Mediation of the Cardioprotective Effects of Mannitol Discovered, with Refutation of Common Protein Kinases
Abstract
:1. Introduction
2. Results
2.1. Animal Characteristics
2.2. Infarct Size
2.3. Cardiac Function
3. Discussion
Limitations
4. Materials and Methods
4.1. Surgical Preparation
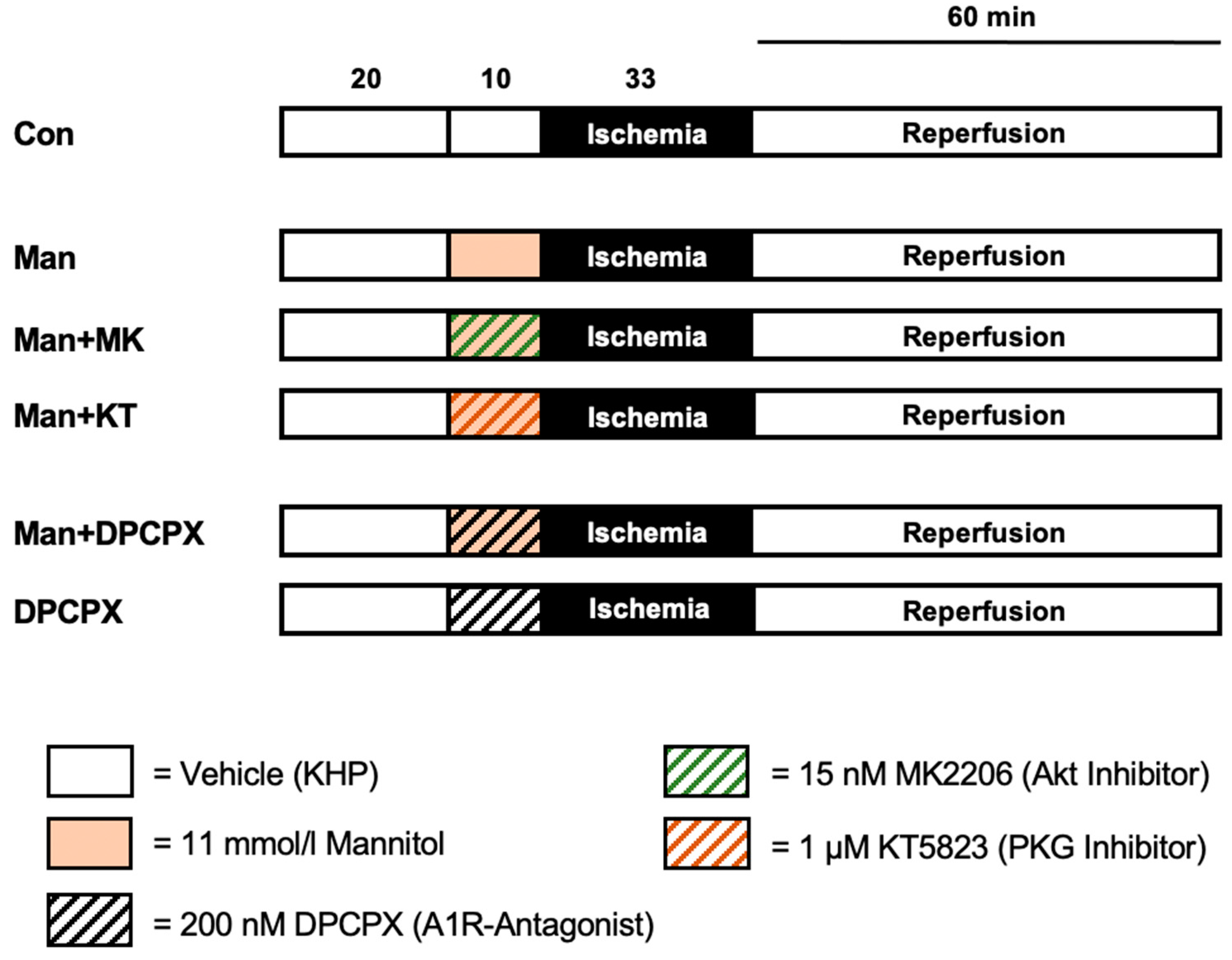
4.2. Experimental Protocol
4.3. Statistical Analysis
4.3.1. Sample Size Analysis
4.3.2. Statistical Approach
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hausenloy, D.J.; Barrabes, J.A.; Botker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016, 111, 70. [Google Scholar] [CrossRef] [Green Version]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Caricati-Neto, A.; Errante, P.R.; Menezes-Rodrigues, F.S. Recent Advances in Pharmacological and Non-Pharmacological Strategies of Cardioprotection. Int. J. Mol. Sci. 2019, 20, 4002. [Google Scholar] [CrossRef] [Green Version]
- Torregroza, C.; Raupach, A.; Feige, K.; Weber, N.C.; Hollmann, M.W.; Huhn, R. Perioperative Cardioprotection: General Mechanisms and Pharmacological Approaches. Anesth. Analg. 2020, 131, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Torregroza, C.; Huhn, R.; Hollmann, M.W.; Preckel, B. Perioperative Cardioprotection: Clinical Implications. Anesth. Analg. 2020, 131, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Poullis, M. Mannitol and cardiac surgery. Thorac Cardiovasc. Surg. 1999, 47, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Lucatorto, M. Mannitol revisited. J. Neurosci. Nurs. 1994, 26, 170–174. [Google Scholar] [CrossRef]
- Schilte, C.; Bouzat, P.; Millet, A.; Boucheix, P.; Pernet-Gallay, K.; Lemasson, B.; Barbier, E.L.; Payen, J.F. Mannitol Improves Brain Tissue Oxygenation in a Model of Diffuse Traumatic Brain Injury. Crit. Care Med. 2015, 43, 2212–2218. [Google Scholar] [CrossRef]
- Farrokh, S.; Cho, S.M.; Suarez, J.I. Fluids and hyperosmolar agents in neurocritical care: An update. Curr. Opin. Crit. Care 2019, 25, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Witherspoon, B.; Ashby, N.E. The Use of Mannitol and Hypertonic Saline Therapies in Patients with Elevated Intracranial Pressure: A Review of the Evidence. Nurs. Clin. N. Am. 2017, 52, 249–260. [Google Scholar] [CrossRef]
