Evolution of the Early Spliceosomal Complex—From Constitutive to Regulated Splicing
Abstract
:1. Introduction
2. The Spliceosome
3. The Evolution of the Spliceosome
4. Regulated and Alternative Splicing
5. Coupling Splicing to Transcription
6. Co-Transcriptional Splicing of Long Introns and Intron Looping
7. The Evolution of Intron Architecture and Intron-Exon Structures
8. Definition of the Exon Intron Boarders by the Spliceosome
9. The Spliceosomal E-Complex
10. Degeneration of Splice Sites I—5′ ss and snU1
11. Degeneration of Splice Sites II—Branchpoint Binding by SF1
12. The U2AF Heterodimer: Beyond Py Tract Binding—A Physical Link between the Branchpoint and 3′ ss
13. PRP40—A Physical Link between Pol II, snU1-RNP, and SF1/U2AF
14. An Evolutionary Derived Model for Co-Transcriptional Formation of the E-Complex
15. Co-Transcriptional Formation of the E-Complex in Regulated and Alternative Splicing
16. Limitations and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vosseberg, J.; Snel, B. Domestication of self-splicing introns during eukaryogenesis: The rise of the complex spliceosomal machinery. Biol. Direct 2017, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Fedorova, L.; Fedorov, A. Introns in gene evolution. Genetica 2003, 118, 123–131. [Google Scholar] [CrossRef]
- Kaufer, N.F.; Potashkin, J. Analysis of the splicing machinery in fission yeast: A comparison with budding yeast and mammals. Nucleic Acids Res. 2000, 28, 3003–3010. [Google Scholar] [CrossRef] [Green Version]
- Burge, C.B.; Padgett, R.A.; Sharp, P.A. Evolutionary fates and origins of U12-type introns. Mol. Cell 1998, 2, 773–785. [Google Scholar] [CrossRef]
- Matlin, A.J.; Moore, M.J. Spliceosome assembly and composition. Adv. Exp. Med. Biol. 2007, 623, 14–35. [Google Scholar] [CrossRef]
- Zhou, Z.; Licklider, L.J.; Gygi, S.P.; Reed, R. Comprehensive proteomic analysis of the human spliceosome. Nature 2002, 419, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, D.A.; Steitz, J.A. Interactions of small nuclear RNA’s with precursor messenger RNA during in vitro splicing. Science 1992, 257, 1918–1925. [Google Scholar] [CrossRef]
- Das, R.; Zhou, Z.; Reed, R. Functional association of U2 snRNP with the ATP-independent spliceosomal complex E. Mol. Cell 2000, 5, 779–787. [Google Scholar] [CrossRef]
- Wahl, M.C.; Will, C.L.; Luhrmann, R. The spliceosome: Design principles of a dynamic RNP machine. Cell 2009, 136, 701–718. [Google Scholar] [CrossRef] [Green Version]
- Wahl, M.C.; Luhrmann, R. SnapShot: Spliceosome Dynamics II. Cell 2015, 162, 456. [Google Scholar] [CrossRef] [Green Version]
- De Conti, L.; Baralle, M.; Buratti, E. Exon and intron definition in pre-mRNA splicing. Wiley Interdiscip. Rev. RNA 2013, 4, 49–60. [Google Scholar] [CrossRef]
- Matera, A.G.; Wang, Z. A day in the life of the spliceosome. Nat. Rev. Mol. Cell Biol. 2014, 15, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Ast, G. How did alternative splicing evolve? Nat. Rev. Genet. 2004, 5, 773–782. [Google Scholar] [CrossRef]
- Keren, H.; Lev-Maor, G.; Ast, G. Alternative splicing and evolution: Diversification, exon definition and function. Nat. Rev. Genet. 2010, 11, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Lustig, A.J.; Lin, R.J.; Abelson, J. The yeast RNA gene products are essential for mRNA splicing in vitro. Cell 1986, 47, 953–963. [Google Scholar] [CrossRef]
- Meyer, M.; Vilardell, J. The quest for a message: Budding yeast, a model organism to study the control of pre-mRNA splicing. Brief. Funct. Genom. Proteom. 2009, 8, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayraghavan, U.; Company, M.; Abelson, J. Isolation and characterization of pre-mRNA splicing mutants of Saccharomyces cerevisiae. Genes Dev. 1989, 3, 1206–1216. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, M.E.; Charenton, C.; Nagai, K. RNA Splicing by the Spliceosome. Annu. Rev. Biochem. 2020, 89, 359–388. [Google Scholar] [CrossRef]
- Fair, B.J.; Pleiss, J.A. The power of fission: Yeast as a tool for understanding complex splicing. Curr. Genet. 2017, 63, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Hang, J.; Wan, R.; Huang, M.; Wong, C.C.; Shi, Y. Structure of a yeast spliceosome at 3.6-angstrom resolution. Science 2015, 349, 1182–1191. [Google Scholar] [CrossRef]
- Burke, J.E.; Longhurst, A.D.; Merkurjev, D.; Sales-Lee, J.; Rao, B.; Moresco, J.J.; Yates, J.R., 3rd; Li, J.J.; Madhani, H.D. Spliceosome Profiling Visualizes Operations of a Dynamic RNP at Nucleotide Resolution. Cell 2018, 173, 1014–1030 e1017. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Moore, J.; Ozadam, H.; Shulha, H.P.; Rhind, N.; Weng, Z.; Moore, M.J. Transcriptome-wide Interrogation of the Functional Intronome by Spliceosome Profiling. Cell 2018, 173, 1031–1044 e1013. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, A.N.; Kaufer, N.F. Pre-mRNA splicing in Schizosaccharomyces pombe: Regulatory role of a kinase conserved from fission yeast to mammals. Curr. Genet. 2003, 42, 241–251. [Google Scholar] [CrossRef]
