Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension
Abstract
1. Introduction
2. Autonomic Dysregulation and the Role of RAAS Signalling in Hypertension
2.1. The Problem of Hypertension
2.2. Actions of Ang II in Hypertension
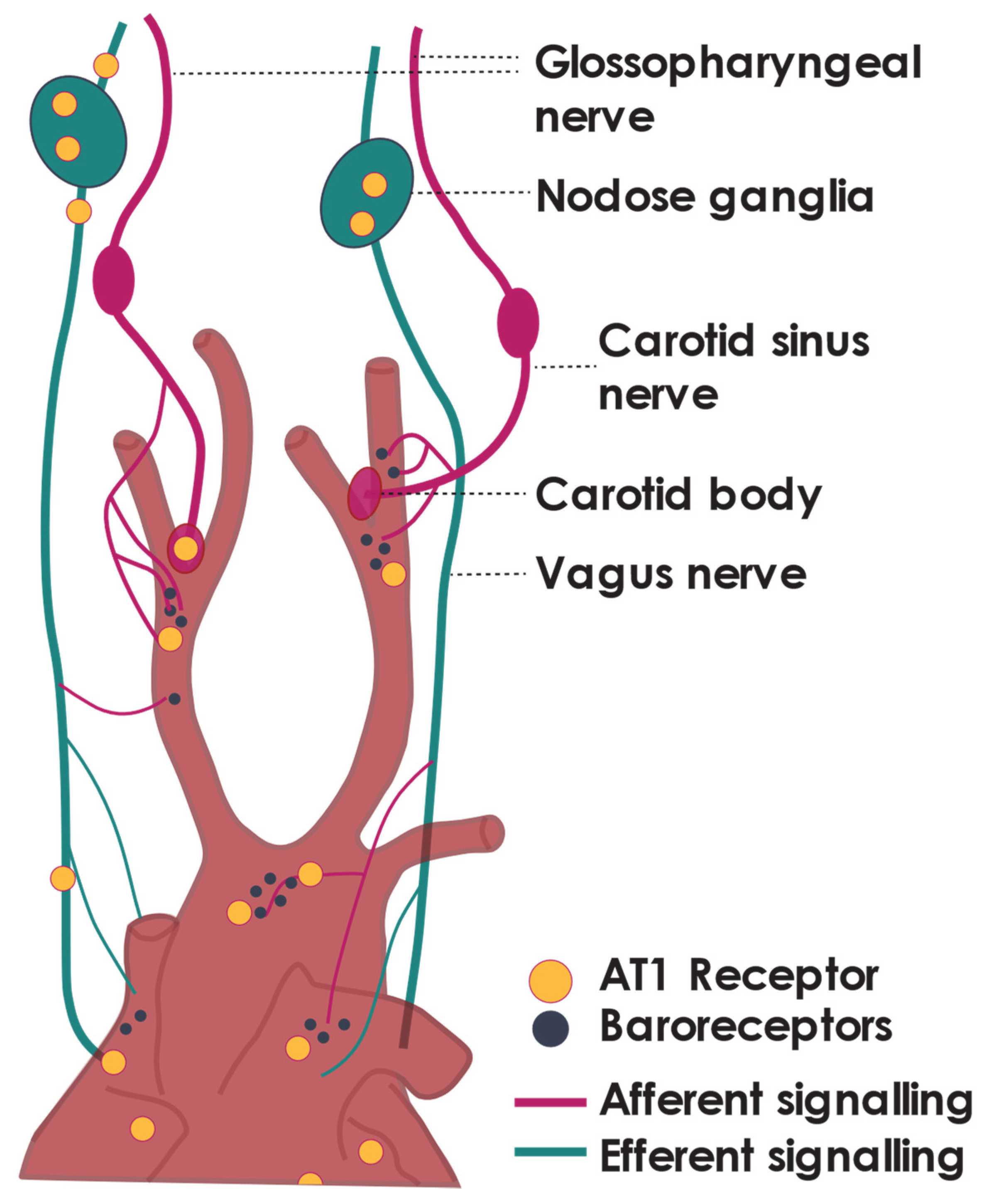
3. Receptors of Ang II Are Found throughout the Parasympathetic Nervous System
4. Ang II Tonically Inhibits Cardiac Vagal Tone—Peripheral Actions
5. Ang II Attenuates Cardiac Vagal Activity—Central Actions
5.1. Ang II within the Brain
5.2. Ang II in Central Brain Regions That Can Modulate Parasympathetic Nerve Activity
6. Ang II and Its Actions on the Baroreflex
6.1. Baroreflex Dysfunction in Hypertension
6.2. Actions of Ang II on the Baroreflex
7. Beyond Ang II
8. Perspectives and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basso, N.; Terragno, N.A. History about the discovery of the renin-angiotensin system. Hypertension 2001, 38, 1246–1249. [Google Scholar] [CrossRef]
- Nehme, A.; Zouein, F.A.; Zayeri, Z.D.; Zibara, K. An update on the tissue renin angiotensin system and its role in physiology and pathology. J. Cardiovasc. Dev. Dis. 2019, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, V.P.; Baker, K.M. The intracellular renin–angiotensin system: A new paradigm. Trends Endocrinol. Metab. 2007, 18, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Re, R.N. Tissue renin angiotensin systems. Med. Clin. N. Am. 2004, 88, 19–38. [Google Scholar] [CrossRef]
- Miller, A.; Arnold, A.C. The renin–angiotensin system in cardiovascular autonomic control: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Haspula, D.; Clark, M.A. Neuroinflammation and sympathetic overactivity: Mechanisms and implications in hypertension. Auton. Neurosci. 2018, 210, 10–17. [Google Scholar] [CrossRef]
- Veerasingham, S.J.; Raizada, M.K. Brain renin-angiotensin system dysfunction in hypertension: Recent advances and perspectives. Br. J. Pharmacol. 2003, 139, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.; Sigmund, C. Minireview: Overview of the Renin-Angiotensin System—An endocrine and paracrine system. Endocrinol. 2003, 144, 2179–2183. [Google Scholar] [CrossRef]
- Browning, K.N.; Verheijden, S.; Boeckxstaens, G.E. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology 2017, 152, 730–744. [Google Scholar] [CrossRef]
- Chang, R.B.; Strochlic, D.E.; Williams, E.K.; Umans, B.; Liberles, S.D. Vagal sensory neuron subtypes that differentially control breathing. Cell 2015, 161, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Campos, I.D.; Pinto, V.; Sousa, N.; Pereira, V.H. A brain within the heart: A review on the intracardiac nervous system. J. Mol. Cell. Cardiol. 2018, 119, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Townend, J.; Al-Ani, M.; West, J.N.; Littler, W.A.; Coote, J.H. Modulation of cardiac autonomic control in humans by angiotensin II. Hypertension 1995, 25, 1270–1275. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Bundy, J.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.N.; Mills, K.T.; He, H.; Chen, J.; Whelton, P.K.; He, J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality. JAMA Cardiol. 2017, 2, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global disparities of hypertension prevalence and control: A systematic analysis of Population-Based studies from 90 countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Saklayen, M.G.; Deshpande, N.V. Timeline of history of hypertension treatment. Front. Cardiovasc. Med. 2016, 3, 3. [Google Scholar] [CrossRef]
- Grassi, G. Assessment of sympathetic cardiovascular drive in human hypertension. Hypertension 2009, 54, 690–697. [Google Scholar] [CrossRef]
- Anderson, E.A.; Sinkey, C.A.; Lawton, W.J.; Mark, A.L. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 1989, 14, 177–183. [Google Scholar] [CrossRef]
