A Systematic Approach to the Development of Cilostazol Nanosuspension by Liquid Antisolvent Precipitation (LASP) and Its Combination with Ultrasound
Abstract
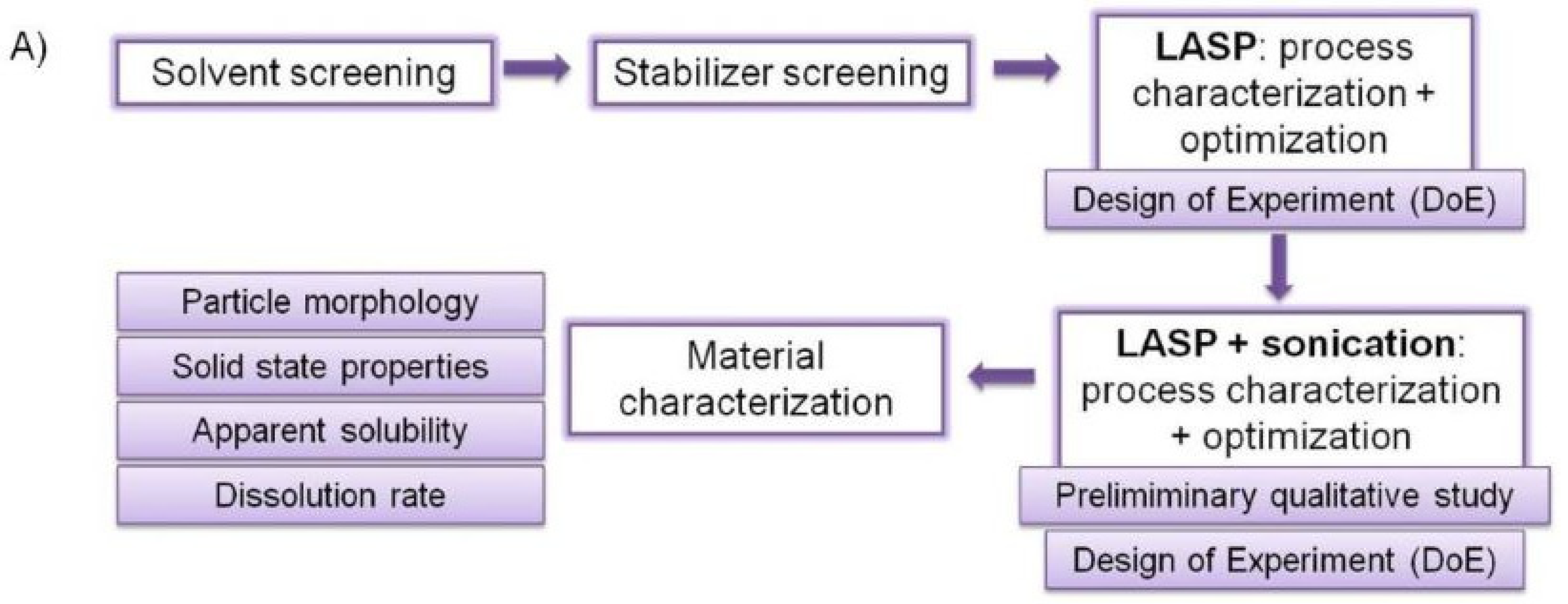
:1. Introduction
2. Results and Discussion
2.1. Solvent Screening
2.2. Stabilizer Screening
2.3. LASP–Study of Factors and Optimization: Influence of Drug Concentration, Stabilizer Amount, Mixing and Feeding Speed
2.4. Sonication–Study of Factors and Optimization
2.4.1. Preliminary Sonication Studies: Moment of Ultrasound Activation, Sonication Pattern, Initial Temperature
2.4.2. LASP+Sonication Study of Factors Using DoE: Influence of Drug Concentration, Sonication Time, and Amplitude
2.4.3. LASP+Sonication Optimization
2.5. Characterization of Micro- and Nanosuspensions
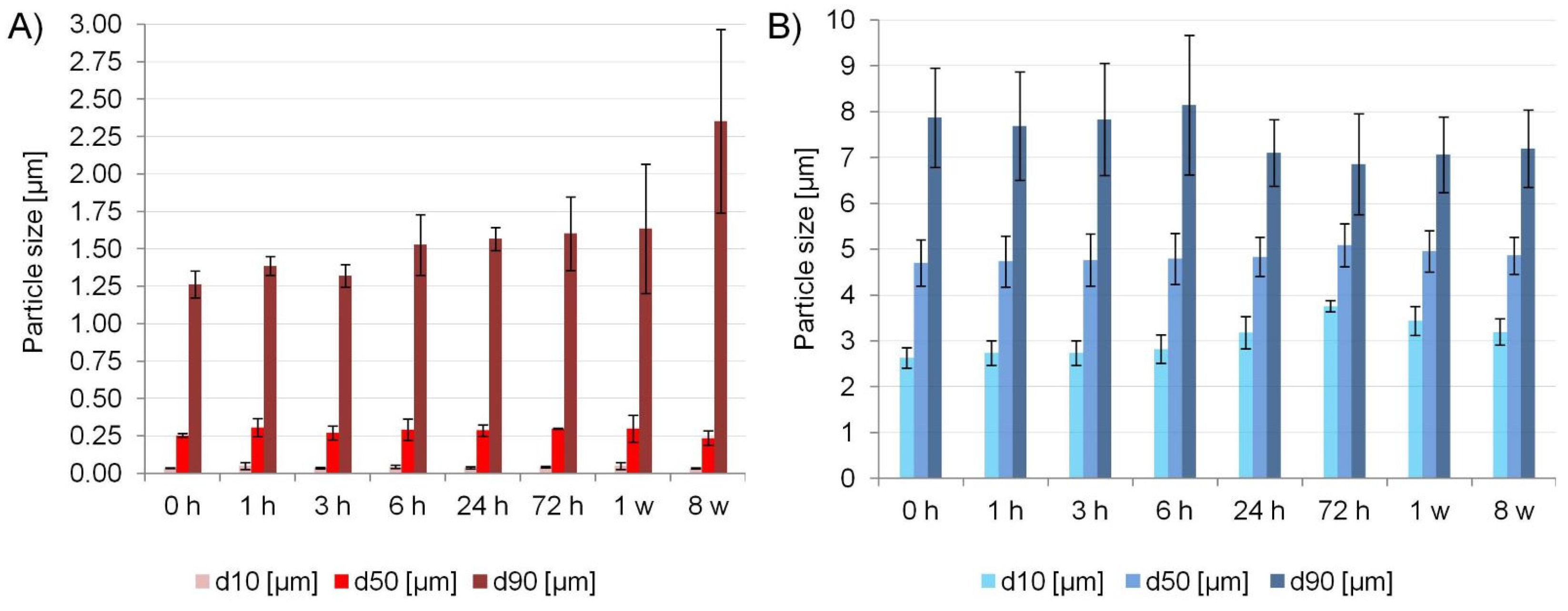
2.5.1. Physical Stability and Process Efficiency
2.5.2. Solid State and Morphology
2.5.3. Solubility and Dissolution Rate
3. Materials and Methods
3.1. Materials
3.2. Solvent Screening
3.3. Stabilizer Screening
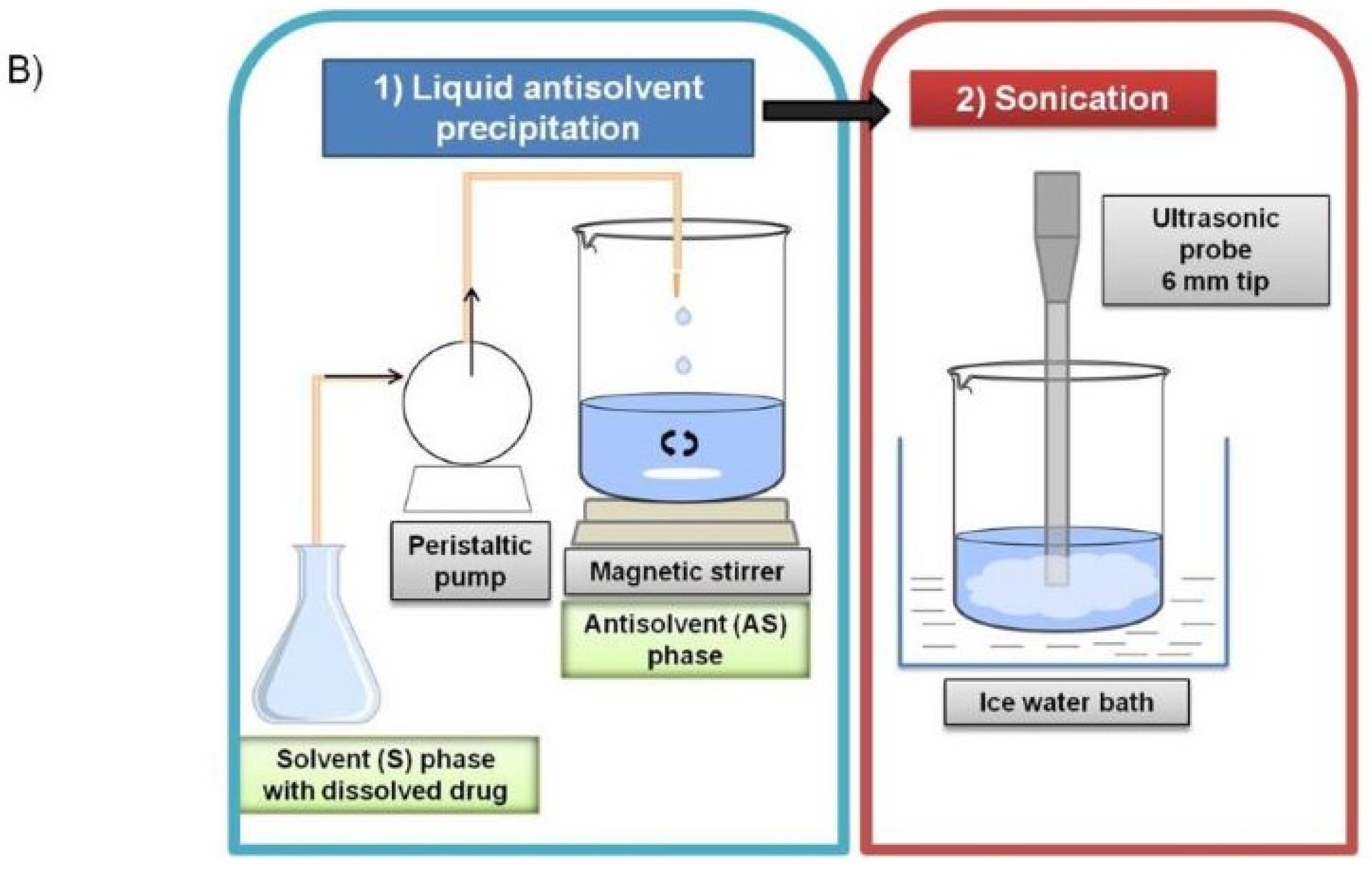
3.4. LASP–Study of Factors and Optimization Using DoE
3.5. Sonication–Study of Factors and Optimization
3.5.1. Preliminary Sonication Studies
3.5.2. LASP+Sonication Study of Factors Using DoE
3.6. Micro- and Nanocrystals Characterization Methods
3.6.1. Particle Size Distribution (PSD)
3.6.2. Physical Stability and Process Efficiency
3.6.3. Solid State and Morphology Characterization
3.6.4. Solubility Study and Dissolution Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Details of Solvent and Stabilizer Screening


| Solvent | MW | Dielectric Constant | Viscosity [mPas] | Density [g/cm3] | Log P | Surface Tension [mN/m] |
|---|---|---|---|---|---|---|
| DMSO | 78.14 | 47 a [99] | 2.47 b | 1.1 | −1.35 | 42.27 c [100] |
| DMF | 73.09 | 38 a [99] | 0.802 a | 0.95 | −1.01 | 36.42 a |
| MeOH | 32.00 | 33 b [101] | 0.544 a | 0.79 | −0.77 | 22.07 a |
| PEG 400 | 400 | 12.50 a [102] | 120 b | 1.13 | n/a | 42.06 c [103] |
| ACN | 41.05 | 38.8 b | 0.35 b | 0.79 | −0.34 | 29.04 b |
| AcOH | 60.05 | 6.15 a [104] | 1.056 a | 1.05 | −0.17 | 27.10 a |

Appendix B. Details of Statistical Analyses in DoE Studies on LASP and LASP+Sonication
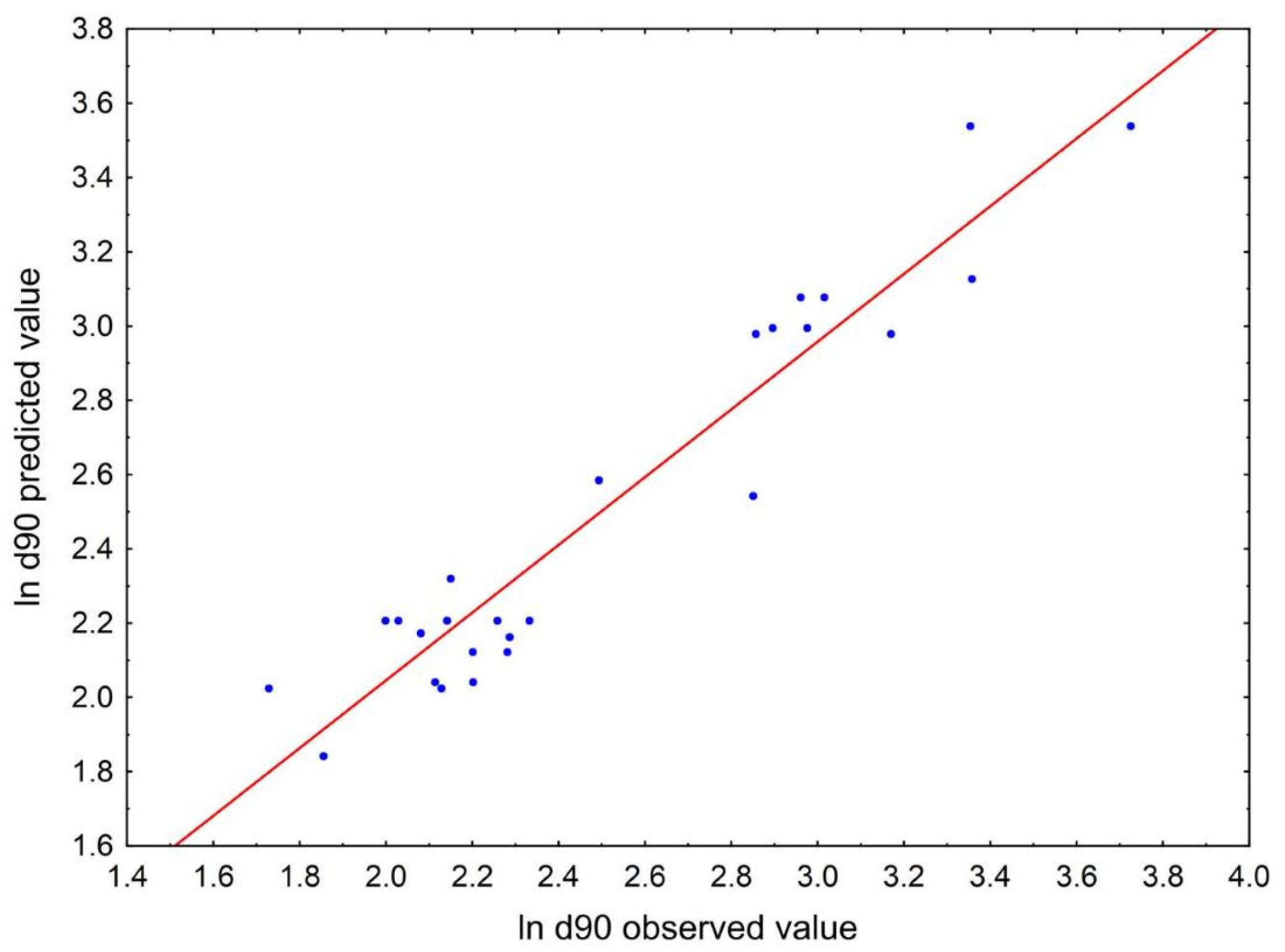
| Settings | Predicted ln d90 (95% Prediction Interval) | Predicted d90 [µm] (95% Prediction Interval) | Observed d90 [µm] (Min–Max) | Prediction Error (PE) [%] |
|---|---|---|---|---|
| 50 mg/mL CIL PVA/CIL = 1.0 750 rpm | 1.72 (1.33–2.10) | 5.56 (3.79–8.13) | 8.74 (7.71–10.8) | 36 |
| 20 mg/mL CIL PVA/CIL = 1.0 620 rpm | 2.13 (1.78–2.48) | 8.44 (5.94–11.97) | 8.39 (7.48–9.13) | 0.6 |


References
- Kherallah, R.Y.; Khawaja, M.; Olson, M.; Angiolillo, D.; Birnbaum, Y. Cilostazol: A Review of Basic Mechanisms and Clinical Uses. Cardiovasc. Drugs Ther. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Motta, N.A.V.; Autran, L.J.; Brazão, S.C.; de Oliveira Lopes, R.; Scaramello, C.B.V.; Lima, G.F.; de Brito, F.C.F. Could Cilostazol Be Beneficial in COVID-19 Treatment? Thinking about Phosphodiesterase-3 as a Therapeutic Target. Int. Immunopharmacol. 2021, 92, 107336. [Google Scholar] [CrossRef] [PubMed]
- Cilostazol Prescribing Information, Reference ID 4099638. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020863s024lbl.pdf (accessed on 21 April 2021).
- Mustapha, O.; Kim, K.S.; Shafique, S.; Kim, D.S.; Jin, S.G.; Seo, Y.G.; Youn, Y.S.; Oh, K.T.; Yong, C.S.; Kim, J.O.; et al. Comparison of Three Different Types of Cilostazol-Loaded Solid Dispersion: Physicochemical Characterization and Pharmacokinetics in Rats. Colloids Surf. B Biointerfaces 2017, 154, 89–95. [Google Scholar] [CrossRef]
- Verma, S.; Rudraraju, V.S. Wetting Kinetics: An Alternative Approach Towards Understanding the Enhanced Dissolution Rate for Amorphous Solid Dispersion of a Poorly Soluble Drug. AAPS PharmSciTech 2015, 16, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.G.; Rajput, S.J. Enhancement of Oral Bioavailability of Cilostazol by Forming Its Inclusion Complexes. AAPS PharmSciTech 2009, 10, 660–669. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Shukr, M.H.; Bendas, E.R. In Vitro and in Vivo Evaluation of Self-Nanoemulsifying Drug Delivery Systems of Cilostazol for Oral and Parenteral Administration. Int. J. Pharm. 2014, 476, 60–69. [Google Scholar] [CrossRef]
