Molecular Evolution of Calcium Signaling and Transport in Plant Adaptation to Abiotic Stress
Abstract
1. Introduction
2. Calcium Signal Transduction in Responses to Abiotic Stress
2.1. Multiplicity of Abiotic Stresses and the Role of the Ca2+-Sensing Network
2.2. Calcium-Mediation of Hormonal Signaling
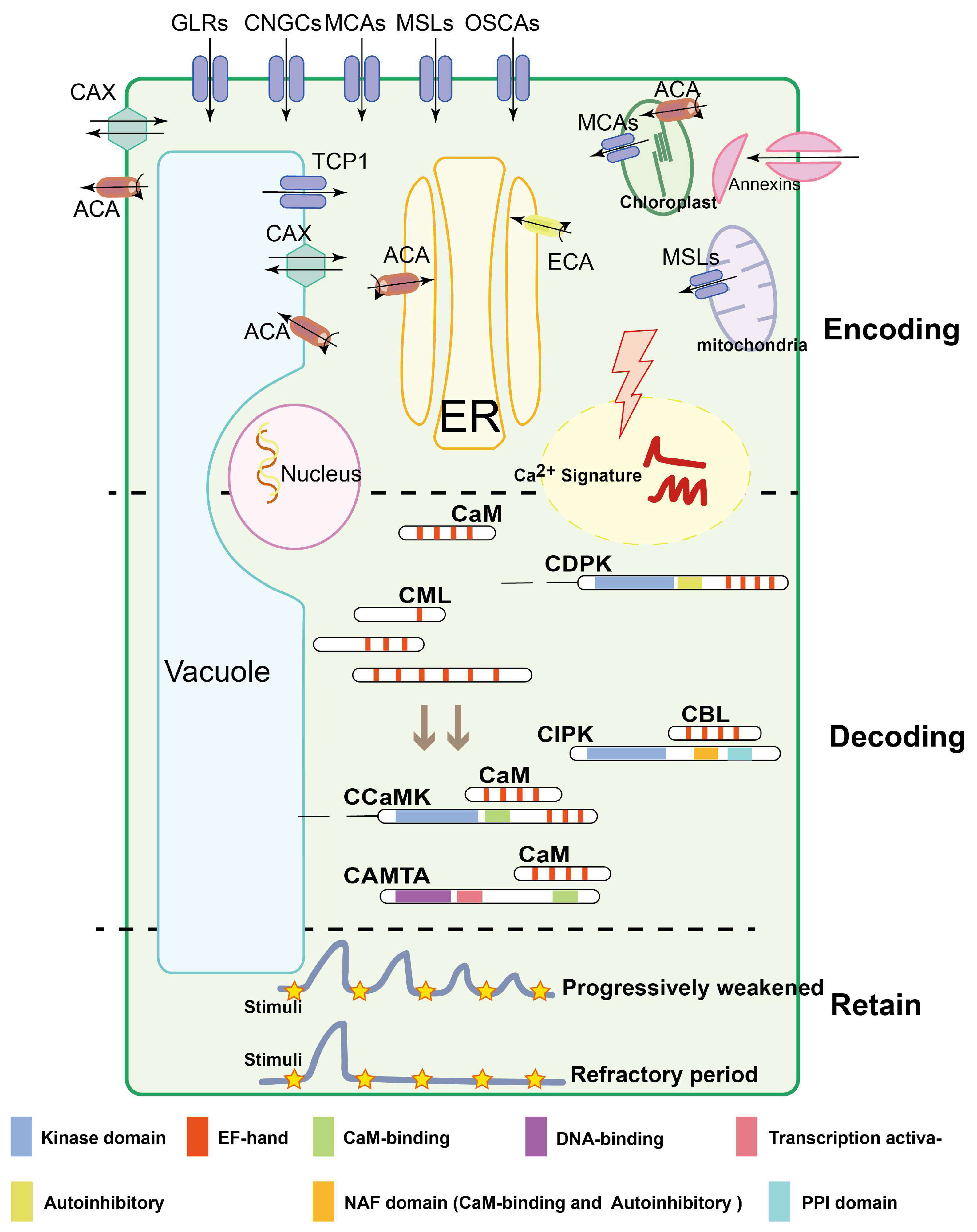
3. Calcium Dynamics in Plant Cells
3.1. Transporters Shape the Ca2+ Signature
3.2. Ca2+-Signaling Sensors
3.2.1. Calmodulins and Calmodulin-Dependent Proteins
3.2.2. Calcium-Dependent Protein Kinases
3.2.3. The CBL–CIPK Signaling Network
3.2.4. Ca2+ Binding Proteins without EF-Hands
3.3. Ca2+ Signature Memory for Abiotic Stresses
4. Evolution of Calcium Signaling for Abiotic Stresses in Green Plants
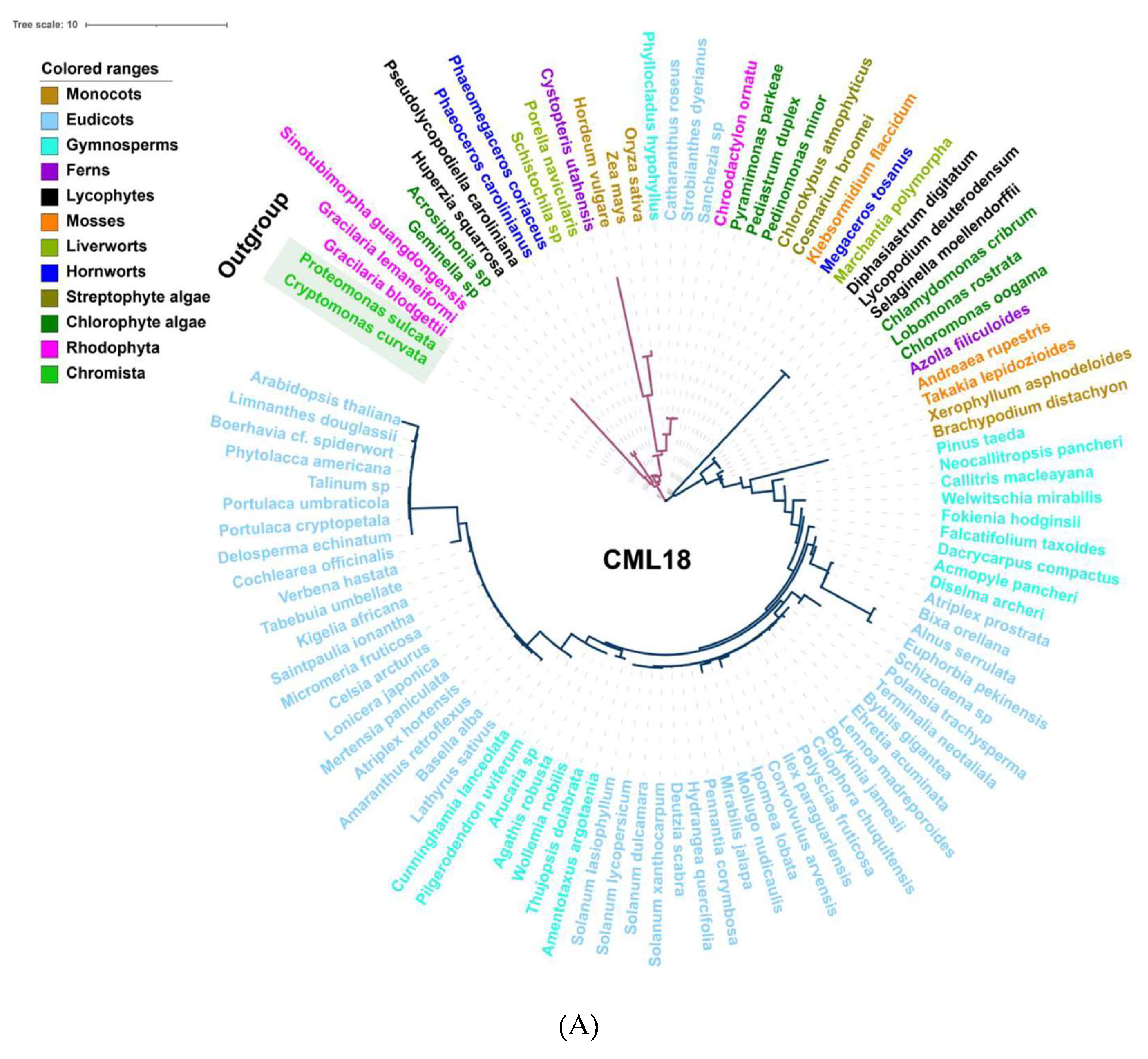
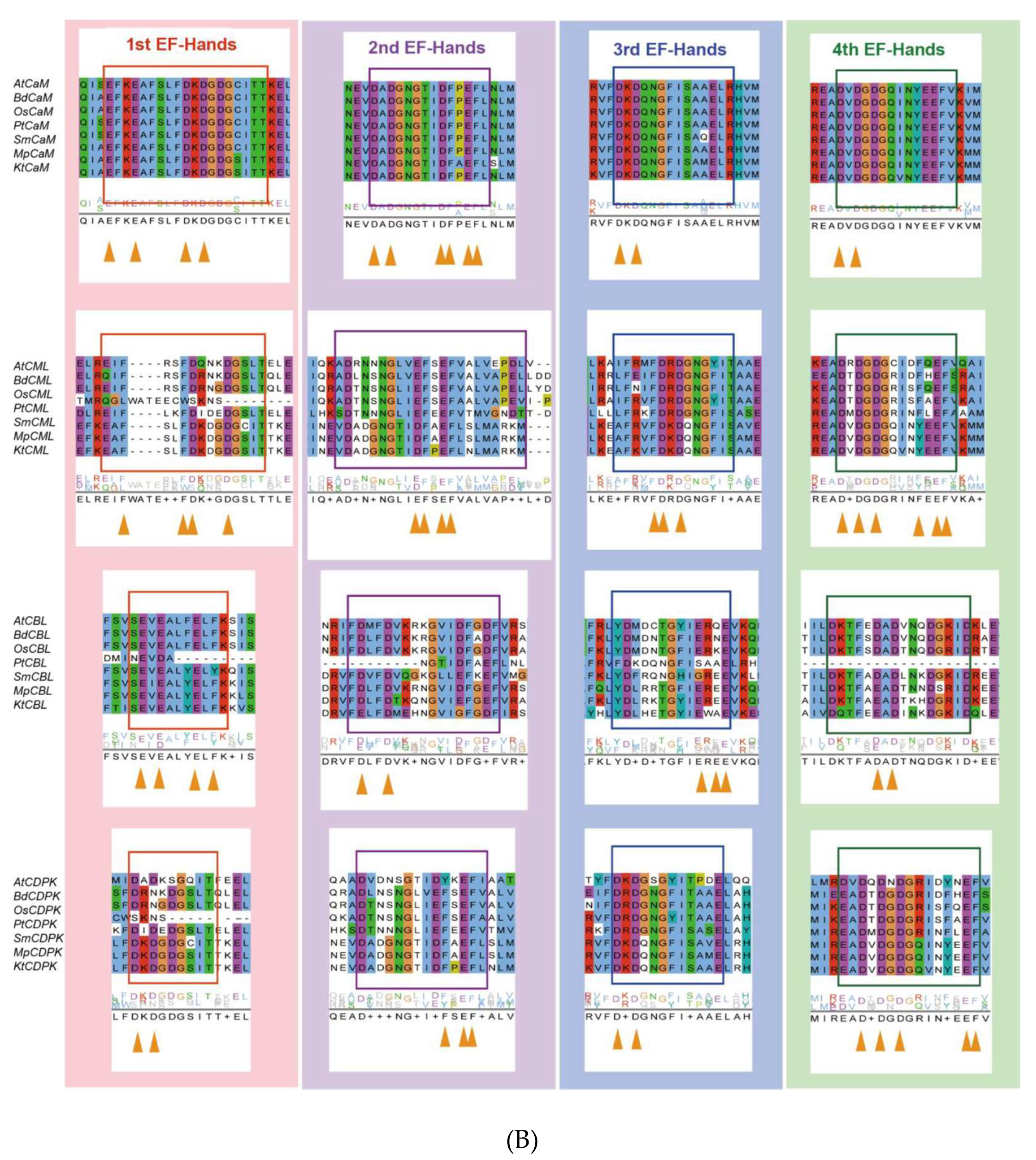
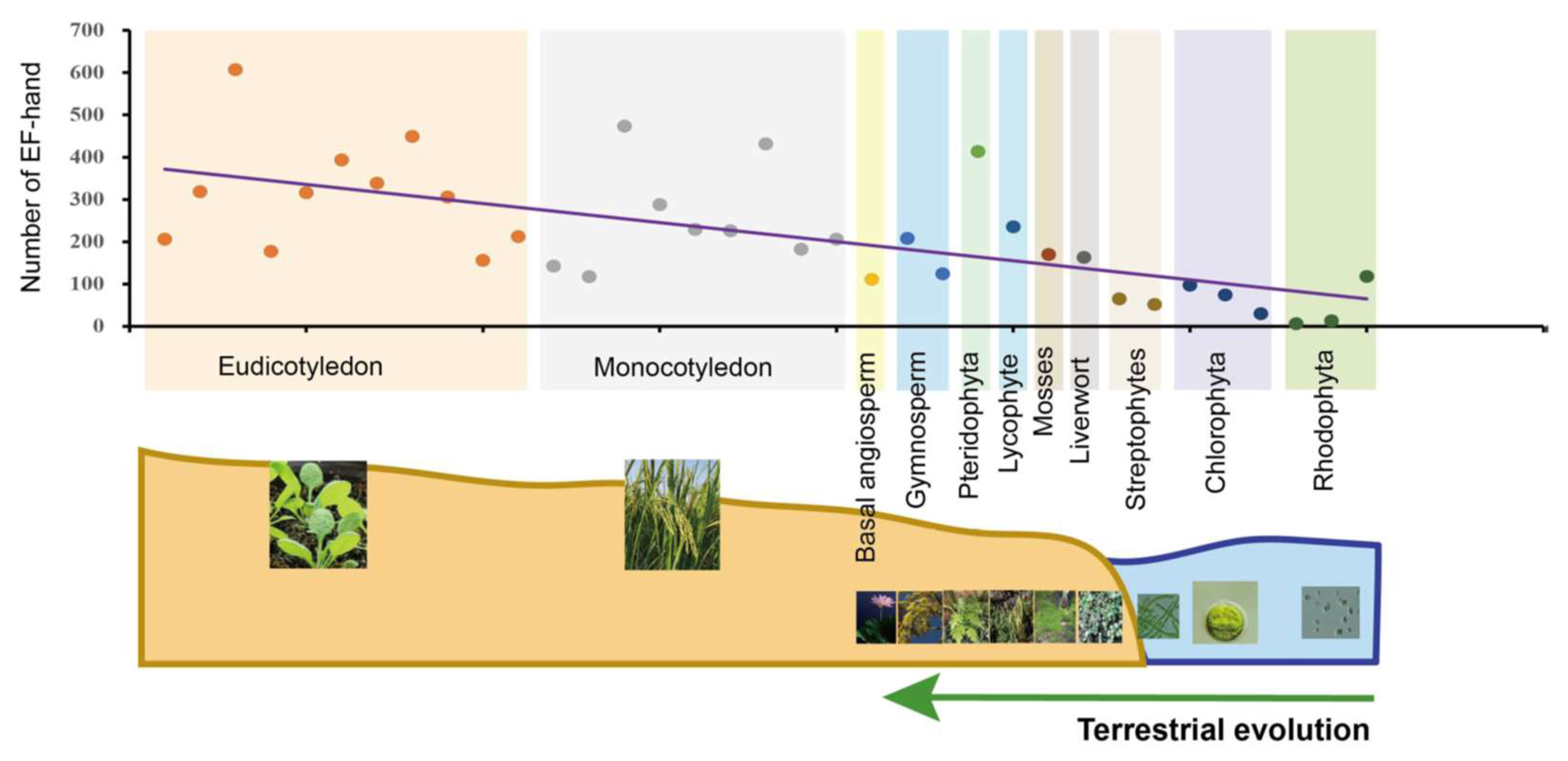
4.1. Comparative Genetic and Evolutionary Analysis of Calcium-Related Gene Families
4.2. Linking Environmental Cues and the Evolution of Calcium-Signaling
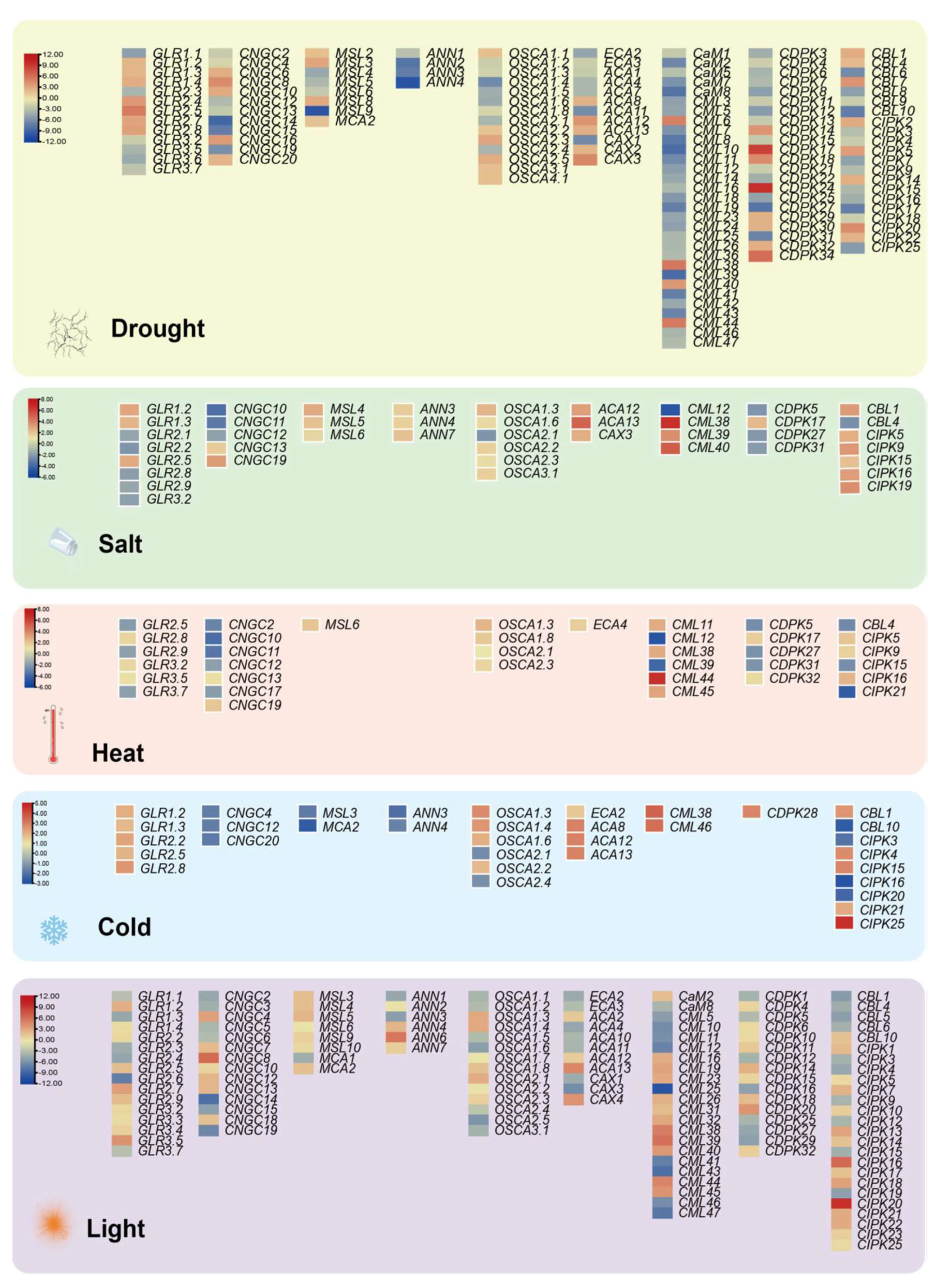
4.3. Transcriptomic Analysis Reveals Ca2+ Regulation under Abiotic Stresses
5. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahuja, I.; de Vos, R.C.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant. Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Env. Sci Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically Rethinking Agriculture for the 21st Century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [PubMed]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. N. Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant. Cell 2002, 14, S165–S183. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, P.J. Ca2+ talyzing Initial Responses to Environmental Stresses. Trends Plant. Sci. 2021, 26, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, W.; Tong, T.; Chen, G.; Zeng, F.; Jang, S.; Gao, W.; Li, Z.H.; Mak, M.; Deng, F.; et al. Molecular Interaction and Evolution of Jasmonate Signaling with Transport and Detoxification of Heavy Metals and Metalloids in Plants. Front. Plant. Sci. 2021, 12, 665842. [Google Scholar] [CrossRef]
- Tang, R.J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant. Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef]
- Marchadier, E.; Oates, M.E.; Fang, H.; Donoghue, P.C.; Hetherington, A.M.; Gough, J. Evolution of the Calcium-Based Intracellular Signaling System. Genome Biol. Evol. 2016, 8, 2118–2132. [Google Scholar] [CrossRef]
- Schaffer, D.E.; Iyer, L.M.; Burroughs, A.M.; Aravind, L. Functional Innovation in the Evolution of the Calcium-Dependent System of the Eukaryotic Endoplasmic Reticulum. Front. Genet. 2020, 11, 34. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Yadav, D.; Khan, A.L.; Hashem, A.; Abd Allah, E.F.; Al-Harrasi, A. Molecular Players of EF-hand Containing Calcium Signaling Event in Plants. Int. J. Mol. Sci. 2019, 20, 1476. [Google Scholar] [CrossRef]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The Evolution of Calcium-Based Signalling in Plants. Curr. Biol. 2017, 27, R667–R679. [Google Scholar] [CrossRef] [PubMed]
- Pivato, M.; Ballottari, M. Chlamydomonas reinhardtii cellular compartments and their contribution to intracellular calcium signalling. J. Exp. Bot. 2021, 72, 5312–5335. [Google Scholar] [CrossRef]
- Batistic, O.; Kudla, J. Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta 2012, 1820, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sadeghnezhad, E.; Guan, P.; Gong, P. Review: Microtubules monitor calcium and reactive oxygen species signatures in signal transduction. Plant. Sci. 2021, 304, 110589. [Google Scholar] [CrossRef]
- Brandt, B.; Munemasa, S.; Wang, C.; Nguyen, D.; Yong, T.; Yang, P.G.; Poretsky, E.; Belknap, T.F.; Waadt, R.; Aleman, F.; et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. Elife 2015, 4, e03599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.K.; Saha, D.; Skalicky, M.; Mishra, U.N.; Chauhan, J.; Behera, L.P.; Lenka, D.; Chand, S.; Kumar, V.; Dey, P.; et al. Crucial Cell Signaling Compounds Crosstalk and Integrative Multi-Omics Techniques for Salinity Stress Tolerance in Plants. Front. Plant. Sci. 2021, 12, 1227. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef]
- Choi, W.G.; Hilleary, R.; Swanson, S.J.; Kim, S.H.; Gilroy, S. Rapid, Long-Distance Electrical and Calcium Signaling in Plants. Annu. Rev. Plant. Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef]