- Fisher, J.P.; Paton, J.F.R. The sympathetic nervous system and blood pressure in humans: Implications for hypertension. J. Hum. Hypertens. 2012, 26, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Grassi, G. The autonomic nervous system and hypertension. Circ. Res. 2014, 114, 1804–1814. [Google Scholar] [CrossRef]
- Flaa, A.; Mundal, H.H.; Eide, I.; Kjeldsen, S.; Rostrup, M. Sympathetic activity and cardiovascular risk factors in young men in the low, normal, and high blood pressure ranges. Hypertension 2006, 47, 396–402. [Google Scholar] [CrossRef]
- Davrath, L.R.; Goren, Y.; Pinhas, I.; Toledo, E.; Akselrod, S. Early autonomic malfunction in normotensive individuals with a genetic predisposition to essential hypertension. Am. J. Physiol. Circ. Physiol. 2003, 285, H1697–H1704. [Google Scholar] [CrossRef] [PubMed]
- Del Colle, S.; Morello, F.; Rabbia, F.; Milan, A.; Naso, D.; Puglisi, E.; Mulatero, P.; Veglio, F. Antihypertensive drugs and the sympathetic nervous system. J. Cardiovasc. Pharmacol. 2007, 50, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Moser, M. Evolution of the treatment of hypertension from the 1940s to JNC V. Am. J. Hypertens. 1997, 10, 2S–8S. [Google Scholar] [CrossRef][Green Version]
- Cai, A.; Calhoun, D.A. Resistant hypertension. Hypertension 2017, 70, 5–9. [Google Scholar] [CrossRef]
- Burnier, M.; Egan, B.M. Adherence in hypertension. Circ. Res. 2019, 124, 1124–1140. [Google Scholar] [CrossRef]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.J. Hypertension. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Dickerson, J.C.; Hingorani, A.D.; Ashby, M.J.; Palmer, C.R.; Brown, M.J. Optimisation of antihypertensive treatment by crossover rotation of four major classes. Lancet 1999, 353, 2008–2013. [Google Scholar] [CrossRef]
- Ye, S.; Zhong, H.; Duong, V.N.; Campese, V.M. Losartan Reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertension 2002, 39, 1101–1106. [Google Scholar] [CrossRef]
- Ramchandra, R.; Hood, S.G.; Watson, A.; Allen, A.M.; May, C.N. Central Angiotensin Type 1 receptor blockade decreases cardiac but not renal sympathetic nerve activity in heart failure. Hypertension 2012, 59, 634–641. [Google Scholar] [CrossRef]
- Ramchandra, R.; Barrett, C.; Malpas, S.C. Nitric oxide and sympathetic nerve activity in the control of blood pressure. Clin. Exp. Pharmacol. Physiol. 2005, 32, 440–446. [Google Scholar] [CrossRef]
- Baltatu, O.; Silva, J.J.A.; Ganten, D.; Bader, M. The brain Renin-Angiotensin system modulates angiotensin II–Induced hypertension and cardiac hypertrophy. Hypertension 2000, 35, 409–412. [Google Scholar] [CrossRef]
- Ganten, D.; Hermann, K.; Bayer, C.; Unger, T.; Lang, R.E. Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science 1983, 221, 869–871. [Google Scholar] [CrossRef]
- Gutkind, J.S.; Kurihara, M.; Castren, E.; Saavedra, J.M. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. J. Hypertens. 1988, 6, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhu, D.-N.; Yu, Z.; Wang, J.Q.; Sun, Z.-J.; Yao, T. Expression of angiotensin II type 1 (AT1) receptor in the rostral ventrolateral medulla in rats. J. Appl. Physiol. 2002, 92, 2153–2161. [Google Scholar] [CrossRef]
- Peng, Y.-J.; Raghuraman, G.; Khan, S.A.; Kumar, G.K.; Prabhakar, N.R. Angiotensin II evokes sensory long-term facilitation of the carotid body via NADPH oxidase. J. Appl. Physiol. 2011, 111, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y. Oxidative stress in the brain causes hypertension via sympathoexcitation. Front. Physiol. 2012, 3, 335. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Kimura, Y.; Ito, K.; Shimokawa, H.; Takeshita, A. Increased Reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 2004, 109, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Griendling, K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Winklewski, P.J.; Radkowski, M.; Wszedybyl-Winklewska, M.; Demkow, U. Brain inflammation and hypertension: The chicken or the egg? J. Neuroinflamm. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Li, Y.-L.; Schultz, H.D.; Liu, D.; Cornish, K.G.; Zucker, I.H. Sympathoexcitation by central ANG II: Roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am. J. Physiol. Circ. Physiol. 2005, 288, H2271–H2279. [Google Scholar] [CrossRef] [PubMed]
