Cabozantinib Is Effective in Melanoma Brain Metastasis Cell Lines and Affects Key Signaling Pathways
Abstract
1. Introduction
2. Results
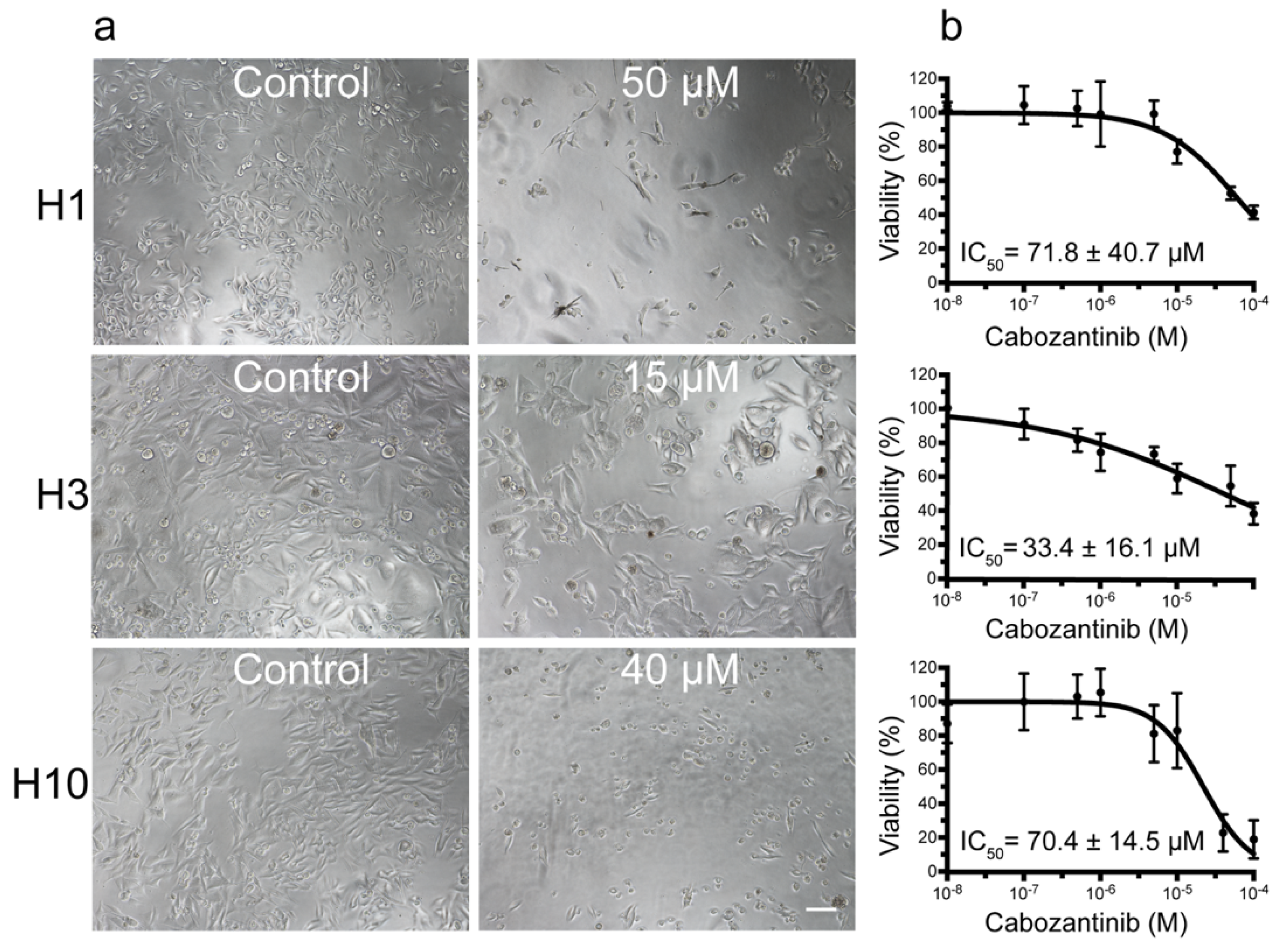
2.1. MBM Cell Viability and Colony Formation Is Decreased after Cabozantinib Treatment
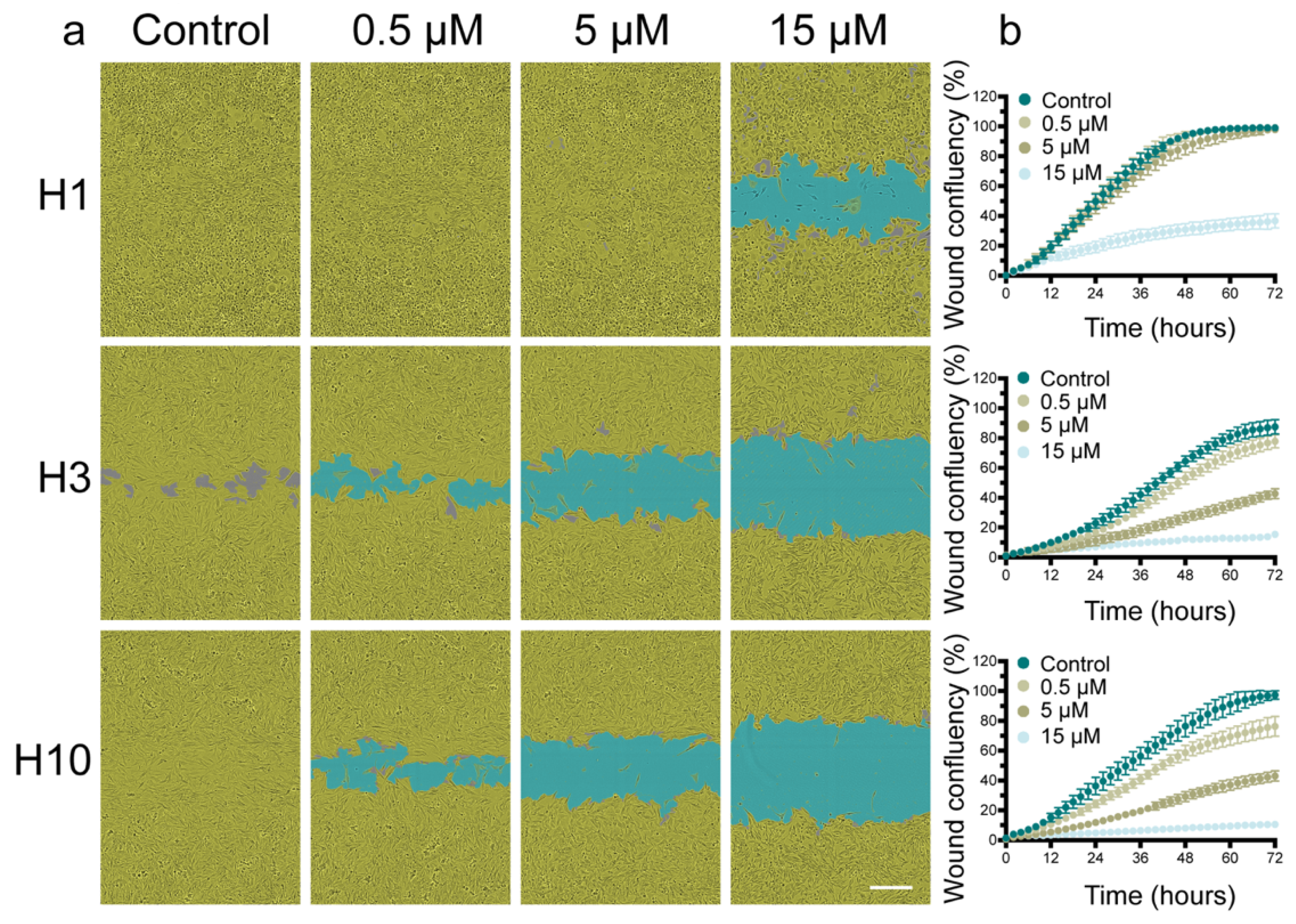
2.2. Cabozantinib Inhibits MBM Cell Migration
2.3. Cabozantinib Induces Apoptosis in MBM Cell Lines
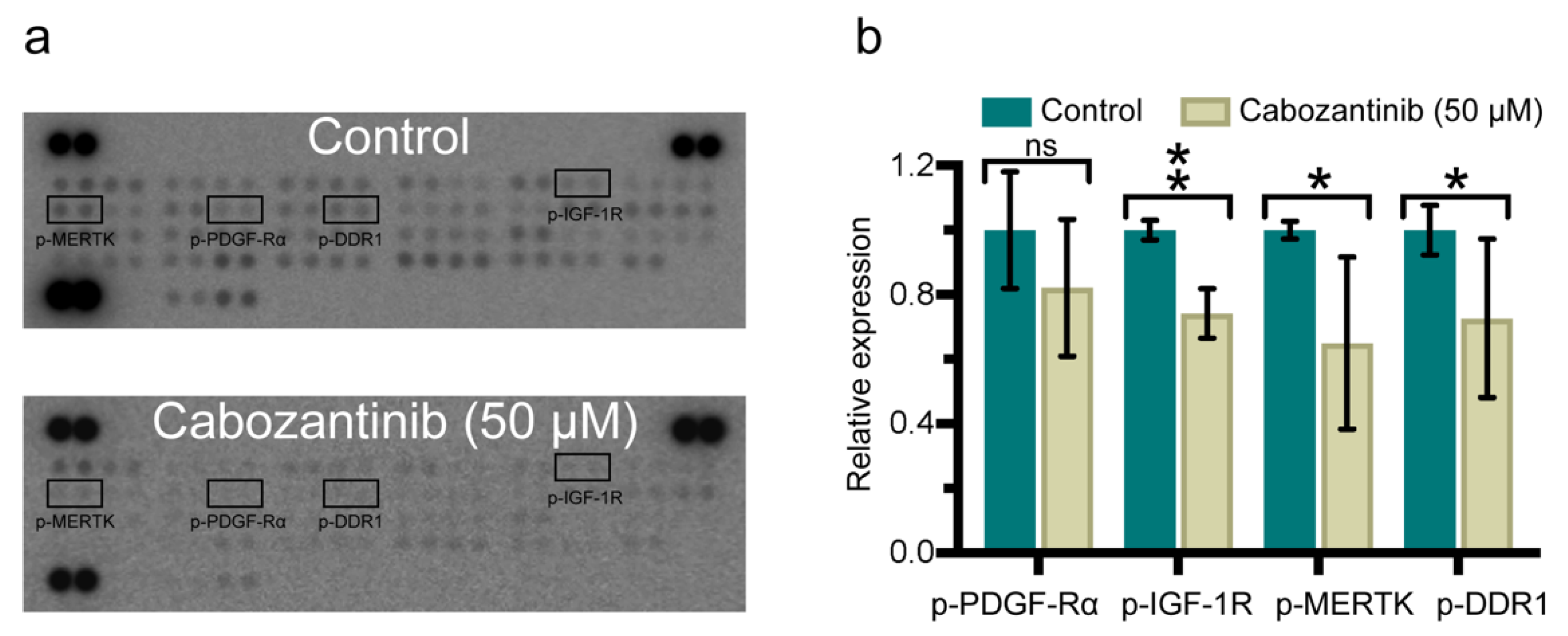
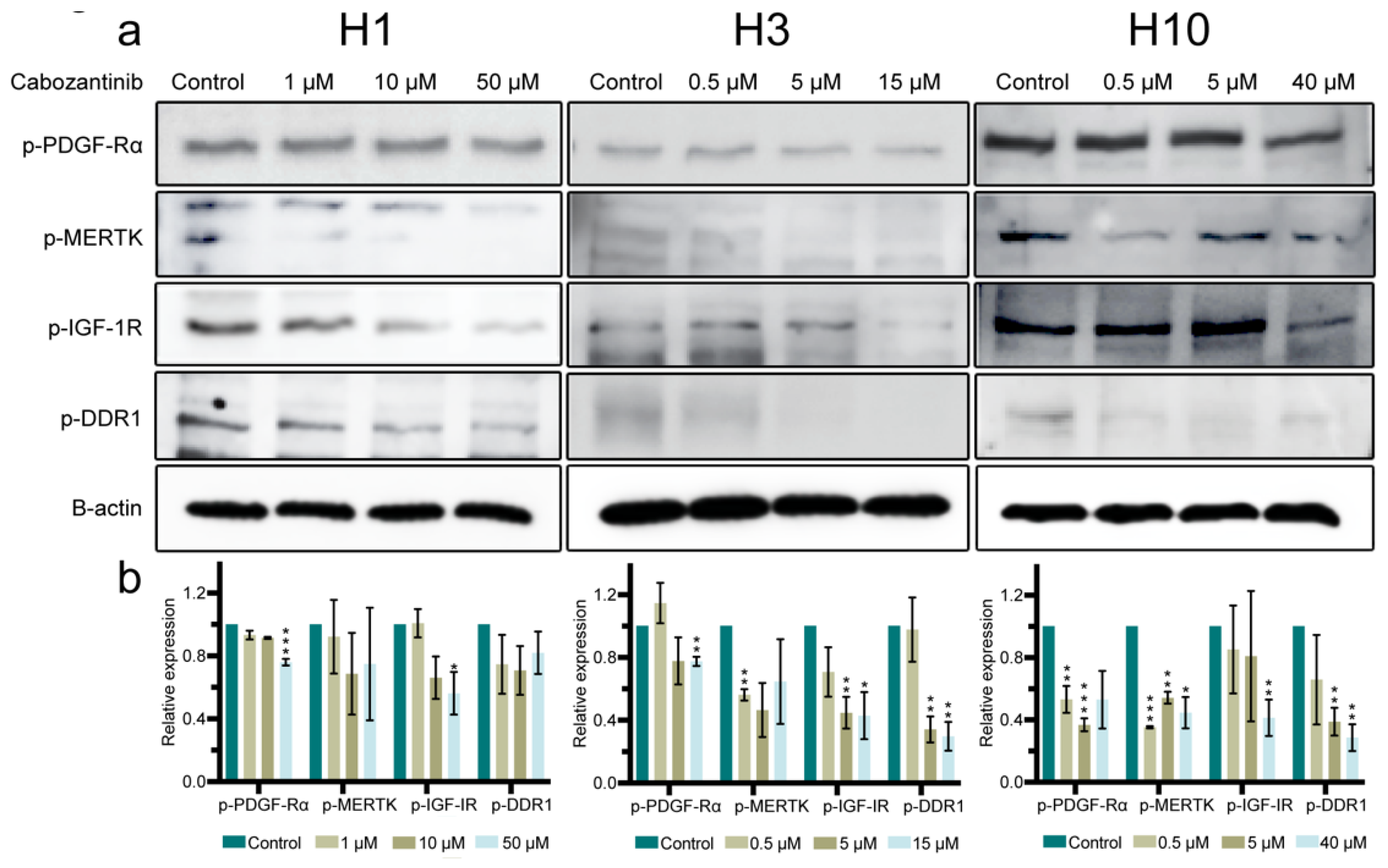
2.4. Cabozantinib Targets a Broad Range of p-RTKs in MBM Cells
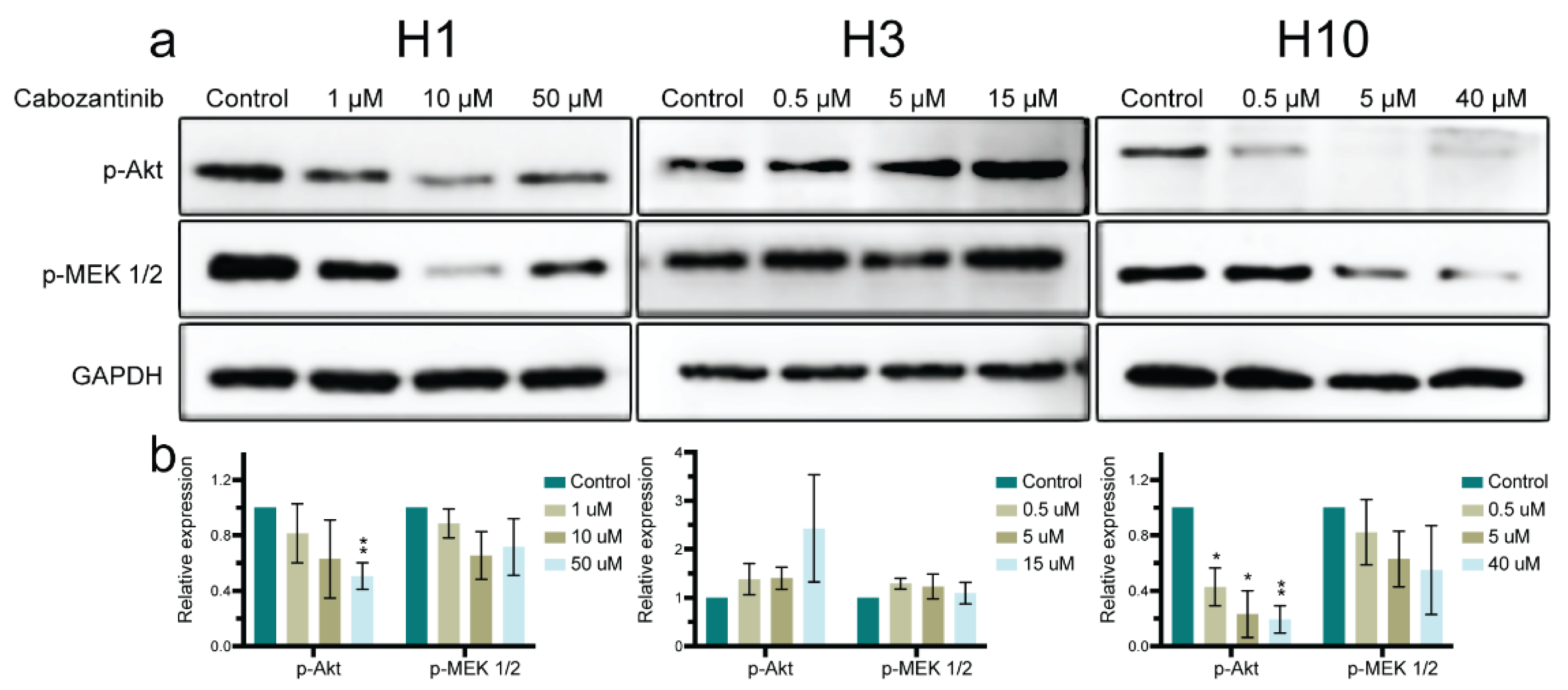
2.5. MAPK and PI3K-Akt Signaling Pathways in MBM Cells Are Affected by Cabozantinib
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Drug
4.3. Monolayer Cell Viability Assay
