Connections between Immune-Derived Mediators and Sensory Nerves for Itch Sensation
Abstract
1. Introduction
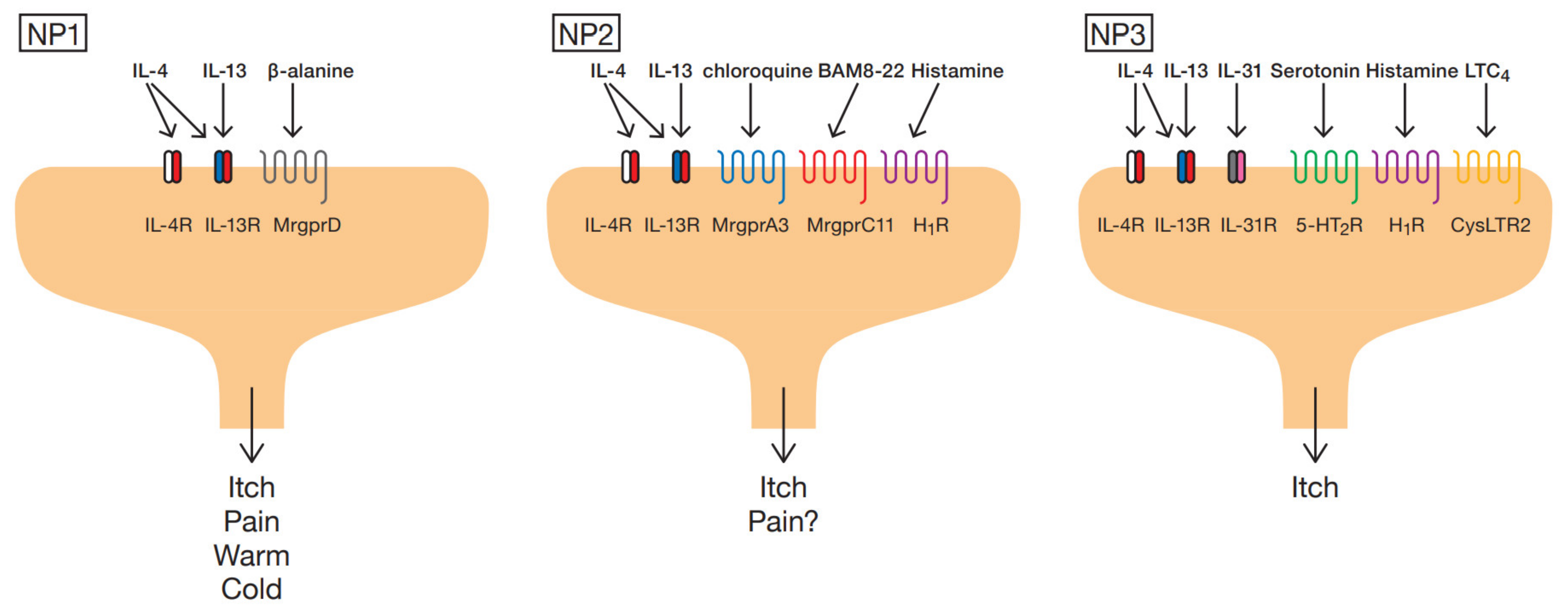
2. Subtype of Sensory Neurons
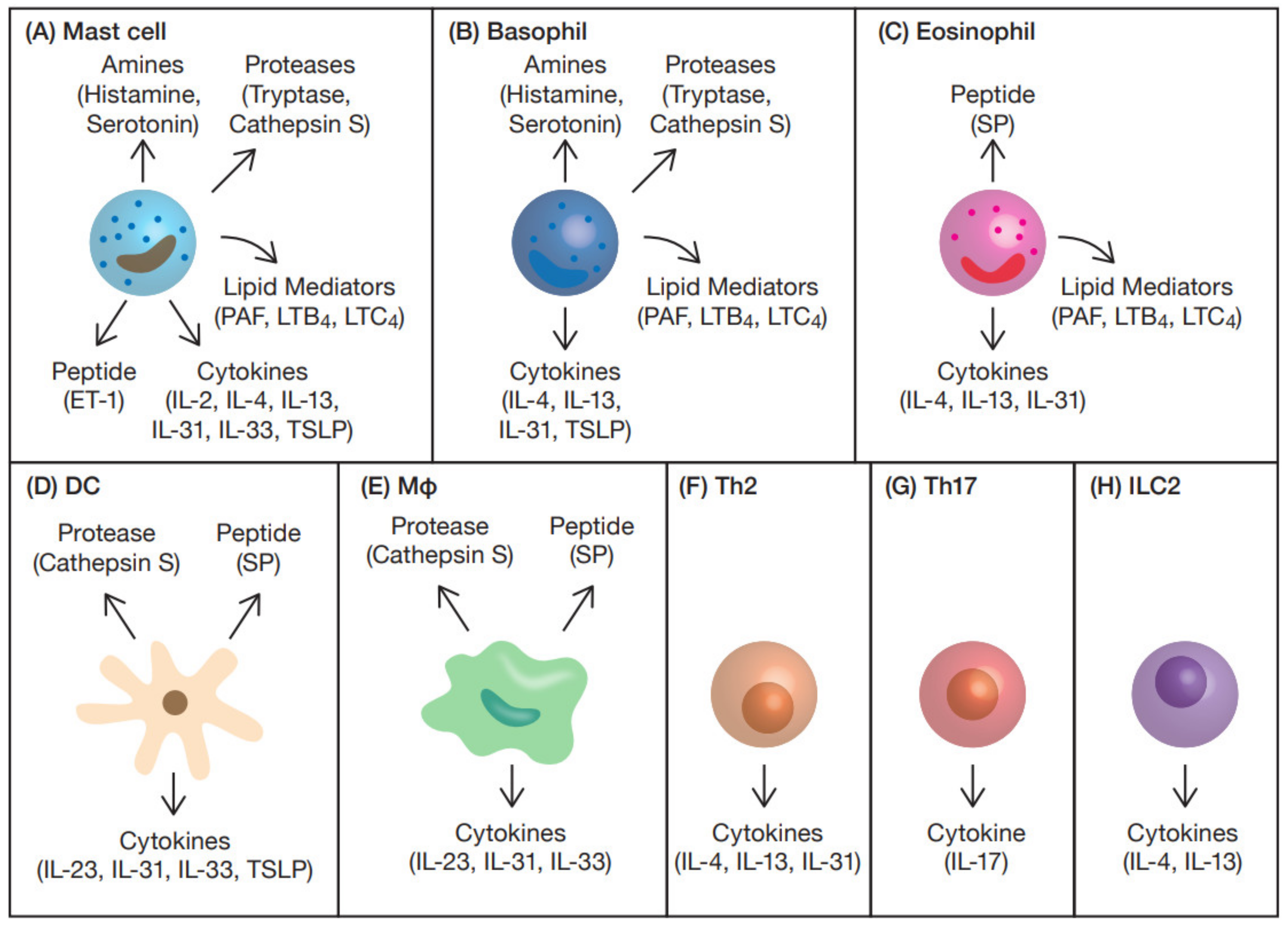
3. Itch Mediators and Modulators from Immune Cells
3.1. Amines
3.1.1. Histamine
3.1.2. Serotonin
3.2. Proteases
3.2.1. Tryptase
3.2.2. Chymase
3.2.3. Cathepsin S
3.3. Peptides
3.3.1. Substance P
3.3.2. Endothelin-1
3.4. Cytokines
3.4.1. IL-2
3.4.2. IL-4
3.4.3. IL-13
3.4.4. IL-17
3.4.5. IL-23
3.4.6. IL-31
3.5. Lipid Mediators
3.5.1. PAF
3.5.2. LTB4
3.5.3. LTC4
3.6. Others
3.6.1. IL-33
3.6.2. TSLP
4. Immune System-Targeted Antipruritic Drugs
4.1. Therapeutic Drugs for Amines
4.2. Therapeutic Drugs for Proteases
4.3. Therapeutic Drugs for Peptides
4.4. Therapeutic Drugs for Cytokines
4.5. Therapeutic Drugs for Lipid Mediators
| Category | Pruritogens | Receptors | Therapeutic Methods | Reference |
|---|---|---|---|---|
| Amines | Histamine | H1R/H4R | Anti-histamine/Anti-inflammatory, immuno-modulatory topical and systemic therapy (Cyclosporine A, Pimecrolimus, Tacrolimus and Corticosteroids) | [6,28] |
| Serotonin | 5-HT2 receptor | Sertraline | [41] | |
| Proteases | Tryptase | PAR-2 | Anti-histamine/Cyclosporine A/Pimecrolimus/Tacrolimus/Corticosteroids | [6] |
| Chymase | PAR-2 | ONO-WH-236/Anti-histamine/Cyclosporine A/Pimecrolimus/Tacrolimus/Corticosteroids | [6,63] | |
| Cathepsin S | PAR-2/PAR-4 | LHVS/Anti-histamine/Cyclosporine A/Pimecrolimus/Tacrolimus/Corticosteroids | [6,70] | |
| Peptides | Substance P | NK-1R | Serlopitant/Gabapentin/Pregabalin/Capsaicin | [6,151] |
| Endothelin-1 | ETA | Bosentan | [82] | |
| cytokines | IL-2 | IL-2R | Cyclosporine A/Delgocitinib/Baricitinib/Abrocitinib | [28,86,87,89,153] |
| IL-4 | IL-4Rα/γC | Dupilumab/Delgocitinib/Baricitinib/Abrocitinib | [28,93,99,152,153] | |
| IL-4Rα/IL-13Rα1 | ||||
| IL-13 | IL-4Rα/IL-13Rα1 | Dupilumab/Tralokinumab/Lebrikizumab | [28,93,99] | |
| IL-17 | IL-17RA/IL-17RC | Brodalumab | [103] | |
| IL-23 | IL-12Rβ1/IL-23R | Delgocitinib/Baricitinib | [28,105,106,153] | |
| IL-31 | IL-31RA/OSMR | Nemolizumab/Delgocitinib/Baricitinib/Abrocitinib | [28,111,112,113,114,153,154] | |
| IL-33 | ST2/IL-1RAcP | Etokimab/Delgocitinib/Baricitinib | [28,140,153] | |
| TSLP | TSLPR | Tezepelumab/Delgocitinib/Baricitinib/Abrocitinib | [28,149,150,153,155] | |
| Lipid mediators | PAF | PAFR | PAF antagonist | [118,156] |
| LTB4 | BLT1/BLT2 | CMHVA | [128,130] | |
| LTC4 | CysLTR1/CysLTR2 | CysLTR2 antagonist | [157] |
| Ligands | Receptors | Source | Modulation |
|---|---|---|---|
| SLIGRL-NH2 | PAR-2 | mast cells, basophils | Enhances CQ and BAM8-22 induced itch |
| IL-4 | IL-4Rα/γC IL-4Rα/IL-13Rα1 | Th2, Tfh, ILC2, mast cells, basophils, eosinophils | Enhanced neuronal responsiveness to histamine, CQ, TSLP and IL-31 |
| IL-13 | IL-13Rα1/IL-13Rα2 | Th2, ILC2, mast cells, basophils, eosinophils | May enhance neuronal responsiveness to histamine, CQ, TSLP and IL-31, as well as IL-4 |
| IL-23 | IL-12Rβ1/IL-23R | DCs, macrophages | Reduced histamine-induced itch |
| IL-33 | ST2/IL-1RAcP | DCs, macrophages, mast cells | Enhanced CQ evoked calcium responses |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ikoma, A. Updated neurophysiology of itch. Biol. Pharm. Bull. 2013, 36, 1235–1240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mettang, T.; Kremer, A.E. Uremic pruritus. Kidney Int. 2015, 87, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Dull, M.M.; Kremer, A.E. Treatment of Pruritus Secondary to Liver Disease. Curr. Gastroenterol. Rep. 2019, 21, 48. [Google Scholar] [CrossRef]
- Iwamoto, S.; Tominaga, M.; Kamata, Y.; Kawakami, T.; Osada, T.; Takamori, K. Association Between Inflammatory Bowel Disease and Pruritus. Crohns Colitis 360 2020, 2, otaa012. [Google Scholar] [CrossRef]
- Greaves, M.W. Itch in systemic disease: Therapeutic options. Dermatol. Ther. 2005, 18, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, A.; Steinhoff, M.; Stander, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef]
