Thermoresponsive Molecular Brushes with a Rigid-Chain Aromatic Polyester Backbone and Poly-2-alkyl-2-oxazoline Side Chains
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
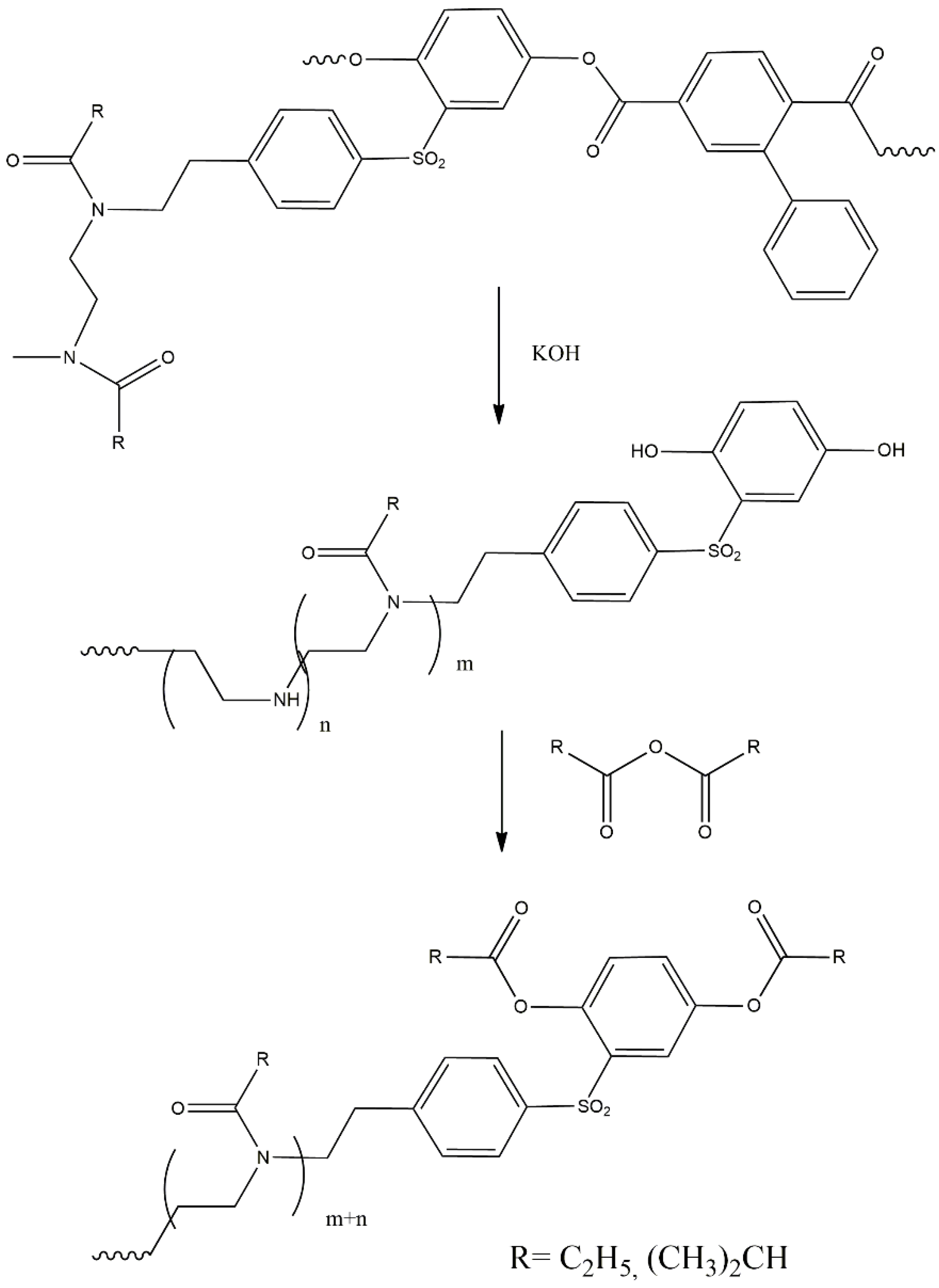
2.1.1. Synthesis of the Polyester Multifunctional Macroinitiator
2.1.2. Synthesis of the APEr.ch.-Graft-PAlOx Copolymers with PEtOx or PiPrOx Side Chains
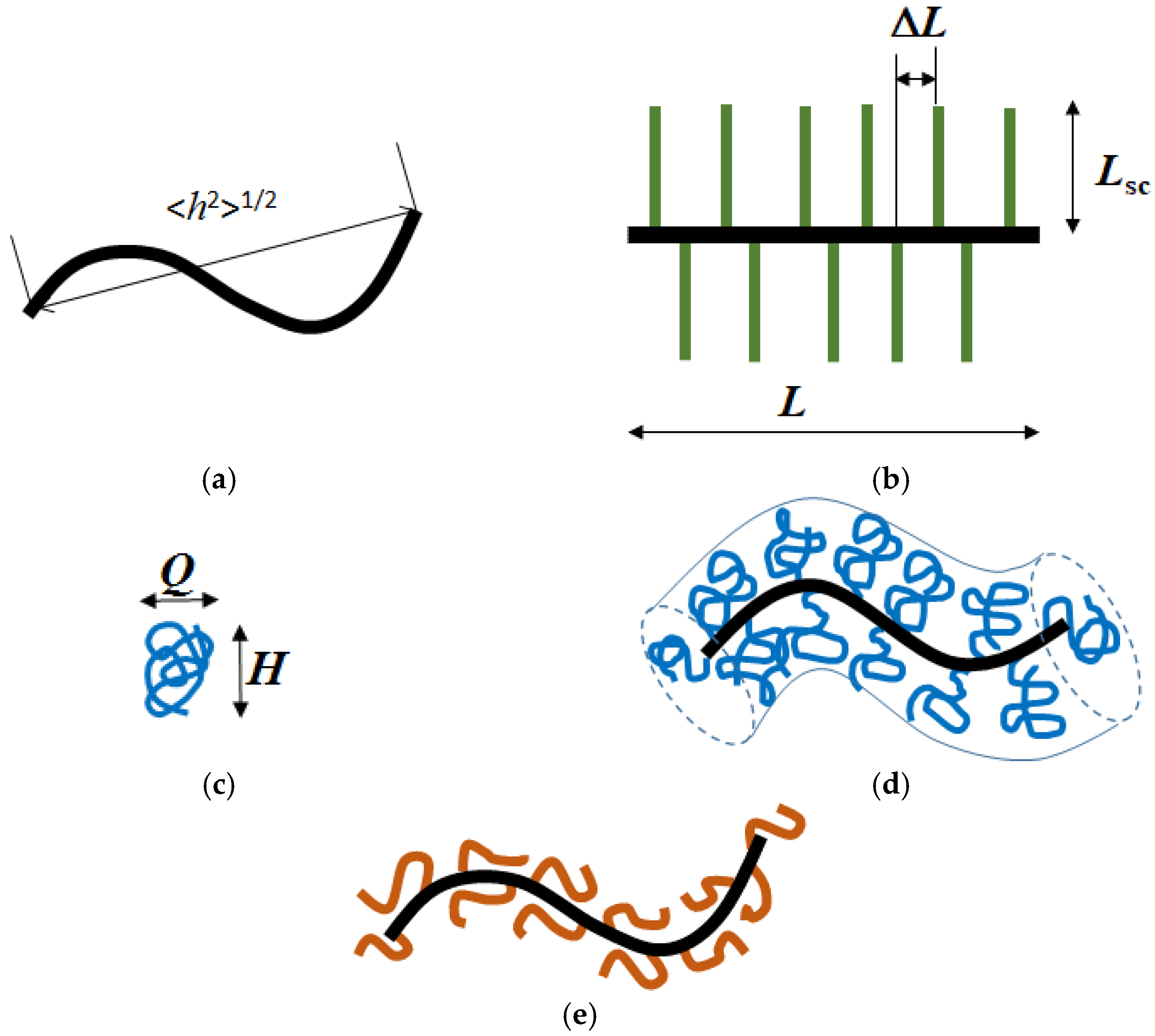
2.2. Molecular Characteristics and Equilibrium Rigidity of the APEr.ch. Macroinitiator and the Grafted Copolymers APEr.ch.-graft-PEtOx and APEr.ch.-graft-PiPrOx
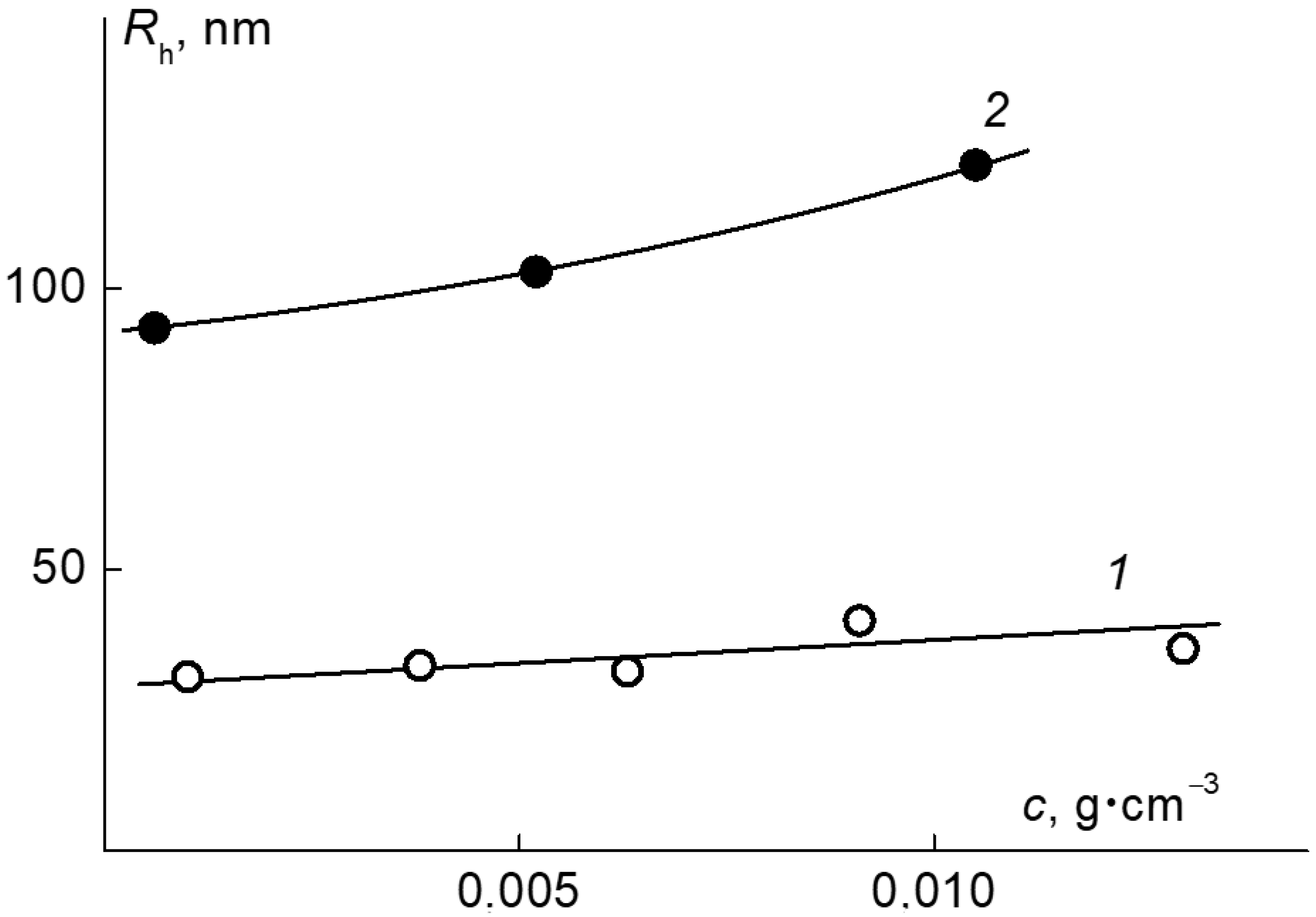
2.3. Self-Organization in Aqueous Solutions of the APEr.ch.-graft-PEtOx and APEr.ch.-graft-PiPrOx Grafted Copolymers
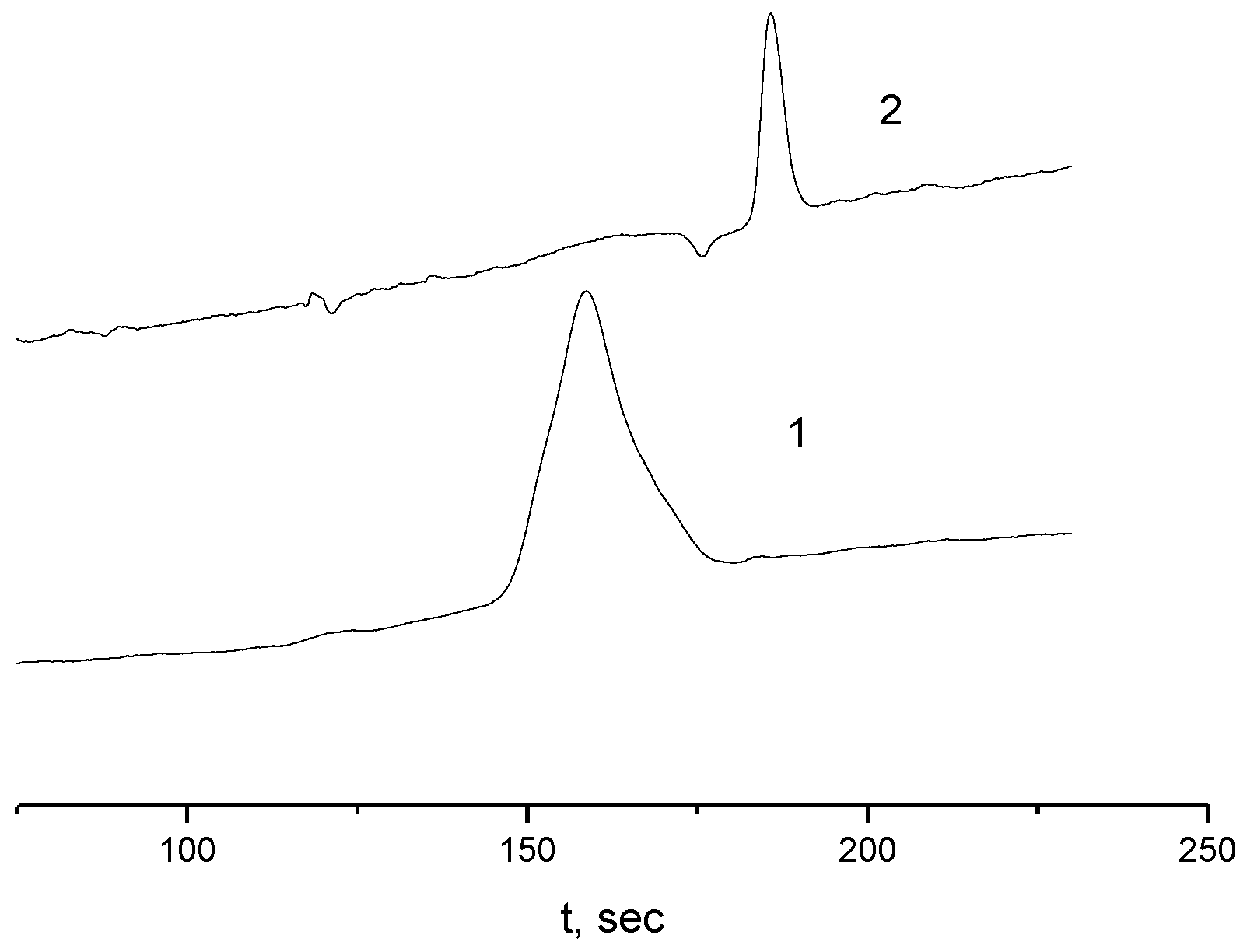
2.4. Kinetics of Aggregation in Aqueous Solutions of the APEr.ch.-graft-PEtOx and the APEr.ch.-graft-PiPrOx
3. Materials and Methods
3.1. Synthesis
3.1.1. Poly(2-[4-(2-Br-ethyl)phenylsulfonyl]-1,4-phenylene-2’,5’-biphenyldicarboxylate Synthesis
3.1.2. Polymerization of 2-Alkyl-2-Oxazolines on the Polyester Macroinitiator
3.1.3. Hydrolysis of Graft-Copolymers
3.2. Determination of Molar Mass and Hydrodynamic Characteristics of Polymers
3.3. Study of the Solutions’ Phase Separation upon Heating
3.4. Microscopic Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guha-Sridhar, C.; Hines, W.A.; Samulski, E.T. Polypeptide liquid crystals: Magnetic susceptibility, twist elastic constant, rotation viscosity coefficient, and poly-γ-benzyl-L-glutamate side chain conformation. J. Chem. Phys. 1974, 61, 947–953. [Google Scholar] [CrossRef]
- Conio, G.; Bianchi, E.; Ciferri, A.; Tealdi, A.; Aden, M.A. Mesophase formation and chain rigidity in cellulose and derivatives. 1. (Hydroxypropyl)cellulose in dimethylacetamide. Macromolecues 1983, 16, 1264–1270. [Google Scholar] [CrossRef]
- Ronova, I.A.; Ponomarev, I.I. Design of monomeric units for rigid aromatic polymers. Struct. Chem. 2019, 30, 1611–1627. [Google Scholar] [CrossRef]
