad, T.; Islam, A.; Hassan, M.I. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188568. [Google Scholar] [CrossRef]
- Mikawa, T.; Lleonart, M.E.; Takaori-Kondo, A.; Inagaki, N.; Yokode, M.; Kondoh, H. Dysregulated glycolysis as an oncogenic event. Cell Mol. Life Sci. 2015, 72, 1881–1892. [Google Scholar] [CrossRef]
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019, 38, 205–222. [Google Scholar] [CrossRef]
- Zuo, L.; Wijegunawardana, D. Redox role of ROS and inflammation in pulmonary Diseases. Adv. Exp. Med. Biol. 2021, 1304, 187–204. [Google Scholar]
- Zilberman-Peled, B.; Bransburg-Zabary, S.; Klein, D.C.; Gothilf, Y. Molecular evolution of multiple arylalkylamine N-acetyltransferase (AANAT) in fish. Mar. Drugs 2011, 9, 906–921. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rosales-Corral, S.; Acuna-Castroviejo, D.; Escames, D. Inhibition of mitochondrial pyruvate dehydrogenase kinase: A proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2019, 2, 105–119. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Liu, C.; Manucha, W.; Abreu-Gonzalez, P.; Dominguez-Rodriguez, A. Plasticity of glucose metabolism in activated immune cells: Advantages for melatonin inhibition of COVID-19 disease. Melatonin Res. 2020, 3, 362–379. [Google Scholar] [CrossRef]
- Schwartz, L.; Peres, S.; Jolicoeur, M.; da Veiga Moreira, J. Cancer and Alzheimer’s disease: Intracellular pH scales the metabolic disorders. Biogerontology 2020, 21, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, X.; Yan, Y.; Li, H. Pyruvate dehydrogenase kinases (PDKs): An overview toward clinical applications. Biosci. Rep. 2021, 41, BSR20204402. [Google Scholar] [CrossRef] [PubMed]
- Prochownik, E.V.; Wang, H. The metabolic fates of pyruvate in normal and neoplastic cells. Cells 2021, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.X.; Li, C.; Yan, X.L.; Qu, Y.; Yang, Y.; Guo, Z.N. Crosstalk between oxidative stress and ferroptosis/oxytosis in ischemic stroke: Possible targets and molecular mechanisms. Oxid Med. Cell Longev. 2021, 2021, 6643382. [Google Scholar] [CrossRef] [PubMed]
- Halladin, N.L. Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan. Med. J. 2015, 62, B5054. [Google Scholar]
- Brito, R.; Castillo, G.; Gonzalez, J.; Valls, N.; Rodrigo, R. Oxidative stress in hypertension: Mechanisms and therapeutic opportunities. Exp. Clin. Endocrinol. Diabetes 2015, 123, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, L.X.; Silva-Almeida, C.; Rondeau, J.D.; Sonveaux, P. Mitochondrial transfer in cancer: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 3245. [Google Scholar] [CrossRef]
- Arun, S.; Liu, L.; Donmez, G. Mitochondrial biology and neurological diseases. Curr. Neuropharmacol. 2016, 14, 143–154. [Google Scholar] [CrossRef]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, J. Increased levels of malondialdehyde and nitrate/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal Res. 2001, 31, 343–349. [Google Scholar] [CrossRef]
- Toro-Perez, J.; Rodrigo, R. Contribution of oxidative stress in mechanisms of postoperative complications and multiple organ dysfunction syndrome. Redox Rep. 2021, 26, 35–44. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, Q.; Wei, J.; Wang, B.; Wang, H.; Meng, L.; Xin, Y.; Jiang, X. Medical prevention and treatment of radiation-induced carotid injury. Biomed. Pharmacother. 2020, 131, 110664. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Vigo, D.E. Melatonin, mitochondria, and the metabolic syndrome. Cell Mol. Life Sci. 2017, 74, 3941–3954. [Google Scholar] [CrossRef]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Roy, P.; Cifani, C.; Amenta, F.; Tayebati, S.K. Cardiovascular changes related to metabolic syndrome: Evidence in obese Zucker rats. Int. J. Mol. Sci. 2020, 21, 2035. [Google Scholar] [CrossRef] [Green Version]
- Wongprayoon, P.; Govitrapong, P. Melatonin as a mitochondrial protector in neurodegenerative diseases. Cell Mol. Life Sci. 2017, 74, 3999–4014. [Google Scholar] [CrossRef]