- Paus, R.; Schmelz, M.; Biro, T.; Steinhoff, M. Frontiers in pruritus research: Scratching the brain for more effective itch therapy. J. Clin. Investig. 2006, 116, 1174–1186. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lonnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Liu, Q.; Sikand, P.; Ma, C.; Tang, Z.; Han, L.; Li, Z.; Sun, S.; LaMotte, R.H.; Dong, X. Mechanisms of itch evoked by beta-alanine. J. Neurosci. 2012, 32, 14532–14537. [Google Scholar] [CrossRef]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin. Immunopathol. 2018, 40, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Voisin, T.; Perner, C.; Messou, M.A.; Shiers, S.; Ualiyeva, S.; Kanaoka, Y.; Price, T.J.; Sokol, C.L.; Bankova, L.G.; Austen, K.F.; et al. The CysLT2R receptor mediates leukotriene C4-driven acute and chronic itch. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef] [PubMed]
- MacGlashan, D. Histamine. J. Allergy Clin. Immunol. 2003, 112, S53–S59. [Google Scholar] [CrossRef]
- Ringvall, M.; Ronnberg, E.; Wernersson, S.; Duelli, A.; Henningsson, F.; Abrink, M.; Garcia-Faroldi, G.; Fajardo, I.; Pejler, G. Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J. Allergy Clin. Immunol. 2008, 121, 1020–1026. [Google Scholar] [CrossRef]
- Shimizu, K.; Andoh, T.; Yoshihisa, Y.; Shimizu, T. Histamine released from epidermal keratinocytes plays a role in alpha-melanocyte-stimulating hormone-induced itching in mice. Am. J. Pathol. 2015, 185, 3003–3010. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Kabashima, K.; Nakashima, C.; Nonomura, Y.; Otsuka, A.; Cardamone, C.; Parente, R.; De Feo, G.; Triggiani, M. Biomarkers for evaluation of mast cell and basophil activation. Immunol. Rev. 2018, 282, 114–120. [Google Scholar] [CrossRef]
- Hashimoto, T.; Rosen, J.D.; Sanders, K.M.; Yosipovitch, G. Possible roles of basophils in chronic itch. Exp. Dermatol. 2019, 28, 1373–1379. [Google Scholar] [CrossRef]
- Nakashima, C.; Ishida, Y.; Kitoh, A.; Otsuka, A.; Kabashima, K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp. Dermatol. 2019, 28, 1405–1411. [Google Scholar] [CrossRef]
- Moriguchi, T.; Takai, J. Histamine and histidine decarboxylase: Immunomodulatory functions and regulatory mechanisms. Genes Cells 2020, 25, 443–449. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, E. Neural processing of itch. Neuroscience 2013, 250, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Fukui, H.; Sugama, K.; Horio, Y.; Ito, S.; Mizuguchi, H.; Wada, H. Expression cloning of a cDNA encoding the bovine histamine H1 receptor. Proc. Natl. Acad. Sci. USA 1991, 88, 11515–11519. [Google Scholar] [CrossRef]
- Oda, T.; Morikawa, N.; Saito, Y.; Masuho, Y.; Matsumoto, S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J. Biol. Chem. 2000, 275, 36781–36786. [Google Scholar] [CrossRef] [PubMed]
- Hough, L.B. Genomics meets histamine receptors: New subtypes, new receptors. Mol. Pharmacol. 2001, 59, 415–419. [Google Scholar] [CrossRef]
- Ohsawa, Y.; Hirasawa, N. The role of histamine H1 and H4 receptors in atopic dermatitis: From basic research to clinical study. Allergol. Int. 2014, 63, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.S.; Tak, M.H.; Lee, M.H.; Kim, M.; Kim, M.; Koo, J.Y.; Lee, C.H.; Kim, M.; Oh, U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 2007, 27, 2331–2337. [Google Scholar] [CrossRef]
- Iannone, M.; Tonini, G.; Janowska, A.; Dini, V.; Romanelli, M. Definition of treatment goals in terms of clinician-reported disease severity and patient-reported outcomes in moderate-to-severe adult atopic dermatitis: A systematic review. Curr. Med. Res. Opin. 2021, 37, 1295–1301. [Google Scholar] [CrossRef]
- Rossbach, K.; Nassenstein, C.; Gschwandtner, M.; Schnell, D.; Sander, K.; Seifert, R.; Stark, H.; Kietzmann, M.; Baumer, W. Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience 2011, 190, 89–102. [Google Scholar] [CrossRef]
- Sommer, C. Serotonin in pain and analgesia: Actions in the periphery. Mol. Neurobiol. 2004, 30, 117–125. [Google Scholar] [CrossRef]
- Conti, P.; Shaik-Dasthagirisaheb, Y.B. Mast Cell Serotonin Immunoregulatory Effects Impacting on Neuronal Function: Implications for Neurodegenerative and Psychiatric Disorders. Neurotox. Res. 2015, 28, 147–153. [Google Scholar] [CrossRef]
- Domocos, D.; Selescu, T.; Ceafalan, L.C.; Iodi Carstens, M.; Carstens, E.; Babes, A. Role of 5-HT1A and 5-HT3 receptors in serotonergic activation of sensory neurons in relation to itch and pain behavior in the rat. J. Neurosci. Res. 2020, 98, 1999–2017. [Google Scholar] [CrossRef]