- Pantoja, O. Recent Advances in the Physiology of Ion Channels in Plants. Annu. Rev. Plant. Biol. 2021, 72, 463–495. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Ghosh, A.; Li, Z.-G.; Siddiqui, M.N.; Fujita, M.; Tran, L.-S.P. Methylglyoxal—A signaling molecule in plant abiotic stress responses. Free Radic. Biol. Med. 2018, 122, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, S.K.; Rao, S.; Mishra, L.K.; Sharma, M.; Pandey, G.K. Chapter two—Plant stress responses mediated by CBL–CIPK phosphorylation network. In The Enzymes; Lin, C., Luan, S., Eds.; Academic Press: Salt Lake City, UT, USA, 2016; Volume 40, pp. 31–64. [Google Scholar]
- Wang, Y.; Blatt, M.R.; Chen, Z.-H. Ion Transport at the Plant Plasma Membrane. eLS 2018, 1–16. [Google Scholar] [CrossRef]
- Galon, Y.; Aloni, R.; Nachmias, D.; Snir, O.; Feldmesser, E.; Scrase-Field, S.; Boyce, J.M.; Bouché, N.; Knight, M.R.; Fromm, H. Calmodulin-binding transcription activator 1 mediates auxin signaling and responds to stresses in Arabidopsis. Planta 2010, 232, 165–178. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Q.R.; Liu, L.L.; Zhang, H.M.; Gao, J.W.; Pei, Z.M. Osmotic stress alters circadian cytosolic Ca2+ oscillations and OSCA1 is required in circadian gated stress adaptation. Plant. Signal. Behav. 2020, 15, 1836883. [Google Scholar] [CrossRef]
- Hou, C.; Tian, W.; Kleist, T.; He, K.; Garcia, V.; Bai, F.; Hao, Y.; Luan, S.; Li, L. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 2014, 24, 632–635. [Google Scholar] [CrossRef]
- Hamilton, E.S.; Jensen, G.S.; Maksaev, G.; Katims, A.; Sherp, A.M.; Haswell, E.S. Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 2015, 350, 438–441. [Google Scholar] [CrossRef]
- Jing, P.; Zou, J.; Kong, L.; Hu, S.; Wang, B.; Yang, J.; Xie, G. OsCCD1, a novel small calcium-binding protein with one EF-hand motif, positively regulates osmotic and salt tolerance in rice. Plant. Sci. 2016, 247, 104–114. [Google Scholar] [CrossRef]
- Franz, S.; Ehlert, B.; Liese, A.; Kurth, J.; Cazalé, A.C.; Romeis, T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol. Plant. 2011, 4, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Wu, W.H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant. Mol. Biol. 2007, 65, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant. Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant. Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Halfter, U.; Ishitani, M.; Zhu, J.-K. Molecular Characterization of Functional Domains in the Protein Kinase SOS2 That Is Required for Plant Salt Tolerance. Plant. Cell 2001, 13, 1383–1400. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Liu, Y.; Zhao, Y.; Han, T.; Miao, X.; Zhang, A. Calcineurin B-like protein 5 (SiCBL5) in Setaria italica enhances salt tolerance by regulating Na+ homeostasis. Crop. J. 2021, (in press). [CrossRef]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant. Cell Env. 2015, 38, 2766–2779. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Honsbein, A.; Sokolovski, S.; Grefen, C.; Campanoni, P.; Pratelli, R.; Paneque, M.; Chen, Z.; Johansson, I.; Blatt, M.R. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant. Cell 2009, 21, 2859–2877. [Google Scholar] [CrossRef]
- Grefen, C.; Chen, Z.; Honsbein, A.; Donald, N.; Hills, A.; Blatt, M.R. A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant. Cell 2010, 22, 3076–3092. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Qi, G.N.; Li, J.; Xu, Z.J.; Wu, W.H.; Wang, Y. The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant. Cell 2014, 26, 3387–3402. [Google Scholar] [CrossRef]
- Tang, R.J.; Zhao, F.G.; Garcia, V.J.; Kleist, T.J.; Yang, L.; Zhang, H.X.; Luan, S. Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Van Kleeff, P.J.M.; Gao, J.; Mol, S.; Zwart, N.; Zhang, H.; Li, K.W.; de Boer, A.H. The Arabidopsis GORK K+-channel is phosphorylated by calcium-dependent protein kinase 21 (CPK21), which in turn is activated by 14-3-3 proteins. Plant. Physiol. Biochem. 2018, 125, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Ronzier, E.; Corratgé-Faillie, C.; Sanchez, F.; Prado, K.; Brière, C.; Leonhardt, N.; Thibaud, J.-B.; Tou Cheu, X. CPK13, a Noncanonical Ca2+-Dependent Protein Kinase, Specifically Inhibits KAT2 and KAT1 Shaker K+ Channels and Reduces Stomatal Opening. Plant. Physiol. 2014, 166, 314–326. [Google Scholar] [CrossRef]
