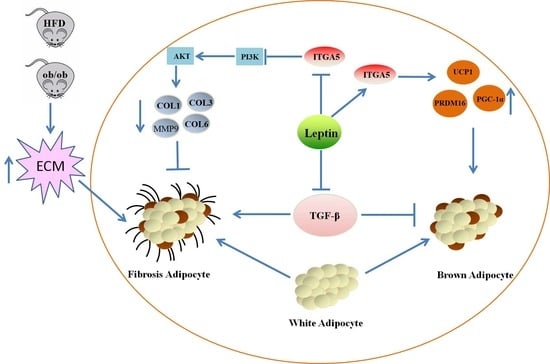
The Mechanism of Leptin on Inhibiting Fibrosis and Promoting Browning of White Fat by Reducing ITGA5 in Mice
Abstract
:1. Introduction
2. Results
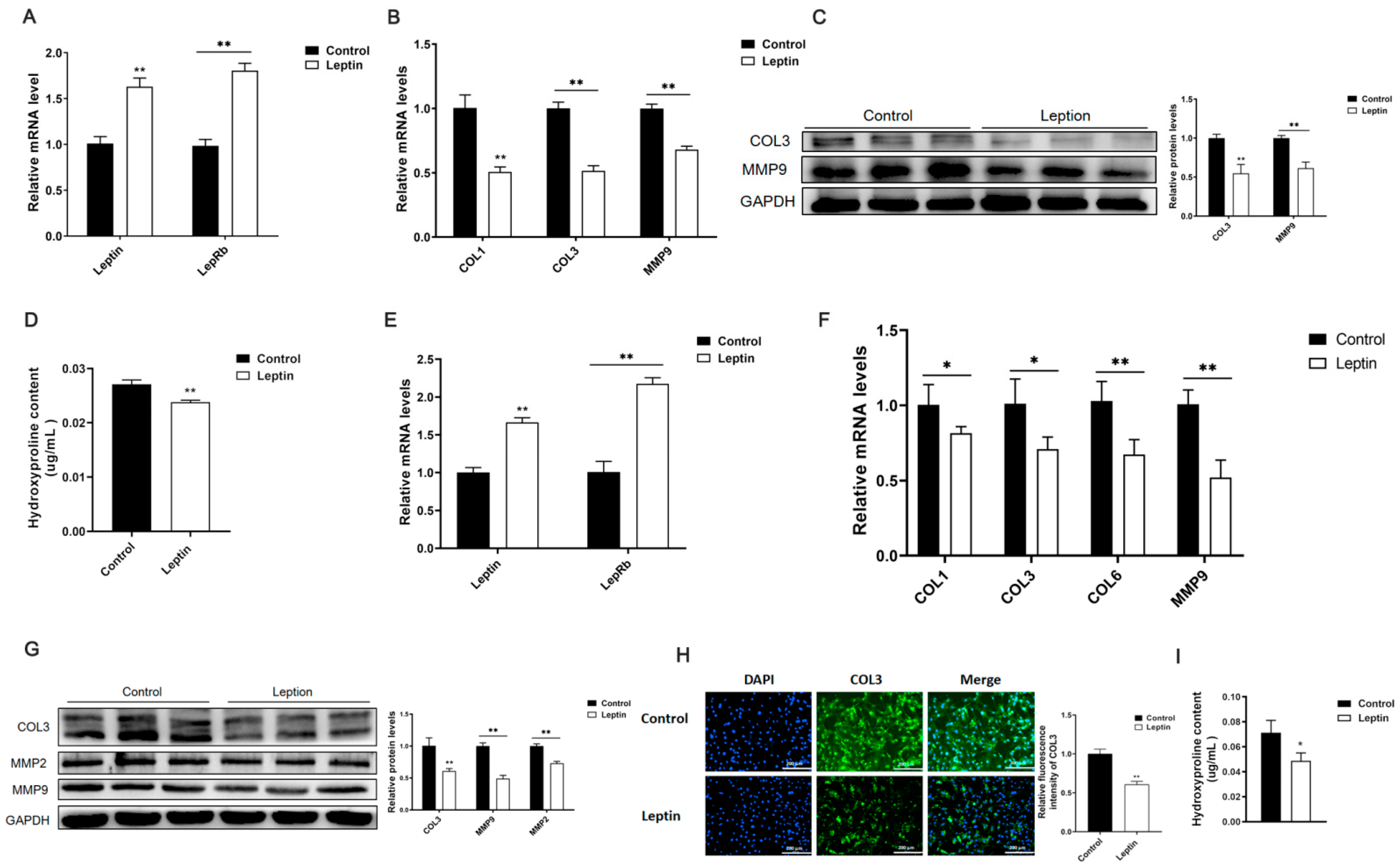
2.1. Leptin Relieves Adipose Tissue Fibrosis in Mice
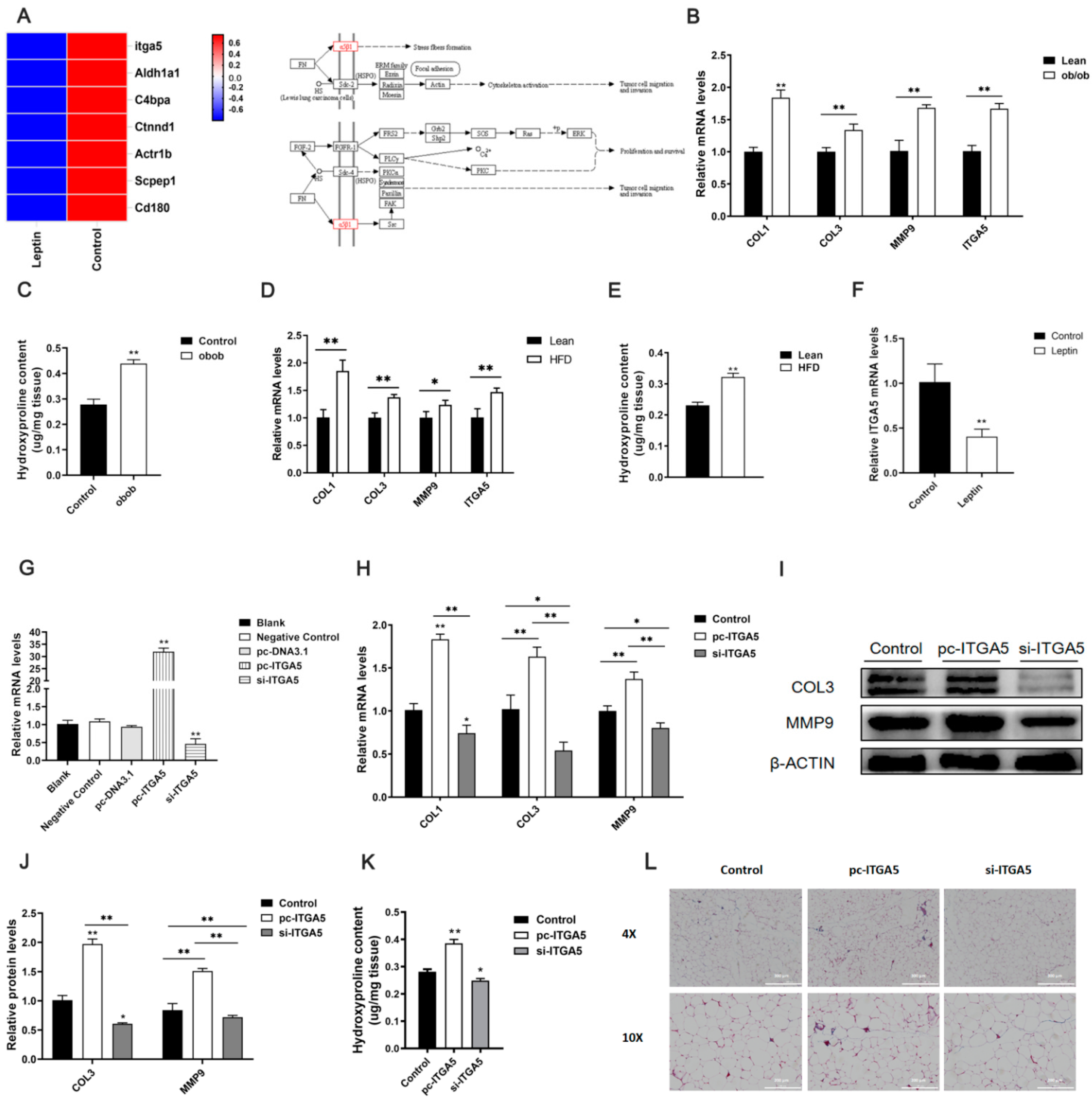
2.2. Leptin Relieves Adipose Tissue Fibrosis by Inhibiting ITGA5