- International Human Genome Sequencing, C. Finishing the euchromatic sequence of the human genome. Nature 2004, 431, 931–945. [Google Scholar] [CrossRef]
- Blencowe, B.J. Alternative splicing: New insights from global analyses. Cell 2006, 126, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Magen, A.; Ast, G. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res. 2007, 35, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakabe, N.J.; de Souza, S.J. Sequence features responsible for intron retention in human. BMC Genom. 2007, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Hatje, K.; Rahman, R.U.; Vidal, R.O.; Simm, D.; Hammesfahr, B.; Bansal, V.; Rajput, A.; Mickael, M.E.; Sun, T.; Bonn, S.; et al. The landscape of human mutually exclusive splicing. Mol. Syst. Biol. 2017, 13, 959. [Google Scholar] [CrossRef]
- Pohl, M.; Bortfeldt, R.H.; Grutzmann, K.; Schuster, S. Alternative splicing of mutually exclusive exons—A review. Bio Syst. 2013, 114, 31–38. [Google Scholar] [CrossRef]
- Koren, E.; Lev-Maor, G.; Ast, G. The emergence of alternative 3′ and 5′ splice site exons from constitutive exons. PLoS Comput. Biol. 2007, 3, e95. [Google Scholar] [CrossRef] [Green Version]
- Pandya-Jones, A.; Black, D.L. Co-transcriptional splicing of constitutive and alternative exons. RNA 2009, 15, 1896–1908. [Google Scholar] [CrossRef] [Green Version]
- Wachutka, L.; Caizzi, L.; Gagneur, J.; Cramer, P. Global donor and acceptor splicing site kinetics in human cells. eLife 2019, 8, e45056. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Mantei, N.; Weissmann, C. Characterization and kinetics of synthesis of 15S beta-globin RNA, a putative precursor of beta-globin mRNA. Cold Spring Harb. Symp. Quant. Biol. 1978, 42 Pt 2, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Audibert, A.; Weil, D.; Dautry, F. In vivo kinetics of mRNA splicing and transport in mammalian cells. Mol. Cell. Biol. 2002, 22, 6706–6718. [Google Scholar] [CrossRef] [Green Version]
- Beyer, A.L.; Bouton, A.H.; Miller, O.L., Jr. Correlation of hnRNP structure and nascent transcript cleavage. Cell 1981, 26, 155–165. [Google Scholar] [CrossRef]
- Beyer, A.L.; Osheim, Y.N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988, 2, 754–765. [Google Scholar] [CrossRef] [Green Version]
- Bentley, D.L. Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 2014, 15, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Tellier, M.; Maudlin, I.; Murphy, S. Transcription and splicing: A two-way street. Wiley Interdiscip. Rev. RNA 2020, 11, e1593. [Google Scholar] [CrossRef]
- Herzel, L.; Ottoz, D.S.M.; Alpert, T.; Neugebauer, K.M. Splicing and transcription touch base: Co-transcriptional spliceosome assembly and function. Nat. Rev. Mol. Cell Biol. 2017, 18, 637–650. [Google Scholar] [CrossRef]
- Giono, L.E.; Kornblihtt, A.R. Linking transcription, RNA polymerase II elongation and alternative splicing. Biochem. J. 2020, 477, 3091–3104. [Google Scholar] [CrossRef]
- De la Mata, M.; Alonso, C.R.; Kadener, S.; Fededa, J.P.; Blaustein, M.; Pelisch, F.; Cramer, P.; Bentley, D.; Kornblihtt, A.R. A slow RNA polymerase II affects alternative splicing in vivo. Mol. Cell 2003, 12, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, G.; Lafaille, C.; de la Mata, M.; Marasco, L.E.; Munoz, M.J.; Le Jossic-Corcos, C.; Corcos, L.; Kornblihtt, A.R. How slow RNA polymerase II elongation favors alternative exon skipping. Mol. Cell 2014, 54, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuryev, A.; Patturajan, M.; Litingtung, Y.; Joshi, R.V.; Gentile, C.; Gebara, M.; Corden, J.L. The C-terminal domain of the largest subunit of RNA polymerase II interacts with a novel set of serine/arginine-rich proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 6975–6980. [Google Scholar] [CrossRef] [Green Version]
- Nojima, T.; Gomes, T.; Grosso, A.R.F.; Kimura, H.; Dye, M.J.; Dhir, S.; Carmo-Fonseca, M.; Proudfoot, N.J. Mammalian NET-Seq Reveals Genome-wide Nascent Transcription Coupled to RNA Processing. Cell 2015, 161, 526–540. [Google Scholar] [CrossRef] [Green Version]
- Misteli, T.; Spector, D.L. RNA polymerase II targets pre-mRNA splicing factors to transcription sites in vivo. Mol. Cell 1999, 3, 697–705. [Google Scholar] [CrossRef]
- Harlen, K.M.; Trotta, K.L.; Smith, E.E.; Mosaheb, M.M.; Fuchs, S.M.; Churchman, L.S. Comprehensive RNA Polymerase II Interactomes Reveal Distinct and Varied Roles for Each Phospho-CTD Residue. Cell Rep. 2016, 15, 2147–2158. [Google Scholar] [CrossRef] [Green Version]