- Mustapha, O.; Kim, K.S.; Shafique, S.; Kim, D.S.; Jin, S.G.; Seo, Y.G.; Youn, Y.S.; Oh, K.T.; Lee, B.-J.; Park, Y.J.; et al. Development of Novel Cilostazol-Loaded Solid SNEDDS Using a SPG Membrane Emulsification Technique: Physicochemical Characterization and in Vivo Evaluation. Colloids Surf. B Biointerfaces 2017, 150, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, D.; Chen, M. Drug Nanocrystals for the Formulation of Poorly Soluble Drugs and Its Application as a Potential Drug Delivery System. J. Nanopart. Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Junghanns, J.-U.A.H.; Müller, R.H. Nanocrystal Technology, Drug Delivery and Clinical Applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar]
- Peltonen, L.; Hirvonen, J. Drug Nanocrystals—Versatile Option for Formulation of Poorly Soluble Materials. Int. J. Pharm. 2018, 537, 73–83. [Google Scholar] [CrossRef]
- Möschwitzer, J.P. Drug Nanocrystals in the Commercial Pharmaceutical Development Process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, C.; Ito, Y.; Nagai, N. An Oral Formulation of Cilostazol Nanoparticles Enhances Intestinal Drug Absorption in Rats. Exp. Ther. Med. 2018, 15, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinno, J.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of Particle Size Reduction on Dissolution and Oral Absorption of a Poorly Water-Soluble Drug, Cilostazol, in Beagle Dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, C.; Ito, Y.; Nagai, N. Enhanced Percutaneous Absorption of Cilostazol Nanocrystals Using Aqueous Gel Patch Systems and Clarification of the Absorption Mechanism. Exp. Ther. Med. 2018, 15, 3501–3508. [Google Scholar] [CrossRef]
- Nagai, N.; Yoshioka, C.; Ito, Y.; Funakami, Y.; Nishikawa, H.; Kawabata, A. Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model. Int. J. Mol. Sci. 2015, 16, 29329–29344. [Google Scholar] [CrossRef] [Green Version]
- Komasaka, T.; Fujimura, H.; Tagawa, T.; Sugiyama, A.; Kitano, Y. Practical Method for Preparing Nanosuspension Formulations for Toxicology Studies in the Discovery Stage: Formulation Optimization and in Vitro/in Vivo Evaluation of Nanosized Poorly Water-Soluble Compounds. Chem. Pharm. Bull. 2014, 62, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, H.; Komasaka, T.; Tomari, T.; Kitano, Y.; Takekawa, K. Nanosuspension Formulations of Poorly Water-Soluble Compounds for Intravenous Administration in Exploratory Toxicity Studies: In Vitro and in Vivo Evaluation. J. Appl. Toxicol. 2016, 36, 1259–1267. [Google Scholar] [CrossRef]
- Aghrbi, I.; Fülöp, V.; Jakab, G.; Kállai-Szabó, N.; Balogh, E.; Antal, I. Nanosuspension with Improved Saturated Solubility and Dissolution Rate of Cilostazol and Effect of Solidification on Stability. J. Drug Deliv. Sci. Technol. 2021, 61, 102165. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up Approaches for Preparing Drug Nanocrystals: Formulations and Factors Affecting Particle Size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Liquid Antisolvent Precipitation and Stabilization of Nanoparticles of Poorly Water Soluble Drugs in Aqueous Suspensions: Recent Developments and Future Perspective. Chem. Eng. J. 2012, 181–182, 1–34. [Google Scholar] [CrossRef]
- D’Addio, S.M.; Prud’homme, R.K. Controlling Drug Nanoparticle Formation by Rapid Precipitation. Adv. Drug Deliv. Rev. 2011, 63, 417–426. [Google Scholar] [CrossRef]
- Ruecroft, G.; Hipkiss, D.; Ly, T.; Maxted, N.; Cains, P.W. Sonocrystallization: The Use of Ultrasound for Improved Industrial Crystallization. Org. Process Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, J.-S.; Hwang, S.-J. Enhancement of Wettability and Dissolution Properties of Cilostazol Using the Supercritical Antisolvent Process: Effect of Various Additives. Chem. Pharm. Bull. 2010, 58, 230–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, N.; Oh, G.-H.; Park, C.; Tran, T.T.T.; Park, Y.J.; Oh, E.; Le, H.; Tran, T.T.D.; Park, J.-B.; Lee, B.-J. Reprecipitation of Poorly Water-Soluble Cilostazol Crystals Using Adsorbing Carriers for Enhanced Dissolution and Physicochemical Modification. J. Drug Deliv. Sci. Technol. 2018, 43, 477–486. [Google Scholar] [CrossRef]
- Sai Gouthami, K.; Kumar, D.; Thipparaboina, R.; Chavan, R.B.; Shastri, N.R. Can Crystal Engineering Be as Beneficial as Micronisation and Overcome Its Pitfalls?: A Case Study with Cilostazol. Int. J. Pharm. 2015, 491, 26–34. [Google Scholar] [CrossRef]
- Tari, T.; Szabó-Révész, P.; Aigner, Z. Comparative Study of Different Crystallization Methods in the Case of Cilostazol Crystal Habit Optimization. Crystals 2019, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Miao, X.; Sun, C.; Jiang, T.; Zheng, L.; Wang, T.; Wang, S. Investigation of Nanosized Crystalline Form to Improve the Oral Bioavailability of Poorly Water Soluble Cilostazol. J. Pharm. Pharm. Sci. 2011, 14, 196–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.-S. Design of Cilostazol Nanocrystals for Improved Solubility. J. Pharm. Innov. 2020, 15, 416–423. [Google Scholar] [CrossRef]