4.4. Tumorsphere Cell Viability Assay
4.5. Cell Migration Assay
4.6. Apoptosis Assay by Flow Cytometry
4.7. Apoptosis Assay by Caspase-3/7 Activity
4.8. Receptor Tyrosine Kinase (RTK) Array
4.9. Western Blots
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BRAF | v-Raf murine sarcoma viral oncogene homolog B1 |
| BBB | Blood–brain barrier |
| RTK | Receptor tyrosine kinase |
| FDA | The United States Food and Drug Administration |
| C-met | Mesenchymal-epithelial transition factor |
| VEGFR | Vascular endothelial growth factor receptor |
| MBM | Melanoma brain metastasis |
| REC | The Regional Ethical Committee |
| STR | Short tandem repeat |
| EDTA | Ethylenediamine tetraacetic acid |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt |
| IC50 | Half maximal inhibitory concentration |
| RT | Room temperature |
| PBS | Phosphate buffered saline |
| p-RTK | Phosphorylated-RTK |
| BCA | Bicinchoninic acid |
| HRP | Horseradish peroxidase |
| WB | Western blot |
| RIPA | Radioimmunoprecipitation assay |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TBS | Tris-buffered saline |
| OS | Overall survival |
| UM | Uveal melanoma |
| GPCR | G protein-coupled receptor |
| PDGF-Rα | Platelet-derived growth factor-receptor α |
| MERTK | Mer tyrosine kinase |
| IGF-1R | Insulin-like growth factor 1 receptor |
| DDR1 | Discoidin domain receptor 1 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphoinositide 3-kinase |
| Akt | Protein kinase B |
| MEK 1/2 | Dual specificity mitogen-activated protein kinase 1/2 |
| PTEN | Phosphatase and tensin homolog |