- Guggilam, A.; Patel, K.P.; Haque, M.; Ebenezer, P.J.; Kapusta, D.R.; Francis, J. Cytokine blockade attenuates sympathoexcitation in heart failure: Cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur. J. Hear. Fail. 2008, 10, 625–634. [Google Scholar] [CrossRef]
- Shi, L.; Fu, A.K.; Ip, N.Y. Multiple roles of the Rho GEF ephexin1 in synapse remodeling. Commun. Integr. Biol. 2010, 3, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Hiley, E.; McMullan, R.; Nurrish, S.J. The Gα12-RGS RhoGEF-RhoA signalling pathway regulates neurotransmitter release in C. elegans. EMBO J. 2006, 25, 5884–5895. [Google Scholar] [CrossRef]
- Takefuji, M.; Yura, Y.; Kaibuchi, K.; Murohara, T. RhoGEF-mediated vasoconstriction in hypertension. Hypertens. Res. 2013, 36, 930–931. [Google Scholar] [CrossRef]
- Allen, A.M.; Lewis, S.J.; Verberne, A.J.; Mendelsohn, F.A. Angiotensin receptors and the vagal system. Clin. Exp. Hypertens. Part. A Theory Pr. 1988, 10, 1239–1249. [Google Scholar] [CrossRef]
- Diz, D.I.; Barnes, K.L.; Ferrario, C.M. Contribution of the vagus nerve to angiotensin II binding sites in the canine medulla. Brain Res Bull 1986, 17, 497–505. [Google Scholar] [CrossRef]
- Allen, A.M.; Chai, S.Y.; Sexton, P.M.; Lewis, S.J.; Verberne, A.J.; Jarrott, B.; Louis, W.J.; Clevers, J.; McKinley, M.J.; Paxinos, G. Angiotensin II receptors and angiotensin converting enzyme in the medulla oblongata. Hypertension 1987, 9, III198–III205. [Google Scholar] [CrossRef]
- Chen, D.; Jancovski, N.; Bassi, J.K.; Nguyen-Huu, T.-P.; Choong, Y.-T.; Palma-Rigo, K.; Davern, P.J.; Gurley, S.B.; Thomas, W.G.; Head, G.A.; et al. Angiotensin Type 1A receptors in C1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress. J. Neurosci. 2012, 32, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.C.; Hart, S.D.; Timmermans, P.B. Effect of angiotensin II antagonism on canine renal sympathetic nerve function. Hypertension 1991, 17, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, M.; Shaltout, H.A.; De Lima, D.C.; Nascimento, K.D.; Chappell, M.C.; Diz, D.I. Central Angiotensin-(1–7) improves vagal function independent of blood pressure in hypertensive (mRen2)27 rats. Hypertension 2012, 60, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Isa, K.; García-Espinosa, M.A.; Arnold, A.C.; Pirro, N.T.; Tommasi, E.N.; Ganten, D.; Chappell, M.C.; Ferrario, C.M.; Diz, D.I. Chronic immunoneutralization of brain angiotensin-(1-12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R111–R115. [Google Scholar] [CrossRef]
- Averill, D.B.; Diz, D.I. Angiotensin peptides and baroreflex control of sympathetic outflow: Pathways and mechanisms of the medulla oblongata. Brain Res. Bull. 2000, 51, 119–128. [Google Scholar] [CrossRef]
- Widdop, R.E.; Krstew, E.; Jarrott, B. Electrophysiological responses of angiotensin peptides on the rat isolated nodose ganglion. Clin. Exp. Hypertens. Part. A Theory Pr. 1992, 14, 597–613. [Google Scholar] [CrossRef]
- Yamaki, F.; Arai, T.; Aoyama, M.; Watanabe, A.; Takata, Y. Angiotensin AT(1)-receptor blockers enhance cardiac responses to parasympathetic nerve stimulation via presynaptic AT(1) receptors in pithed rats. J. Pharmacol. Sci. 2013, 122, 28–33. [Google Scholar] [CrossRef]
- Rechtman, M.; Majewski, H. A facilitatory effect of anti-angiotensin drugs on vagal bradycardia in the pithed rat and guinea-pig. Br. J. Pharmacol. 1993, 110, 289–296. [Google Scholar] [CrossRef]
- Gierthmuehlen, M.; Stieglitz, T.; Zentner, J.; Plachta, D.T.T. Haemodynamic responses to selective vagal nerve stimulation under enalapril medication in rats. PLoS ONE 2016, 11, e0147045. [Google Scholar] [CrossRef][Green Version]