- Shimizu, T.; Osumi, T.; Niimi, K.; Nakagawa, K. Physico-Chemical Properties and Stability of Cilostazol. Arzneimittelforschung 1985, 35, 1117–1123. [Google Scholar] [PubMed]
- Chung, H.-R.; Kwon, E.; Oikawa, H.; Kasai, H.; Nakanishi, H. Effect of Solvent on Organic Nanocrystal Growth Using the Reprecipitation Method. J. Cryst. Growth 2006, 294, 459–463. [Google Scholar] [CrossRef]
- Stowell, G.W.; Behme, R.J.; Denton, S.M.; Pfeiffer, I.; Sancilio, F.D.; Whittall, L.B.; Whittle, R.R. Thermally-Prepared Polymorphic Forms of Cilostazol. J. Pharm. Sci. 2002, 91, 2481–2488. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical Nanocrystals by Nanomilling: Critical Process Parameters, Particle Fracturing and Stabilization Methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef]
- Verma, S.; Gokhale, R.; Burgess, D.J. A Comparative Study of Top-down and Bottom-up Approaches for the Preparation of Micro/Nanosuspensions. Int. J. Pharm. 2009, 380, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Natarajan, U. Factors Responsible for the Aggregation of Poly(Vinyl Alcohol) in Aqueous Solution as Revealed by Molecular Dynamics Simulations. Ind. Eng. Chem. Res. 2020, 59, 16099–16111. [Google Scholar] [CrossRef]
- Tierney, T.B.; Guo, Y.; Beloshapkin, S.; Rasmuson, Å.C.; Hudson, S.P. Investigation of the Particle Growth of Fenofibrate Following Antisolvent Precipitation and Freeze–Drying. Cryst. Growth Des. 2015, 15, 5213–5222. [Google Scholar] [CrossRef]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Evaluation of the Inhibitory Potential of HPMC, PVP and HPC Polymers on Nucleation and Crystal Growth. RSC Adv. 2016, 6, 77569–77576. [Google Scholar] [CrossRef]
- Teżyk, M.; Milanowski, B.; Ernst, A.; Lulek, J. Recent Progress in Continuous and Semi-Continuous Processing of Solid Oral Dosage Forms: A Review. Drug Dev. Ind. Pharm. 2016, 42, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Sahoo, N.G.; Li, L.; Judeh, Z. Fabrication of Quercetin Nanoparticles by Anti-Solvent Precipitation Method for Enhanced Dissolution. Powder Technol. 2012, 223, 59–64. [Google Scholar] [CrossRef]
- Zu, Y.; Li, N.; Zhao, X.; Li, Y.; Ge, Y.; Wang, W.; Wang, K.; Liu, Y. In Vitro Dissolution Enhancement of Micronized L-Nimodipine by Antisolvent Re-Crystallization from Its Crystal Form H. Int. J. Pharm. 2014, 464, 1–9. [Google Scholar] [CrossRef]
- Mohyeldin, S.M.; Mehanna, M.M.; Elgindy, N.A. The Relevancy of Controlled Nanocrystallization on Rifampicin Characteristics and Cytotoxicity. Int. J. Nanomed. 2016, 11, 2209–2222. [Google Scholar] [CrossRef] [Green Version]
- Malkani, A.; Date, A.A.; Hegde, D. Celecoxib Nanosuspension: Single-Step Fabrication Using a Modified Nanoprecipitation Method and in Vivo Evaluation. Drug Deliv. Transl. Res. 2014, 4, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Reddy, V.A.; Arora, S.; Patel, K. Development of Surface Stabilized Candesartan Cilexetil Nanocrystals with Enhanced Dissolution Rate, Permeation Rate across CaCo-2, and Oral Bioavailability. Drug Deliv. Transl. Res. 2016, 6, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Gao, Y.; Zhao, J.; Zhang, J.; Li, Q.; Zhao, Z.; Liu, J. Preparation and Optimization of Resveratrol Nanosuspensions by Antisolvent Precipitation Using Box-Behnken Design. AAPS PharmSciTech 2015, 16, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.R.; Parikh, R.H.; Chavda, J.R.; Sheth, N.R. Application of Plackett–Burman Screening Design for Preparing Glibenclamide Nanoparticles for Dissolution Enhancement. Powder Technol. 2013, 235, 405–411. [Google Scholar] [CrossRef]
- Sultana, S.; Talegaonkar, S.; Ali, R.; Mittal, G.; Bhatnagar, A.; Ahmad, F.J. Formulation Development and Optimization of Alpha Ketoglutarate Nanoparticles for Cyanide Poisoning. Powder Technol. 2011, 211, 1–9. [Google Scholar] [CrossRef]
- Mishra, B.; Sahoo, J.; Dixit, P.K. Enhanced Bioavailability of Cinnarizine Nanosuspensions by Particle Size Engineering: Optimization and Physicochemical Investigations. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Mao, S.; Shi, Y.; Li, L.C.; Fang, L. Nanonization of Itraconazole by High Pressure Homogenization: Stabilizer Optimization and Effect of Particle Size on Oral Absorption. J. Pharm. Sci. 2011, 100, 3365–3373. [Google Scholar] [CrossRef]