- David, C.J.; Boyne, A.R.; Millhouse, S.R.; Manley, J.L. The RNA polymerase II C-terminal domain promotes splicing activation through recruitment of a U2AF65-Prp19 complex. Genes Dev. 2011, 25, 972–983. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Dufu, K.; Romney, B.; Feldt, M.; Elenko, M.; Reed, R. Functional coupling of RNAP II transcription to spliceosome assembly. Genes Dev. 2006, 20, 1100–1109. [Google Scholar] [CrossRef] [Green Version]
- Hicks, M.J.; Yang, C.R.; Kotlajich, M.V.; Hertel, K.J. Linking splicing to Pol II transcription stabilizes pre-mRNAs and influences splicing patterns. PLoS Biol. 2006, 4, e147. [Google Scholar] [CrossRef] [Green Version]
- Natalizio, B.J.; Robson-Dixon, N.D.; Garcia-Blanco, M.A. The Carboxyl-terminal Domain of RNA Polymerase II Is Not Sufficient to Enhance the Efficiency of Pre-mRNA Capping or Splicing in the Context of a Different Polymerase. J. Biol. Chem. 2009, 284, 8692–8702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentley, D.L. Rules of engagement: Co-transcriptional recruitment of pre-mRNA processing factors. Curr. Opin. Cell Biol. 2005, 17, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Tacke, R.; Manley, J.L. Phosphorylated RNA polymerase II stimulates pre-mRNA splicing. Genes Dev. 1999, 13, 1234–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Erickson, B.; Luo, W.; Seward, D.; Graber, J.H.; Pollock, D.D.; Megee, P.C.; Bentley, D.L. Gene-specific RNA polymerase II phosphorylation and the CTD code. Nat. Struct. Mol. Biol. 2010, 17, 1279–1286. [Google Scholar] [CrossRef]
- Neves, L.T.; Douglass, S.; Spreafico, R.; Venkataramanan, S.; Kress, T.L.; Johnson, T.L. The histone variant H2A.Z promotes efficient cotranscriptional splicing in S. cerevisiae. Genes Dev. 2017, 31, 702–717. [Google Scholar] [CrossRef] [Green Version]
- Nissen, K.E.; Homer, C.M.; Ryan, C.J.; Shales, M.; Krogan, N.J.; Patrick, K.L.; Guthrie, C. The histone variant H2A.Z promotes splicing of weak introns. Genes Dev. 2017, 31, 688–701. [Google Scholar] [CrossRef] [Green Version]
- Wiesner, S.; Stier, G.; Sattler, M.; Macias, M.J. Solution structure and ligand recognition of the WW domain pair of the yeast splicing factor Prp40. J. Mol. Biol. 2002, 324, 807–822. [Google Scholar] [CrossRef]
- Dujardin, G.; Lafaille, C.; Petrillo, E.; Buggiano, V.; Gomez Acuna, L.I.; Fiszbein, A.; Godoy Herz, M.A.; Nieto Moreno, N.; Munoz, M.J.; Allo, M.; et al. Transcriptional elongation and alternative splicing. Biochim. Biophys. Acta 2013, 1829, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Schor, I.E.; Gomez Acuna, L.I.; Kornblihtt, A.R. Coupling between transcription and alternative splicing. Cancer Treat. Res. 2013, 158, 1–24. [Google Scholar] [CrossRef]
- Zhang, S.; Aibara, S.; Vos, S.M.; Agafonov, D.E.; Luhrmann, R.; Cramer, P. Structure of a transcribing RNA polymerase II-U1 snRNP complex. Science 2021, 371, 305–309. [Google Scholar] [CrossRef]
- Leader, Y.; Lev Maor, G.; Sorek, M.; Shayevitch, R.; Hussein, M.; Hameiri, O.; Tammer, L.; Zonszain, J.; Keydar, I.; Hollander, D.; et al. The upstream 5′ splice site remains associated to the transcription machinery during intron synthesis. Nat. Commun. 2021, 12, 4545. [Google Scholar] [CrossRef] [PubMed]
- Wood, V.; Gwilliam, R.; Rajandream, M.A.; Lyne, M.; Lyne, R.; Stewart, A.; Sgouros, J.; Peat, N.; Hayles, J.; Baker, S.; et al. The genome sequence of Schizosaccharomyces pombe. Nature 2002, 415, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Kupfer, D.M.; Drabenstot, S.D.; Buchanan, K.L.; Lai, H.; Zhu, H.; Dyer, D.W.; Roe, B.A.; Murphy, J.W. Introns and splicing elements of five diverse fungi. Eukaryot. Cell 2004, 3, 1088–1100. [Google Scholar] [CrossRef] [Green Version]
- Plaschka, C.; Newman, A.J.; Nagai, K. Structural Basis of Nuclear pre-mRNA Splicing: Lessons from Yeast. Cold Spring Harb. Perspect. Biol. 2019, 11, a032391. [Google Scholar] [CrossRef] [Green Version]
- Will, C.L.; Luhrmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 2011, 3, a003707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Yang, Z.; Kibukawa, M.; Paddock, M.; Passey, D.A.; Wong, G.K. Minimal introns are not “junk”. Genome Res. 2002, 12, 1185–1189. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.P.; Burge, C.B. A computational analysis of sequence features involved in recognition of short introns. Proc. Natl. Acad. Sci. USA 2001, 98, 11193–11198. [Google Scholar] [CrossRef] [Green Version]
- Robberson, B.L.; Cote, G.J.; Berget, S.M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol. Cell. Biol. 1990, 10, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Berget, S.M. Exon recognition in vertebrate splicing. J. Biol. Chem. 1995, 270, 2411–2414. [Google Scholar] [CrossRef] [Green Version]