- O’Kane, D.; Baldwin, G.S.; Bolton, D.M.; Ischia, J.J.; Patel, O. Preconditioning against renal ischaemia reperfusion injury: The failure to translate to the clinic. J. Nephrol. 2019, 32, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Feige, K.; Rubbert, J.; Raupach, A.; Stroethoff, M.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Torregroza, C. Cardioprotective Properties of Mannitol-Involvement of Mitochondrial Potassium Channels. Int. J. Mol. Sci. 2021, 22, 2395. [Google Scholar] [CrossRef] [PubMed]
- Falck, G.; Schjott, J.; Jynge, P. Hyperosmotic pretreatment reduces infarct size in the rat heart. Physiol. Res. 1999, 48, 331–340. [Google Scholar]
- Gardner, T.J.; Stewart, J.R.; Casale, A.S.; Downey, J.M.; Chambers, D.E. Reduction of myocardial ischemic injury with oxygen-derived free radical scavengers. Surgery 1983, 94, 423–427. [Google Scholar] [PubMed]
- Magovern, G.J., Jr.; Bolling, S.F.; Casale, A.S.; Bulkley, B.H.; Gardner, T.J. The mechanism of mannitol in reducing ischemic injury: Hyperosmolarity or hydroxyl scavenger? Circulation 1984, 70, I91–I95. [Google Scholar]
- Zálešák, M.; BlaŽíček, P.; Pancza, D.; Gablovský, I.; Štrbák, V.; Ravingerová, T. Hyperosmotic environment blunts effectivity of ischemic preconditioning against ischemia-reperfusion injury and improves ischemic tolerance in non-preconditioned isolated rat hearts. Physiol. Res. 2016, 65, 1045–1051. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Tsang, A.; Yellon, D.M. The reperfusion injury salvage kinase pathway: A common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc. Med. 2005, 15, 69–75. [Google Scholar] [CrossRef]
- Rossello, X.; Yellon, D.M. The RISK pathway and beyond. Basic Res. Cardiol. 2017, 113, 2. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.O.; Nehrke, K.; Brookes, P.S. The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection. Biochem. J. 2017, 474, 2067–2094. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef]
- Boengler, K.; Lochnit, G.; Schulz, R. Mitochondria “THE” target of myocardial conditioning. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1215–H1231. [Google Scholar] [CrossRef] [Green Version]
- Rotko, D.; Kunz, W.S.; Szewczyk, A.; Kulawiak, B. Signaling pathways targeting mitochondrial potassium channels. Int. J. Biochem. Cell Biol. 2020, 125, 105792. [Google Scholar] [CrossRef]