- Dong, Y.; Ng, W.K.; Hu, J.; Shen, S.; Tan, R.B.H. A Continuous and Highly Effective Static Mixing Process for Antisolvent Precipitation of Nanoparticles of Poorly Water-Soluble Drugs. Int. J. Pharm. 2010, 386, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, S.V.; Dave, R.N. Controlling Particle Size of a Poorly Water-Soluble Drug Using Ultrasound and Stabilizers in Antisolvent Precipitation. Ind. Eng. Chem. Res. 2009, 48, 7581–7593. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Dave, R.N. Analysis of Nucleation Kinetics of Poorly Water-Soluble Drugs in Presence of Ultrasound and Hydroxypropyl Methyl Cellulose during Antisolvent Precipitation. Int. J. Pharm. 2010, 387, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-W.; Yeo, S.-D. Antisolvent Crystallization of Carbamazepine from Organic Solutions. Chem. Eng. Res. Des. 2012, 90, 2202–2208. [Google Scholar] [CrossRef]
- Park, M.-W.; Yeo, S.-D. Antisolvent Crystallization of Roxithromycin and the Effect of Ultrasound. Separ. Sci. Technol. 2010, 45, 1402–1410. [Google Scholar] [CrossRef]
- Hatkar, U.N.; Gogate, P.R. Process Intensification of Anti-Solvent Crystallization of Salicylic Acid Using Ultrasonic Irradiations. Chem. Eng. Process Process Intensif. 2012, 57–58, 16–24. [Google Scholar] [CrossRef]
- Xia, D.; Quan, P.; Piao, H.; Piao, H.; Sun, S.; Yin, Y.; Cui, F. Preparation of Stable Nitrendipine Nanosuspensions Using the Precipitation–Ultrasonication Method for Enhancement of Dissolution and Oral Bioavailability. Eur. J. Pharm. Sci. 2010, 40, 325–334. [Google Scholar] [CrossRef]
- Alshweiat, A.; Katona, G.; Csóka, I.; Ambrus, R. Design and Characterization of Loratadine Nanosuspension Prepared by Ultrasonic-Assisted Precipitation. Eur. J. Pharm. Sci. 2018, 122, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Yu, P.; He, J.-H.; Zhang, S.; Xia, Y.-L.; Zhang, W.-L.; Liu, J.-P. Enhanced Dissolution and Oral Bioavailability of Lurasidone Hydrochloride Nanosuspensions Prepared by Antisolvent Precipitation–Ultrasonication Method. RSC Adv. 2016, 6, 49052–49059. [Google Scholar] [CrossRef]
- Jiang, T.; Han, N.; Zhao, B.; Xie, Y.; Wang, S. Enhanced Dissolution Rate and Oral Bioavailability of Simvastatin Nanocrystal Prepared by Sonoprecipitation. Drug Dev. Ind. Pharm. 2012, 38, 1230–1239. [Google Scholar] [CrossRef]
- Liu, D.; Xu, H.; Tian, B.; Yuan, K.; Pan, H.; Ma, S.; Yang, X.; Pan, W. Fabrication of Carvedilol Nanosuspensions Through the Anti-Solvent Precipitation–Ultrasonication Method for the Improvement of Dissolution Rate and Oral Bioavailability. AAPS PharmSciTech 2012, 13, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, M.; Hironaka, S. Effect of Temperature on Antisolvent Crystallization and Transformation Behaviors of Thiazole-Derivative Polymorphs. Cryst. Growth Des. 2006, 6, 1214–1218. [Google Scholar] [CrossRef]
- Iurian, S.; Tomuţa, I.; Rus, L.; Achim, M.; Leucuta, S.E. Optimization of the Sonication Process for Meloxicam Nanocrystals Preparation. Clujul Med. 2015, 88, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Sander, J.R.G.; Zeiger, B.W.; Suslick, K.S. Sonocrystallization and Sonofragmentation. Ultrason. Sonochem. 2014, 21, 1908–1915. [Google Scholar] [CrossRef]
- Kim, H.N.; Suslick, K.S. The Effects of Ultrasound on Crystals: Sonocrystallization and Sonofragmentation. Crystals 2018, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Rahim, H.; Sadiq, A.; Khan, S.; Khan, M.A.; Shah, S.M.H.; Hussain, Z.; Ullah, R.; Shahat, A.A.; Ibrahim, K. Aceclofenac Nanocrystals with Enhanced in Vitro, in Vivo Performance: Formulation Optimization, Characterization, Analgesic and Acute Toxicity Studies. Drug Des. Devel. Ther. 2017, 11, 2443–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taneja, S.; Shilpi, S.; Khatri, K. Formulation and Optimization of Efavirenz Nanosuspensions Using the Precipitation-Ultrasonication Technique for Solubility Enhancement. Artif. Cells Nanomed. Biotechnol. 2016, 44, 978–984. [Google Scholar] [CrossRef]
- Belkacem, N.; Sheikh Salem, M.A.; AlKhatib, H.S. Effect of Ultrasound on the Physico-Chemical Properties of Poorly Soluble Drugs: Antisolvent Sonocrystallization of Ketoprofen. Powder Technol. 2015, 285, 16–24. [Google Scholar] [CrossRef]
- Kassem, M.A.A.; ElMeshad, A.N.; Fares, A.R. Enhanced Solubility and Dissolution Rate of Lacidipine Nanosuspension: Formulation Via Antisolvent Sonoprecipitation Technique and Optimization Using Box–Behnken Design. AAPS PharmSciTech 2017, 18, 983–996. [Google Scholar] [CrossRef]