- Potter, E.K. Angiotensin inhibits action of vagus nerve at the heart. Br. J. Pharmacol. 1982, 75, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Yamazaki, T.; Akiyama, T.; Li, M.; Zheng, C.; Shishido, T.; Mori, H.; Sugimachi, M. Angiotensin II attenuates myocardial interstitial acetylcholine release in response to vagal stimulation. Am. J. Physiol. Circ. Physiol. 2007, 293, H2516–H2522. [Google Scholar] [CrossRef]
- Jackson-Cowan, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the brain: The renin angiotensin system. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef]
- Huang, J.; Hara, Y.; Anrather, J.; Speth, R.; Iadecola, C.; Pickel, V. Angiotensin II subtype 1A (AT1A) receptors in the rat sensory vagal complex: Subcellular localization and association with endogenous angiotensin. Neuroscience 2003, 122, 21–36. [Google Scholar] [CrossRef]
- Guo, D.F.; Sun, Y.L.; Hamet, P.; Inagami, T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001, 11, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Ganten, D.; Minnich, J.L.; Grenger, P.; Hayduk, K.; Brecht, H.M.; Barbeau, A.; Boucher, R.; Genest, J. Angiotensin-Forming enzyme in brain tissue. Science 1971, 173, 64–65. [Google Scholar] [CrossRef]
- Paul, M.; Mehr, A.P.; Kreutz, R. Physiology of local Renin-Angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef] [PubMed]
- De Morais, S.D.B.; Shanks, J.; Zucker, I.H. Integrative physiological aspects of brain RAS in hypertension. Curr. Hypertens. Rep. 2018, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stornetta, R.L.; Hawelu-Johnson, C.L.; Guyenet, P.G.; Lynch, K.R. Astrocytes synthesize angiotensinogen in brain. Science 1988, 242, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Haspula, D.; Clark, M.A. MAPK activation patterns of AT1R and CB1R in SHR versus Wistar astrocytes: Evidence of CB1R hypofunction and crosstalk between AT1R and CB1R. Cell. Signal. 2017, 40, 81–90. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, A.; Liu, M.; Rodríguez, V.; Krause, E.; Sumners, C. Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R444–R458. [Google Scholar] [CrossRef]
- Stern, J.E.; Son, S.; Biancardi, V.C.; Zheng, H.; Sharma, N.; Patel, K.P. Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 2016, 68, 1483–1493. [Google Scholar] [CrossRef]
- Herrera, M.; Sparks, M.; Alfonso-Pecchio, A.R.; Harrison-Bernard, L.M.; Coffman, T.M. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 2013, 61, 253–258. [Google Scholar] [CrossRef]
- Biancardi, V.; Son, S.J.; Ahmadi, S.; Filosa, J.A.; Stern, J.E. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood–brain barrier. Hypertension 2014, 63, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, A.; Korim, W.S.; Elsaafien, K.; Yao, S.T. The role of the blood–brain barrier in hypertension. Exp. Physiol. 2018, 103, 337–342. [Google Scholar] [CrossRef]
- Yao, S.; May, C. Intra-carotid angiotensin II activates tyrosine hydroxylase-expressing rostral ventrolateral medulla neurons following blood–brain barrier disruption in rats. Neuroscience 2013, 245, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, V.C.; Stern, J.E. Compromised blood-brain barrier permeability: Novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. J. Physiol. 2015, 594, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.; Lonergan, T.; Deuchars, J.; James, P.; Kasparov, S. Detection of angiotensin II mediated nitric oxide release within the nucleus of the solitary tract using electron-paramagnetic resonance (EPR) spectroscopy. Auton. Neurosci. 2006, 126–127, 193–201. [Google Scholar] [CrossRef]
- Young, C.N.; Davisson, R.L. Angiotensin-II, the brain, and hypertension. Hypertension 2015, 66, 920–926. [Google Scholar] [CrossRef]
- Kumagai, H.; Oshima, N.; Matsuura, T.; Iigaya, K.; Imai, M.; Onimaru, H.; Sakata, K.; Osaka, M.; Onami, T.; Takimoto, C.; et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens. Res. 2011, 35, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Stornetta, R.L.; Holloway, B.B.; Souza, G.M.; Abbott, S.B. Rostral ventrolateral medulla and hypertension. Hypertension 2018, 72, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Spyer, K.M. Neural organisation and control of the baroreceptor reflex. Ergeb. der Physiol. 1981, 88, 24–124. [Google Scholar]