- Tadevosyan, A.; Vaniotis, G.; Allen, B.G.; Hébert, T.E.; Nattel, S. G protein-coupled receptor signalling in the cardiac nuclear membrane: Evidence and possible roles in physiological and pathophysiological function. J. Physiol. 2012, 590, 1313–1330. [Google Scholar] [CrossRef]
- Lasley, R.D. Adenosine Receptor-Mediated Cardioprotection-Current Limitations and Future Directions. Front. Pharmacol. 2018, 9, 310. [Google Scholar] [CrossRef]
- Chen, J.F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets—What are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef] [Green Version]
- Headrick, J.P.; Lasley, R.D. Adenosine receptors and reperfusion injury of the heart. Handb. Exp. Pharmacol. 2009, 193, 189–214. [Google Scholar] [CrossRef] [Green Version]
- Pingle, S.C.; Mishra, S.; Marcuzzi, A.; Bhat, S.G.; Sekino, Y.; Rybak, L.P.; Ramkumar, V. Osmotic diuretics induce adenosine A1 receptor expression and protect renal proximal tubular epithelial cells against cisplatin-mediated apoptosis. J. Biol. Chem. 2004, 279, 43157–43167. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Dorado, D.; Oliveras, J. Myocardial oedema: A preventable cause of reperfusion injury? Cardiovasc. Res. 1993, 27, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Ouriel, K.; Ginsburg, M.E.; Patti, C.S.; Pearce, F.J.; Hicks, G.L. Preservation of myocardial function with mannitol reperfusate. Circulation 1985, 72, Ii254–Ii258. [Google Scholar] [PubMed]
- Dos Santos, P.; Kowaltowski, A.J.; Laclau, M.N.; Seetharaman, S.; Paucek, P.; Boudina, S.; Thambo, J.B.; Tariosse, L.; Garlid, K.D. Mechanisms by which opening the mitochondrial ATP-sensitive K(+) channel protects the ischemic heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H284–H295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, G.J.; Peart, J.N. KATP channels and myocardial preconditioning: An update. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H921–H930. [Google Scholar] [CrossRef] [Green Version]
- Perrelli, M.G.; Pagliaro, P.; Penna, C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J. Cardiol. 2011, 3, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Behmenburg, F.; Dorsch, M.; Huhn, R.; Mally, D.; Heinen, A.; Hollmann, M.W.; Berger, M.M. Impact of Mitochondrial Ca2+-Sensitive Potassium (mBKCa) Channels in Sildenafil-Induced Cardioprotection in Rats. PLoS ONE 2015, 10, e0144737. [Google Scholar] [CrossRef]
- Yan, L. Abstract #DDT01-1: MK-2206: A potent oral allosteric AKT inhibitor. Cancer Res. 2009, 69, DDT01. [Google Scholar]