- Tran, T.T.-D.; Tran, P.H.-L.; Nguyen, M.N.U.; Tran, K.T.M.; Pham, M.N.; Tran, P.C.; Vo, T.V. Amorphous Isradipine Nanosuspension by the Sonoprecipitation Method. Int. J. Pharm. 2014, 474, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, G.; Li, Y.; Wang, J.; Yin, J.; Ren, L. Enhanced Dissolution and Oral Bioavailbility of Cinacalcet Hydrochlorde Nanocrystals with No Food Effect. Nanotechnology 2019, 30, 055102. [Google Scholar] [CrossRef]
- Rangaraj, N.; Pailla, S.R.; Chowta, P.; Sampathi, S. Fabrication of Ibrutinib Nanosuspension by Quality by Design Approach: Intended for Enhanced Oral Bioavailability and Diminished Fast Fed Variability. AAPS PharmSciTech 2019, 20, 326. [Google Scholar] [CrossRef]
- Gajera, B.Y.; Shah, D.A.; Dave, R.H. Development of an Amorphous Nanosuspension by Sonoprecipitation-Formulation and Process Optimization Using Design of Experiment Methodology. Int. J. Pharm. 2019, 559, 348–359. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Zhu, J.; Wang, J.; Xu, Y.; Luan, H.; Zhu, Z.; Hu, M.; Wang, H. Preparation, Optimization of Intravenous ZL-004 Nanosuspensions by the Precipitation Method, Effect of Particle Size on in Vivo Pharmacokinetics and Tissue Distribution. J. Drug Deliv. Sci. Technol. 2019, 50, 313–320. [Google Scholar] [CrossRef]
- Mishra, B.; Sahoo, J.; Dixit, P.K. Formulation and Process Optimization of Naproxen Nanosuspensions Stabilized by Hydroxy Propyl Methyl Cellulose. Carbohyd. Polym. 2015, 127, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Ma, Y.; Cao, G.; Wang, J.; Zhang, X.; Feng, J.; Wang, W. Improved Oral Bioavailability for Lutein by Nanocrystal Technology: Formulation Development, in Vitro and in Vivo Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.; Desai, M.A.; Patel, S.R. Anti-Solvent Sonocrystallization for Nano-Range Particle Size of Telmisartan through Taguchi and Box–Behnken Design. Chem. Pap. 2020, 74, 323–331. [Google Scholar] [CrossRef]
- Wong, J.; Brugger, A.; Khare, A.; Chaubal, M.; Papadopoulos, P.; Rabinow, B.; Kipp, J.; Ning, J. Suspensions for Intravenous (IV) Injection: A Review of Development, Preclinical and Clinical Aspects. Adv. Drug Deliv. Rev. 2008, 60, 939–954. [Google Scholar] [CrossRef]
- Yue, P.-F.; Li, Y.; Wan, J.; Yang, M.; Zhu, W.-F.; Wang, C.-H. Study on Formability of Solid Nanosuspensions during Nanodispersion and Solidification: I. Novel Role of Stabilizer/Drug Property. Int. J. Pharm. 2013, 454, 269–277. [Google Scholar] [CrossRef]
- Malamatari, M.; Somavarapu, S.; Taylor, K.M.G.; Buckton, G. Solidification of Nanosuspensions for the Production of Solid Oral Dosage Forms and Inhalable Dry Powders. Expert Opin. Drug Deliv. 2016, 13, 435–450. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and Chemical Stability of Drug Nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.B.; Benameur, H.; Porter, C.J.H.; Pouton, C.W. Using Polymeric Precipitation Inhibitors to Improve the Absorption of Poorly Water-Soluble Drugs: A Mechanistic Basis for Utility. J. Drug Target 2010, 18, 704–731. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M. Strategy for Control of Crystallization of Polymorphs. CrystEngComm 2009, 11, 949–964. [Google Scholar] [CrossRef]
- Pandeeswaran, M.; El-Mossalamy, E.H.; Elango, K.P. Spectroscopic Studies on the Interaction of Cilostazole with Iodine and 2,3-Dichloro-5,6-Dicyanobenzoquinone. Spectrochim. Acta A 2011, 78, 375–382. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Vermant, J.; Martens, J.A.; Froyen, L.; Humbeeck, J.V.; Van den Mooter, G.; Augustijns, P. Solubility Increases Associated with Crystalline Drug Nanoparticles: Methodologies and Significance. Mol. Pharm. 2010, 7, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Juenemann, D.; Jantratid, E.; Wagner, C.; Reppas, C.; Vertzoni, M.; Dressman, J.B. Biorelevant in Vitro Dissolution Testing of Products Containing Micronized or Nanosized Fenofibrate with a View to Predicting Plasma Profiles. Eur. J. Pharm. Biopharm. 2011, 77, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.C. Comparison of Methods for Predicting Dissolution and the Theoretical Implications of Particle-Size-Dependent Solubility. J. Pharm. Sci. 2012, 101, 681–689. [Google Scholar] [CrossRef]
- Murdande, S.B.; Shah, D.A.; Dave, R.H. Impact of Nanosizing on Solubility and Dissolution Rate of Poorly Soluble Pharmaceuticals. J. Pharm. Sci. 2015, 104, 2094–2102. [Google Scholar] [CrossRef]