- Proietti, S.; Cucina, A.; Minini, M.; Bizzarri, M. Melatonin, mitochondria, and the cancer cell. Cell Mol. Life Sci. 2017, 74, 4015–4025. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Baez-Ferrer, N.; Reiter, R.J.; Avanzas, P.; Hernandez-Vaquero, D. Melatonin and cardioprotection in humans: A systematic and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2021, 8, 635083. [Google Scholar] [CrossRef]
- Rusanova, I.; Martinez-Ruiz, L.; Florido, J.; Rodriguez-Santana, C.; Guerra-Librero, A.; Acuna-Castroviejo, D.; Escames, G. Protective effects of melatonin on the skin: Future perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef] [Green Version]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Lu, H.; Forbes, R.A.; Verma, A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 2002, 277, 23111–23115. [Google Scholar] [CrossRef] [Green Version]
- Robey, I.F.; Lien, A.D.; Welsh, S.J.; Baggett, B.K.; Gillies, R.J. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia 2005, 7, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, M.; Cui, P.; Yu, M.; Han, J.; Li, H.; Xiu, R. Melatonin modulates the expression of VEGF and HIF-1 alpha induced by CoCl2 in cultured cancer cells. J. Pineal Res. 2008, 44, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, M.S.; Suh, S.I.; Baek, W.K. Melatonin down-regulates HIF-1 alpha expression through inhibition of protein translation in prostate cancer cells. J. Pineal Res. 2009, 46, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Wang, F.; Ling, E.A.; Liu, S.; Wang, L.; Yang, Y.; Yao, L.; Chen, X.; Wang, F.; et al. Melatonin antagonizes hypoxia-mediated glioblastoma cell migration and invasion via inhibition of HIF-1α. J. Pineal Res. 2013, 55, 121–130. [Google Scholar] [CrossRef]
- Mota, A.L.; Jardim, B.V.; Castro, T.B.; Colombo, J.; Sonehara, N.M.; Nishiyama, V.K.G.; Pierri, V.A.G.; de Campos Zuccari, D.A.P. Melatonin modifies tumor hypoxia and metabolism by inhibiting HIF-1α and energy metabolic pathway in the in vitro and in vivo models of breast cancer. Melatonin Res. 2019, 2, 83–98. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; de Las Heras, N.; Ferder, L.; Lahera, V.; Reiter, R.J.; Manucha, W. Potential effects of mealtonin and mirconutrients on mitochondrial dysfunction during a cytokine storm typical of oxidative/inflammatory diseases. Diseases 2021, 9, 30. [Google Scholar] [CrossRef]
- Hosseini, A.; Esmaeili Gouvarchin Ghaleh, H.; Aghamollaei, H.; Fasihi Ramandi, M.; Alishiri, G.; Shahriary, A.; Hassanpour, K.; Tat, M.; Farnoosh, G. Evaluation of Th1 and Th2 mediated cellular and humoral immunity in patients with COVID-19 following the use of melatonin as an adjunctive treatment. Eur. J. Pharmacol. 2021, 904, 174193. [Google Scholar] [CrossRef]
- Rahim, I.; Sayed, R.K.; Fernández-Ortiz, M.; Aranda-Martínez, P.; Guerra-Librero, A.; Fernández-Martínez, J.; Rusanova, I.; Escames, G.; Djerdjouri, B.; Acuña-Castroviejo, D. Melatonin alleviates sepsis-induced heart injury through activating the Nrf2 pathway ad inhibition the NLRP3 inflammasome. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 261–277. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal Res. 2015, 58, 1–11. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin and the von Hippel-Lindau/HIF-1 oxygen sensing mechanism: A review. Biochim. Biophys. Acta 2016, 1865, 176–183. [Google Scholar] [CrossRef]
- Brune, B.; Zhou, Z. The role of nitric oxide (NO) in stability regulation of hypoxia inducible factor-1α (HIF-1α). Curr. Med. Chem. 2003, 10, 248–261. [Google Scholar] [CrossRef]
- Tataranni, T.; Piccoli, C. Dichloroacetate (DCA) and cancer: An overview towards clinical applications. Oxid. Med. Cell Longev. 2019, 2019, 8201079. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Martyniuk, C.J.; James, M.O.; Calcutt, N.A. Dichloroacetate-induced peripheral neuropathy. Int. Rev. Neurobiol. 2019, 145, 211–238. [Google Scholar]