- Kakali, F.; Kallitsis, J.K. Chain Rigidity of Substituted Aromatic Polyesters Containing Oligophenyl Units in the Main Chain. Macromolecules 1996, 29, 4759–4763. [Google Scholar] [CrossRef]
- Ronova, I. Structural aspects in polymers: Interconnections between conformational parameters of the polymers with their physical properties. Struct. Chem. 2010, 21, 541–553. [Google Scholar] [CrossRef]
- Kwolek, S.L.; Morgan, P.W.; Schaefgen, J.R.; Gulrich, L.W. Synthesis, Anisotropic Solutions, and Fibers of Poly(1,4-benzamide). Macromolecues 1977, 10, 1390–1396. [Google Scholar] [CrossRef]
- Valenti, B.; Alfonso, G.C.; Ciferri, A.; Giordani, P.; Marrucci, G. Solution spinning of a semirigid chain polymer forming ultrahigh modulus fibers. J. Appl. Polym. Sci. 1981, 26, 3643–3655. [Google Scholar] [CrossRef]
- Ozawa, S. A New Approach to High Modulus, High Tenacity Fibers. Polym. J. 1987, 19, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Eduard, P.; Thiyagarajan, S.; Noordover, B.A.J.; van Es, D.S.; Koning, C.E. Semi-Aromatic Polyesters Based on a Carbohydrate-Derived Rigid Diol for Engineering Plastics. ChemSusChem 2015, 8, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsvetkov, V. Structure and properties of rigid chain polymer molecules in solutions. Polym. Sci. USSR 1979, 21, 2879–2899. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers. Hydrodynamic and Optical Properties in Solution, 1st ed.; Plenum Press: New York, NY, USA, 1989; pp. 1–512. [Google Scholar]
- Tsvetkov, V.; Tarasova, G.; Vinogradov, Y.; Kuprianova, N.; Yamshchikov, V.; Skazka, V.; Ivanov, V.; Smirnova, V.; Migunova, I. Hydrodynamic and optical properties of poly-N-2,4-dimethylphenylmaleimide molecules in solution. Polym. Sci. USSR 1971, 13, 705–714. [Google Scholar] [CrossRef]
- Wolfe, J.F.; Loo, B.H.; Arnold, F.E. Rigid-rod polymers. 2. Synthesis and thermal properties of para-aromatic polymers with 2,6-benzobisthiazole units in the main chain. Macromolecues 1981, 14, 915–920. [Google Scholar] [CrossRef]