- Akiyama, T.; Ivanov, M.; Nagamine, M.; Davoodi, A.; Carstens, M.I.; Ikoma, A.; Cevikbas, F.; Kempkes, C.; Buddenkotte, J.; Steinhoff, M.; et al. Involvement of TRPV4 in Serotonin-Evoked Scratching. J. Investig. Dermatol. 2016, 136, 154–160. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nagasawa, T.; Satoh, M.; Kuraishi, Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci. Res. 1999, 35, 77–83. [Google Scholar] [CrossRef]
- Thomsen, J.S.; Petersen, M.B.; Benfeldt, E.; Jensen, S.B.; Serup, J. Scratch induction in the rat by intradermal serotonin: A model for pruritus. Acta Derm. Venereol. 2001, 81, 250–254. [Google Scholar] [CrossRef]
- Jinks, S.L.; Carstens, E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: Comparison with scratching behavior. J. Neurophysiol. 2002, 87, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Nojima, H.; Carstens, E. 5-Hydroxytryptamine (5-HT)2 receptor involvement in acute 5-HT-evoked scratching but not in allergic pruritus induced by dinitrofluorobenzene in rats. J. Pharmacol. Exp. Ther. 2003, 306, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.P.; Guan, B.C.; Ru, L.Q.; Chen, J.G.; Li, Z.W. Potentiation of 5-HT3 receptor function by the activation of coexistent 5-HT2 receptors in trigeminal ganglion neurons of rats. Neuropharmacology 2004, 47, 833–840. [Google Scholar] [CrossRef]
- Machida, T.; Iizuka, K.; Hirafuji, M. Recent Advances in 5-Hydroxytryptamine (5-HT) Receptor Research: How Many Pathophysiological Roles Does 5-HT Play via Its Multiple Receptor Subtypes? Biol. Pharm. Bull 2013, 36, 1416–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cortes-Altamirano, J.L.; Olmos-Hernandez, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodriguez, A. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 Receptors and their Role in the Modulation of Pain Response in the Central Nervous System. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef]
- Bolier, A.R.; Peri, S.; Oude Elferink, R.P.; Beuers, U. The challenge of cholestatic pruritus. Acta Gastroenterol. Belg. 2012, 75, 399–404. [Google Scholar]
- Caughey, G.H.; Raymond, W.W.; Blount, J.L.; Hau, L.W.; Pallaoro, M.; Wolters, P.J.; Verghese, G.M. Characterization of human gamma-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J. Immunol. 2000, 164, 6566–6575. [Google Scholar] [CrossRef]
- Wong, G.W.; Yasuda, S.; Madhusudhan, M.S.; Li, L.; Yang, Y.; Krilis, S.A.; Sali, A.; Stevens, R.L. Human tryptase epsilon (PRSS22), a new member of the chromosome 16p13.3 family of human serine proteases expressed in airway epithelial cells. J. Biol. Chem. 2001, 276, 49169–49182. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Tryptase genetics and anaphylaxis. J. Allergy Clin. Immunol. 2006, 117, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, L.; Sanz, C.; Garcia-Solaesa, V.; Padron, J.; Garcia-Sanchez, A.; Davila, I.; Isidoro-Garcia, M.; Lorente, F. Tryptase: Genetic and functional considerations. Allergol. Immunopathol. 2012, 40, 385–389. [Google Scholar] [CrossRef]
- Caughey, G.H. The structure and airway biology of mast cell proteinases. Am. J. Respir. Cell. Mol. Biol. 1991, 4, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.A. Biologic effects of mast cell enzymes. Am. Rev. Respir. Dis. 1992, 145, S37–S41. [Google Scholar] [CrossRef]
- Xia, H.Z.; Kepley, C.L.; Sakai, K.; Chelliah, J.; Irani, A.M.; Schwartz, L.B. Quantitation of Tryptase, Chymase, Fc~Rlcu, and FcεRlγ mRNAs in Human Mast Cells and Basophils by Competitive Reverse Transcription-Polymerase Chain Reaction. J. Immunol. 1995, 154, 5472–5480. [Google Scholar] [PubMed]
- Jogie-Brahim, S.; Min, H.K.; Fukuoka, Y.; Xia, H.Z.; Schwartz, L.B. Expression of alpha-tryptase and beta-tryptase by human basophils. J. Allergy Clin. Immunol. 2004, 113, 1086–1092. [Google Scholar] [CrossRef]
- Ui, H.; Andoh, T.; Lee, J.B.; Nojima, H.; Kuraishi, Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur. J. Pharmacol. 2006, 530, 172–178. [Google Scholar] [CrossRef]
- Lee, S.E.; Jeong, S.K.; Lee, S.H. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med. J. 2010, 51, 808–822. [Google Scholar] [CrossRef]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Carstens, M.I.; Carstens, E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J. Neurophysiol. 2011, 106, 1078–1088. [Google Scholar] [CrossRef]