- Maniatis, T.; Reed, R. An extensive network of coupling among gene expression machines. Nature 2002, 416, 499–506. [Google Scholar] [CrossRef]
- Schellenberg, M.J.; Ritchie, D.B.; MacMillan, A.M. Pre-mRNA splicing: A complex picture in higher definition. Trends Biochem. Sci. 2008, 33, 243–246. [Google Scholar] [CrossRef]
- Soller, M. Pre-messenger RNA processing and its regulation: A genomic perspective. Cell. Mol. Life Sci. CMLS 2006, 63, 796–819. [Google Scholar] [CrossRef] [PubMed]
- Reed, R. Initial splice-site recognition and pairing during pre-mRNA splicing. Curr. Opin. Genet. Dev. 1996, 6, 215–220. [Google Scholar] [CrossRef]
- Niu, D.K. Exon definition as a potential negative force against intron losses in evolution. Biol. Direct 2008, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Jamison, S.F.; Crow, A.; Garcia-Blanco, M.A. The spliceosome assembly pathway in mammalian extracts. Mol. Cell. Biol. 1992, 12, 4279–4287. [Google Scholar] [CrossRef]
- Kent, O.A.; Ritchie, D.B.; Macmillan, A.M. Characterization of a U2AF-independent commitment complex (E’) in the mammalian spliceosome assembly pathway. Mol. Cell. Biol. 2005, 25, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Legrain, P.; Seraphin, B.; Rosbash, M. Early commitment of yeast pre-mRNA to the spliceosome pathway. Mol. Cell. Biol. 1988, 8, 3755–3760. [Google Scholar] [CrossRef] [PubMed]
- Seraphin, B.; Rosbash, M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell 1989, 59, 349–358. [Google Scholar] [CrossRef]
- Lerner, M.R.; Boyle, J.A.; Mount, S.M.; Wolin, S.L.; Steitz, J.A. Are snRNPs involved in splicing? Nature 1980, 283, 220–224. [Google Scholar] [CrossRef]
- Siliciano, P.G.; Guthrie, C. 5′ splice site selection in yeast: Genetic alterations in base-pairing with U1 reveal additional requirements. Genes Dev. 1988, 2, 1258–1267. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Weiner, A.M. A compensatory base change in U1 snRNA suppresses a 5′ splice site mutation. Cell 1986, 46, 827–835. [Google Scholar] [CrossRef]
- Peled-Zehavi, H.; Berglund, J.A.; Rosbash, M.; Frankel, A.D. Recognition of RNA branch point sequences by the KH domain of splicing factor 1 (mammalian branch point binding protein) in a splicing factor complex. Mol. Cell. Biol. 2001, 21, 5232–5241. [Google Scholar] [CrossRef] [Green Version]
- Ruskin, B.; Zamore, P.D.; Green, M.R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell 1988, 52, 207–219. [Google Scholar] [CrossRef]
- Zamore, P.D.; Green, M.R. Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc. Natl. Acad. Sci. USA 1989, 86, 9243–9247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merendino, L.; Guth, S.; Bilbao, D.; Martinez, C.; Valcarcel, J. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature 1999, 402, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Romfo, C.M.; Nilsen, T.W.; Green, M.R. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature 1999, 402, 832–835. [Google Scholar] [CrossRef]
- Zorio, D.A.; Blumenthal, T. U2AF35 is encoded by an essential gene clustered in an operon with RRM/cyclophilin in Caenorhabditis elegans. RNA 1999, 5, 487–494. [Google Scholar] [CrossRef]
- Berglund, J.A.; Abovich, N.; Rosbash, M. A cooperative interaction between U2AF65 and mBBP/SF1 facilitates branchpoint region recognition. Genes Dev. 1998, 12, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Vilardell, J.; Query, C.C. Pre-spliceosome formation in S.pombe requires a stable complex of SF1-U2AF(59)-U2AF(23). EMBO J. 2002, 21, 5516–5526. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Maucuer, A.; Gupta, A.; Manceau, V.; Thickman, K.R.; Bauer, W.J.; Kennedy, S.D.; Wedekind, J.E.; Green, M.R.; Kielkopf, C.L. Structure of phosphorylated SF1 bound to U2AF(6)(5) in an essential splicing factor complex. Structure 2013, 21, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Gozani, O.; Potashkin, J.; Reed, R. A potential role for U2AF-SAP 155 interactions in recruiting U2 snRNP to the branch site. Mol. Cell. Biol. 1998, 18, 4752–4760. [Google Scholar] [CrossRef] [Green Version]
- Rutz, B.; Seraphin, B. Transient interaction of BBP/ScSF1 and Mud2 with the splicing machinery affects the kinetics of spliceosome assembly. RNA 1999, 5, 819–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, Y.; Oubridge, C.; van Roon, A.M.; Nagai, K. Crystal structure of human U1 snRNP, a small nuclear ribonucleoprotein particle, reveals the mechanism of 5′ splice site recognition. eLife 2015, 4, e04986. [Google Scholar] [CrossRef]
- Hinterberger, M.; Pettersson, I.; Steitz, J.A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J. Biol. Chem. 1983, 258, 2604–2613. [Google Scholar] [CrossRef]