- Sicree, A.J.; Arnold, F.E.; Van Deusen, R.L. New imidazoisoquinoline ladder polymers. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 265–272. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Andrianov, K.A.; Vinogradov, E.L.; Shtennikova, I.N.; Yakushkina, S.E.; Pakhomov, V.I. Hydrodynamic properties and optical anisotropy of molecules of linear and cyclolinear polyphenylsiloxanes in solution. J. Polym. Sci. Part C Polym. Symp. 1968, 23, 385–391. [Google Scholar] [CrossRef]
- Doty, P.; Holtzer, A.; Bradbury, J.H.; Blout, E. Polypeptides. II. Configuration of polymers of γ-benzyl-L- glutamate in solution. J. Am. Chem. Soc. 1954, 76, 4493–4494. [Google Scholar] [CrossRef]
- Doty, P.; Bradbury, J.H.; Holtze, A.H. Polypeptides. IV. Molecular weight, configuration and association of poly-γ-benzyl-Lglutamate in various solvent. J. Am. Chem. Soc. 1956, 78, 947–954. [Google Scholar] [CrossRef]
- Gray, H.B., Jr.; Bloomfield, V.A.; Hearst, J.E. Sedimentation Coefficients of Linear and Cyclic Wormlike Coils with Excluded-Volume Effects. J. Chem. Phys. 1967, 46, 1493–1498. [Google Scholar] [CrossRef]
- Tsvetkov, V.; Tsepelevich, S. Light scattering of solutions of polyamidohydrazide and conformation characteristics of its molecules. Polym. Sci. USSR 1983, 25, 2221–2230. [Google Scholar] [CrossRef]
- Meyerhoff, G.; Sutterlin, N. Eihylzellulosen in losungen. Hydrodynamische eigenschaften. Makromol. Chem. 1965, 87, 258–270. [Google Scholar] [CrossRef]
- Pogodina, N.V.; Mel’nikov, A.B.; Mikryukova, O.I.; Didenko, S.A.; Marchenko, G.N. Conformational characteristics of low-molecular weight colloids as shown by diffusion-sedimentation analysis. Polym. Sci. Ser. A 1984, 26, 2819–2825. [Google Scholar] [CrossRef]
- Tsvethov, V.; Andreyeva, L.; Korneyeva, Y.; Lavrenko, P.; Plate, N.; Shibayev, V.; Petruhhin, B. Conformational and optical properties of poly(decyl acrylate). Polym. Sci. USSR 1972, 14, 1944–1954. [Google Scholar] [CrossRef]