- Zhao, Y.Y.; Tian, Y.; Zhang, J.; Xu, F.; Yang, Y.P.; Huang, Y.; Zhao, H.Y.; Zhang, J.W.; Xue, C.; Lam, M.H.; et al. Effects of an oral allosteric AKT inhibitor (MK-2206) on human nasopharyngeal cancer in vitro and in vivo. Drug Des. Devel. Ther. 2014, 8, 1827–1837. [Google Scholar] [CrossRef] [Green Version]
- Torregroza, C.; Jalajel, O.; Raupach, A.; Feige, K.; Bunte, S.; Heinen, A.; Mathes, A.; Hollmann, M.W.; Huhn, R.; Stroethoff, M. Activation of PKG and Akt Is Required for Cardioprotection by Ramelteon-Induced Preconditioning and Is Located Upstream of mKCa-Channels. Int. J. Mol. Sci. 2020, 21, 2585. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Xi, L.; Kukreja, R.C. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J. Biol. Chem. 2008, 283, 29572–29585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hirai, K.; Ashraf, M. Activation of mitochondrial ATP-sensitive K(+) channel for cardiac protection against ischemic injury is dependent on protein kinase C activity. Circ. Res. 1999, 85, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, K.; Churchill, E.; Mochly-Rosen, D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc. Res. 2006, 70, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Spahn, D.R.; Zhang, X.; Liu, Y.; Chu, H.; Liu, Z. Morphine Postconditioning Protects Against Reperfusion Injury: The Role of Protein Kinase C-Epsilon, Extracellular Signal-Regulated Kinase 1/2 and Mitochondrial Permeability Transition Pores. Cell Physiol. Biochem. 2016, 39, 1930–1940. [Google Scholar] [CrossRef] [Green Version]
- Hassouna, A.; Matata, B.M.; Galiñanes, M. PKC-epsilon is upstream and PKC-alpha is downstream of mitoKATP channels in the signal transduction pathway of ischemic preconditioning of human myocardium. Am. J. Physiol. Cell Physiol. 2004, 287, C1418–C1425. [Google Scholar] [CrossRef]
- Pravdic, D.; Sedlic, F.; Mio, Y.; Vladic, N.; Bienengraeber, M.; Bosnjak, Z.J. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology 2009, 111, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasley, R.D.; Rhee, J.W.; Van Wylen, D.G.; Mentzer, R.M., Jr. Adenosine A1 receptor mediated protection of the globally ischemic isolated rat heart. J. Mol. Cell Cardiol. 1990, 22, 39–47. [Google Scholar] [CrossRef]