- Bringmann, P.; Luhrmann, R. Purification of the individual snRNPs U1, U2, U5 and U4/U6 from HeLa cells and characterization of their protein constituents. EMBO J. 1986, 5, 3509–3516. [Google Scholar] [CrossRef]
- Porter, G.; Brennwald, P.; Wise, J.A. U1 small nuclear RNA from Schizosaccharomyces pombe has unique and conserved features and is encoded by an essential single-copy gene. Mol. Cell. Biol. 1990, 10, 2874–2881. [Google Scholar] [CrossRef]
- Seraphin, B.; Rosbash, M. Mutational analysis of the interactions between U1 small nuclear RNA and pre-mRNA of yeast. Gene 1989, 82, 145–151. [Google Scholar] [CrossRef]
- Siliciano, P.G.; Jones, M.H.; Guthrie, C. Saccharomyces cerevisiae has a U1-like small nuclear RNA with unexpected properties. Science 1987, 237, 1484–1487. [Google Scholar] [CrossRef]
- Hümmer, S.; Borao, S.; Guerra-Moreno, A.; Cozzuto, L.; Hidalgo, E.; Ayté, J. Cross talk between the upstream exon-intron junction and U2AF65 facilitates splicing of non-consensus introns. Cell Rep. 2021, 37, 109893. [Google Scholar] [CrossRef]
- Yeo, G.; Burge, C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. J. Comput. Mol. Cell Biol. 2004, 11, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Sorek, R.; Shamir, R.; Ast, G. How prevalent is functional alternative splicing in the human genome? Trends Genet. 2004, 20, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Mertins, P.; Gallwitz, D. Nuclear pre-mRNA splicing in the fission yeast Schizosaccharomyces pombe strictly requires an intron-contained, conserved sequence element. EMBO J. 1987, 6, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Drabenstot, S.D.; Kupfer, D.M.; White, J.D.; Dyer, D.W.; Roe, B.A.; Buchanan, K.L.; Murphy, J.W. FELINES: A utility for extracting and examining EST-defined introns and exons. Nucleic Acids Res. 2003, 31, e141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitton, D.A.; Rallis, C.; Jeffares, D.C.; Smith, G.C.; Chen, Y.Y.; Codlin, S.; Marguerat, S.; Bahler, J. LaSSO, a strategy for genome-wide mapping of intronic lariats and branch points using RNA-seq. Genome Res. 2014, 24, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Masuda, A.; Matsuura, T.; Ohno, K. Human branch point consensus sequence is yUnAy. Nucleic Acids Res. 2008, 36, 2257–2267. [Google Scholar] [CrossRef] [Green Version]
- Corioni, M.; Antih, N.; Tanackovic, G.; Zavolan, M.; Kramer, A. Analysis of in situ pre-mRNA targets of human splicing factor SF1 reveals a function in alternative splicing. Nucleic Acids Res. 2011, 39, 1868–1879. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Luyten, I.; Bottomley, M.J.; Messias, A.C.; Houngninou-Molango, S.; Sprangers, R.; Zanier, K.; Kramer, A.; Sattler, M. Structural basis for recognition of the intron branch site RNA by splicing factor 1. Science 2001, 294, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Loerch, S.; Kielkopf, C.L. Unmasking the U2AF homology motif family: A bona fide protein-protein interaction motif in disguise. RNA 2016, 22, 1795–1807. [Google Scholar] [CrossRef] [Green Version]
- Abovich, N.; Rosbash, M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell 1997, 89, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, N.; Andoh, T.; Frendewey, D.; Tani, T. Mutations in the SF1-U2AF59-U2AF23 complex cause exon skipping in Schizosaccharomyces pombe. J. Biol. Chem. 2007, 282, 2221–2228. [Google Scholar] [CrossRef] [Green Version]
- Shitashige, M.; Satow, R.; Honda, K.; Ono, M.; Hirohashi, S.; Yamada, T. Increased susceptibility of Sf1(+/−) mice to azoxymethane-induced colon tumorigenesis. Cancer Sci. 2007, 98, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Rutz, B.; Seraphin, B. A dual role for BBP/ScSF1 in nuclear pre-mRNA retention and splicing. EMBO J. 2000, 19, 1873–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guth, S.; Valcarcel, J. Kinetic role for mammalian SF1/BBP in spliceosome assembly and function after polypyrimidine tract recognition by U2AF. J. Biol. Chem. 2000, 275, 38059–38066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanackovic, G.; Kramer, A. Human splicing factor SF3a, but not SF1, is essential for pre-mRNA splicing in vivo. Mol. Biol. Cell 2005, 16, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielkopf, C.L.; Rodionova, N.A.; Green, M.R.; Burley, S.K. A novel peptide recognition mode revealed by the X-ray structure of a core U2AF35/U2AF65 heterodimer. Cell 2001, 106, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Corsini, L.; Bonnal, S.; Basquin, J.; Hothorn, M.; Scheffzek, K.; Valcarcel, J.; Sattler, M. U2AF-homology motif interactions are required for alternative splicing regulation by SPF45. Nat. Struct. Mol. Biol. 2007, 14, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Rudner, D.Z.; Kanaar, R.; Breger, K.S.; Rio, D.C. Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo. Mol. Cell. Biol. 1998, 18, 1765–1773. [Google Scholar] [CrossRef] [Green Version]