- Filippov, A.; Kozlov, A.; Tarabukina, E.; Obrezkova, M.; Muzafarov, A. Solution properties of comb-like polymers consisting of dimethylsiloxane monomer units. Polym. Int. 2016, 65, 393–399. [Google Scholar] [CrossRef]
- Zhang, M.; Müller, A.H.E. Cylindrical polymer brushes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3461–3481. [Google Scholar] [CrossRef]
- Wintermantel, M.; Gerle, M.; Fischer, K.; Schmidt, M.; Wataoka, I.; Urakawa, H.; Kajiwara, K.; Tsukahara, Y. Molecular bottlebrushes. Macromolecules 1996, 29, 978–983. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Sumerlin, B.S.; Matyjaszewski, K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33, 759–785. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with Complex Architecture by Living Anionic Polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef]
- Yilmaz, G.; Toiserkani, H.; Demirkol, D.O.; Sakarya, S.; Timur, S.; Yagci, Y.; Torun, L. Modification of polysulfones by click chemistry: Amphiphilic graft copolymers and their protein adsorption and cell adhesion properties. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 110–117. [Google Scholar] [CrossRef]
- Ilgach, D.; Meleshko, T.K.; Yakimansky, A.V. Methods of controlled radical polymerization for the synthesis of polymer brushes. Polym. Sci. Ser. C 2015, 57, 3–19. [Google Scholar] [CrossRef]
- Birshtein, T.; Borisov, O.; Zhulina, Y.; Khokhlov, A.; Yurasova, T. Conformations of comb-like macromolecules. Polym. Sci. USSR 1987, 29, 1293–1300. [Google Scholar] [CrossRef]
- I Popov, K.; Palyulin, V.V.; Möller, M.; Khokhlov, A.R.; I Potemkin, I. Surface induced self-organization of comb-like macromolecules. Beilstein J. Nanotechnol. 2011, 2, 569–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Wade, M.A.; Walsh, D.; Guironnet, D.; Rogers, S.A.; Sing, C.E. Dilute solution structure of bottlebrush polymers. Soft Matter 2019, 15, 2928–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalsin, S.J.; Rions-Maehren, T.G.; Beam, M.D.; Bates, F.S.; Hillmyer, M.A.; Matsen, M.W. Bottlebrush Block Polymers: Quantitative Theory and Experiments. ACS Nano 2015, 9, 12233–12245. [Google Scholar] [CrossRef]