2.3. ITGA5 Promotes Fibrosis in Adipocytes, Inhibition of ITGA5 Alleviates Fibrosis in Adipocytes
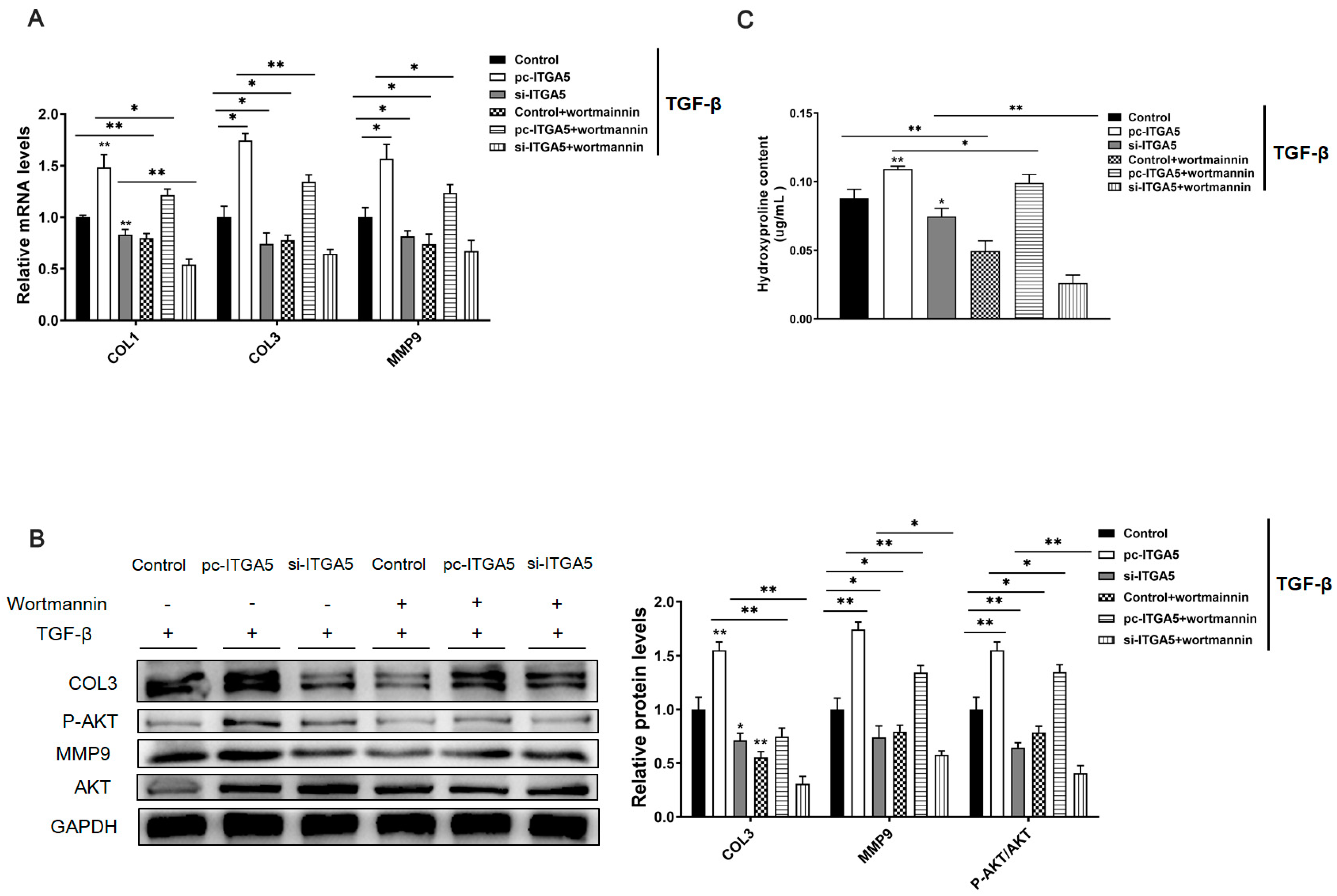
2.4. ITGA5 Promotes Fibrosis in Adipocytes by Activating the PI3K-AKT Signaling Pathway
2.5. Leptin Promotes White Fat Browning and Relieves the Inhibitory Effect of TGF-β on Adipocyte Browning
2.6. Down regulation of ITGA5 Alleviates the Inhibitory Effect of Browning Caused by Fibrosis
3. Research Highlights
- (1)
- Leptin relieves adipose fibrosis by inhibiting ITGA5;
- (2)
- ITGA5 promotes adipocyte fibrosis by activating the PI3K-AKT signaling pathway, inhibiting ITGA5 could alleviate adipocyte fibrosis;
- (3)
- Down regulation of ITGA5 alleviates the inhibitory effect of browning caused by fibrosis.