- Chen, F.; Dong, G.; Wang, F.; Shi, Y.; Zhu, J.; Zhang, Y.; Ruan, B.; Wu, Y.; Feng, X.; Zhao, C.; et al. A beta-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice. N. Phytol. 2021, 232, 655–672. [Google Scholar] [CrossRef]
- Mori, K.; Renhu, N.; Naito, M.; Nakamura, A.; Shiba, H.; Yamamoto, T.; Suzaki, T.; Iida, H.; Miura, K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018, 8, 550. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Catalá, R.; Santos, E.; Alonso, J.M.; Ecker, J.R.; Martinez-Zapater, J.M.; Salinas, J. Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant. Cell 2003, 15, 2940–2951. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Liu, X.; Luo, S.; Zhang, X.; Liu, X.; Lin, Q.; Zhu, S.; Wan, H.; Yang, Y.; et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant. 2021, 14, 315–329. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Poovaiah, B.W. Calcium Signaling-Mediated Plant Response to Cold Stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant. Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Fu, G.; Chen, X.; Zhu, J.; Zhang, Z. Functional Characterization of a Tomato Calcium-dependent Protein Kinase Gene, LeCPK2, Involved in Heat (Light) Stress. Genom. Appl. Biol. 2011, 30, 338–345. [Google Scholar]
- Liu, H.T.; Gao, F.; Li, G.L.; Han, J.L.; Liu, D.L.; Sun, D.Y.; Zhou, R.G. The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant. J. 2008, 55, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Saidi, Y.; Finka, A.; Goloubinoff, P. Heat perception and signalling in plants: A tortuous path to thermotolerance. New Phytol. 2011, 190, 556–565. [Google Scholar] [CrossRef]
- Hu, B.; Deng, F.; Chen, G.; Chen, X.; Gao, W.; Long, L.; Xia, J.; Chen, Z.H. Evolution of Abscisic Acid Signaling for Stress Responses to Toxic Metals and Metalloids. Front. Plant. Sci. 2020, 11, 909. [Google Scholar] [CrossRef]
- Soma, F.; Takahashi, F.; Suzuki, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Plant Raf-like kinases regulate the mRNA population upstream of ABA-unresponsive SnRK2 kinases under drought stress. Nat. Commun. 2020, 11, 1373. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.H.; Zhang, B.; Hills, A.; Blatt, M.R. PYR/PYL/RCAR abscisic acid receptors regulate K+ and Cl- channels through reactive oxygen species-mediated activation of Ca2+ channels at the plasma membrane of intact Arabidopsis guard cells. Plant. Physiol. 2013, 163, 566–577. [Google Scholar] [CrossRef]
- Geiger, D.; Scherzer, S.; Mumm, P.; Marten, I.; Ache, P.; Matschi, S.; Liese, A.; Wellmann, C.; Al-Rasheid, K.A.; Grill, E.; et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 2010, 107, 8023–8028. [Google Scholar] [CrossRef]
- Choi, H.I.; Park, H.J.; Park, J.H.; Kim, S.; Im, M.Y.; Seo, H.H.; Kim, Y.W.; Hwang, I.; Kim, S.Y. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant. Physiol. 2005, 139, 1750–1761. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yu, X.C.; Wang, X.J.; Zhao, R.; Li, Y.; Fan, R.C.; Shang, Y.; Du, S.Y.; Wang, X.F.; Wu, F.Q.; et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant. Cell 2007, 19, 3019–3036. [Google Scholar] [CrossRef]
- Edel, K.H.; Kudla, J. Integration of calcium and ABA signaling. Curr. Opin. Plant. Biol. 2016, 33, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Drerup, M.M.; Schlucking, K.; Hashimoto, K.; Manishankar, P.; Steinhorst, L.; Kuchitsu, K.; Kudla, J. The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant. 2013, 6, 559–569. [Google Scholar] [CrossRef]
- Xiong, T.; Tan, Q.; Li, S.; Mazars, C.; Galaud, J.P.; Zhu, X. Interactions between calcium and ABA signaling pathways in the regulation of fruit ripening. J. Plant. Physiol 2021, 256, 153309. [Google Scholar] [CrossRef] [PubMed]
- Delk, N.A.; Johnson, K.A.; Chowdhury, N.I.; Braam, J. CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant. Physiol. 2005, 139, 240–253. [Google Scholar] [CrossRef]
- Magnan, F.; Ranty, B.; Charpenteau, M.; Sotta, B.; Galaud, J.P.; Aldon, D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant. J. 2008, 56, 575–589. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Du, L.; Wang, H.; Yang, T. Recent advances in calcium/calmodulin-mediated signaling with an emphasis on plant-microbe interactions. Plant. Physiol. 2013, 163, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Reichelt, M.; Vadassery, J.; Mithofer, A. Calmodulin-like protein CML37 is a positive regulator of ABA during drought stress in Arabidopsis. Plant. Signal. Behav. 2015, 10, e1011951. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, K.; Nguyen, L.; Stael, S.; Audenaert, D.; Beeckman, T.; Vanneste, S. The Screening for novel inhibitors of Auxin-induced Ca2+ signaling. In Plant Chemical Genomics: Methods and Protocols; Hicks, G.R., Zhang, C., Eds.; Springer: New York, NY, USA, 2021; pp. 89–98. [Google Scholar]