- Gupta, K.; Harvima, I.T. Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef]
- Thapaliya, M.; Chompunud Na Ayudhya, C.; Amponnawarat, A.; Roy, S.; Ali, H. Mast Cell-Specific MRGPRX2: A Key Modulator of Neuro-Immune Interaction in Allergic Diseases. Curr. Allergy Asthma. Rep. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Carstens, M.I.; Ikoma, A.; Cevikbas, F.; Steinhoff, M.; Carstens, E. Mouse model of touch-evoked itch (alloknesis). J. Investig. Dermatol. 2012, 132, 1886–1891. [Google Scholar] [CrossRef]
- Gallwitz, M.; Enoksson, M.; Hellman, L. Expression profile of novel members of the rat mast cell protease (rMCP)-2 and (rMCP)-8 families, and functional analyses of mouse mast cell protease (mMCP)-8. Immunogenetics 2007, 59, 391–405. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem. Cell Biol. 2019, 152, 253–269. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell proteases as protective and inflammatory mediators. Adv. Exp. Med. Biol. 2011, 716, 212–234. [Google Scholar] [CrossRef] [PubMed]
- Wasse, H.; Naqvi, N.; Husain, A. Impact of Mast Cell Chymase on Renal Disease Progression. Curr. Hypertens. Rev. 2012, 8, 15–23. [Google Scholar] [CrossRef]
- De Souza Junior, D.A.; Santana, A.C.; da Silva, E.Z.; Oliver, C.; Jamur, M.C. The Role of Mast Cell Specific Chymases and Tryptases in Tumor Angiogenesis. Biomed. Res. Int. 2015, 2015, 142359. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Prasad, V.; McCarthy, E.T.; Savin, V.J.; Dileepan, K.N.; Stechschulte, D.J.; Lianos, E.; Wiegmann, T.; Sharma, M. Chymase increases glomerular albumin permeability via protease-activated receptor-2. Mol. Cell. Biochem. 2007, 297, 161–169. [Google Scholar] [CrossRef]
- Nabe, T.; Kijitani, Y.; Kitagawa, Y.; Sakano, E.; Ueno, T.; Fujii, M.; Nakao, S.; Sakai, M.; Takai, S. Involvement of chymase in allergic conjunctivitis of guinea pigs. Exp. Eye Res. 2013, 113, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Imada, T.; Komorita, N.; Kobayashi, F.; Naito, K.; Yoshikawa, T.; Miyazaki, M.; Nakamura, N.; Kondo, T. Therapeutic potential of a specific chymase inhibitor in atopic dermatitis. Jpn. J. Pharmacol. 2002, 90, 214–217. [Google Scholar] [CrossRef][Green Version]
- Schwarz, G.; Boehncke, W.H.; Braun, M.; Schroter, C.J.; Burster, T.; Flad, T.; Dressel, D.; Weber, E.; Schmid, H.; Kalbacher, H. Cathepsin S activity is detectable in human keratinocytes and is selectively upregulated upon stimulation with interferon-gamma. J. Investig. Dermatol. 2002, 119, 44–49. [Google Scholar] [CrossRef]
- Viode, C.; Lejeune, O.; Turlier, V.; Rouquier, A.; Casas, C.; Mengeaud, V.; Redoules, D.; Schmitt, A.M. Cathepsin S, a new pruritus biomarker in clinical dandruff/seborrhoeic dermatitis evaluation. Exp. Dermatol. 2014, 23, 274–275. [Google Scholar] [CrossRef]
- Reddy, V.B.; Shimada, S.G.; Sikand, P.; Lamotte, R.H.; Lerner, E.A. Cathepsin S elicits itch and signals via protease-activated receptors. J. Investig. Dermatol. 2010, 130, 1468–1470. [Google Scholar] [CrossRef]
- Reddy, V.B.; Sun, S.; Azimi, E.; Elmariah, S.B.; Dong, X.; Lerner, E.A. Redefining the concept of protease-activated receptors: Cathepsin S evokes itch via activation of Mrgprs. Nat. Commun. 2015, 6, 7864. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Pitcher, T.; Grant, A.D.; Hewitt, E.; Lindstrom, E.; Malcangio, M. Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour. Neurobiol. Pain 2019, 6, 100032. [Google Scholar] [CrossRef]
- Patricio, E.S.; Costa, R.; Figueiredo, C.P.; Gers-Barlag, K.; Bicca, M.A.; Manjavachi, M.N.; Segat, G.C.; Gentry, C.; Luiz, A.P.; Fernandes, E.S.; et al. Mechanisms Underlying the Scratching Behavior Induced by the Activation of Proteinase-Activated Receptor-4 in Mice. J. Investig. Dermatol. 2015, 135, 2484–2491. [Google Scholar] [CrossRef]
- Lotts, T.; Stander, S. Research in practice: Substance P antagonism in chronic pruritus. J. Dtsch. Dermatol. Ges. 2014, 12, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Stander, S.; Yosipovitch, G. Substance P and neurokinin 1 receptor are new targets for the treatment of chronic pruritus. Br. J. Dermatol. 2019, 181, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- Andoh, T.; Nagasawa, T.; Satoh, M.; Kuraishi, Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J. Pharmacol. Exp. Ther. 1998, 286, 1140–1145. [Google Scholar] [PubMed]