- Almond, S.M.; Warren, M.J.; Shealy, K.M.; Threatt, T.B.; Ward, E.D. A systematic review of the efficacy and safety of over-the-counter medications used in older people for the treatment of primary insomnia. Sr. Care Pharm. 2021, 36, 83–91. [Google Scholar] [CrossRef]
- Simko, F.; Pechanova, O. Potential roles of melatonin and chronotherapy among the new trends in hypertension treatment. J. Pineal Res. 2009, 47, 127–133. [Google Scholar] [CrossRef]
- Ohdo, A. Chrono-drug discovery a development of circadian rhythm of molecular, cellular and organ level. Biol. Pharm. Bull. 2021, 44, 101–124. [Google Scholar] [CrossRef]
- Mocayar Maron, F.J.; Ferder, L.; Reiter, R.J.; Manucha, W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J. Steroid Biochem. Mol. Biol. 2020, 199, 105595. [Google Scholar] [CrossRef]
- Sion, B.; Bégou, M. Can chronopharmacology improve the therapeutic management of neurological diseases? Fundam. Clin. Pharmacol. 2021, 35, 564–581. [Google Scholar] [CrossRef]
- Govender, J.; Loos, B.; Marais, E.; Engelbrecht, A.M. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: A review of the protective role of melatonin. J. Pineal. Res. J. 2014, 57, 367–380. [Google Scholar] [CrossRef]
- Pariente, R.; Pariente, J.A.; Rodríguez, A.B.; Espino, J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: Effects on oxidative stress and DNA fragmentation. J. Pineal Res. 2016, 60, 55–64. [Google Scholar] [CrossRef]
- Huang, J.; Shan, W.; Li, N.; Zhou, B.; Guo, E.; Xia, M.; Lu, W.; Wu, Y.; Chen, J.; Wang, B.; et al. Melatonin provides protection against cisplatin-induced ovarian damage and loss of fertility in mice. Reprod. Biomed. Online 2021, 42, 505–519. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Xiang, S.; Mao, L.; Brimer, S.; Wren, M.A.; Yuan, L.; Anbalagan, M.; Hauch, A.; Frasch, T.; Rowan, B.G.; et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014, 74, 4099–4110. [Google Scholar] [CrossRef] [Green Version]
- Chuffa, L.G.; Ferreira Selva, F.R.; Alonso Novais, A.A.; Simko, V.A.; Martin Gimenez, V.M.; Manucha, W.; Zuccari, D.A.P.C.; Reiter, R.J. Melatonin-loaded nanocarriers: New horizons for therapeutic applications. Molecules 2021, 26, 3562. [Google Scholar] [CrossRef]
- Sumsuzzman, D.M.; Khan, Z.A.; Choi, J.; Hong, Y. Differential role of melatonin in healthy brain aging: A systematic review and meta-analysis of the SAMP8 model. Aging 2021, 13, 9373–9397. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [Green Version]
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in cancer treatment: Current knowledge and future opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef]
- De Castro, T.B.; Mota, A.L.; Bordin-Junior, N.A.; Neto, D.S.; Zuccari, D.A.P.C. Immunohistochemical expression of melatonin receptor MT1 and glucose transporter GLUT1 in human breast cancer. Anticancer Agents Med. Chem. 2018, 18, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Pistioli, L.; Katsarelias, D.; Audisio, R.A.; Olofsson Bagge, R. The intricate relationship between melatonin and breast cancer: A short review. Chirurgia 2021, 116, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Maleki, D.P.; Reiter, R.J.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B. Melatonin as a potential inhibitor of kidney cancer: A survey of the molecular processes. IUBMB Life 2020, 72, 2355–2365. [Google Scholar] [CrossRef]
- Hardeland, R. Divergent importance of chronobiological considerations in high- and low-dose melatonin therapies. Diseases 2021, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.J.; Casarotto, M.; Calvo, J.P.; Mazzei, L.; Ponce Zumino, A.Z.; Garcia, I.M.; Cuello-Carrion, F.D.; Fornes, M.W.; Ferder, L.; Diez, E.R.; et al. Antiarrhythmic effect linked to melatonin cardiorenal protection involves AT(1) reduction and Hsp70-VDR increase. J. Pineal Res. 2018, 65, e12513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.Y.; Ganner, J.L.; Gordon, C.J.; Phillips, C.L.; Grunstein, R.R.; Comas, M. The efficacy of combined bright light and melatonin therapies on sleep and circadian outcomes: A systematic review. Sleep Med. Rev. 2021, 58, 101491. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Lincet, H.; Wu, Z.; Coquerel, A.; Forgez, P.; Alifano, M.; Fournel, L. The key role of Warburg effect in SARS-CoV-2 replication and associated inflammatory response. Biochimie 2021, 180, 169–177. [Google Scholar] [CrossRef]