- Jurišić-Knežev, D.; Bergougnoux, V.; Milde, D.; Fellner, M. Auxin binding protein 4 is involved in the Ca2+/auxin-regulated expression of ZCAX3 gene in maize (Zea mays). Botany 2014, 92, 332–339. [Google Scholar] [CrossRef]
- Ma, X.; Li, Q.H.; Yu, Y.N.; Qiao, Y.M.; Haq, S.U.; Gong, Z.H. The CBL-CIPK Pathway in Plant Response to Stress Signals. Int. J. Mol. Sci. 2020, 21, 5668. [Google Scholar] [CrossRef] [PubMed]
- Rietz, S.; Dermendjiev, G.; Oppermann, E.; Tafesse, F.G.; Effendi, Y.; Holk, A.; Parker, J.E.; Teige, M.; Scherer, G.F. Roles of Arabidopsis patatin-related phospholipases a in root development are related to auxin responses and phosphate deficiency. Mol. Plant. 2010, 3, 524–538. [Google Scholar] [CrossRef]
- Xu, W.; Huang, W. Calcium-Dependent Protein Kinases in Phytohormone Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 2436. [Google Scholar] [CrossRef] [PubMed]
- Hazak, O.; Mamon, E.; Lavy, M.; Sternberg, H.; Behera, S.; Schmitz-Thom, I.; Bloch, D.; Dementiev, O.; Gutman, I.; Danziger, T.; et al. A novel Ca2+-binding protein that can rapidly transduce auxin responses during root growth. PLoS Biol. 2019, 17, e3000085. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Murata, Y.; Yang, Y.; Munemasa, S.; Wang, Y.F.; Andreoli, S.; Tiriac, H.; Alonso, J.M.; Harper, J.F.; Ecker, J.R.; et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, S.; Hossain, M.A.; Nakamura, Y.; Mori, I.C.; Murata, Y. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant. Physiol. 2011, 155, 553–561. [Google Scholar] [CrossRef]
- Matschi, S.; Hake, K.; Herde, M.; Hause, B.; Romeis, T. The calcium-dependent protein kinase CPK28 regulates development by inducing growth phase-specific, spatially restricted alterations in jasmonic acid levels independent of defense responses in Arabidopsis. Plant. Cell 2015, 27, 591–606. [Google Scholar] [CrossRef]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.-H.; Obermeyer, G.; Feijó, J.A. Glutamate Receptor–Like Genes Form Ca2+ Channels in Pollen Tubes and Are Regulated by Pistil d-Serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef]
- Chen, Z.H.; Hills, A.; Lim, C.K.; Blatt, M.R. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant. J. 2010, 61, 816–825. [Google Scholar] [CrossRef]
- Hills, A.; Chen, Z.H.; Amtmann, A.; Blatt, M.R.; Lew, V.L. OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant. Physiol. 2012, 159, 1026–1042. [Google Scholar] [CrossRef]
- Chen, Z.H.; Hills, A.; Batz, U.; Amtmann, A.; Lew, V.L.; Blatt, M.R. Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant. Physiol. 2012, 159, 1235–1251. [Google Scholar] [CrossRef]
- Jezek, M.; Silva-Alvim, F.A.L.; Hills, A.; Donald, N.; Ishka, M.R.; Shadbolt, J.; He, B.; Lawson, T.; Harper, J.F.; Wang, Y.; et al. Guard cell endomembrane Ca2+-ATPases underpin a ’carbon memory’ of photosynthetic assimilation that impacts on water-use efficiency. Nat. Plants 2021, 7, 1301–1313. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. N. Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Niu, Y.; Zhang, J.; Zhou, Y.; Ma, Z.; Huang, X. Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: Recent advances. Plant. Cell Tissue Organ. Cult. (PCTOC) 2017, 132, 413–424. [Google Scholar] [CrossRef]
- Jaślan, D.; Dreyer, I.; Lu, J.; O’Malley, R.; Dindas, J.; Marten, I.; Hedrich, R. Voltage-dependent gating of SV channel TPC1 confers vacuole excitability. Nat. Commun. 2019, 10, 2659. [Google Scholar] [CrossRef]
- Garciadeblas, B.; Benito, B.; Rodríguez-Navarro, A. Plant cells express several stress calcium ATPases but apparently no sodium ATPase. Plant. Soil 2001, 235, 181–192. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.H.; Liu, X.; Colmer, T.D.; Zhou, M.; Shabala, S. Tissue-specific root ion profiling reveals essential roles of the CAX and ACA calcium transport systems in response to hypoxia in Arabidopsis. J. Exp. Bot. 2016, 67, 3747–3762. [Google Scholar] [CrossRef]
- Hirschi, K. Vacuolar H+/Ca2+ transport: Who’s directing the traffic? Trends Plant. Sci. 2001, 6, 100–104. [Google Scholar] [CrossRef]