| APC | Adenomatous polyposis coli |
| NRAS | Neuroblastoma rat sarcoma protein |
| KRAS | Kirsten rat sarcoma protein |
| Erk1/2 | Extracellular signal-regulated kinase 1/2 |
References
- Zhang, D.; Wang, Z.; Shang, D.; Yu, J.; Yuan, S. Incidence and prognosis of brain metastases in cutaneous melanoma patients: A population-based study. Melanoma Res. 2019, 29, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Choi, J.-W.; Kim, Y.-S. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: A meta-analysis. Br. J. Dermatol. 2010, 164, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Fattore, L.; Ruggiero, C.F.; Liguoro, D.; Castaldo, V.; Catizone, A.; Ciliberto, G.; Mancini, R. The Promise of Liquid Biopsy to Predict Response to Immunotherapy in Metastatic Melanoma. Front. Oncol. 2021, 11, 5069. [Google Scholar] [CrossRef]
- Mittapalli, R.K.; Vaidhyanathan, S.; Dudek, A.Z.; Elmquist, W.F. Mechanisms Limiting Distribution of the Threonine-Protein Kinase B-RaFV600E Inhibitor Dabrafenib to the Brain: Implications for the Treatment of Melanoma Brain Metastases. J. Pharmacol. Exp. Ther. 2013, 344, 655–664. [Google Scholar] [CrossRef]
- Gampa, G.; Vaidhyanathan, S.; Sarkaria, J.N.; Elmquist, W.F. Drug delivery to melanoma brain metastases: Can current challenges lead to new opportunities? Pharmacol. Res. 2017, 123, 10–25. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- National Cancer Institute. FDA Approvals for Cabozantinib. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/cabozantinib-s-malate (accessed on 9 December 2020).