- Kumar, S.; Shen, J.; Zolnik, B.; Sadrieh, N.; Burgess, D.J. Optimization and Dissolution Performance of Spray-Dried Naproxen Nano-Crystals. Int. J. Pharm. 2015, 486, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, R.S.; Biradar, S.V.; Yamamura, S.; Paradkar, A.R.; York, P. Preparation of Amorphous Cefuroxime Axetil Nanoparticles by Sonoprecipitation for Enhancement of Bioavailability. Eur. J. Pharm. Biopharm. 2008, 70, 109–115. [Google Scholar] [CrossRef]
- Keck, C.M. Particle Size Analysis of Nanocrystals: Improved Analysis Method. Int. J. Pharm. 2010, 390, 3–12. [Google Scholar] [CrossRef]
- Keck, C.M.; Müller, R.H. Size Analysis of Submicron Particles by Laser Diffractometry—90% of the Published Measurements Are False. Int. J. Pharm. 2008, 355, 150–163. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of Particle Size on Solubility, Dissolution Rate, and Oral Bioavailability: Evaluation Using Coenzyme Q10 as Naked Nanocrystals. Int. J. Nanomed. 2012, 7, 5733–5744. [Google Scholar] [CrossRef] [Green Version]
- Peltonen, L.; Strachan, C. Understanding Critical Quality Attributes for Nanocrystals from Preparation to Delivery. Molecules 2015, 20, 22286–22300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, A.; Ray, P. Studies on Surface Tension of Poly(Vinyl Alcohol): Effect of Concentration, Temperature, and Addition of Chaotropic Agents. J. Appl. Polym. Sci. 2004, 93, 122–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Beck, C.; Dalvi, S.V.; Dave, R.N. Controlled Liquid Antisolvent Precipitation Using a Rapid Mixing Device. Chem. Eng. Sci. 2010, 65, 5669–5675. [Google Scholar] [CrossRef]
- Desai, C.; Meng, X.; Yang, D.; Wang, X.; Akkunuru, V.; Mitra, S. Effect of Solvents on Stabilization of Micro Drug Particles. J. Cryst. Growth 2011, 314, 353–358. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of Process and Formulation Parameters on the Formation of Submicron Particles by Solvent Displacement and Emulsification–Diffusion Methods: Critical Comparison. Adv. Colloid. Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Can the Cavi-Precipitation Process Be Exploited to Generate Smaller Size Drug Nanocrystal? Drug Dev. Ind. Pharm. 2021, 47, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hunger, J.; Buchner, R.; Kandil, M.E.; May, E.F.; Marsh, K.N.; Hefter, G. Relative Permittivity of Dimethylsulfoxide and N,N-Dimethylformamide at Temperatures from (278 to 328) K and Pressures from (0.1 to 5) MPa. J. Chem. Eng. Data 2010, 55, 2055–2065. [Google Scholar] [CrossRef]
- Lü, P.; Zhao, G.; Zhang, X.; Yin, J.; Bao, J. Measurement and Prediction on the Surface Properties of Dimethyl Sulfoxide/Water Mixtures. Chem. Res. Chin. Univ. 2016, 32, 100–105. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric Constants of Water, Methanol, Ethanol, Butanol and Acetone: Measurement and Computational Study. J. Solut. Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Mohammadian, E.; Rahimpour, E.; Martinez, F.; Jouyban, A. Budesonide Solubility in Polyethylene Glycol 400+ water at Different Temperatures: Experimental Measurement and Mathematical Modelling. J. Mol. Liq. 2019, 274, 418–425. [Google Scholar] [CrossRef]
- Sequeira, M.C.M.; Pereira, M.F.V.; Avelino, H.M.N.T.; Caetano, F.J.P.; Fareleira, J.M.N.A. Viscosity Measurements of Poly(Ethyleneglycol) 400 [PEG 400] at Temperatures from 293 K to 348 K and at Pressures up to 50 MPa Using the Vibrating Wire Technique. Fluid Phase Equilib. 2019, 496, 7–16. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Amiri, H. Measurement and Modelling of Static Dielectric Constants of Aqueous Solutions of Methanol, Ethanol and Acetic Acid at T = 293.15 K and 91.3 kPa. J. Chem. Thermodyn. 2013, 57, 67–70. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Yadav, M.D. Effect of Ultrasound and Stabilizers on Nucleation Kinetics of Curcumin during Liquid Antisolvent Precipitation. Ultrason. Sonochem. 2015, 24, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.-Y.; Park, C.H. Characteristics of Polymers Enabling Nano-Comminution of Water-Insoluble Drugs. Int. J. Pharm. 2008, 355, 328–336. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole Nanosuspensions: Influence of Formulation Variables on Particle Size Reduction and Physical Stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Viitala, T.; Kartal-Hodzic, A.; Liang, H.; Laaksonen, T.; Hirvonen, J.; Peltonen, L. Interaction Studies between Indomethacin Nanocrystals and PEO/PPO Copolymer Stabilizers. Pharm. Res. 2015, 32, 628–639. [Google Scholar] [CrossRef]