- Tuteja, N. Chapter twenty-four—Mechanisms of high salinity tolerance in plants. In Methods in Enzymology; Häussinger, D., Sies, H., Eds.; Academic Press: Salt Lake City, UT, USA, 2007; Volume 428, pp. 419–438. [Google Scholar]
- Gleason, C.; Chaudhuri, S.; Yang, T.; Munoz, A.; Poovaiah, B.W.; Oldroyd, G.E. Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 2006, 441, 1149–1152. [Google Scholar] [CrossRef]
- Harper, J.F.; Harmon, A. Plants, symbiosis and parasites: A calcium signalling connection. Nat. Rev. Mol. Cell Biol. 2005, 6, 555. [Google Scholar] [CrossRef]
- Batisti, O.; Kudla, J. Plant calcineurin B-like proteins and their interacting protein kinases. Biochim. Biophys. Acta 2009, 1793, 985–992. [Google Scholar] [CrossRef]
- Snedden, W.A.; Fromm, H. Calmodulin as a versatile calcium signal transducer in plants. N. Phytol. 2010, 151, 35–66. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2009, 425, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Kim, M.C.; Yoo, J.H.; Moon, B.C.; Koo, S.C.; Park, B.O.; Lee, J.H.; Koo, Y.D.; Han, H.J.; Lee, S.Y.; et al. Isolation of a calmodulin-binding transcription factor from rice (Oryza sativa L.). J. Biol. Chem. 2005, 280, 40820–40831. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Park, C.Y.; Kim, J.C.; Heo, W.D.; Cheong, M.S.; Park, H.C.; Kim, M.C.; Moon, B.C.; Choi, M.S.; Kang, Y.H.; et al. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J. Biol. Chem. 2005, 280, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kudla, J. Calcium decoding mechanisms in plants. Biochimie 2011, 93, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dunand, C.; Snedden, W.; Galaud, J.P. CaM and CML emergence in the green lineage. Trends Plant. Sci. 2015, 20, 483–489. [Google Scholar] [CrossRef]
- Chigri, F.; Flosdorff, S.; Pilz, S.; Kölle, E.; Dolze, E.; Gietl, C.; Vothknecht, U.C. The Arabidopsis calmodulin-like proteins AtCML30 and AtCML3 are targeted to mitochondria and peroxisomes, respectively. Plant. Mol. Biol. 2012, 78, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Calmodulin-binding protein kinases in plants. Trends Plant. Sci. 2003, 8, 123–127. [Google Scholar] [CrossRef]
- Singh, S.; Parniske, M. Activation of calcium- and calmodulin-dependent protein kinase (CCaMK), the central regulator of plant root endosymbiosis. Curr. Opin. Plant. Biol. 2012, 15, 444–453. [Google Scholar] [CrossRef]
- Yang, T.; Shad Ali, G.; Yang, L.; Du, L.; Reddy, A.S.; Poovaiah, B.W. Calcium/calmodulin-regulated receptor-like kinase CRLK1 interacts with MEKK1 in plants. Plant. Signal. Behav. 2010, 5, 991–994. [Google Scholar] [CrossRef]
- Galon, Y.; Finkler, A.; Fromm, H. Calcium-regulated transcription in plants. Mol. Plant. 2010, 3, 653–669. [Google Scholar] [CrossRef]
- Reddy, A.S.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant. Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA Transcription Factors in Cold-Regulated Gene Expression and Freezing Tolerance. Plant. Cell 2009, 21, 972–984. [Google Scholar] [CrossRef]
- Shen, Q.; Fu, L.; Su, T.; Ye, L.; Huang, L.; Kuang, L.; Wu, L.; Wu, D.; Chen, Z.H.; Zhang, G. Calmodulin HvCaM1 Negatively Regulates Salt Tolerance via Modulation of HvHKT1s and HvCAMTA4. Plant. Physiol 2020, 183, 1650–1662. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. N. Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kurenda, A.; Stolz, S.; Chetelat, A.; Farmer, E.E. Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. USA 2018, 115, 10178–10183. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.H.; Liu, X.; Colmer, T.D.; Shabala, L.; Salih, A.; Zhou, M.; Shabala, S. Revealing the roles of GORK channels and NADPH oxidase in acclimation to hypoxia in Arabidopsis. J. Exp. Bot. 2017, 68, 3191–3204. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.-H.; Liu, X.; Shabala, L.; Yu, M.; Zhou, M.; Salih, A.; Shabala, S. The loss of RBOHD function modulates root adaptive responses to combined hypoxia and salinity stress in Arabidopsis. Env. Exp. Bot. 2019, 158, 125–135. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, H.; Yang, L.; Chen, C.; Shabala, L.; Xiong, M.; Niu, M.; Liu, J.; Zheng, Z.; Zhou, L.; et al. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 2019, 70, 5879–5893. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef] [PubMed]