- Négrier, S.; Moriceau, G.; Attignon, V.; Haddad, V.; Pissaloux, D.; Guerin, N.; Carrie, C. Activity of cabozantinib in radioresistant brain metastases from renal cell carcinoma: Two case reports. J. Med. Case Rep. 2018, 12, 351. [Google Scholar] [CrossRef]
- Sun, T.Y.; Niu, X.; Chakraborty, A.; Neal, J.W.; Wakelee, H.A. Lengthy Progression-Free Survival and Intracranial Activity of Cabozantinib in Patients with Crizotinib and Ceritinib-Resistant ROS1-Positive Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 14, e21–e24. [Google Scholar] [CrossRef]
- Wen, P.Y.; Drappatz, J.; De Groot, J.; Prados, M.D.; Reardon, A.D.; Schiff, D.; Chamberlain, M.; Mikkelsen, T.; Desjardins, A.; Holland, J.; et al. Phase II study of cabozantinib in patients with progressive glioblastoma: Subset analysis of patients naive to antiangiogenic therapy. Neuro-Oncology 2017, 20, 249–258. [Google Scholar] [CrossRef]
- Hirsch, L.; Chanza, N.M.; Farah, S.; Xie, W.; Flippot, R.; Braun, D.A.; Rathi, N.; Thouvenin, J.; Collier, K.A.; Seront, E.; et al. Clinical Activity and Safety of Cabozantinib for Brain Metastases in Patients With Renal Cell Carcinoma. JAMA Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a Novel MET and VEGFR2 Inhibitor, Simultaneously Suppresses Metastasis, Angiogenesis, and Tumor Growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Chiarion-Sileni, V.; Del Vecchio, M.; Ferrucci, P.F.; Guida, M.; Quaglino, P.; Guidoboni, M.; Marchetti, P.; Cutaia, O.; Amato, G.; et al. Primary Analysis and 4-Year Follow-Up of the Phase III NIBIT-M2 Trial in Melanoma Patients with Brain Metastases. Clin. Cancer Res. 2021, 27, 4737–4745. [Google Scholar] [CrossRef]
- Daud, A.; Kluger, H.M.; Kurzrock, R.; Schimmoller, F.; Weitzman, A.L.; Samuel, T.A.; Moussa, A.H.; Gordon, M.S.; Shapiro, G.I. Phase II randomised discontinuation trial of the MET/VEGF receptor inhibitor cabozantinib in metastatic melanoma. Br. J. Cancer 2017, 116, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Olson, D.J.; Allred, J.B.; Strand, C.A.; Bao, R.; Zha, Y.; Carll, T.; Labadie, B.W.; Bastos, B.R.; Butler, M.O.; et al. Randomized Phase II Trial and Tumor Mutational Spectrum Analysis from Cabozantinib versus Chemotherapy in Metastatic Uveal Melanoma (Alliance A091201). Clin. Cancer Res. 2020, 26, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhuo, X.; Fu, X.; Wu, Y.; Mao, C. Targeting PAR2 Overcomes Gefitinib Resistance in Non-Small-Cell Lung Cancer Cells Through Inhibition of EGFR Transactivation. Front. Pharmacol. 2021, 12, 625289. [Google Scholar] [CrossRef]
- Shang, R.; Song, X.; Wang, P.; Zhou, Y.; Lu, X.; Wang, J.; Xu, M.; Chen, X.; Utpatel, K.; Che, L.; et al. Cabozantinib-based combination therapy for the treatment of hepatocellular carcinoma. Gut 2020, 70, 1746–1757. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, H.; Sun, Y.; Qin, M.; Gong, P.; Hou, Y.; Zhao, Y. Design, synthesis and biological evaluation of novel c-Met/HDAC dual inhibitors. Bioorganic Med. Chem. Lett. 2020, 30, 127610. [Google Scholar] [CrossRef]
- Starenki, D.; Sosonkina, N.; Hong, S.-K.; Lloyd, R.V.; Park, J.-I. Mortalin (GRP75/HSPA9) Promotes Survival and Proliferation of Thyroid Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2069. [Google Scholar] [CrossRef]
- Zhang, L.; Scorsone, A.K.; Woodfield, S.E.; Zage, P.E. Sensitivity of neuroblastoma to the novel kinase inhibitor cabozantinib is mediated by ERK inhibition. Cancer Chemother. Pharmacol. 2015, 76, 977–987. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Karuppusamy Rathinam, M.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The Soft Agar Colony Formation Assay. J. Vis. Exp. 2014, 92, e51998. [Google Scholar] [CrossRef]