- Pesek, S.L.; Li, X.; Hammouda, B.; Hong, K.; Verduzco, R. Small-Angle Neutron Scattering Analysis of Bottlebrush Polymers Prepared via Grafting-Through Polymerization. Macromolecues 2013, 46, 6998–7005. [Google Scholar] [CrossRef]
- Zhang, B.; Gröhn, F.; Pedersen, J.S.; Fischer, K.; Schmidt, M. Conformation of Cylindrical Brushes in Solution: Effect of Side Chain Length. Macromolecues 2006, 39, 8440–8450. [Google Scholar] [CrossRef]
- Terao, K.; Hokajo, T.; Nakamura, A.Y.; Norisuye, T. Solution Properties of Polymacromonomers Consisting of Polystyrene. 3. Viscosity Behavior in Cyclohexane and Toluene. Macromolecues 1999, 32, 3690–3694. [Google Scholar] [CrossRef]
- Wataoka, I.; Urakawa, H.; Kobayashi, K.; Akaike, T.; Schmidt, M.; Kajiwara, K. Structural Characterization of Glycoconjugate Polystyrene in Aqueous Solution. Macromolecues 1999, 32, 1816–1821. [Google Scholar] [CrossRef]
- Nakamura, Y. Stiffness parameter of brush-like polymers with rod-like side chains. J. Chem. Phys. 2016, 145, 14903. [Google Scholar] [CrossRef] [PubMed]
- Filippov, A.P.; Krasova, A.S.; Tarabukina, E.B.; Kashina, A.; Meleshko, T.K.; Yakimansky, A.V. The effect of side chain length on hydrodynamic and conformational characteristics of polyimide-graft-polymethylmethacrylate copolymers in thermodynamically good solutions. J. Polym. Res. 2016, 23, 219. [Google Scholar] [CrossRef]
- Theodorakis, P.E.; Paul, W.; Binder, K. Microphase separation in bottlebrush polymers under poor-solvent conditions. EPL (Europhys. Lett.) 2009, 88, 63002. [Google Scholar] [CrossRef] [Green Version]