4. Discussion
5. Materials and Methods
5.1. Animal Experiment
5.2. Adipocyte Culture
5.3. Determination of Adipose Tissue Hydroxyproline
5.4. Real-Time Quantitative PCR Analysis
5.5. Western Blotting Analysis
5.6. Immunofluorescence Assay
5.7. HE Staining
5.8. Immunohistochemistry
5.9. Masson Stain
5.10. Oil Red O Staining
5.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Name |
| ITGA5 | integrin alpha 5 |
| TGF-β | transforming growth factor-β |
| PI3K | phosphatidylinositol 3 kinase |
| AKT/PKB | protein kinase B |
| UCP1 | uncoupling protein 1 |
| NPY | neuropeptide Y |
| PRDM16 | PR domain-containing 16 |
| JAK2 | janus kinase 2 |
| STAT3 | signal transducer and activator of transcription |
| Hh | hedgehog |
| ECM | extracellular matrix |
| HFD | high-fat diets |
| DMEM | Dulbecco’s Modified Eagle Medium |
| GTF2IRD1 | general transcription factor II-I repeat domain-containing protein 1 |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| PVDF | polyvinylidene fluoride |
| COL1 | collagen i |
| COL3 | collagen iii |
| COL6 | collagen vi |
| MMP9 | matrix metalloproteinase 9 |
| PGC-1α | proliferator-activated receptor-gamma coactivator-1-alpha |
| IBMX | 3-isobutyl-1-methylxanthine |
| MAPK | mitogen-activated protein kinase |
| DEX | dexamethasone |
References
- Suárez-Zamorano, N.; Fabbiano, S.; Chevalier, C.; Stojanović, O.; Colin, D.J.; Stevanović, A.; Veyrat-Durebex, C.; Tarallo, V.; Rigo, D.; Germain, S.; et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat. Med. 2015, 21, 1497–1501. [Google Scholar] [CrossRef]
- de Medeiros, S.F.; Rodgers, R.J.; Norman, R.J. Adipocyte and steroidogenic cell cross-talk in polycystic ovary syndrome. Hum. Reprod. Update 2021, 27, 771–796. [Google Scholar] [CrossRef]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, H.; Yu, H.; Gong, J.; Jiang, J.; Qiao, X.; Perkey, E.; Kim, D.I.; Emont, M.P.; Zestos, A.G.; Cho, J.S.; et al. An immune-beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat. Med. 2018, 24, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Friedman-Einat, M.; Cogburn, L.A.; Yosefi, S.; Hen, G.; Shinder, D.; Shirak, A.; Seroussi, E. Discovery and characterization of the first genuine avian leptin gene in the rock dove (Columba livia). Endocrinology 2014, 155, 3376–3384. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Shen, Y.; Hsu, C.W.; Fong, J.P.; Uang, S.N.; Chang, J.W. Reduced adiponectin:leptin ratio associated with inhalation exposure to vinyl chloride monomer. Sci. Total Environ. 2020, 703, 135488. [Google Scholar] [CrossRef]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef]
- Caron, A.; Lee, S.; Elmquist, J.K.; Gautron, L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 2018, 19, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Zhou, B.O.; Shimada, I.S.; Zhao, Z.; Morrison, S.J. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 2016, 18, 782–796. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hansen, M.J.; Jones, J.E.; Vlahos, R.; Bozinovski, S.; Anderson, G.P.; Morris, M.J. Cigarette smoke exposure reprograms the hypothalamic neuropeptide Y axis to promote weight loss. Am. J. Respir. Crit. Care Med. 2006, 173, 1248–1254. [Google Scholar] [CrossRef]
- Decara, J.; Rivera, P.; Arrabal, S.; Vargas, A.; Serrano, A.; Pavón, F.J.; Dieguez, C.; Nogueiras, R.; Rodríguez de Fonseca, F.; Suárez, J. Cooperative role of the glucagon-like peptide-1 receptor and β3-adrenergic-mediated signalling on fat mass reduction through the downregulation of PKA/AKT/AMPK signalling in the adipose tissue and muscle of rats. Acta Physiol. 2018, 222, e13008. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Feng, F.; Wu, T.; Luo, D.; Hu, C.; Sun, C. Foxc2 coordinates inflammation and browning of white adipose by leptin-STAT3-PRDM16 signal in mice. Int. J. Obes. 2018, 42, 252–259. [Google Scholar] [CrossRef]