- Mahesh, K.V.; Singh, S.K.; Gulati, M. A Comparative Study of Top-down and Bottom-up Approaches for the Preparation of Nanosuspensions of Glipizide. Powder Technol. 2014, 256, 436–449. [Google Scholar] [CrossRef]
- Sharma, O.P.; Patel, V.; Mehta, T. Design of Experiment Approach in Development of Febuxostat Nanocrystal: Application of Soluplus® as Stabilizer. Powder Technol. 2016, 302, 396–405. [Google Scholar] [CrossRef]
- Singare, D.S.; Marella, S.; Gowthamrajan, K.; Kulkarni, G.T.; Vooturi, R.; Rao, P.S. Optimization of Formulation and Process Variable of Nanosuspension: An Industrial Perspective. Int. J. Pharm. 2010, 402, 213–220. [Google Scholar] [CrossRef]
















| Run No. (Randomized Order) | Independent Variables Settings | Dependent Variables Results | ||||||
|---|---|---|---|---|---|---|---|---|
| CIL Conc. [mg/mL] | PVA/CIL | Solvent Phase Flow Rate [mL/min] | Mixing [rpm] | d10 [µm] | d50 [µm] | d90 [µm] | Span | |
| 23 | 55 | 0.75 | 3 | 250 | 3.22 | 5.74 | 9.84 | 1.153 |
| 22 | 55 | 0.75 | 5 | 500 | 3.07 | 5.59 | 9.56 | 1.161 |
| 21 | 55 | 0.75 | 1 | 500 | 2.42 | 5.31 | 10.30 | 1.482 |
| 25 (C) | 55 | 0.75 | 3 | 500 | 2.81 | 4.82 | 7.60 | 0.994 |
| 27 (C) | 55 | 0.75 | 3 | 500 | 2.32 | 4.27 | 7.38 | 1.183 |
| 5 | 10 | 1.25 | 1 | 250 | 2.39 | 5.40 | 12.10 | 1.806 |
| 13 | 100 | 1.25 | 1 | 250 | 3.16 | 5.48 | 9.03 | 1.069 |
| 1 | 10 | 0.25 | 1 | 250 | 5.22 | 15.00 | 28.60 | 1.563 |
| 4 | 10 | 0.25 | 5 | 750 | 4.47 | 9.49 | 17.40 | 1.368 |
| 26 (C) | 55 | 0.75 | 3 | 500 | 3.20 | 5.32 | 8.51 | 0.999 |
| 15 | 100 | 1.25 | 5 | 250 | 2.91 | 5.52 | 9.79 | 1.247 |
| 24 | 55 | 0.75 | 3 | 750 | 2.38 | 4.05 | 6.39 | 0.991 |
| 9 | 100 | 0.25 | 1 | 250 | 4.88 | 10.80 | 20.40 | 1.433 |
| 10 | 100 | 0.25 | 1 | 750 | 4.43 | 9.53 | 18.10 | 1.439 |
| 3 | 10 | 0.25 | 5 | 250 | 6.54 | 23.00 | 41.50 | 1.518 |
| 12 | 100 | 0.25 | 5 | 750 | 5.77 | 11.40 | 19.60 | 1.220 |
| 14 | 100 | 1.25 | 1 | 750 | 3.02 | 5.15 | 8.28 | 1.021 |
| 11 | 100 | 0.25 | 5 | 250 | 5.35 | 11.20 | 19.30 | 1.253 |
| 8 | 10 | 1.25 | 5 | 750 | 2.75 | 4.97 | 8.40 | 1.136 |
| 20 | 55 | 1.25 | 3 | 500 | 2.79 | 4.89 | 8.01 | 1.066 |
| 7 | 10 | 1.25 | 5 | 250 | 3.28 | 6.39 | 12.10 | 1.384 |
| 16 | 100 | 1.25 | 5 | 750 | 2.82 | 5.30 | 9.04 | 1.174 |
| 18 | 100 | 0.75 | 3 | 500 | 2.81 | 5.11 | 8.58 | 1.130 |
| 17 | 10 | 0.75 | 3 | 500 | 4.03 | 8.69 | 17.30 | 1.526 |
| 2 | 10 | 0.25 | 1 | 750 | 4.93 | 12.70 | 23.80 | 1.486 |
| 19 | 55 | 0.25 | 3 | 500 | 8.67 | 16.80 | 28.70 | 1.194 |
| 6 | 10 | 1.25 | 1 | 750 | 2.06 | 3.45 | 5.63 | 1.032 |
| Setup No. | Moment of Ultrasound Application | Sonication Pattern | Initial AS Temperature [°C] | PSD [µm] | ||
|---|---|---|---|---|---|---|
| d10 | d50 | d90 | ||||
| 1 | single-step sonoprecipitation | continuous | 11 | 2.74 ± 0.53 | 4.81 ± 1.14 | 8.21 ± 2.48 |
| 2 | single-step sonoprecipitation | continuous | 25 | 2.79 ± 0.02 | 5.09 ± 0.31 | 9.04 ± 0.88 |
| 3 n = 2 | two-step LASP+sonication | continuous | 11 | 2.54 ± 0.63 | 3.84 ± 0.63 | 5.73 ± 0.33 |
| 4 | two-step LASP+sonication | continuous | 25 | 2.10 ± 0.06 | 3.70 ± 0.10 | 6.25 ± 0.13 |
| 5 | two-step LASP+sonication | pulse 5 s + pause 5 s | 11 | 5.26 ± 0.23 | 8.58 ± 0.38 | 13.20 ± 0.70 |
| 6 | two-step LASP+sonication | pulse 5 s + pause 5 s | 25 | 2.16 ± 0.20 | 3.96 ± 0.43 | 6.80 ± 0.64 |
| Run No. (Randomized Order) | Independent Variables Settings | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rep. | CIL Conc. [mg/mL] | Amplitude [%] | Time [min] | a Energy [J] | d10 [µm] | d50 [µm] | d90 [µm] | Span | |
| 39 | 3 | 90 | 90 | 10 | 13,808 | 2.220 | 3.950 | 6.60 | 1.11 |
| 17 | 2 | 20 | 30 | 10 | 2994 | 2.070 | 3.790 | 6.67 | 1.21 |
| 5 | 1 | 90 | 30 | 10 | 2995 | 2.520 | 4.550 | 7.54 | 1.12 |
| 26 | 2 | 100 | 60 | 25 | 19,394 | 2.350 | 4.210 | 6.96 | 1.09 |
| 33 | 3 | 20 | 30 | 10 | 2981 | 2.330 | 4.480 | 7.98 | 1.26 |
| 21 | 2 | 90 | 30 | 10 | 2994 | 2.410 | 4.230 | 6.94 | 1.07 |
| 12 | 1 | 55 | 99 | 25 | 40,472 | 1.840 | 3.170 | 5.47 | 1.15 |
| 24 | 2 | 90 | 90 | 40 | n/a | 2.120 | 3.800 | 6.48 | 1.15 |
| 14 | 1 | 55 | 60 | 44 | 31,862 | 1.940 | 3.370 | 5.74 | 1.13 |
| 7 | 1 | 90 | 90 | 10 | 11,802 | 2.460 | 4.750 | 8.12 | 1.19 |
| 3 | 1 | 20 | 90 | 10 | 13,254 | 1.830 | 3.180 | 5.49 | 1.15 |
| 28 | 2 | 55 | 99 | 25 | 39,596 | 1.830 | 3.180 | 5.49 | 1.51 |
| 15 (C) | 1 | 55 | 60 | 25 | 19,497 | 2.050 | 3.630 | 6.20 | 1.14 |
| 47 (C) | 3 | 55 | 60 | 25 | 19,494 | 2.080 | 3.650 | 6.15 | 1.12 |
| 30 | 2 | 55 | 60 | 44 | 33,469 | 1.970 | 3.530 | 6.10 | 1.17 |
| 27 | 2 | 55 | 21 | 25 | 4504 | 2.490 | 4.570 | 7.65 | 1.13 |
| * 20 | 2 | 20 | 90 | 40 | 54,540 | * 0.032 | * 0.306 | * 4.43 | 14.36 |