- Felder, R.B.; Mifflin, S.W. Modulation of carotid sinus afferent input to nucleus tractus solitarius by parabrachial nucleus stimulation. Circ. Res. 1988, 63, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.A.; Menani, J.V.; Lopes, O.U.; Colombari, E. Commissural NTS lesions and cardiovascular responses in aortic Baroreceptor–Denervated rats. Hypertension 1999, 34, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Dampney, R.A. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef]
- Wang, J.; Irnaten, M.; Neff, R.A.; Venkatesan, P.; Evans, C.; Loewy, A.D.; Mettenleiter, T.C.; Mendelowitz, D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann. New York Acad. Sci. 2006, 940, 237–246. [Google Scholar] [CrossRef]
- Machhada, A.; Ang, R.; Ackland, G.; Ninkina, N.; Buchman, V.L.; Lythgoe, M.; Trapp, S.; Tinker, A.; Marina, N.; Gourine, A.V. Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Hear. Rhythm. 2015, 12, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Diz, D.I.; Barnes, K.L.; Ferrario, C.M. Hypotensive actions of microinjections of angiotensin II into the dorsal motor nucleus of the vagus. J. Hypertens Suppl. 1984, 2, 53–56. [Google Scholar]
- Averill, D.B.; Diz, D.I.; Barnes, K.L.; Ferrario, C.M. Pressor responses of angiotensin II microinjected into the dorsomedial medulla of the dog. Brain Res. 1987, 414, 294–300. [Google Scholar] [CrossRef]
- Shigematsu, H.; Hirooka, Y.; Eshima, K.; Shihara, M.; Tagawa, T.; Takeshita, A. Endogenous angiotensin II in the NTS contributes to sympathetic activation in rats with aortocaval shunt. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1665–R1673. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, S.M.; Marks, D.; Kobayashi, K.; Okano, H.; Low, M.J.; Andresen, M.C. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J. Neurosci. 2007, 27, 13292–13302. [Google Scholar] [CrossRef]
- Blanch, G.T.; Freiria-Oliveira, A.H.; Speretta, G.F.F.; Carrera, E.J.; Li, H.; Speth, R.C.; Colombari, E.; Sumners, C.; Colombari, D.S.A. Increased expression of angiotensin II type 2 receptors in the solitary-vagal complex blunts renovascular hypertension. Hypertension 2014, 64, 777–783. [Google Scholar] [CrossRef]
- Roulston, C.L.; Lawrence, A.J.; Jarrott, B.; Widdop, R.E. Localization of AT2 receptors in the nucleus of the solitary tract of spontaneously hypertensive and Wistar Kyoto rats using [125I] CGP42112: Upregulation of a non-angiotensin II binding site following unilateral nodose ganglionectomy. Brain Res. 2003, 968, 139–155. [Google Scholar] [CrossRef]
- Armstrong, M.; Kerndt, C.C.; Moore, R.A. Physiology, Baroreceptors; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Standish, A.; Enquist, L.; Schwaber, J. Innervation of the heart and its central medullary origin defined by viral tracing. Science 1994, 263, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Brognara, F.; Felippe, I.S.; Salgado, H.C.; Paton, J.F.R. Autonomic innervation of the carotid body as a determinant of its sensitivity: Implications for cardiovascular physiology and pathology. Cardiovasc. Res. 2021, 117, 1015–1032. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Zhang, D.; Li, Y.-L. Cellular and Molecular mechanisms underlying arterial baroreceptor remodeling in cardiovascular diseases and diabetes. Neurosci. Bull. 2018, 35, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Van Bilsen, M.; Patel, H.C.; Bauersachs, J.; Böhm, M.; Borggrefe, M.; Brutsaert, D.; Coats, A.J.; De Boer, R.A.; De Keulenaer, G.W.; Filippatos, G.S.; et al. The autonomic nervous system as a therapeutic target in heart failure: A scientific position statement from the translational research committee of the heart failure association of the European society of cardiology. Eur. J. Hear. Fail. 2017, 19, 1361–1378. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Robbi, E.; Caporotondi, A.; Guazzotti, G.; Sleight, P.; Febo, O. Prognostic implications of baroreflex sensitivity in heart failure patients in the Beta-Blocking Era. J. Am. Coll. Cardiol. 2009, 53, 193–199. [Google Scholar] [CrossRef]