- Sundstrøm, T.; Prestegarden, L.; Azuaje, F.; Aasen, S.N.; Røsland, G.V.; Varughese, J.K.; Bahador, M.; Bernatz, S.; Braun, Y.; Harter, P.N.; et al. Inhibition of mitochondrial respiration prevents BRAF-mutant melanoma brain metastasis. Acta Neuropathol. Commun. 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Didolkar, M.; Pickren, J.; Moore, R. Metastatic pattern of malignant melanoma: A study of 216 autopsy cases. Am. J. Surg. 1978, 135, 807–810. [Google Scholar] [CrossRef]
- Xiang, Q.; Chen, W.; Ren, M.; Wang, J.; Zhang, H.; Deng, D.Y.; Zhang, L.; Shang, C.; Chen, Y. Cabozantinib Suppresses Tumor Growth and Metastasis in Hepatocellular Carcinoma by a Dual Blockade of VEGFR2 and MET. Clin. Cancer Res. 2014, 20, 2959–2970. [Google Scholar] [CrossRef]
- Broussard, L.; Howland, A.; Ryu, S.; Song, K.; Norris, D.; Armstrong, C.A.; Song, P.I. Melanoma Cell Death Mechanisms. Chonnam Med. J. 2018, 54, 135–142. [Google Scholar] [CrossRef][Green Version]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-Da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Wu, Z.; Ng, R.C.-L.; Garcia-Barceló, M.-M.; Yuan, Z.-W.; Wong, K.K.Y.; Tam, P.K.H.; Lui, V.C.H. Conditional deletion of platelet derived growth factor receptor alpha (Pdgfra) in urorectal mesenchyme causes mesenchyme apoptosis and urorectal developmental anomalies in mice. Cell Death Differ. 2018, 26, 1396–1410. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 2006, 17, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.S.; McMahon, E.J.; Pop, S.M.; Reap, E.A.; Caricchio, R.; Cohen, P.L.; Earp, H.S.; Matsushima, G.K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001, 411, 207–211. [Google Scholar] [CrossRef]
- Assent, D.; Bourgot, I.; Hennuy, B.; Geurts, P.; Noel, A.; Foidart, J.-M.; Maquoi, E. A Membrane-Type-1 Matrix Metalloproteinase (MT1-MMP)—Discoidin Domain Receptor 1 Axis Regulates Collagen-Induced Apoptosis in Breast Cancer Cells. PLoS ONE 2015, 10, e0116006. [Google Scholar] [CrossRef] [PubMed]
- Willenberg, A.; Saalbach, A.; Simon, J.C.; Anderegg, U. Melanoma Cells Control HA Synthesis in Peritumoral Fibroblasts via PDGF-AA and PDGF-CC: Impact on Melanoma Cell Proliferation. J. Investig. Dermatol. 2012, 132, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sinnberg, T.; Niessner, H.; Dölker, R.; Sauer, B.; Kempf, W.E.; Meier, F.; Leslie, N.; Schittek, B. PTEN regulates IGF-1R-mediated therapy resistance in melanoma. Pigment Cell Melanoma Res. 2015, 28, 572–589. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Oliver, J.W.; Hazra, S.; Benzaghou, F.; Yau, T.; Cheng, A.-L.; Rimassa, L. Cabozantinib in combination with atezolizumab versus sorafenib in treatment-naive advanced hepatocellular carcinoma: COSMIC-312 Phase III study design. Futur. Oncol. 2020, 16, 1525–1536. [Google Scholar] [CrossRef]
- Schlegel, J.; Sambade, M.J.; Sather, S.; Moschos, S.J.; Tan, A.C.; Winges, A.; DeRyckere, D.; Carson, C.C.; Trembath, D.G.; Tentler, J.J.; et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J. Clin. Investig. 2013, 123, 2257–2267. [Google Scholar] [CrossRef]
- Tworkoski, K.A.; Platt, J.T.; Bacchiocchi, A.; Bosenberg, M.; Boggon, T.J.; Stern, D.F. MERTK controls melanoma cell migration and survival and differentially regulates cell behavior relative to AXL. Pigment Cell Melanoma Res. 2013, 26, 527–541. [Google Scholar] [CrossRef]
- Fukunaga-Kalabis, M.; Martinez, G.; Liu, Z.-J.; Kalabis, J.; Mrass, P.; Weninger, W.; Firth, S.M.; Planque, N.; Perbal, B.; Herlyn, M. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J. Cell Biol. 2006, 175, 563–569. [Google Scholar] [CrossRef]
- Reger de Moura, C.; Battistella, M.; Sohail, A.; Caudron, A.; Feugeas, J.P.; Podgorniak, M.P.; Pages, C.; Mazouz Dorval, S.; Marco, O.; Menashi, S.; et al. Discoidin domain receptors: A promising target in melanoma. Pigment Cell Melanoma Res. 2019, 32, 697–707. [Google Scholar] [CrossRef]
- Romayor, I.; Badiola, I.; Olaso, E. Inhibition of DDR1 reduces invasive features of human A375 melanoma, HT29 colon carcinoma and SK-HEP hepatoma cells. Cell Adhes. Migr. 2020, 14, 69–81. [Google Scholar] [CrossRef]