- Borisov, O.V.; Zhulina, E. Amphiphilic Graft Copolymer in a Selective Solvent: Intramolecular Structures and Conformational Transitions. Macromolecues 2005, 38, 2506–2514. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Hao, X.; Zeng, G.; Wang, W.; Liu, R.; Huang, Y. Backbone-collapsed intra- and inter-molecular self-assembly of cellulose-based dense graft copolymer. Carbohydr. Polym. 2012, 88, 290–298. [Google Scholar] [CrossRef]
- Meleshko, T.K.; Ivanov, I.V.; Kashina, A.V.; Bogorad, N.N.; Simonova, M.A.; Zakharova, N.V.; Filippov, A.P.; Yakimansky, A.V. Diphilic macromolecular brushes with a polyimide backbone and [G9.poly(methacrylic acid) blocks in side chains. Polym. Sci. Ser. B 2018, 60, 35–50. [Google Scholar] [CrossRef]
- Ishizu, K.; Tsubaki, K.; Mori, A.; Uchida, S. Architecture of nanostructured polymers. Prog. Polym. Sci. 2003, 28, 27–54. [Google Scholar] [CrossRef]
- Simonova, M.; Ivanov, I.; Meleshko, T.; Kopyshev, A.; Santer, S.; Yakimansky, A.; Filippov, A. Self-Assembly of Molecular Brushes with Polyimide Backbone and Amphiphilic Block Copolymer Side Chains in Selective Solvents. Polymers 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Filippov, A.P.; Belyaeva, E.V.; Krasova, A.S.; Simonova, M.; Meleshko, T.K.; Ilgach, D.; Bogorad, N.N.; Yakimansky, A.; Larin, S.; Darinskii, A.A. Conformations of molecular brushes based on polyimide and poly(methyl methacrylate) in selective solvents: Experiment and computer simulation. Polym. Sci. Ser. A 2014, 56, 393–404. [Google Scholar] [CrossRef]
- Krasova, A.; Belyaeva, E.; Tarabukina, E.; Filippov, A.; Meleshko, T.; Ilgach, D.; Bogorad, N.; Yakimansky, A. Synthesis and Solution Properties of Loose Polymer Brushes Having Polyimide Backbone and Methylmethacrylate Side Chains. Macromolecues Symp. 2012, 316, 32–42. [Google Scholar] [CrossRef]
- Filippov, A.P.; Krasova, A.S.; Tarabukina, E.B.; Meleshko, T.K.; Yakimansky, A.V.; Sheiko, S.S. The Behavior of Amphiphilic Molecular Brushes with Polyimide Backbone and Poly(methyl methacrylate) and Polystyrene Side Chains in the Vicinity of Θ Point. Polym. Sci. Ser. C 2018, 60, 219–227. [Google Scholar] [CrossRef]