- Wang, J.; Ge, J.; Cao, H.; Zhang, X.; Guo, Y.; Li, X.; Xia, B.; Yang, G.; Shi, X. Leptin Promotes White Adipocyte Browning by Inhibiting the Hh Signaling Pathway. Cells 2019, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Decker, M.; Martinez-Morentin, L.; Wang, G.; Lee, Y.; Liu, Q.; Leslie, J.; Ding, L. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat. Cell Biol. 2017, 19, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, P.; Li, W.; Fang, F.; Zhu, W.; Fan, J.; Wang, F.; Gao, Y.; Zhao, Q.; Wang, Q.; et al. Perturbation of Specific Signaling Pathways Is Involved in Initiation of Mouse Liver Fibrosis. Hepatology 2021, 73, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Shook, B.; Rodeheffer, M.S. Forecasting Fat Fibrosis. Cell Metab. 2017, 25, 493–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta 2014, 1842, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Becerril, S.; Rodríguez, A.; Catalán, V.; Méndez-Giménez, L.; Ramírez, B.; Sáinz, N.; Llorente, M.; Unamuno, X.; Gómez-Ambrosi, J.; Frühbeck, G. Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: Role of tenascin C. Int. J. Obes. 2018, 42, 1458–1470. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, L.J.; Rawling, T.J.; Sjöholm, K.; Franck, N.; Dankel, S.N.; Price, E.J.; Knight, B.; Liversedge, N.H.; Mellgren, G.; Nystrom, F.; et al. COL6A3 is regulated by leptin in human adipose tissue and reduced in obesity. Endocrinology 2015, 156, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Wu, R.; Zhu, W.; Jiang, Z.; Xu, Y.; Chen, H.; Zhang, Z.; Chen, H.; Zhang, L.; Yu, H.; et al. Hypoxia preconditioned mesenchymal stem cells prevent cardiac fibroblast activation and collagen production via leptin. PLoS ONE 2014, 9, e103587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elinav, E.; Ali, M.; Bruck, R.; Brazowski, E.; Phillips, A.; Shapira, Y.; Katz, M.; Solomon, G.; Halpern, Z.; Gertler, A. Competitive inhibition of leptin signaling results in amelioration of liver fibrosis through modulation of stellate cell function. Hepatology 2009, 49, 278–286. [Google Scholar] [CrossRef]
- Wang, W.; Ishibashi, J.; Trefely, S.; Shao, M.; Cowan, A.J.; Sakers, A.; Lim, H.W.; O’Connor, S.; Doan, M.T.; Cohen, P.; et al. A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate. Cell Metab. 2019, 30, 174–189 e175. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ikeda, K.; Chen, Y.; Alba, D.L.; Stifler, D.; Shinoda, K.; Hosono, T.; Maretich, P.; Yang, Y.; Ishigaki, Y.; et al. Repression of Adipose Tissue Fibrosis through a PRDM16-GTF2IRD1 Complex Improves Systemic Glucose Homeostasis. Cell Metab. 2018, 27, 180–194 e186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Wan, Q.; Yan, W. Integrin α5/ITGA5 Promotes The Proliferation, Migration, Invasion And Progression Of Oral Squamous Carcinoma By Epithelial-Mesenchymal Transition. Cancer Manag. Res. 2019, 11, 9609–9620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Zhou, T.; Chen, Z.; Yan, M.; Li, B.; Lv, H.; Wang, C.; Xiang, S.; Shi, L.; Zhu, Y.; et al. Coupling of Integrin α5 to Annexin A2 by Flow Drives Endothelial Activation. Circ. Res. 2020, 127, 1074–1090. [Google Scholar] [CrossRef]
- Fromigué, O.; Brun, J.; Marty, C.; Da Nascimento, S.; Sonnet, P.; Marie, P.J. Peptide-based activation of alpha5 integrin for promoting osteogenesis. J. Cell. Biochem. 2012, 113, 3029–3038. [Google Scholar] [CrossRef]
- Xu, L.; Yin, L.; Tao, X.; Qi, Y.; Han, X.; Xu, Y.; Song, S.; Li, L.; Sun, P.; Peng, J. Dioscin, a potent ITGA5 inhibitor, reduces the synthesis of collagen against liver fibrosis: Insights from SILAC-based proteomics analysis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 107, 318–328. [Google Scholar] [CrossRef]