- Ormancey, M.; Thuleau, P.; Mazars, C.; Cotelle, V. CDPKs and 14-3-3 Proteins: Emerging Duo in Signaling. Trends Plant. Sci. 2017, 22, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, X.; Chang, S.; Chu, Z.; Wang, H.; Han, S.; Wang, Y. Calcium-dependent protein kinase 21 phosphorylates 14-3-3 proteins in response to ABA signaling and salt stress in rice. Biochem. Biophys. Res. Commun. 2017, 493, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, F.; Li, K. The 14-3-3 proteins: Regulators of plant metabolism and stress responses. Plant. Biol. 2021, 23, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Yip Delormel, T.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. N. Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef]
- Rigo, G.; Ayaydin, F.; Tietz, O.; Zsigmond, L.; Kovacs, H.; Pay, A.; Salchert, K.; Darula, Z.; Medzihradszky, K.F.; Szabados, L.; et al. Inactivation of plasma membrane-localized CDPK-RELATED KINASE5 decelerates PIN2 exocytosis and root gravitropic response in Arabidopsis. Plant. Cell 2013, 25, 1592–1608. [Google Scholar] [CrossRef] [PubMed]
- Cseplo, A.; Zsigmond, L.; Andrasi, N.; Baba, A.I.; Labhane, N.M.; Peto, A.; Kolbert, Z.; Kovacs, H.E.; Steinbach, G.; Szabados, L.; et al. The AtCRK5 Protein Kinase Is Required to Maintain the ROS NO Balance Affecting the PIN2-Mediated Root Gravitropic Response in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 5979. [Google Scholar] [CrossRef]
- Baba, A.I.; Valkai, I.; Labhane, N.M.; Koczka, L.; Andrasi, N.; Klement, E.; Darula, Z.; Medzihradszky, K.F.; Szabados, L.; Feher, A.; et al. CRK5 Protein Kinase Contributes to the Progression of Embryogenesis of Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 6120. [Google Scholar] [CrossRef]
- Kim, K.-N. Stress responses mediated by the CBL calcium sensors in plants. Plant. Bio. Rep. 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Dong, Q.; Bai, B.; Almutairi, B.O.; Kudla, J. Emerging roles of the CBL-CIPK calcium signaling network as key regulatory hub in plant nutrition. J. Plant. Physiol. 2021, 257, 153335. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Wang, C.; Li, K.; Luan, S. The CBL-CIPK Calcium Signaling Network: Unified Paradigm from 20 Years of Discoveries. Trends Plant. Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Sanyal, S.K.; Pandey, G.K. Ca2+-CBL-CIPK: A modulator system for efficient nutrient acquisition. Plant Cell Rep. 2021, 40, 2111–2122. [Google Scholar] [CrossRef]
- Weinl, S.; Kudla, J. The CBL-CIPK Ca2+-decoding signaling network: Function and perspectives. N. Phytol. 2009, 184, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barrena, M.J.; Fujii, H.; Angulo, I.; Martinez-Ripoll, M.; Zhu, J.K.; Albert, A. The structure of the C-terminal domain of the protein kinase AtSOS2 bound to the calcium sensor AtSOS3. Mol. Cell 2007, 26, 427–435. [Google Scholar] [CrossRef]
- Sanyal, S.K.; Mahiwal, S.; Nambiar, D.M.; Pandey, G.K. CBL-CIPK module-mediated phosphoregulation: Facts and hypothesis. Biochem. J. 2020, 477, 853–871. [Google Scholar] [CrossRef]
- Meena, M.K.; Sardar, A.; Chattopadhyay, D. Decoding and Relay of Calcium Signals by CBL-CIPK Module in Plants. Proc. Indian Natl. Sci. Acad. 2018, 85, 143–156. [Google Scholar] [CrossRef]
- Clark, G.B.; Roux, S.J. Annexins of Plant Cells. Plant. Physiol. 1995, 109, 1133–1139. [Google Scholar] [CrossRef]
- Wang, X. Plant phospholipases. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2001, 52, 211–231. [Google Scholar] [CrossRef]
- Menegazzi, P.; Guzzo, F.; Baldan, B.; Mariani, P.; Treves, S. Purification of Calreticulin-like Protein(s) from Spinach Leaves. Biochem. Biophys. Res. Commun. 1993, 190, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Franklin, A.E.; Hoffman, N.E. Primary structure and characterization of an Arabidopsis thaliana calnexin-like protein. J. Biol. Chem. 1993, 268, 6560–6566. [Google Scholar] [CrossRef]
- Furuyama, T.; Dzelzkalns, V.A. A novel calcium-binding protein is expressed in Brassica pistils and anthers late in flower development. Plant. Mol. Biol. 1999, 39, 729–737. [Google Scholar] [CrossRef]
- Mortimer, J.C.; Laohavisit, A.; Macpherson, N.; Webb, A.; Brownlee, C.; Battey, N.H.; Davies, J.M. Annexins: Multifunctional components of growth and adaptation. J. Exp. Bot. 2008, 59, 533–544. [Google Scholar] [CrossRef]
- Clark, G.B.; Morgan, R.O.; Fernandez, M.P.; Roux, S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. N. Phytol. 2012, 196, 695–712. [Google Scholar] [CrossRef]