- Sato, E.; Tamari, N.; Horibe, H. Facile synthesis of graft copolymers containing rigid poly(dialkyl fumarate) branches by macromonomer method. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2474–2480. [Google Scholar] [CrossRef]
- Song, H.; Dotrong, M.; Price, G.; Vakil, U.; Santhosh, U.; Evers, R. Rod aggregation in graft rigid-rod copolymers for single-component molecular composites. Polymer 1994, 35, 675–680. [Google Scholar] [CrossRef]
- Sui, K.; Zhao, X.; Wu, Z.; Xia, Y.; Liang, H.; Li, Y. Synthesis, Rapid Responsive Thickening, and Self-Assembly of Brush Copolymer Poly(ethylene oxide)-graft-Poly(N,N-dimethylaminoethyl methacrylate) in Aqueous Solutions. Langmuir 2012, 28, 153–160. [Google Scholar] [CrossRef]
- Xu, Y.; Bolisetty, S.; Drechsler, M.; Jiayin, B.; Matthias, Y.; Axel, B.; Müller, H.E. pH and salt responsive poly(N,N-dimethylaminoethyl methacrylate) cylindrical brushes and their quaternized derivatives. Polymer 2008, 49, 3957–3964. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Zhu, K.; Kjøniksen, A.-L.; Nyström, B. Temperature-responsive self-assembly of charged and uncharged hydroxyethylcellulose-graft-poly(N-isopropylacrylamide) copolymer in aqueous solution. Colloid Polym. Sci. 2011, 289, 993–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasile, C.; Bumbu, G.-G.; Mylonas, I.; Bokias, G.; Staikos, G. Thermoresponsive behaviour in aqueous solution of poly(maleic acid-alt-vinyl acetate) grafted with poly(N-isopropylacrylamide). Polym. Int. 2004, 53, 1176–1179. [Google Scholar] [CrossRef]
- Köseli, V.; Rzaev, Z.M.O.; Pişkin, E. Bioengineering functional copolymers. III. Synthesis of biocompatible poly[(N-isopropylacrylamide-co-maleic anhydride)-g-poly(ethylene oxide)]/poly(ethylene imine) macrocomplexes and their thermostabilization effect on the activity of the enzyme penicilli. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1580–1593. [Google Scholar] [CrossRef]