- Varma, G.; Seth, P.; Coutinho de Souza, P.; Callahan, C.; Pinto, J.; Vaidya, M.; Sonzogni, O.; Sukhatme, V.; Wulf, G.M.; Grant, A.K. Visualizing the effects of lactate dehydrogenase (LDH) inhibition and LDH-A genetic ablation in breast and lung cancer with hyperpolarized pyruvate NMR. NMR Biomed. 2021, 34, e4560. [Google Scholar] [CrossRef]
- Da Veiga Moreira, J.; De Staercke, L.; Cesar Martinez-Basilio, P.; Gauthier-Thibodeau, S.; Montegut, L.; Schwartz, L.; Jolicoeur, M. Hyperosmolarity triggers the Warburg effect in Chinese hamster ovary cells and reveals a reduced mitochondria horsepower. Metabolites 2021, 11, 344. [Google Scholar] [CrossRef]
- Bueno, M.; Papazoglou, A.; Valenzi, E.; Rojas, M.; Lafyatis, R.; Mora, A.L. Mitochondria, aging, and cellular senescence: Implications for scleroderma. Curr. Rheumatol. Rep. 2020, 22, 37. [Google Scholar] [CrossRef]
- Bottani, E.; Lamperti, C.; Prigione, A.; Tiranti, V.; Persico, N.; Brunetti, D. Therapeutic approaches to treat mitochondrial diseases: “One-size-fits-all” and “Precision Medicine” Strategies. Pharmaceutics 2020, 12, 1083. [Google Scholar] [CrossRef]
- Orsucci, D.; Caldarazzo, I.E.; Rossi, A.; Siciliano, G.; Mancuso, M. Mitochondrial syndromes revisited. J. Clin. Med. 2021, 10, 1249. [Google Scholar] [CrossRef]
- Pendleton, A.L.; Wesolowski, S.R.; Regnault, T.R.H.; Lynch, R.M.; Limesand, S.W. Dimming the powerhouse: Mitochondrial dysfunction in the liver and skeletal muscle of intrauterine growth restricted fetuses. Front. Endocrinol. 2021, 12, 612888. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Schulz, P.; Steimer, T. Neurobiology of circadian systems. CNS Drugs 2009, 23, 3–13. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Perreau-Lenz, S.; Buijs, R.M. A network of (autonomic) clock outputs. Chronobiol. Int. 2006, 23, 521–535. [Google Scholar] [CrossRef]
- Stehle, J.H.; von Gall, C.; Schomerus, C.; Korf, H.W. Of rodents and ungulates and melatonin: Creating a uniform code for darkness by different signaling mechanisms. J. Biol. Rhythm. 2001, 16, 312–325. [Google Scholar] [CrossRef]
- Stevens, R.G. Artificial lighting in the industrialized world: Circadian disruption and breast cancer. Cancer Causes Control 2006, 17, 501–507. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut clock: Implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 2011, 62, 139–150. [Google Scholar]
- Zeman, M.; Herichova, I. Melatonin and clock genes expression in the cardiovascular system. Front. Biosci. Schol. Ed. 2013, 5, 743–753. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; Manucha, W.; Chuffa, L.G.d.A.; Zuccari, D.A.P.d.C. Melatonin and Pathological Cell Interactions: Mitochondrial Glucose Processing in Cancer Cells. Int. J. Mol. Sci. 2021, 22, 12494. https://doi.org/10.3390/ijms222212494
Reiter RJ, Sharma R, Rosales-Corral S, Manucha W, Chuffa LGdA, Zuccari DAPdC. Melatonin and Pathological Cell Interactions: Mitochondrial Glucose Processing in Cancer Cells. International Journal of Molecular Sciences. 2021; 22(22):12494. https://doi.org/10.3390/ijms222212494
Chicago/Turabian StyleReiter, Russel J, Ramaswamy Sharma, Sergio Rosales-Corral, Walter Manucha, Luiz Gustavo de Almeida Chuffa, and Debora Aparecida Pires de Campos Zuccari. 2021. "Melatonin and Pathological Cell Interactions: Mitochondrial Glucose Processing in Cancer Cells" International Journal of Molecular Sciences 22, no. 22: 12494. https://doi.org/10.3390/ijms222212494
APA StyleReiter, R. J., Sharma, R., Rosales-Corral, S., Manucha, W., Chuffa, L. G. d. A., & Zuccari, D. A. P. d. C. (2021). Melatonin and Pathological Cell Interactions: Mitochondrial Glucose Processing in Cancer Cells. International Journal of Molecular Sciences, 22(22), 12494. https://doi.org/10.3390/ijms222212494