- Huh, S.M.; Noh, E.K.; Kim, H.G.; Jeon, B.W.; Bae, K.; Hu, H.C.; Kwak, J.M.; Park, O.K. Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses. Plant. Cell Physiol. 2010, 51, 1499–1514. [Google Scholar] [CrossRef]
- Gorecka, K.M.; Thouverey, C.; Buchet, R.; Pikula, S. Potential role of annexin AnnAt1 from Arabidopsis thaliana in pH-mediated cellular response to environmental stimuli. Plant. Cell Physiol. 2007, 48, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Shang, Z.; Rubio, L.; Cuin, T.A.; Very, A.A.; Wang, A.; Mortimer, J.C.; Macpherson, N.; Coxon, K.M.; Battey, N.H.; et al. Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+- and K+-permeable conductance in root cells. Plant. Cell 2012, 24, 1522–1533. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 1–16. (in press). [CrossRef] [PubMed]
- Sharma, D.; Kumar, A. Chapter 18—Calcium signaling network in abiotic stress tolerance in plants. In Calcium Transport Elements in Plants; Upadhyay, S.K., Ed.; Academic Press: Salt Lake City, UT, USA, 2021; pp. 297–314. [Google Scholar]
- Tuteja, N. Integrated calcium signaling in plants. In Signaling in Plants; Signaling and Communication in Plants; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–49. [Google Scholar]
- Katano, K.; Honda, K.; Suzuki, N. Integration between ROS Regulatory Systems and Other Signals in the Regulation of Various Types of Heat Responses in Plants. Int. J. Mol. Sci. 2018, 19, 3370. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium Signaling Network in Plants. Plant. Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Kumar, P.; Bae, H. Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant. Biol. 2017, 17, 38. [Google Scholar] [CrossRef]
- Yang, T.; Chaudhuri, S.; Yang, L.; Du, L.; Poovaiah, B.W. A calcium/calmodulin-regulated member of the receptor-like kinase family confers cold tolerance in plants. J. Biol. Chem. 2010, 285, 7119–7126. [Google Scholar] [CrossRef]
- Deng, F.; Zeng, F.; Chen, G.; Feng, X.; Riaz, A.; Wu, X.; Gao, W.; Wu, F.; Holford, P.; Chen, Z.H. Metalloid hazards: From plant molecular evolution to mitigation strategies. J. Hazard. Mater. 2021, 409, 124495. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Huang, Y.; Chen, F.; Zhang, X.; Sessa, E.; Zhao, C.; Marchant, D.B.; Xue, D.; Chen, G.; Dai, F.; et al. Evolution of rapid blue-light response linked to explosive diversification of ferns in angiosperm forests. N. Phytol. 2021, 230, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Y.; Chan, K.X.; Marchant, D.B.; Franks, P.J.; Randall, D.; Tee, E.E.; Chen, G.; Ramesh, S.; Phua, S.Y.; et al. Evolution of chloroplast retrograde signaling facilitates green plant adaptation to land. Proc. Natl. Acad. Sci. USA 2019, 116, 5015–5020. [Google Scholar] [CrossRef] [PubMed]
- Kass, G.E.; Orrenius, S. Calcium signaling and cytotoxicity. Env. Health Perspect 1999, 107, 25–35. [Google Scholar] [CrossRef]
- Davenport, R. Glutamate receptors in plants. Ann. Bot. 2002, 90, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. N. Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Maksaev, G.; Haswell, E.S. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc. Natl. Acad. Sci. USA 2012, 109, 19015–19020. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-H.; Yang, M.; Sui, J.-L.; Qi, J.-Y.; Fang, Y.-J.; Hu, S.-N.; Tang, C.-R. The calcium-dependent protein kinase (CDPK) and CDPK-related kinase gene families in Hevea brasiliensis: Comparison with five other plant species in structure, evolution, and expression. FEBS Open Bio. 2017, 7, 4–24. [Google Scholar] [CrossRef]
- Valmonte, G.R.; Arthur, K.; Higgins, C.M.; MacDiarmid, R.M. Calcium-Dependent Protein Kinases in Plants: Evolution, Expression and Function. Plant. Cell Physiol. 2014, 55, 551–569. [Google Scholar] [CrossRef]
- McCormack, E.; Braam, J. Calmodulins and related potential calcium sensors of Arabidopsis. N. Phytol. 2003, 159, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Aharon, G.S.; Sottosanto, J.B.; Blumwald, E. Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc. Natl. Acad. Sci. USA 2005, 102, 16107. [Google Scholar] [CrossRef] [PubMed]
- Jaslan, D.; Mueller, T.D.; Becker, D.; Schultz, J.; Cuin, T.A.; Marten, I.; Dreyer, I.; Schonknecht, G.; Hedrich, R. Gating of the two-pore cation channel AtTPC1 in the plant vacuole is based on a single voltage-sensing domain. Plant. Biol. 2016, 18, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Mohanta, N.; Mohanta, Y.K.; Parida, P.; Bae, H. Genome-wide identification of Calcineurin B-Like (CBL) gene family of plants reveals novel conserved motifs and evolutionary aspects in calcium signaling events. BMC Plant. Biol. 2015, 15, 189. [Google Scholar] [CrossRef]