| 29 | 2 | 55 | 60 | 6 | 4318 | 2.220 | 3.850 | 6.33 | 1.10 |
| * 36 | 3 | 20 | 90 | 40 | 54,438 | * 0.031 | * 0.314 | * 3.91 | 12.37 |
| 45 | 3 | 55 | 60 | 6 | 4312 | 2.200 | 3.830 | 6.32 | 1.08 |
| 31 (C) | 2 | 55 | 60 | 25 | 19,495 | 2.160 | 3.820 | 6.39 | 1.11 |
| * 41 | 3 | 10 | 60 | 25 | 17,993 | * 0.029 | * 0.233 | * 4.09 | 17.45 |
| 34 | 3 | 20 | 30 | 40 | 12,009 | 1.970 | 3.440 | 5.90 | 1.14 |
| 48 (C) | 3 | 55 | 60 | 25 | 18,002 | 2.030 | 3.700 | 6.44 | 1.19 |
| 37 | 3 | 90 | 30 | 10 | 2995 | 2.640 | 4.820 | 8.18 | 1.15 |
| * 9 | 1 | 10 | 60 | 25 | 18,008 | * 0.029 | * 0.245 | * 4.62 | 18.81 |
| 18 | 2 | 20 | 30 | 40 | 12,007 | 2.280 | 4.090 | 6.81 | 1.11 |
| 32 (C) | 2 | 55 | 60 | 25 | 18,005 | 2.170 | 3.800 | 6.32 | 1.09 |
| 43 | 3 | 55 | 21 | 25 | 4503 | 2.120 | 3.630 | 6.00 | 1.07 |
| * 25 | 2 | 10 | 60 | 25 | 18,010 | * 0.028 | * 0.206 | * 4.26 | 20.56 |
| 16 (C) | 1 | 55 | 60 | 25 | 17,998 | 2.190 | 3.870 | 6.43 | 1.10 |
| 38 | 3 | 90 | 30 | 40 | 12,006 | 2.200 | 3.950 | 6.66 | 1.13 |
| 22 | 2 | 90 | 30 | 40 | 11,997 | 2.290 | 4.150 | 7.01 | 1.14 |
| 40 | 3 | 90 | 90 | 40 | 54,223 | 2.150 | 3.790 | 6.39 | 1.12 |
| 13 | 1 | 55 | 60 | 6 | 4317 | 2.240 | 3.970 | 6.60 | 1.10 |
| 11 | 1 | 55 | 21 | 25 | 4487 | 2.440 | 4.330 | 7.12 | 1.08 |
| 42 | 3 | 100 | 60 | 25 | 18,014 | 2.380 | 4.120 | 6.61 | 1.03 |
| 23 | 2 | 90 | 90 | 10 | 13,215 | 2.170 | 4.050 | 7.10 | 1.22 |
| 2 | 1 | 20 | 30 | 40 | 11,998 | 1.800 | 3.550 | 6.56 | 1.34 |
| 10 | 1 | 100 | 60 | 25 | 18,002 | 2.780 | 5.260 | 8.89 | 1.16 |
| 8 | 1 | 90 | 90 | 40 | 54,578 | 2.070 | 3.610 | 6.08 | 1.11 |
| 19 | 2 | 20 | 90 | 10 | 13,546 | 1.900 | 3.250 | 5.52 | 1.11 |
| 46 | 3 | 55 | 60 | 44 | 31,717 | 1.950 | 3.450 | 5.96 | 1.16 |
| 35 | 3 | 20 | 90 | 10 | 13,216 | 2.090 | 3.490 | 5.67 | 1.03 |
| 1 | 1 | 20 | 30 | 10 | 2999 | 2.090 | 3.620 | 6.07 | 1.10 |
| * 4 | 1 | 20 | 90 | 40 | 53,803 | * 0.033 | * 0.385 | * 4.21 | 10.87 |
| 44 | 3 | 55 | 99 | 25 | 39,061 | 2.040 | 3.620 | 6.23 | 1.16 |
| 6 | 1 | 90 | 30 | 40 | 12,007 | 2.250 | 3.930 | 6.44 | 1.07 |
| Setup No. | CIL Concentration [mg/mL] | Moment of Ultrasound Application | Initial AS Temperature [°C] | PSD [µm] | ||
|---|---|---|---|---|---|---|
| d10 | d50 | d90 | ||||
| 1 | 10 | single-step sonoprecipitation | 25 | 0.030 ± 0.004 | 0.191 ± 0.05 | 3.49 ± 0.21 |
| 2 | 10 | single-step sonoprecipitation | 11 | 0.030 ± 0.004 | 0.181 ± 0.05 | 3.48 ± 0.39 |
| 3 | 10 | two-step LASP+sonication | 11 | 0.028 ± 0.01 | 0.162 ± 0.02 | 3.09 ± 0.56 |
| 4 | 10 | two-step LASP+sonication | 25 | 0.045 ± 0.05 | 0.582 ± 0.15 | 3.71 ± 0.29 |
| 5 | 20 | single-step sonoprecipitation | 11 | 1.28 ± 1.06 | 2.85 ± 0.48 | 5.04 ± 0.16 |
| 6 | 20 | two-step LASP+sonication | 11 | 0.74 ± 1.12 | 2.82 ± 0.46 | 5.16 ± 0.32 |
| Independent Variable | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| CIL concentration in solvent phase [mg/mL] | 10 | 55 | 100 |
| PVA/CIL ratio [w/w] | 0.25 | 0.75 | 1.25 |
| Solvent phase flow rate [mL/min] | 1 | 3 | 5 |
| Mixing speed [rpm] | 250 | 500 | 750 |
| Independent Variable | Level | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| CIL concentration in S phase [mg/mL] | 10 | 20 | 55 | 90 | 100 |
| Ultrasound amplitude [%] | 21 | 30 | 60 | 90 | 99 |
| Sonication time [min] | 6 | 10 | 25 | 40 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubowska, E.; Milanowski, B.; Lulek, J. A Systematic Approach to the Development of Cilostazol Nanosuspension by Liquid Antisolvent Precipitation (LASP) and Its Combination with Ultrasound. Int. J. Mol. Sci. 2021, 22, 12406. https://doi.org/10.3390/ijms222212406
Jakubowska E, Milanowski B, Lulek J. A Systematic Approach to the Development of Cilostazol Nanosuspension by Liquid Antisolvent Precipitation (LASP) and Its Combination with Ultrasound. International Journal of Molecular Sciences. 2021; 22(22):12406. https://doi.org/10.3390/ijms222212406
Chicago/Turabian StyleJakubowska, Emilia, Bartłomiej Milanowski, and Janina Lulek. 2021. "A Systematic Approach to the Development of Cilostazol Nanosuspension by Liquid Antisolvent Precipitation (LASP) and Its Combination with Ultrasound" International Journal of Molecular Sciences 22, no. 22: 12406. https://doi.org/10.3390/ijms222212406
APA StyleJakubowska, E., Milanowski, B., & Lulek, J. (2021). A Systematic Approach to the Development of Cilostazol Nanosuspension by Liquid Antisolvent Precipitation (LASP) and Its Combination with Ultrasound. International Journal of Molecular Sciences, 22(22), 12406. https://doi.org/10.3390/ijms222212406