- McQueen, D.S.; Noble, M.A.; Bond, S.M. Endothelin-1 activates ETA receptors to cause reflex scratching in BALB/c mice. Br. J. Pharmacol. 2007, 151, 278–284. [Google Scholar] [CrossRef]
- Gomes, L.O.; Hara, D.B.; Rae, G.A. Endothelin-1 induces itch and pain in the mouse cheek model. Life Sci. 2012, 91, 628–633. [Google Scholar] [CrossRef]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Hori, S.; Aramoti, I.; Ohkubo, H.; Nakanishi, S. Cloning and expression og a cDNA encoding an endothelin receptor. Nature 1990, 348, 730–732. [Google Scholar] [CrossRef]
- Sakurai, T.; Yanagisawa, M.; Takuwa, Y.; Miyazaki, H.; Kimura, S.; Goto, K.; Masaki, T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 1990, 348, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P. International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol. Rev. 2002, 54, 219–226. [Google Scholar] [CrossRef]
- Kido-Nakahara, M.; Wang, B.; Ohno, F.; Tsuji, G.; Ulzii, D.; Takemura, M.; Furue, M.; Nakahara, T. Inhibition of mite-induced dermatitis, pruritus, and nerve sprouting in mice by the endothelin receptor antagonist bosentan. Allergy 2021, 76, 291–301. [Google Scholar] [CrossRef]
- Sim, G.C.; Radvanyi, L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef]
- Salamon, P.; Shefler, I.; Moshkovits, I.; Munitz, A.; Horwitz Klotzman, D.; Mekori, Y.A.; Hershko, A.Y. IL-33 and IgE stimulate mast cell production of IL-2 and regulatory T cell expansion in allergic dermatitis. Clin. Exp. Allergy 2017, 47, 1409–1416. [Google Scholar] [CrossRef]
- Mitra, S.; Leonard, W.J. Biology of IL-2 and its therapeutic modulation: Mechanisms and strategies. J. Leukoc. Biol. 2018, 103, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Wrangle, J.M.; Patterson, A.; Johnson, C.B.; Neitzke, D.J.; Mehrotra, S.; Denlinger, C.E.; Paulos, C.M.; Li, Z.; Cole, D.J.; Rubinstein, M.P. IL-2 and Beyond in Cancer Immunotherapy. J. Interferon Cytokine Res. 2018, 38, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, M.K.; Roozbeh, J.; Geramizadeh, B.; Namazi, M.R. Interleukin-2 serum levels are elevated in patients with uremic pruritus: A novel finding with practical implications. Nephrol. Dial. Transplant. 2011, 26, 3338–3344. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Smith, P.K.; Yosipovitch, G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin. Rev. Allergy Immunol. 2016, 51, 263–292. [Google Scholar] [CrossRef]
- Darsow, U.; Scharen, E.; Bromm, B.; Ring, J. Skin testing of the pruritogenic activity of histamine and cytoldnes (interIeukin-2 and tumour necrosis factor-a) at the dermal-epidermal junction. Br. J. Dermatol. 1997, 137, 415–417. [Google Scholar]
- Mack, M.R.; Kim, B.S. The Itch–Scratch Cycle: A Neuroimmune Perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Trier, A.M.; Mack, M.R.; Kim, B.S. The Neuroimmune Axis in Skin Sensation, Inflammation, and Immunity. J. Immunol. 2019, 202, 2829–2835. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4JAKSTAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J. Clin. Med. 2020, 9, 3741. [Google Scholar] [CrossRef] [PubMed]
- Garcovich, S.; Maurelli, M.; Gisondi, P.; Peris, K.; Yosipovitch, G.; Girolomoni, G. Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines 2021, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef] [PubMed]
- Campion, M.; Smith, L.; Gatault, S.; Metais, C.; Buddenkotte, J.; Steinhoff, M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp. Dermatol. 2019, 28, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Ichimasu, N.; Chen, Y.; Kobayashi, K.; Suzuki, S.; Chikazawa, S.; Shimura, S.; Katagiri, K. Possible involvement of type 2 cytokines in alloknesis in mouse models of menopause and dry skin. Exp. Dermatol. 2021, 30, 1745–1753. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Tabata, Y.; Hershey, G.K.K. IL-13 receptor isoforms: Breaking through the complexity. Curr. Allergy Asthm. Rep. 2007, 7, 338–345. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Z.; Steinhoff, M.; Li, Y.; Buhl, T.; Fischer, M.; Chen, W.; Cheng, W.; Zhu, R.; Yan, X.; et al. Innate immune regulates cutaneous sensory IL-13 receptor alpha 2 to promote atopic dermatitis. Brain Behav. Immun. 2021, 98, 28–39. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Gordon, K.; Hsu, S.; Elewski, B.; Eichenfield, L.F.; Kircik, L.; Rastogi, S.; Pillai, R.; Israel, R. Improvement in itch and other psoriasis symptoms with brodalumab in phase 3 randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1305–1313. [Google Scholar] [CrossRef]