- Joseph, C.N.; Porta, C.; Casucci, G.; Casiraghi, N.; Maffeis, M.; Rossi, M.; Bernardi, L. Slow Breathing Improves Arterial Baroreflex Sensitivity and Decreases Blood Pressure in Essential Hypertension. Hypertension 2005, 46, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Ormezzano, O.; Cracowski, J.-L.; Quesada, J.-L.; Pierre, H.; Mallion, J.-M.; Baguet, J.-P. EVAluation of the prognostic value of BARoreflex sensitivity in hypertensive patients: The EVABAR study. J. Hypertens. 2008, 26, 1373–1378. [Google Scholar] [CrossRef]
- Johansson, M.; Gao, S.A.; Friberg, P.; Annerstedt, M.; Carlström, J.; Ivarsson, T.; Jensen, G.; Ljungman, S.; Mathillas, Ö.; Nielsen, F.-D.; et al. Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J. Hypertens. 2007, 25, 163–168. [Google Scholar] [CrossRef]
- Takeshita, A.; Tanaka, S.; Kuroiwa, A.; Nakamura, M. Reduced baroreceptor sensitivity in borderline hypertension. Circulation 1975, 51, 738–742. [Google Scholar] [CrossRef]
- Parmer, R.J.; Cervenka, J.H.; Stone, R.A. Baroreflex sensitivity and heredity in essential hypertension. Circulation 1992, 85, 497–503. [Google Scholar] [CrossRef]
- Grassi, G.; Cattaneo, B.M.; Seravalle, G.; Lanfranchi, A.; Mancia, G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 1998, 31, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Rea, R.F.; Hamdan, M. Baroreflex control of muscle sympathetic nerve activity in borderline hypertension. Circulation 1990, 82, 856–862. [Google Scholar] [CrossRef]
- Tromp, T.; Mahesh, D.; Joles, J.A.; Ramchandra, R. Direct recording of cardiac and renal sympathetic nerve activity shows differential control in renovascular hypertension. Hypertension 2018, 71, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Wallbach, M.; Koziolek, M.J. Baroreceptors in the carotid and hypertension—systematic review and meta-analysis of the effects of baroreflex activation therapy on blood pressure. Nephrol. Dial. Transplant. 2017, 33, 1485–1493. [Google Scholar] [CrossRef]
- Abdala, A.P.; McBryde, F.D.; Marina, N.; Hendy, E.B.; Engelman, Z.J.; Fudim, M.; Sobotka, P.A.; Gourine, A.V.; Paton, J.F.R. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J. Physiol. 2012, 590, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Laterza, M.C.; De Matos, L.D.; Trombetta, I.C.; Braga, A.M.; Roveda, F.; Alves, M.J.; Krieger, E.M.; Negrão, C.E.; Rondon, M.U. Exercise training restores baroreflex sensitivity in Never-Treated hypertensive patients. Hypertension 2007, 49, 1298–1306. [Google Scholar] [CrossRef]
- Bernardi, L.; Porta, C.; Spicuzza, L.; Bellwon, J.; Spadacini, G.; Frey, A.W.; Yeung, L.Y.; Sanderson, J.E.; Pedretti, R.; Tramarin, R. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002, 105, 143–145. [Google Scholar] [CrossRef]
- Ramchandra, R.; Yao, S.T.; May, C.N. Organ selective regulation of sympathetic outflow by the brain angiotensin system. Curr. Hypertens. Rep. 2013, 15, 401–408. [Google Scholar] [CrossRef]
- Reid, I.A. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am. J. Physiol. Metab. 1992, 262, E763–E778. [Google Scholar] [CrossRef]
- Huber, G.; Schuster, F.; Raasch, W. Brain renin-angiotensin system in the pathophysiology of cardiovascular diseases. Pharmacol. Res. 2017, 125, 72–90. [Google Scholar] [CrossRef]
- Garner, M.G.; Phippard, A.F.; Fletcher, P.J.; Maclean, J.M.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Effect of angiotensin II on baroreceptor reflex control of heart rate in conscious baboons. Hypertension 1987, 10, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.A.; Chou, L. Analysis of the action of angiotensin II on the baroreflex control of heart rate in conscious rabbits *. Endocrinology 1990, 126, 2749–2756. [Google Scholar] [CrossRef]