- Sun, Y.B.; Qu, X.; Caruana, G.; Li, J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differ. Res. Biol. Divers. 2016, 92, 102–107. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Halberg, N.; Khan, M.; Magalang, U.J.; Scherer, P.E. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 2013, 33, 904–917. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, M.A.; Del Rio Barquero, L.M.; Ortez, C.I.; Jou, C.; Vigo, M.; Medina, J.; Febrer, A.; Ramon-Krauel, M.; Diaz-Manera, J.; Olive, M.; et al. Differences in Adipose Tissue and Lean Mass Distribution in Patients with Collagen VI Related Myopathies Are Associated with Disease Severity and Physical Ability. Front. Aging Neurosci. 2017, 9, 268. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Struik, D.; Nies, V.J.; Jurdzinski, A.; Harkema, L.; de Bruin, A.; Verkade, H.J.; Downes, M.; Evans, R.M.; van Zutphen, T.; et al. Effective treatment of steatosis and steatohepatitis by fibroblast growth factor 1 in mouse models of nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2016, 113, 2288–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.Y.; Zhang, W.; Luk, C.T.; Sivasubramaniyam, T.; Brunt, J.J.; Schroer, S.A.; Desai, H.R.; Majerski, A.; Woo, M. JAK2 promotes brown adipose tissue function and is required for diet- and cold-induced thermogenesis in mice. Diabetologia 2016, 59, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Jiang, C.; Li, R. Integrin and gene network analysis reveals that ITGA5 and ITGB1 are prognostic in non-small-cell lung cancer. OncoTargets Ther. 2016, 9, 2317–2327. [Google Scholar] [CrossRef] [Green Version]
- Parray, H.A.; Yun, J.W. Cannabidiol promotes browning in 3T3-L1 adipocytes. Mol. Cell. Biochem. 2016, 416, 131–139. [Google Scholar] [CrossRef]
- Patin, E.C.; Orr, S.J.; Schaible, U.E. Macrophage Inducible C-Type Lectin As a Multifunctional Player in Immunity. Front. Immunol. 2017, 8, 861. [Google Scholar] [CrossRef] [Green Version]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.; Apovian, C. Macrophage functions in lean and obese adipose tissue. Metab. Clin. Exp. 2017, 72, 120–143. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Saxena, N.K.; Anania, F.A. Adipocytokines and hepatic fibrosis. Trends Endocrinol. Metab. TEM 2015, 26, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, G.; Ziyadeh, F.N. Leptin and renal fibrosis. Contrib. Nephrol. 2006, 151, 175–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Kotzbeck, P.; Giordano, A.; Mondini, E.; Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018, 59, 784–794. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, Y.; Liang, J.; Sun, Z.; Wu, Q.; Liu, Y.; Sun, C. The Mechanism of Leptin on Inhibiting Fibrosis and Promoting Browning of White Fat by Reducing ITGA5 in Mice. Int. J. Mol. Sci. 2021, 22, 12353. https://doi.org/10.3390/ijms222212353
Liu Y, Li Y, Liang J, Sun Z, Wu Q, Liu Y, Sun C. The Mechanism of Leptin on Inhibiting Fibrosis and Promoting Browning of White Fat by Reducing ITGA5 in Mice. International Journal of Molecular Sciences. 2021; 22(22):12353. https://doi.org/10.3390/ijms222212353
Chicago/Turabian StyleLiu, Yuexia, Yizhou Li, Juntong Liang, Zhuwen Sun, Qiong Wu, Yongnian Liu, and Chao Sun. 2021. "The Mechanism of Leptin on Inhibiting Fibrosis and Promoting Browning of White Fat by Reducing ITGA5 in Mice" International Journal of Molecular Sciences 22, no. 22: 12353. https://doi.org/10.3390/ijms222212353
APA StyleLiu, Y., Li, Y., Liang, J., Sun, Z., Wu, Q., Liu, Y., & Sun, C. (2021). The Mechanism of Leptin on Inhibiting Fibrosis and Promoting Browning of White Fat by Reducing ITGA5 in Mice. International Journal of Molecular Sciences, 22(22), 12353. https://doi.org/10.3390/ijms222212353