- Pavlenko, D.; Funahashi, H.; Sakai, K.; Hashimoto, T.; Lozada, T.; Yosipovitch, G.; Akiyama, T. IL-23 modulates histamine-evoked itch and responses of pruriceptors in mice. Exp. Dermatol. 2020, 29, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Parham, C.; Chirica, M.; Timans, J.; Vaisberg, E.; Travis, M.; Cheung, J.; Pflanz, S.; Zhang, R.; Singh, K.P.; Vega, F.; et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002, 168, 5699–5708. [Google Scholar] [CrossRef]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Kunsleben, N.; Rudrich, U.; Gehring, M.; Novak, N.; Kapp, A.; Raap, U. IL-31 Induces Chemotaxis, Calcium Mobilization, Release of Reactive Oxygen Species, and CCL26 in Eosinophils, Which Are Capable to Release IL-31. J. Investig. Dermatol. 2015, 135, 1908–1911. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kursewicz, C.D.; Fayne, R.A.; Nanda, S.; Shah, S.M.; Nattkemper, L.; Yokozeki, H.; Yosipovitch, G. Mechanisms of Itch in Stasis Dermatitis: Significant Role of IL-31 from Macrophages. J. Investig. Dermatol. 2020, 140, 850–859.e3. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zanvit, P.; Hu, L.; Tseng, P.Y.; Liu, N.; Wang, F.; Liu, O.; Zhang, D.; Jin, W.; Guo, N.; et al. The Cytokine TGF-beta Induces Interleukin-31 Expression from Dermal Dendritic Cells to Activate Sensory Neurons and Stimulate Wound Itching. Immunity 2020, 53, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Ruppenstein, A.; Limberg, M.M.; Loser, K.; Kremer, A.E.; Homey, B.; Raap, U. Involvement of Neuro-Immune Interactions in Pruritus With Special Focus on Receptor Expressions. Front. Med. 2021, 8, 627985. [Google Scholar] [CrossRef]
- Datsi, A.; Steinhoff, M.; Ahmad, F.; Alam, M.; Buddenkotte, J. Interleukin-31: The “itchy” cytokine in inflammation and therapy. Allergy 2021, 76, 2982–2997. [Google Scholar] [CrossRef]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018, 73, 29–36. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e524. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.G.; Cho, T.S.; McBride, M.L.; Feng, J.; Manivannan, B.; Madura, C.; Klein, N.E.; Wright, E.B.; Wickstead, E.S.; Garcia-Verdugo, H.D.; et al. Transmembrane protein TMEM184B is necessary for interleukin-31–induced itch. Pain 2021. publish ahead of print. [Google Scholar] [CrossRef]
- Palgan, K.; Bartuzi, Z. Platelet activating factor in allergies. Int. J. Immunopathol. Pharmacol. 2015, 28, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shields, L.B.E.; Gao, Z.; Wang, Y.; Zhang, Y.P.; Chu, T.; Zhu, Q.; Shields, C.B.; Cai, J. Current Understanding of Platelet-Activating Factor Signaling in Central Nervous System Diseases. Mol. Neurobiol. 2017, 54, 5563–5572. [Google Scholar] [CrossRef]
- Thomsen, J.S.; Sonne, M.; Benfeldt, E.; Jensen, S.B.; Serup, J.; Menne, T. Experimental itch in sodium lauryl sulphate-inflamed and normal skin in humans: A randomized, double-blind, placebo-controlled study of histamine and other inducers of itch. Br. J. Dermatol. 2002, 146, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.J.; Church, M.K.; Skov, P.S. Platelet-activating factor Induces histamine release from human skin mast cells in vivo, which is reduced by local nerve blockade. J. Allergy Clin. Immunol. 1997, 99, 640–647. [Google Scholar] [CrossRef]
- Andoh, T.; Haza, S.; Saito, A.; Kuraishi, Y. Involvement of leukotriene B4 in spontaneous itch-related behaviour in NC mice with atopic dermatitis-like skin lesions. Exp. Dermatol. 2011, 20, 894–898. [Google Scholar] [CrossRef]
- Miyahara, N.; Ohnishi, H.; Miyahara, S.; Takeda, K.; Matsubara, S.; Matsuda, H.; Okamoto, M.; Loader, J.E.; Joetham, A.; Tanimoto, M.; et al. Leukotriene B4 release from mast cells in IgE-mediated airway hyperresponsiveness and inflammation. Am. J. Respir. Cell Mol. Biol. 2009, 40, 672–682. [Google Scholar] [CrossRef]
- Bando, T.; Fujita, S.; Nagano, N.; Yoshikawa, S.; Yamanishi, Y.; Minami, M.; Karasuyama, H. Differential usage of COX-1 and COX-2 in prostaglandin production by mast cells and basophils. Biochem. Biophys. Rep. 2017, 10, 82–87. [Google Scholar] [CrossRef]