- Allen, A.M.; McKinley, M.J.; Oldfield, B.; Dampney, R.A.L.; Mendelsohn, F.A.O. Angiotensin II Receptor binding and the baroreflex pathway. Clin. Exp. Hypertens. Part. A Theory Pr. 1988, 10, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.D.; Stephenson, R.B.; Weaver, L.C. Central actions of angiotensin II oppose baroreceptor-induced sympathoinhibition. Am. J. Physiol. Integr. Comp. Physiol. 1984, 246, R13–R19. [Google Scholar] [CrossRef] [PubMed]
- Lumbers, E.R.; McCloskey, D.I.; Potter, E.K. Inhibition by angiotensin II of baroreceptor-evoked activity in cardiac vagal efferent nerves in the dog. J. Physiol. 1979, 294, 69–80. [Google Scholar] [CrossRef]
- Matsukawa, S.; Reid, I.A. Role of the area postrema in the modulation of the baroreflex control of heart rate by angiotensin II. Circ. Res. 1990, 67, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Gole, H.; Pamidimukkala, J.; Hay, M. Role of the area postrema in angiotensin II modulation of baroreflex control of heart rate in conscious mice. Am. J. Physiol. Circ. Physiol. 2003, 284, H1003–H1007. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Su, C.-H.; Baudrie, V.; Laude, D.; Weng, J.-C.; Chang, A.Y.; Chan, J.Y.; Elghozi, J.-L.; Chan, S.H. Visualizing oxidative stress-induced depression of cardiac vagal baroreflex by MRI/DTI in a mouse neurogenic hypertension model. NeuroImage 2013, 82, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Lohmeier, T.; Lohmeier, J.R.; Warren, S.; May, P.J.; Cunningham, J.T. Sustained activation of the central baroreceptor pathway in angiotensin hypertension. Hypertension 2002, 39, 550–556. [Google Scholar] [CrossRef]
- McMullan, S.; Goodchild, A.K.; Pilowsky, P.M. Circulating angiotensin II attenuates the sympathetic baroreflex by reducing the barosensitivity of medullary cardiovascular neurones in the rat. J. Physiol. 2007, 582, 711–722. [Google Scholar] [CrossRef]
- Huang, C.; Yoshimoto, M.; Miki, K.; Johns, E.J. The contribution of brain angiotensin II to the baroreflex regulation of renal sympathetic nerve activity in conscious normotensive and hypertensive rats. J. Physiol. 2006, 574, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.S.P.; Killinger, S.; Horiuchi, J.; Dampney, R.A.L. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am. J. Physiol. Integr. Comp. Physiol. 2007, 293, R2267–R2278. [Google Scholar] [CrossRef]
- Luoh, S.H.; Chan, S.H. Inhibition of baroreflex by angiotensin II via Fos expression in nucleus tractus solitarii of the rat. Hypertension 2001, 38, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Averill, D.B.; Ferrario, C.M. Angiotensin II acts at AT1receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R1611–R1619. [Google Scholar] [CrossRef] [PubMed]
- Palma-Rigo, K.; Bassi, J.K.; Nguyen-Huu, T.-P.; Jackson, K.L.; Davern, P.J.; Chen, D.; Elghozi, J.-L.; Thomas, W.G.; Allen, A.M.; Head, G.A. Angiotensin 1A receptors transfected into caudal ventrolateral medulla inhibit baroreflex gain and stress responses. Cardiovasc. Res. 2012, 96, 330–339. [Google Scholar] [CrossRef]
- Isa, K.; Arnold, A.C.; Westwood, B.M.; Chappell, M.C.; Diz, D.I. Angiotensin-converting enzyme inhibition, but not AT1 receptor blockade, in the solitary tract nucleus improves baroreflex sensitivity in anesthetized transgenic hypertensive (mRen2)27 rats. Hypertens. Res. 2011, 34, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.M.; Barnes, K.L.; Block, C.H.; Brosnihan, K.B.; Diz, D.I.; Khosla, M.C.; Santos, R.A. Pathways of angiotensin formation and function in the brain. Hypertension 1990, 15, I13–I19. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Gao, L.; Belatti, D.; Curry, P.L.; Zucker, I.H. Central Angiotensin (1–7) Enhances Baroreflex Gain in Conscious Rabbits With Heart Failure. Hypertension 2011, 58, 627–634. [Google Scholar] [CrossRef]