- Solenkova, N.V.; Solodushko, V.; Cohen, M.V.; Downey, J.M. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H441–H449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohse, M.J.; Klotz, K.N.; Lindenborn-Fotinos, J.; Reddington, M.; Schwabe, U.; Olsson, R.A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)—A selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn. Schmiedebergs Arch. Pharmacol. 1987, 336, 204–210. [Google Scholar] [CrossRef]
- Yao, L.; Kato, R.; Foe¨x, P. Isoflurane-induced protection against myocardial stunning is independent of adenosine 1 (A1) receptor in isolated rat heart. Br. J. Anaesth. 2001, 87, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Lasley, R.D.; Anderson, G.M.; Mentzer, R.M., Jr. Ischaemic and hypoxic preconditioning enhance postischaemic recovery of function in the rat heart. Cardiovasc. Res. 1993, 27, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, W.; Hu, K. Molecular mechanism underlying adenosine receptor-mediated mitochondrial targeting of protein kinase C. Biochim. Biophys. Acta 2012, 1823, 950–958. [Google Scholar] [CrossRef] [Green Version]
- Mubagwa, K.; Flameng, W. Adenosine, adenosine receptors and myocardial protection: An updated overview. Cardiovasc. Res. 2001, 52, 25–39. [Google Scholar] [CrossRef]
- Torregroza, C.; Feige, K.; Schneider, L.; Bunte, S.; Stroethoff, M.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Raupach, A. Influence of Hyperglycemia on Dexmedetomidine-Induced Cardioprotection in the Isolated Perfused Rat Heart. J. Clin. Med. 2020, 9, 1445. [Google Scholar] [CrossRef]
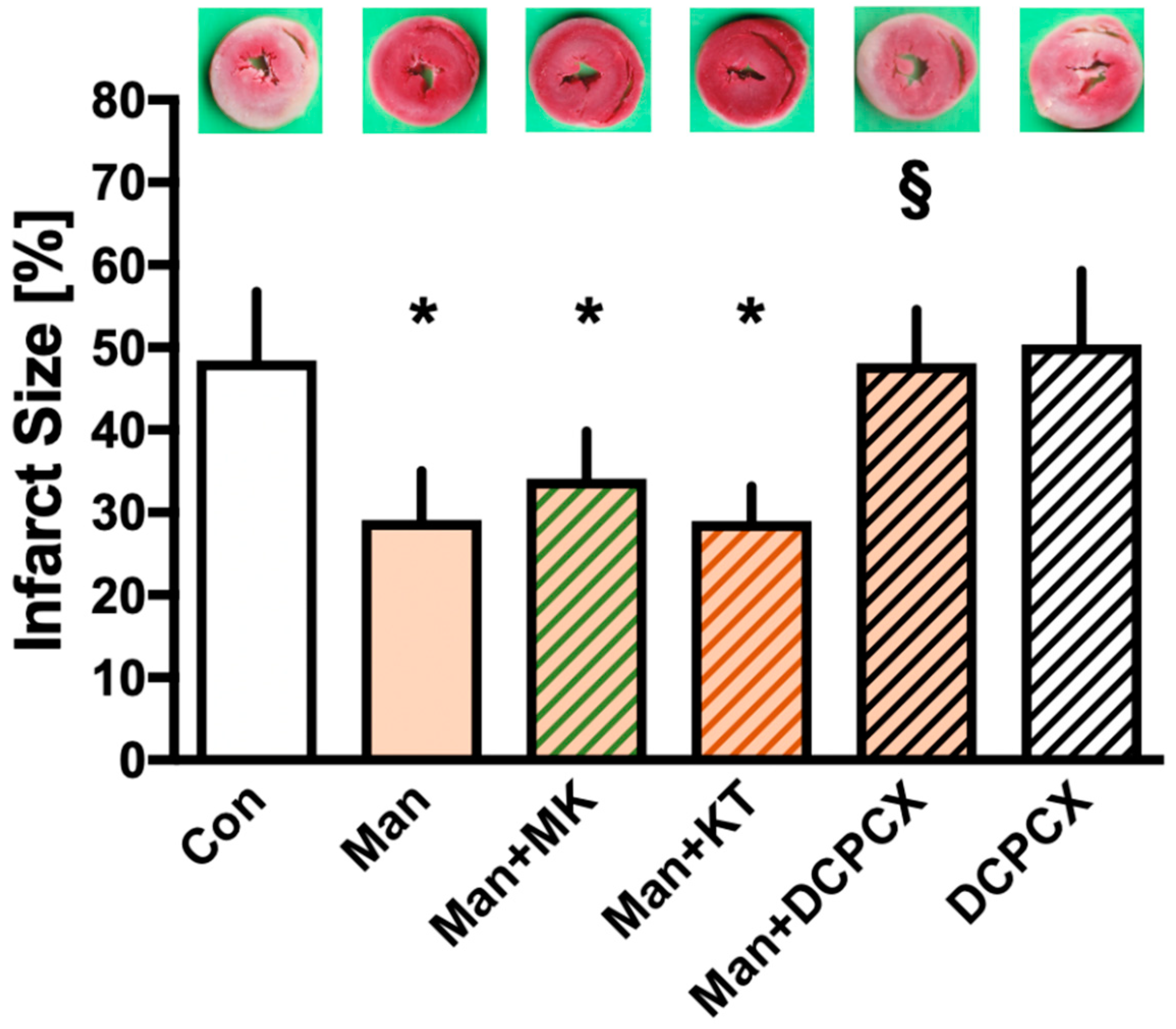
| n | Body Weight (g) | Heart Weight Dry (g) | Heart Weight Wet (g) | Time of Max. Ischemic Contracture (min) | Level of Max. Ischemic Contracture (mmHg) | |
|---|---|---|---|---|---|---|
| Con | 7 | 304 ± 29 | 0.17 ± 0.02 | 1.13 ± 0.08 | 15 ± 1 | 66 ± 14 |
| Man | 7 | 313 ± 28 | 0.17 ± 0.02 | 1.10 ± 0.08 | 16 ± 2 | 56 ± 10 |
| Man + MK | 7 | 308 ± 12 | 0.16 ± 0.01 | 1.07 ± 0.04 | 15 ± 1 | 57 ± 8 |
| Man + KT | 7 | 312 ± 10 | 0.17 ± 0.01 | 1.08 ± 0.05 | 16 ± 2 | 60 ± 9 |
| Man + DPCPX | 7 | 278 ± 10 | 0.15 ± 0.01 | 1.05 ± 0.04 | 14 ± 2 | 65 ± 19 |
| DPCPX | 7 | 290 ± 17 * | 0.16 ± 0.01 | 1.09 ± 0.04 | 15 ± 2 | 71 ± 10 |
| Baseline | PC | Reperfusion | ||
|---|---|---|---|---|
| 30 | 60 | |||
| Heart Rate (bpm) | ||||
| Con | 306 ± 26 | 281 ± 37 | 273 ± 43 | 255 ± 41 |
| Man | 296 ± 27 | 297 ± 22 | 278 ± 62 | 263 ± 21 |