- Zuo, P.; Maniatis, T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996, 10, 1356–1368. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Park, S.Y.; Oda, T.; Akiyoshi, T.; Sato, M.; Shirouzu, M.; Tsuda, K.; Kuwasako, K.; Unzai, S.; Muto, Y.; et al. A novel 3′ splice site recognition by the two zinc fingers in the U2AF small subunit. Genes Dev. 2015, 29, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Birney, E.; Kumar, S.; Krainer, A.R. Analysis of the RNA-recognition motif and RS and RGG domains: Conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993, 21, 5803–5816. [Google Scholar] [CrossRef] [Green Version]
- Worthington, M.T.; Amann, B.T.; Nathans, D.; Berg, J.M. Metal binding properties and secondary structure of the zinc-binding domain of Nup475. Proc. Natl. Acad. Sci. USA 1996, 93, 13754–13759. [Google Scholar] [CrossRef] [Green Version]
- Wentz-Hunter, K.; Potashkin, J. The small subunit of the splicing factor U2AF is conserved in fission yeast. Nucleic Acids Res. 1996, 24, 1849–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.M.; Castle, J.; Garrett-Engele, P.; Kan, Z.; Loerch, P.M.; Armour, C.D.; Santos, R.; Schadt, E.E.; Stoughton, R.; Shoemaker, D.D. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science 2003, 302, 2141–2144. [Google Scholar] [CrossRef] [Green Version]
- Coolidge, C.J.; Seely, R.J.; Patton, J.G. Functional analysis of the polypyrimidine tract in pre-mRNA splicing. Nucleic Acids Res. 1997, 25, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guth, S.; Martinez, C.; Gaur, R.K.; Valcarcel, J. Evidence for substrate-specific requirement of the splicing factor U2AF(35) and for its function after polypyrimidine tract recognition by U2AF(65). Mol. Cell. Biol. 1999, 19, 8263–8271. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, H.; Rahn, A.; Gawande, B.; Guth, S.; Valcarcel, J.; Singh, R. The conserved RNA recognition motif 3 of U2 snRNA auxiliary factor (U2AF 65) is essential in vivo but dispensable for activity in vitro. RNA 2004, 10, 240–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, T.R.; Coelho, M.B.; Desterro, J.M.; Mollet, I.; Carmo-Fonseca, M. In vivo requirement of the small subunit of U2AF for recognition of a weak 3′ splice site. Mol. Cell. Biol. 2006, 26, 8183–8190. [Google Scholar] [CrossRef] [Green Version]
- Abovich, N.; Liao, X.C.; Rosbash, M. The yeast MUD2 protein: An interaction with PRP11 defines a bridge between commitment complexes and U2 snRNP addition. Genes Dev. 1994, 8, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Fouser, L.A.; Friesen, J.D. Effects on mRNA splicing of mutations in the 3′ region of the Saccharomyces cerevisiae actin intron. Mol. Cell. Biol. 1987, 7, 225–230. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Salsi, E.; Chatrikhi, R.; Henderson, S.; Jenkins, J.L.; Green, M.R.; Ermolenko, D.N.; Kielkopf, C.L. An extended U2AF(65)-RNA-binding domain recognizes the 3′ splice site signal. Nat. Commun. 2016, 7, 10950. [Google Scholar] [CrossRef] [Green Version]
- Kielkopf, C.L.; Lucke, S.; Green, M.R. U2AF homology motifs: Protein recognition in the RRM world. Genes Dev. 2004, 18, 1513–1526. [Google Scholar] [CrossRef] [Green Version]
- Potashkin, J.; Naik, K.; Wentz-Hunter, K. U2AF homolog required for splicing in vivo. Science 1993, 262, 573–575. [Google Scholar] [CrossRef]
- Zamore, P.D.; Green, M.R. Biochemical characterization of U2 snRNP auxiliary factor: An essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991, 10, 207–214. [Google Scholar] [CrossRef]
- Shao, C.; Yang, B.; Wu, T.; Huang, J.; Tang, P.; Zhou, Y.; Zhou, J.; Qiu, J.; Jiang, L.; Li, H.; et al. Mechanisms for U2AF to define 3′ splice sites and regulate alternative splicing in the human genome. Nat. Struct. Mol. Biol. 2014, 21, 997–1005. [Google Scholar] [CrossRef]
- Reed, R. The organization of 3′ splice-site sequences in mammalian introns. Genes Dev. 1989, 3, 2113–2123. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.J. Intron recognition comes of AGe. Nat. Struct. Biol. 2000, 7, 14–16. [Google Scholar] [CrossRef]
- Patterson, B.; Guthrie, C. A U-rich tract enhances usage of an alternative 3′ splice site in yeast. Cell 1991, 64, 181–187. [Google Scholar] [CrossRef]