- Pal, K.; Feng, X.; Steinke, J.W.; Burdick, M.D.; Shim, Y.M.; Sung, S.S.; Teague, W.G.; Borish, L. Leukotriene A4 Hydrolase Activation and Leukotriene B4 Production by Eosinophils in Severe Asthma. Am. J. Respir. Cell Mol. Biol. 2019, 60, 413–419. [Google Scholar] [CrossRef]
- Finney-Hayward, T.K.; Bahra, P.; Li, S.; Poll, C.T.; Nicholson, A.G.; Russell, R.E.; Ford, P.A.; Westwick, J.; Fenwick, P.S.; Barnes, P.J.; et al. Leukotriene B4 release by human lung macrophages via receptor- not voltage-operated Ca2+ channels. Eur. Respir. J. 2009, 33, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, T.; Izumi, T.; Chang, K.; Takuwa, Y.; Shimizu, T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997, 387, 620–624. [Google Scholar] [CrossRef]
- Yokomizo, T.; Kato, K.; Terawaki, K.; Izumi, T.; Shimizu, T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 2000, 192, 421–432. [Google Scholar] [CrossRef]
- Andoh, T.; Kuraishi, Y. Expression of BLT1 leukotriene B4 receptor on the dorsal root ganglion neurons in mice. Mol. Brain Res. 2005, 137, 263–266. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Vong, C.T.; Quek, S.; Cheong, J.; Awal, S.; Gentry, C.; Aubdool, A.A.; Liang, L.; Bodkin, J.V.; Bevan, S.; et al. Superoxide generation and leukocyte accumulation: Key elements in the mediation of leukotriene B(4)-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J. 2013, 27, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Harada, A.; Kuraishi, Y. Involvement of Leukotriene B4 Released from Keratinocytes in Itch-associated Response to Intradermal Interleukin-31 in Mice. Acta Derm. Venereol. 2017, 97, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Matsumoto, R.; Urade, Y.; Austen, K.F.; Arm, J.P. c-kit ligand mediates increased expression of cytosolic phospholipase A2, prostaglandin endoperoxide synthase-1, and hematopoietic prostaglandin D2 synthase and increased IgE-dependent prostaglandin D2 generation in immature mouse mast cells. J. Biol. Chem. 1995, 270, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Trier, A.M.; Li, F.; Kim, S.; Chen, Z.; Chai, J.N.; Mack, M.R.; Morrison, S.A.; Hamilton, J.D.; Baek, J.; et al. A basophil-neuronal axis promotes itch. Cell 2021, 184, 422–440.e417. [Google Scholar] [CrossRef]
- Takafuji, S.; Bischoff, S.C.; De Weck, A.L.; Dahinden, C.A. IL-3 and IL-5 prime normal human eosinophils to produce leukotriene C4 in response to soluble agonists. J. Immunol. 1991, 147, 3855–3861. [Google Scholar]
- Ohno, T.; Morita, H.; Arae, K.; Matsumoto, K.; Nakae, S. Interleukin-33 in allergy. Allergy 2012, 67, 1203–1214. [Google Scholar] [CrossRef]
- Nakae, S.; Morita, H.; Ohno, T.; Arae, K.; Matsumoto, K.; Saito, H. Role of interleukin-33 in innate-type immune cells in allergy. Allergol. Int. 2013, 62, 13–20. [Google Scholar] [CrossRef]
- Takeda, T.; Unno, H.; Morita, H.; Futamura, K.; Emi-Sugie, M.; Arae, K.; Shoda, T.; Okada, N.; Igarashi, A.; Inoue, E.; et al. Platelets constitutively express IL-33 protein and modulate eosinophilic airway inflammation. J. Allergy Clin. Immunol. 2016, 138, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Toyama, S.; Moniaga, C.S.; Nakae, S.; Kurosawa, M.; Ogawa, H.; Tominaga, M.; Takamori, K. Regulatory T Cells Exhibit Interleukin-33-Dependent Migratory Behavior during Skin Barrier Disruption. Int. J. Mol. Sci. 2021, 22, 7443. [Google Scholar] [CrossRef] [PubMed]
- Trier, A.M.; Mack, M.R.; Fredman, A.; Tamari, M.; Ver Heul, A.M.; Zhao, Y.; Guo, C.J.; Avraham, O.; Ford, Z.K.; Oetjen, L.K.; et al. IL-33 Signaling in Sensory Neurons Promotes Dry Skin Itch. J. Allergy Clin. Immunol. 2021, in press. [Google Scholar] [CrossRef]
- Peng, G.; Mu, Z.; Cui, L.; Liu, P.; Wang, Y.; Wu, W.; Han, X. Anti-IL-33 Antibody Has a Therapeutic Effect in an Atopic Dermatitis Murine Model Induced by 2, 4-Dinitrochlorobenzene. Inflammation 2018, 41, 154–163. [Google Scholar] [CrossRef]
- Du, L.; Hu, X.; Yang, W.; Yasheng, H.; Liu, S.; Zhang, W.; Zhou, Y.; Cui, W.; Zhu, J.; Qiao, Z.; et al. Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia 2019, 67, 1680–1693. [Google Scholar] [CrossRef]
- Kahremany, S.; Hofmann, L.; Gruzman, A.; Cohen, G. Advances in Understanding the Initial Steps of Pruritoceptive Itch: How the Itch Hits the Switch. Int. J. Mol. Sci. 2020, 21, 4883. [Google Scholar] [CrossRef] [PubMed]