| Man + MK | 306 ± 24 | 300 ± 34 | 308 ± 58 | 293 ± 69 |
| Man + KT | 318 ± 28 | 313 ± 28 | 273 ± 43 | 287 ± 33 |
| Man + DPCPX | 305 ± 37 | 311 ± 36 | 285 ± 68 | 248 ± 52 |
| DPCPX | 290 ± 34 | 298 ± 30 | 290 ± 18 | 246 ± 61 |
| Left Ventricular Developed Pressure (mmHg) | ||||
| Con | 139 ± 16 | 146 ± 14 | 22 ± 15 * | 28 ± 10 * |
| Man | 137 ± 16 | 135 ± 18 | 25 ± 6 * | 34 ± 4 * |
| Man + MK | 143 ± 16 | 141 ± 18 | 29 ± 14 * | 34 ± 19 * |
| Man + KT | 134 ± 6 | 137 ± 6 | 20 ± 10 * | 31 ± 11 * |
| Man + DPCPX | 141 ± 23 | 136 ± 23 | 18 ± 16 * | 31 ± 11 * |
| DPCPX | 136 ± 18 | 136 ± 17 | 23 ± 16 * | 33 ± 17 * |
| Coronary flow (mL/min) | ||||
| Con | 15 ± 2 | 15 ± 1 | 8 ± 1 * | 7 ± 1 * |
| Man | 18 ± 3 | 18 ± 4 | 10 ± 4 * | 9 ± 3 * |
| Man + MK | 16 ± 2 | 16 ± 2 | 10 ± 3 * | 9 ± 2 * |
| Man + KT | 14 ± 2 | 15 ± 1 | 8 ± 2 * | 7 ± 2 * |
| Man + DPCPX | 15 ± 1 | 15 ± 1 | 8 ± 1 * | 8 ± 1 * |
| DPCPX | 15 ± 3 | 15 ± 3 | 8 ± 3 * | 7 ± 3 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torregroza, C.; Glashoerster, C.O.; Feige, K.; Stroethoff, M.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Huhn, R. Mediation of the Cardioprotective Effects of Mannitol Discovered, with Refutation of Common Protein Kinases. Int. J. Mol. Sci. 2021, 22, 12471. https://doi.org/10.3390/ijms222212471
Torregroza C, Glashoerster CO, Feige K, Stroethoff M, Raupach A, Heinen A, Hollmann MW, Huhn R. Mediation of the Cardioprotective Effects of Mannitol Discovered, with Refutation of Common Protein Kinases. International Journal of Molecular Sciences. 2021; 22(22):12471. https://doi.org/10.3390/ijms222212471
Chicago/Turabian StyleTorregroza, Carolin, Chiara O. Glashoerster, Katharina Feige, Martin Stroethoff, Annika Raupach, André Heinen, Markus W. Hollmann, and Ragnar Huhn. 2021. "Mediation of the Cardioprotective Effects of Mannitol Discovered, with Refutation of Common Protein Kinases" International Journal of Molecular Sciences 22, no. 22: 12471. https://doi.org/10.3390/ijms222212471
APA StyleTorregroza, C., Glashoerster, C. O., Feige, K., Stroethoff, M., Raupach, A., Heinen, A., Hollmann, M. W., & Huhn, R. (2021). Mediation of the Cardioprotective Effects of Mannitol Discovered, with Refutation of Common Protein Kinases. International Journal of Molecular Sciences, 22(22), 12471. https://doi.org/10.3390/ijms222212471