- Sridharan, V.; Singh, R. A conditional role of U2AF in splicing of introns with unconventional polypyrimidine tracts. Mol. Cell. Biol. 2007, 27, 7334–7344. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.I.; Voelker, R.B.; Henscheid, K.L.; Warf, M.B.; Berglund, J.A. Identification of motifs that function in the splicing of non-canonical introns. Genome Biol. 2008, 9, R97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roscigno, R.F.; Weiner, M.; Garcia-Blanco, M.A. A mutational analysis of the polypyrimidine tract of introns. Effects of sequence differences in pyrimidine tracts on splicing. J. Biol. Chem. 1993, 268, 11222–11229. [Google Scholar] [CrossRef]
- Kang, H.S.; Sanchez-Rico, C.; Ebersberger, S.; Sutandy, F.X.R.; Busch, A.; Welte, T.; Stehle, R.; Hipp, C.; Schulz, L.; Buchbender, A.; et al. An autoinhibitory intramolecular interaction proof-reads RNA recognition by the essential splicing factor U2AF2. Proc. Natl. Acad. Sci. USA 2020, 117, 7140–7149. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Heimiller, J.; Singh, R. Genomic mRNA profiling reveals compensatory mechanisms for the requirement of the essential splicing factor U2AF. Mol. Cell. Biol. 2011, 31, 652–661. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Fu, X.D. Genomic functions of U2AF in constitutive and regulated splicing. RNA Biol. 2015, 12, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistler, A.L.; Guthrie, C. Deletion of MUD2, the yeast homolog of U2AF65, can bypass the requirement for sub2, an essential spliceosomal ATPase. Genes Dev. 2001, 15, 42–49. [Google Scholar] [CrossRef]
- Tang, J.; Abovich, N.; Rosbash, M. Identification and characterization of a yeast gene encoding the U2 small nuclear ribonucleoprotein particle B” protein. Mol. Cell. Biol. 1996, 16, 2787–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taggart, A.J.; DeSimone, A.M.; Shih, J.S.; Filloux, M.E.; Fairbrother, W.G. Large-scale mapping of branchpoints in human pre-mRNA transcripts in vivo. Nat. Struct. Mol. Biol. 2012, 19, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Sudol, M. Structure and function of the WW domain. Prog. Biophys. Mol. Biol. 1996, 65, 113–132. [Google Scholar] [CrossRef]
- Allen, M.; Friedler, A.; Schon, O.; Bycroft, M. The structure of an FF domain from human HYPA/FBP11. J. Mol. Biol. 2002, 323, 411–416. [Google Scholar] [CrossRef]
- Montes, M.; Becerra, S.; Sanchez-Alvarez, M.; Sune, C. Functional coupling of transcription and splicing. Gene 2012, 501, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Kao, H.Y.; Siliciano, P.G. Identification of Prp40, a novel essential yeast splicing factor associated with the U1 small nuclear ribonucleoprotein particle. Mol. Cell. Biol. 1996, 16, 960–967. [Google Scholar] [CrossRef] [Green Version]
- Newo, A.N.; Lutzelberger, M.; Bottner, C.A.; Wehland, J.; Wissing, J.; Jansch, L.; Kaufer, N.F. Proteomic analysis of the U1 snRNP of Schizosaccharomyces pombe reveals three essential organism-specific proteins. Nucleic Acids Res. 2007, 35, 1391–1401. [Google Scholar] [CrossRef]
- Makarov, E.M.; Owen, N.; Bottrill, A.; Makarova, O.V. Functional mammalian spliceosomal complex E contains SMN complex proteins in addition to U1 and U2 snRNPs. Nucleic Acids Res. 2012, 40, 2639–2652. [Google Scholar] [CrossRef] [Green Version]
- Becerra, S.; Montes, M.; Hernandez-Munain, C.; Sune, C. Prp40 pre-mRNA processing factor 40 homolog B (PRPF40B) associates with SF1 and U2AF65 and modulates alternative pre-mRNA splicing in vivo. RNA 2015, 21, 438–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzini, P.A.; Chew, R.S.E.; Tan, C.W.; Yong, J.Y.; Zhang, F.; Zheng, J.; Roca, X. Human PRPF40B regulates hundreds of alternative splicing targets and represses a hypoxia expression signature. RNA 2019, 25, 905–920. [Google Scholar] [CrossRef] [Green Version]
- Sune, C.; Hayashi, T.; Liu, Y.; Lane, W.S.; Young, R.A.; Garcia-Blanco, M.A. CA150, a nuclear protein associated with the RNA polymerase II holoenzyme, is involved in Tat-activated human immunodeficiency virus type 1 transcription. Mol. Cell. Biol. 1997, 17, 6029–6039. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.T.; Lu, R.M.; Tarn, W.Y. The WW domain-containing proteins interact with the early spliceosome and participate in pre-mRNA splicing in vivo. Mol. Cell. Biol. 2004, 24, 9176–9185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstrohm, A.C.; Albrecht, T.R.; Sune, C.; Bedford, M.T.; Garcia-Blanco, M.A. The transcription elongation factor CA150 interacts with RNA polymerase II and the pre-mRNA splicing factor SF1. Mol. Cell. Biol. 2001, 21, 7617–7628. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Alvarez, M.; Goldstrohm, A.C.; Garcia-Blanco, M.A.; Sune, C. Human transcription elongation factor CA150 localizes to splicing factor-rich nuclear speckles and assembles transcription and splicing components into complexes through its amino and carboxyl regions. Mol. Cell. Biol. 2006, 26, 4998–5014. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Cote, J.; Shaaban, S.; Bedford, M.T. The arginine methyltransferase CARM1 regulates the coupling of transcription and mRNA processing. Molecular cell 2007, 25, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.L.; Robinson, T.J.; Munoz, M.J.; Kornblihtt, A.R.; Garcia-Blanco, M.A. Identification of the cellular targets of the transcription factor TCERG1 reveals a prevalent role in mRNA processing. J. Biol. Chem. 2008, 283, 7949–7961. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Alvarez, M.; Montes, M.; Sanchez-Hernandez, N.; Hernandez-Munain, C.; Sune, C. Differential effects of sumoylation on transcription and alternative splicing by transcription elongation regulator 1 (TCERG1). J. Biol. Chem. 2010, 285, 15220–15233. [Google Scholar] [CrossRef] [Green Version]
- Montes, M.; Cloutier, A.; Sanchez-Hernandez, N.; Michelle, L.; Lemieux, B.; Blanchette, M.; Hernandez-Munain, C.; Chabot, B.; Sune, C. TCERG1 regulates alternative splicing of the Bcl-x gene by modulating the rate of RNA polymerase II transcription. Mol. Cell. Biol. 2012, 32, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Hernandez, N.; Ruiz, L.; Sanchez-Alvarez, M.; Montes, M.; Macias, M.J.; Hernandez-Munain, C.; Sune, C. The FF4 and FF5 domains of transcription elongation regulator 1 (TCERG1) target proteins to the periphery of speckles. J. Biol. Chem. 2012, 287, 17789–17800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, B.; Marx, O.; Norris, A.D. Spliceosomal component PRP-40 is a central regulator of microexon splicing. Cell Rep. 2021, 36, 109464. [Google Scholar] [CrossRef] [PubMed]
- Spingola, M.; Ares, M., Jr. A yeast intronic splicing enhancer and Nam8p are required for Mer1p-activated splicing. Mol. Cell 2000, 6, 329–338. [Google Scholar] [CrossRef]
- Malapeira, J.; Moldon, A.; Hidalgo, E.; Smith, G.R.; Nurse, P.; Ayte, J. A meiosis-specific cyclin regulated by splicing is required for proper progression through meiosis. Mol. Cell. Biol. 2005, 25, 6330–6337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldon, A.; Malapeira, J.; Gabrielli, N.; Gogol, M.; Gomez-Escoda, B.; Ivanova, T.; Seidel, C.; Ayte, J. Promoter-driven splicing regulation in fission yeast. Nature 2008, 455, 997–1000. [Google Scholar] [CrossRef] [Green Version]
- Stepankiw, N.; Raghavan, M.; Fogarty, E.A.; Grimson, A.; Pleiss, J.A. Widespread alternative and aberrant splicing revealed by lariat sequencing. Nucleic Acids Res. 2015, 43, 8488–8501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, D.; Andree, N.; Razanau, A.; Zock-Emmenthal, S.; Lutzelberger, M.; Plath, S.; Schmidt, H.; Guerra-Moreno, A.; Cozzuto, L.; Ayte, J.; et al. Prp4 Kinase Grants the License to Splice: Control of Weak Splice Sites during Spliceosome Activation. PLoS Genet. 2016, 12, e1005768. [Google Scholar] [CrossRef]
- Lipp, J.J.; Marvin, M.C.; Shokat, K.M.; Guthrie, C. SR protein kinases promote splicing of nonconsensus introns. Nat. Struct. Mol. Biol. 2015, 22, 611–617. [Google Scholar] [CrossRef]
- Tang, Z.; Kuo, T.; Shen, J.; Lin, R.J. Biochemical and genetic conservation of fission yeast Dsk1 and human SR protein-specific kinase 1. Mol. Cell. Biol. 2000, 20, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Gross, T.; Lutzelberger, M.; Weigmann, H.; Klingenhoff, A.; Shenoy, S.; Kaufer, N.F. Functional analysis of the fission yeast Prp4 protein kinase involved in pre-mRNA splicing and isolation of a putative mammalian homologue. Nucleic Acids Res. 1997, 25, 1028–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]

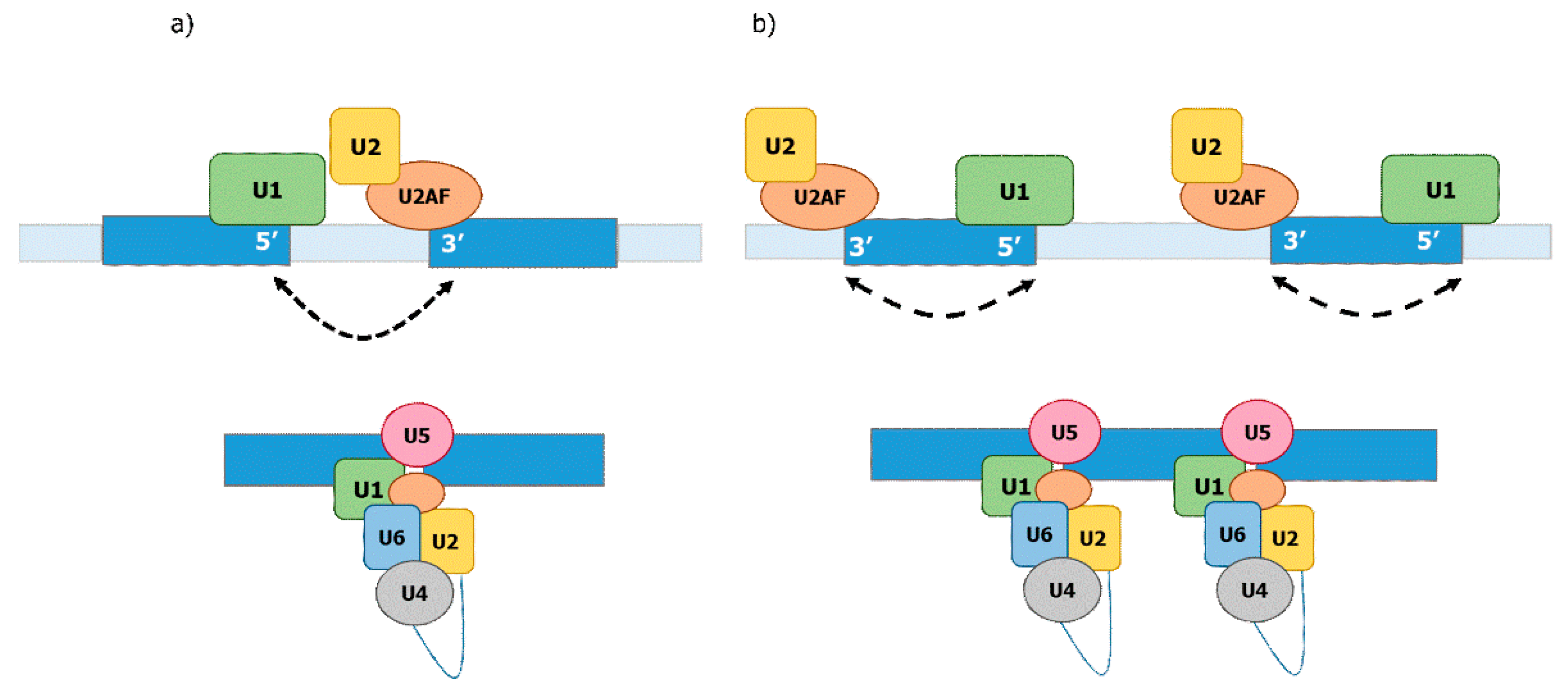
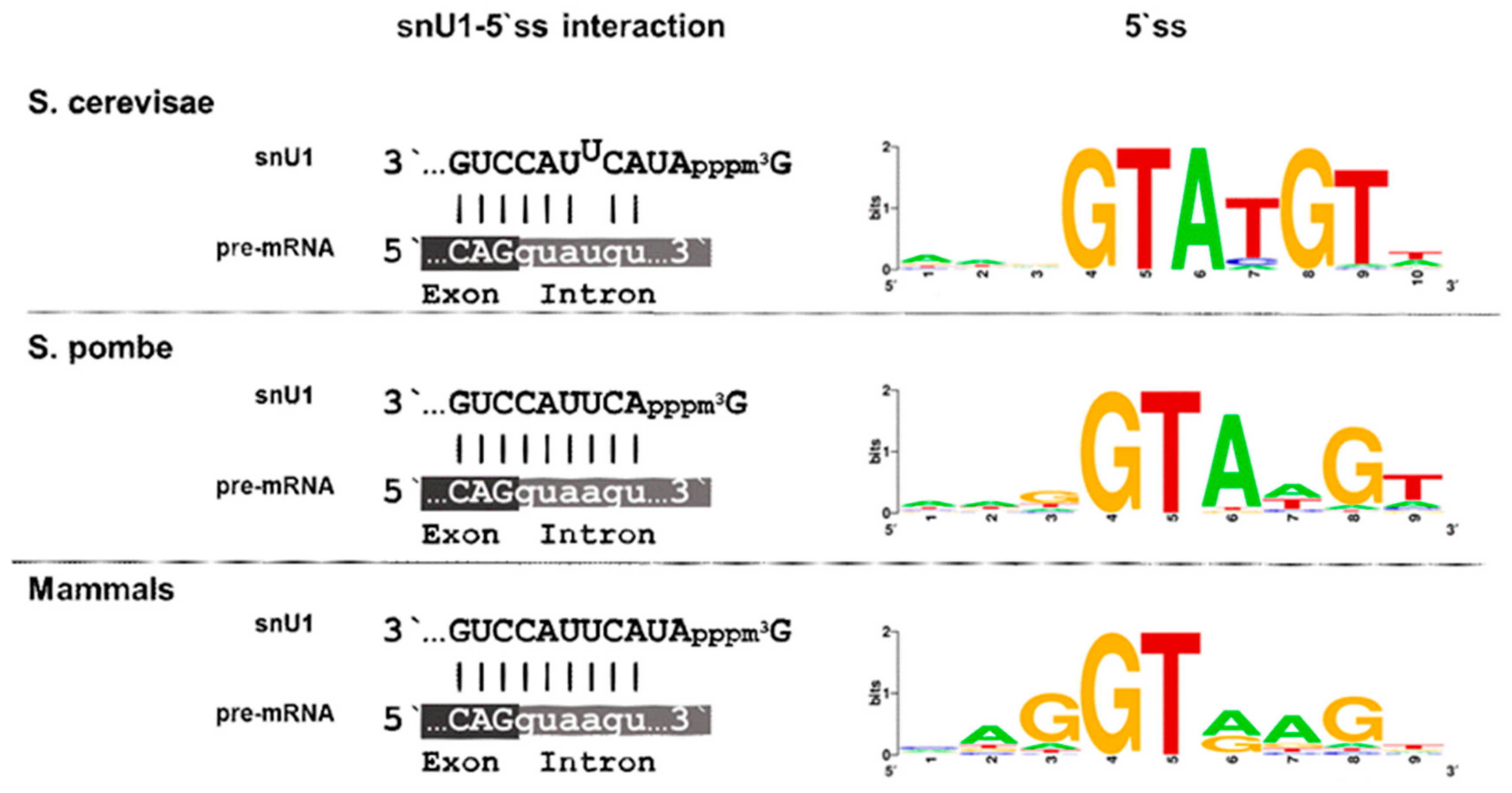
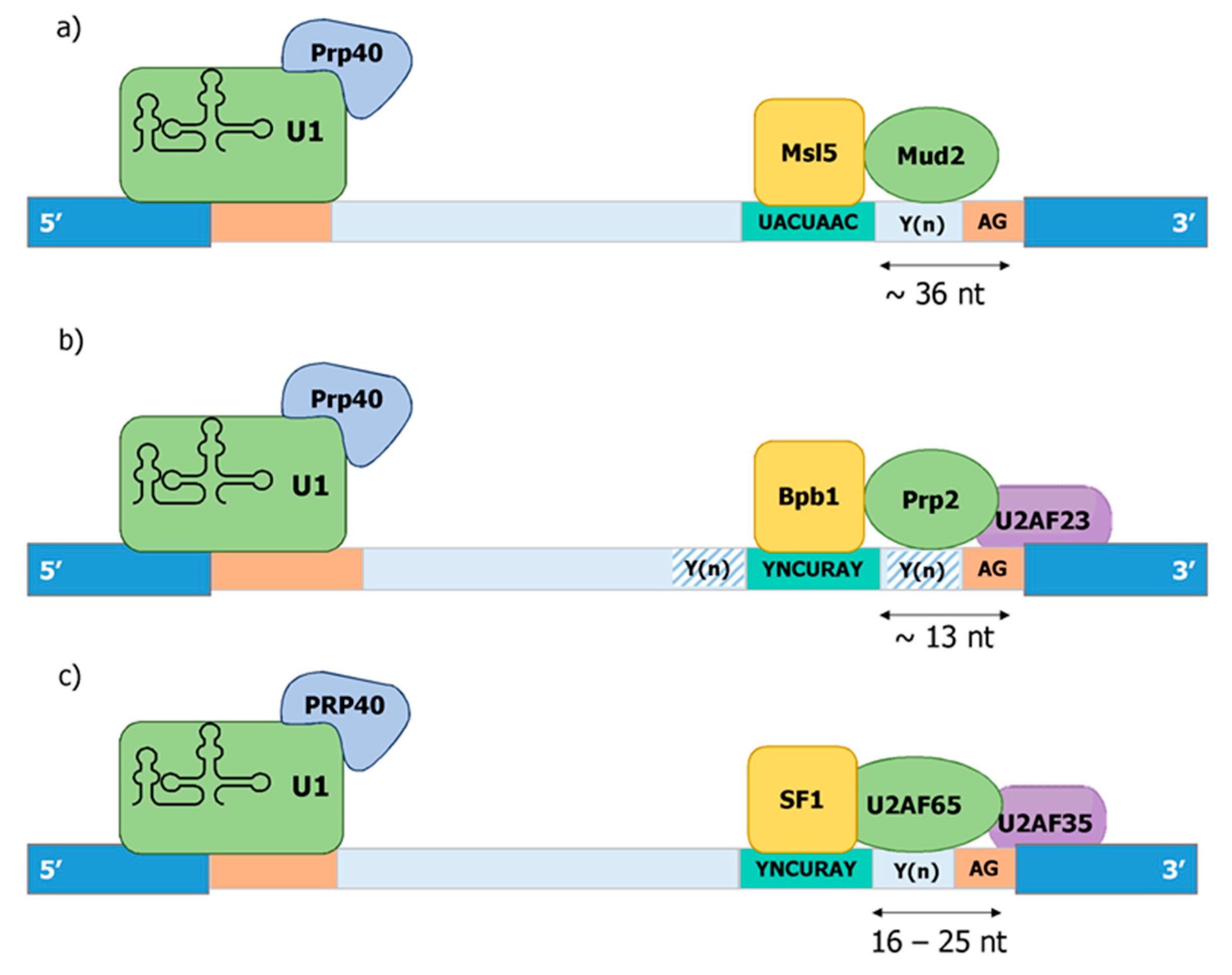
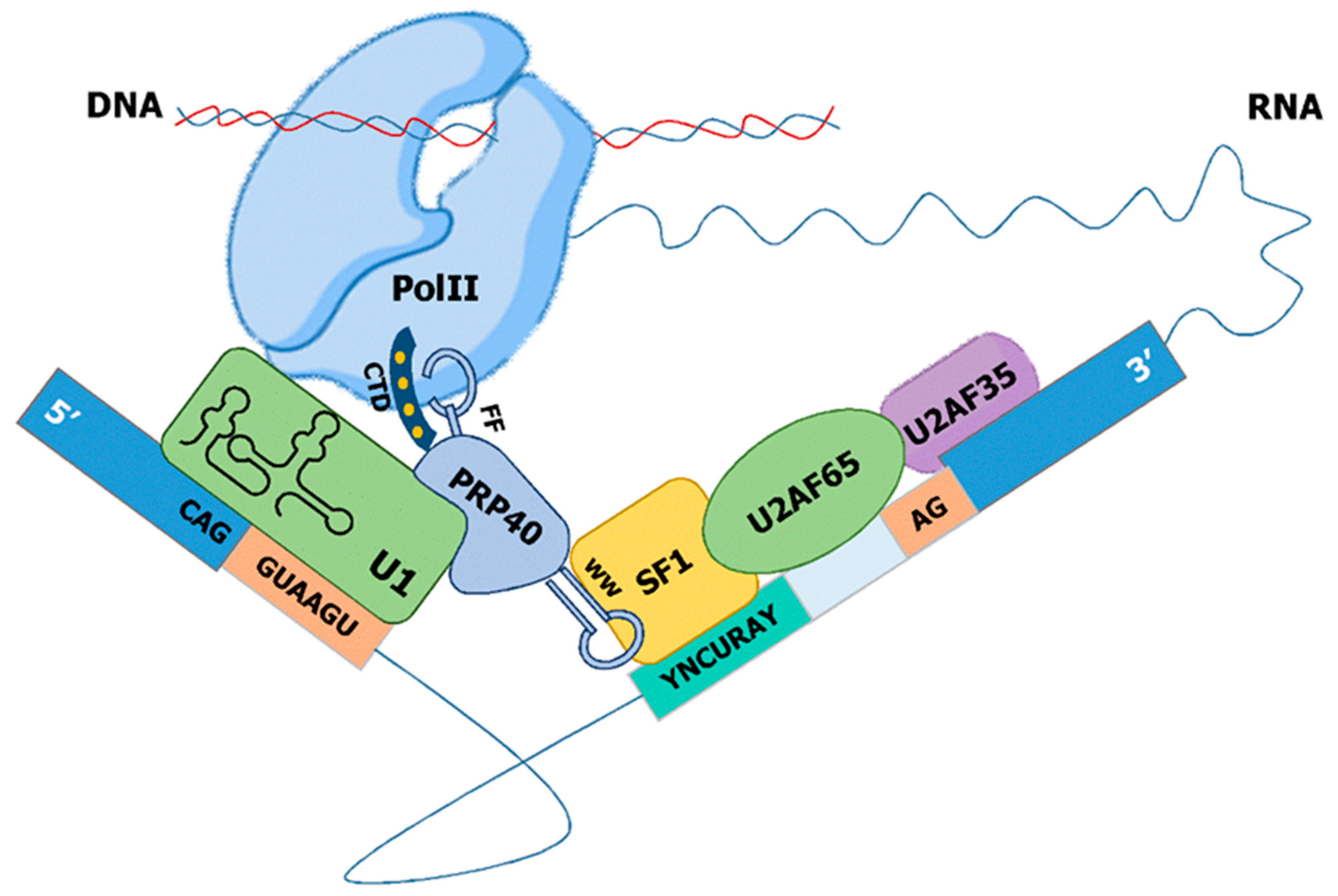
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borao, S.; Ayté, J.; Hümmer, S. Evolution of the Early Spliceosomal Complex—From Constitutive to Regulated Splicing. Int. J. Mol. Sci. 2021, 22, 12444. https://doi.org/10.3390/ijms222212444
Borao S, Ayté J, Hümmer S. Evolution of the Early Spliceosomal Complex—From Constitutive to Regulated Splicing. International Journal of Molecular Sciences. 2021; 22(22):12444. https://doi.org/10.3390/ijms222212444
Chicago/Turabian StyleBorao, Sonia, José Ayté, and Stefan Hümmer. 2021. "Evolution of the Early Spliceosomal Complex—From Constitutive to Regulated Splicing" International Journal of Molecular Sciences 22, no. 22: 12444. https://doi.org/10.3390/ijms222212444
APA StyleBorao, S., Ayté, J., & Hümmer, S. (2021). Evolution of the Early Spliceosomal Complex—From Constitutive to Regulated Splicing. International Journal of Molecular Sciences, 22(22), 12444. https://doi.org/10.3390/ijms222212444