- Batistic, O.; Waadt, R.; Steinhorst, L.; Held, K.; Kudla, J. CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant. J. 2010, 61, 211–222. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, R.; Zhou, Y.; Jiao, Y. Evolutionary strategies drive a balance of the interacting gene products for the CBL and CIPK gene families. N. Phytol. 2020, 226, 1506–1516. [Google Scholar] [CrossRef]
- Grabarek, Z. Structural basis for diversity of the EF-hand calcium-binding proteins. J. Mol. Biol. 2006, 359, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Kretsinger, R.H. Structural differences among subfamilies of EF-hand proteins--a view from the pseudo two-fold symmetry axis. Proteins 2014, 82, 2915–2924. [Google Scholar] [CrossRef]
- Edel, K.H.; Kudla, J. Increasing complexity and versatility: How the calcium signaling toolkit was shaped during plant land colonization. Cell Calcium 2015, 57, 231–246. [Google Scholar] [CrossRef]
- Han, S.; Wang, C.; Wang, W.; Jiang, J. Mitogen-activated protein kinase 6 negatively regulates NaCl-inhibited root growth in Arabidopsis by controlling cytosolic Ca2+ and Na+ homeostasis in root cells. J. Plant Physiol. 2013, 171, 26–34. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Anandhan, S.; Pande, V.; Ahmed, Z.; Qin, Y. Ectopic Expression of Cold Responsive LlaCIPK Gene Enhances Cold Stress Tolerance in Nicotiana tabacum. Genes 2019, 10, 446. [Google Scholar] [CrossRef]
- Amarasinghe, S.; Watson-Haigh, N.S.; Gilliham, M.; Roy, S.; Baumann, U. The evolutionary origin of CIPK16: A gene involved in enhanced salt tolerance. Mol. Phylogenet. Evol. 2016, 100, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; DeFalco, T.A.; Karia, P.; Snedden, W.A.; Moeder, W.; Yoshioka, K.; Dietrich, P. Calmodulin as a Ca2+ -Sensing Subunit of Arabidopsis Cyclic Nucleotide-Gated Channel Complexes. Plant. Cell Physiol. 2017, 58, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Marshall, C.B.; Munro, K.; Kang, H.G.; Moeder, W.; Ikura, M.; Snedden, W.A.; Yoshioka, K. Multiple Calmodulin-Binding Sites Positively and Negatively Regulate Arabidopsis cyclic nucleotide-gated channel12. Plant. Cell 2016, 28, 1738–1751. [Google Scholar] [CrossRef][Green Version]
- Campos, W.F.; Dressano, K.; Ceciliato, P.H.O.; Guerrero-Abad, J.C.; Silva, A.L.; Fiori, C.S.; Morato do Canto, A.; Bergonci, T.; Claus, L.A.N.; Silva-Filho, M.C.; et al. Arabidopsis thaliana rapid alkalinization factor 1-mediated root growth inhibition is dependent on calmodulin-like protein 38. J. Biol. Chem. 2018, 293, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Thor, K.; Jiang, S.; Michard, E.; George, J.; Scherzer, S.; Huang, S.; Dindas, J.; Derbyshire, P.; Leitao, N.; DeFalco, T.A.; et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 2020, 585, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Li, G.L.; Chang, H.; Sun, D.Y.; Zhou, R.G.; Li, B. Calmodulin-binding protein phosphatase PP7 is involved in thermotolerance in Arabidopsis. Plant. Cell Env. 2007, 30, 156–164. [Google Scholar] [CrossRef]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, T.; Li, Q.; Jiang, W.; Chen, G.; Xue, D.; Deng, F.; Zeng, F.; Chen, Z.-H. Molecular Evolution of Calcium Signaling and Transport in Plant Adaptation to Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12308. https://doi.org/10.3390/ijms222212308
Tong T, Li Q, Jiang W, Chen G, Xue D, Deng F, Zeng F, Chen Z-H. Molecular Evolution of Calcium Signaling and Transport in Plant Adaptation to Abiotic Stress. International Journal of Molecular Sciences. 2021; 22(22):12308. https://doi.org/10.3390/ijms222212308
Chicago/Turabian StyleTong, Tao, Qi Li, Wei Jiang, Guang Chen, Dawei Xue, Fenglin Deng, Fanrong Zeng, and Zhong-Hua Chen. 2021. "Molecular Evolution of Calcium Signaling and Transport in Plant Adaptation to Abiotic Stress" International Journal of Molecular Sciences 22, no. 22: 12308. https://doi.org/10.3390/ijms222212308
APA StyleTong, T., Li, Q., Jiang, W., Chen, G., Xue, D., Deng, F., Zeng, F., & Chen, Z.-H. (2021). Molecular Evolution of Calcium Signaling and Transport in Plant Adaptation to Abiotic Stress. International Journal of Molecular Sciences, 22(22), 12308. https://doi.org/10.3390/ijms222212308