- McKay, M.M.; Morrison, D.K. Integrating signals from RTKs to ERK/MAPK. Oncogene 2007, 26, 3113–3121. [Google Scholar] [CrossRef]
- Haddadi, N.; Lin, Y.; Travis, G.; Simpson, A.M.; Nassif, N.T.; McGowan, E.M. PTEN/PTENP1: ’Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol. Cancer 2018, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Devji, T.; Levine, O.; Neupane, B.; Beyene, J.; Xie, F. Systemic Therapy for Previously Untreated Advanced BRAF-Mutated Melanoma: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. JAMA Oncol. 2017, 3, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.L.; Martinka, M.; Li, G. Prognostic Significance of Activated Akt Expression in Melanoma: A Clinicopathologic Study of 292 Cases. J. Clin. Oncol. 2005, 23, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, X.; Qu, H.; Qu, B.; Yin, X.; Zhao, H. Cabozantinib induces PUMA-dependent apoptosis in colon cancer cells via AKT/GSK-3β/NF-κB signaling pathway. Cancer Gene Ther. 2020, 27, 368–377. [Google Scholar] [CrossRef]
- Gajos-Michniewicz, A.; Czyz, M. WNT Signaling in Melanoma. Int. J. Mol. Sci. 2020, 21, 4852. [Google Scholar] [CrossRef]
- Jeong, W.-J.; Ro, E.J.; Choi, K.-Y. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. Npj Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef]
- Bergström, J.D.; Westermark, B.; Heldin, N.-E. Epidermal Growth Factor Receptor Signaling Activates Met in Human Anaplastic Thyroid Carcinoma Cells. Exp. Cell Res. 2000, 259, 293–299. [Google Scholar] [CrossRef]
- Janssen, J.A. New Insights from IGF-IR Stimulating Activity Analyses: Pathological Considerations. Cells 2020, 9, 862. [Google Scholar] [CrossRef]
- Vella, V.; Malaguarnera, R.; Nicolosi, M.L.; Morrione, A.; Belfiore, A. Insulin/IGF signaling and discoidin domain receptors: An emerging functional connection. Biochim. Biophys. Acta (BBA)-Bioenerg. 2019, 1866, 118522. [Google Scholar] [CrossRef]
- Malaguarnera, R.; Nicolosi, M.L.; Sacco, A.; Morcavallo, A.; Vella, V.; Voci, C.; Spatuzza, M.; Xu, S.Q.; Iozzo, R.V.; Vigneri, R.; et al. Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget 2015, 6, 16084–16105. [Google Scholar] [CrossRef]
- Bischof, K.; Knappskog, S.; Hjelle, S.M.; Stefansson, I.; Woie, K.; Salvesen, H.B.; Gjertsen, B.T.; Bjorge, L. Influence of p53 Isoform Expression on Survival in High-Grade Serous Ovarian Cancers. Sci. Rep. 2019, 9, 5244. [Google Scholar] [CrossRef] [PubMed]
- Sundstrøm, T.; Espedal, H.; Harter, P.N.; Fasmer, K.E.; Skaftnesmo, K.O.; Horn, S.; Hodneland, E.; Mittelbronn, M.; Weide, B.; Beschorner, R.; et al. Melanoma brain metastasis is independent of lactate dehydrogenase A expression. Neuro-Oncology 2015, 17, 1374–1385. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannsåker, T.A.; Hoang, T.; Aasen, S.N.; Bjørnstad, O.V.; Parajuli, H.; Sundstrøm, T.; Thorsen, F.A. Cabozantinib Is Effective in Melanoma Brain Metastasis Cell Lines and Affects Key Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 12296. https://doi.org/10.3390/ijms222212296
Mannsåker TA, Hoang T, Aasen SN, Bjørnstad OV, Parajuli H, Sundstrøm T, Thorsen FA. Cabozantinib Is Effective in Melanoma Brain Metastasis Cell Lines and Affects Key Signaling Pathways. International Journal of Molecular Sciences. 2021; 22(22):12296. https://doi.org/10.3390/ijms222212296
Chicago/Turabian StyleMannsåker, Trond Are, Tuyen Hoang, Synnøve Nymark Aasen, Ole Vidhammer Bjørnstad, Himalaya Parajuli, Terje Sundstrøm, and Frits Alan Thorsen. 2021. "Cabozantinib Is Effective in Melanoma Brain Metastasis Cell Lines and Affects Key Signaling Pathways" International Journal of Molecular Sciences 22, no. 22: 12296. https://doi.org/10.3390/ijms222212296
APA StyleMannsåker, T. A., Hoang, T., Aasen, S. N., Bjørnstad, O. V., Parajuli, H., Sundstrøm, T., & Thorsen, F. A. (2021). Cabozantinib Is Effective in Melanoma Brain Metastasis Cell Lines and Affects Key Signaling Pathways. International Journal of Molecular Sciences, 22(22), 12296. https://doi.org/10.3390/ijms222212296