- Chaves, G.Z.; Caligiorne, S.M.; Santos, R.A.; Khosla, M.C.; Campagnole-Santos, M.J. Modulation of the baroreflex control of heart rate by angiotensin-(1–7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J. Hypertens. 2000, 18, 1841–1848. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, H.; Cai, Y.; Halabi, C.M.; Becker, L.K.; Santos, R.A.; Speth, R.C.; Sigmund, C.; Lazartigues, E. Brain-Selective Overexpression of Human Angiotensin-Converting Enzyme Type 2 Attenuates Neurogenic Hypertension. Circ. Res. 2010, 106, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Yamazato, M.; Ferreira, A.J.; Yamazato, Y.; Diez-Freire, C.; Yuan, L.; Gillies, R.; Raizada, M.K. Gene transfer of angiotensin-converting enzyme 2 in the nucleus tractus solitarius improves baroreceptor heart rate reflex in spontaneously hypertensive rats. J. Renin-Angiotensin-Aldosterone Syst. 2011, 12, 456–461. [Google Scholar] [CrossRef]
- Arnold, A.C.; Isa, K.; Shaltout, H.A.; Nautiyal, M.; Ferrario, C.M.; Chappell, M.C.; Diz, D.I. Angiotensin-(1–12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am. J. Physiol. Circ. Physiol. 2010, 299, H763–H771. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.A.; Worker, C.J.; Li, W.; Trebak, F.; Watkins, T.; Gayban, A.J.B.; Yamasaki, E.; Cooper, S.G.; Drumm, B.; Feng, Y. (Pro)renin receptor knockdown in the paraventricular nucleus of the hypothalamus attenuates hypertension development and AT1 receptor-mediated calcium events. Am. J. Physiol. Circ. Physiol. 2019, 316, H1389–H1405. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, A.; Mastitskaya, S.; Aristovich, K. Selective Neuromodulation of the Vagus Nerve. Front. Neurosci. 2021, 15, 685872. [Google Scholar] [CrossRef]
- Ardell, J.L.; Rajendran, P.S.; Nier, H.A.; KenKnight, B.H.; Armour, J.A. Central-peripheral neural network interactions evoked by vagus nerve stimulation: Functional consequences on control of cardiac function. Am. J. Physiol. Circ. Physiol. 2015, 309, H1740–H1752. [Google Scholar] [CrossRef]
- Booth, L.C.; Yao, S.T.; Korsak, A.; Farmer, D.G.; Hood, S.G.; McCormick, D.; Boesley, Q.; Connelly, A.A.; McDougall, S.J.; Korim, W.S.; et al. Selective optogenetic stimulation of efferent fibers in the vagus nerve of a large mammal. Brain Stimul. 2021, 14, 88–96. [Google Scholar] [CrossRef]
- Takayama, Y.; Kushige, H.; Akagi, Y.; Suzuki, Y.; Kumagai, Y.; Kida, Y.S. Selective Induction of Human Autonomic Neurons Enables Precise Control of Cardiomyocyte Beating. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Winbo, A.; Ramanan, S.; Eugster, E.; Jovinge, S.; Skinner, J.R.; Montgomery, J.M. Functional coculture of sympathetic neurons and cardiomyocytes derived from human-induced pluripotent stem cells. Am. J. Physiol. Circ. Physiol. 2020, 319, H927–H937. [Google Scholar] [CrossRef] [PubMed]
- Ruchaya, P.J.; Speretta, G.F.; Blanch, G.T.; Li, H.; Sumners, C.; Menani, J.V.; Colombari, E.; Colombari, D.S. Overexpression of AT2R in the solitary-vagal complex improves baroreflex in the spontaneously hypertensive rat. Neuropeptides 2016, 60, 29–36. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanks, J.; Ramchandra, R. Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension. Int. J. Mol. Sci. 2021, 22, 12305. https://doi.org/10.3390/ijms222212305
Shanks J, Ramchandra R. Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension. International Journal of Molecular Sciences. 2021; 22(22):12305. https://doi.org/10.3390/ijms222212305
Chicago/Turabian StyleShanks, Julia, and Rohit Ramchandra. 2021. "Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension" International Journal of Molecular Sciences 22, no. 22: 12305. https://doi.org/10.3390/ijms222212305
APA StyleShanks, J., & Ramchandra, R. (2021). Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension. International Journal of Molecular Sciences, 22(22), 12305. https://doi.org/10.3390/ijms222212305