- Dewas, C.; Chen, X.; Honda, T.; Junttila, I.; Linton, J.; Udey, M.C.; Porcella, S.F.; Sturdevant, D.E.; Feigenbaum, L.; Koo, L.; et al. TSLP expression: Analysis with a ZsGreen TSLP reporter mouse. J. Immunol. 2015, 194, 1372–1380. [Google Scholar] [CrossRef]
- Reche, P.A.; Soumelis, V.; Gorman, D.M.; Clifford, T.; Liu, M.; Travis, M.; Zurawski, S.M.; Johnston, J.; Liu, Y.J.; Spits, H.; et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 2001, 167, 336–343. [Google Scholar] [CrossRef]
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.R.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007, 204, 253–258. [Google Scholar] [CrossRef]
- Hirano, R.; Hasegawa, S.; Hashimoto, K.; Haneda, Y.; Ohsaki, A.; Ichiyama, T. Human thymic stromal lymphopoietin enhances expression of CD80 in human CD14+ monocytes/macrophages. Inflamm. Res. 2011, 60, 605–610. [Google Scholar] [CrossRef]
- Cook, E.B.; Stahl, J.L.; Schwantes, E.A.; Fox, K.E.; Mathur, S.K. IL-3 and TNFalpha increase Thymic Stromal Lymphopoietin Receptor (TSLPR) expression on eosinophils and enhance TSLP-stimulated degranulation. Clin. Mol. Allergy 2012, 10, 8. [Google Scholar] [CrossRef]
- Kubo, T.; Kamekura, R.; Kumagai, A.; Kawata, K.; Yamashita, K.; Mitsuhashi, Y.; Kojima, T.; Sugimoto, K.; Yoneta, A.; Sumikawa, Y.; et al. DeltaNp63 controls a TLR3-mediated mechanism that abundantly provides thymic stromal lymphopoietin in atopic dermatitis. PLoS ONE 2014, 9, e105498. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Rochman, Y.; Kashyap, M.; Robinson, G.W.; Sakamoto, K.; Gomez-Rodriguez, J.; Wagner, K.U.; Leonard, W.J. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 19455–19460. [Google Scholar] [CrossRef]
- Arima, K.; Watanabe, N.; Hanabuchi, S.; Chang, M.; Sun, S.C.; Liu, Y.J. Distinct signal codes generate dendritic cell functional plasticity. Sci. Signal. 2010, 3, ra4. [Google Scholar] [CrossRef]
- Pariser, D.M.; Bagel, J.; Lebwohl, M.; Yosipovitch, G.; Chien, E.; Spellman, M.C. Serlopitant for psoriatic pruritus: A phase 2 randomized, double-blind, placebo-controlled clinical trial. J. Am. Acad. Dermatol. 2020, 82, 1314–1320. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Yosipovitch, G.; Simpson, E.L.; Kim, B.S.; Wu, J.J.; Eckert, L.; Guillemin, I.; Chen, Z.; Ardeleanu, M.; Bansal, A.; et al. Dupilumab treatment results in early and sustained improvements in itch in adolescents and adults with moderate to severe atopic dermatitis: Analysis of the randomized phase 3 studies SOLO 1 and SOLO 2, AD ADOL, and CHRONOS. J. Am. Acad. Dermatol. 2020, 82, 1328–1336. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M.; Nemolizumab, J.P.S.G. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect. J. Allergy Clin. Immunol. Pract. 2021, 9, 1053–1065. [Google Scholar] [CrossRef]
- Abeck, D.; Andersson, T.; Grosshans, E.; Jablonska, S.; Kragballe, K.; Vahlquist, A.; Schmidt, T.; Dupuy, P.; Ring, J. Topical application of a platelet-activating factor (PAF) antagonist in atopic dermatitis. Acta Derm. Venereol. 1997, 77, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Itadani, S.; Takahashi, S.; Ima, M.; Sekiguchi, T.; Fujita, M.; Nakayama, Y.; Takeuchi, J. Discovery of Highly Potent Dual CysLT1 and CysLT2 Antagonist. ACS Med. Chem. Lett. 2014, 5, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyama, S.; Tominaga, M.; Takamori, K. Connections between Immune-Derived Mediators and Sensory Nerves for Itch Sensation. Int. J. Mol. Sci. 2021, 22, 12365. https://doi.org/10.3390/ijms222212365
Toyama S, Tominaga M, Takamori K. Connections between Immune-Derived Mediators and Sensory Nerves for Itch Sensation. International Journal of Molecular Sciences. 2021; 22(22):12365. https://doi.org/10.3390/ijms222212365
Chicago/Turabian StyleToyama, Sumika, Mitsutoshi Tominaga, and Kenji Takamori. 2021. "Connections between Immune-Derived Mediators and Sensory Nerves for Itch Sensation" International Journal of Molecular Sciences 22, no. 22: 12365. https://doi.org/10.3390/ijms222212365
APA StyleToyama, S., Tominaga, M., & Takamori, K. (2021). Connections between Immune-Derived Mediators and Sensory Nerves for Itch Sensation. International Journal of Molecular Sciences, 22(22), 12365. https://doi.org/10.3390/ijms222212365