- Li, X.; ShamsiJazeyi, H.; Pesek, S.L.; Agrawal, A.; Hammouda, B.; Verduzco, R. Thermoresponsive PNIPAAM bottlebrush polymers with tailored side-chain length and end-group structure. Soft Matter 2014, 10, 2008–2015. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.X.; Li, W.; Liu, K.; Yan, J.T.; Hu, G.X.; Zhang, A.F. Comblike thermoresponsive polymers with sharp transitions: Synthesis, characterization, and their use as sensitive colorimetric sensors. Macromolecules 2011, 44, 8614–8621. [Google Scholar] [CrossRef]
- Weber, C.; Rogers, S.; Vollrath, A.; Hoeppener, S.; Rudolph, T.; Fritz, N.; Hoogenboom, R.; Schubert, U.S. Aqueous solution behavior of comb-shaped poly(2-ethyl-2-oxazoline). J. Polym. Sci. Part A Polym. Chem. 2013, 51, 139–148. [Google Scholar] [CrossRef]
- Weber, C.; Wagner, M.; Baykal, D.; Hoeppener, S.; Paulus, R.M.; Festag, G.; Altuntas, E.; Schacher, F.H.; Schubert, U.S. Easy Access to Amphiphilic Heterografted Poly(2-oxazoline) Comb Copolymers. Macromolecues 2013, 46, 5107–5116. [Google Scholar] [CrossRef]
- Zhang, N.; Luxenhofer, R.; Jordan, R. Thermoresponsive Poly(2-oxazoline) Molecular Brushes by Living Ionic Polymerization: Kinetic Investigations of Pendant Chain Grafting and Cloud Point Modulation by Backbone and Side Chain Length Variation. Macromol. Chem. Phys. 2012, 213, 973–981. [Google Scholar] [CrossRef]
- Guillerm, B.; Darcos, V.; Lapinte, V.; Monge, S.; Coudane, J.; Robin, J.-J. Synthesis and evaluation of triazole-linked poly(ε-caprolactone)-graft-poly(2-methyl-2-oxazoline) copolymers as potential drug carriers. Chem. Commun. 2012, 48, 2879–2881. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Rueda, J.C.; Uhlmann, P.; Müller, M.; Simon, F.; Stamm, M. Facile Approach to Grafting of Poly(2-oxazoline) Brushes on Macroscopic Surfaces and Applications Thereof. ACS Appl. Mater. Interfaces 2012, 4, 1357–1364. [Google Scholar] [CrossRef]
- Adams, N.; Schubert, U.S. Poly(2-oxazolines) in biological and biomedical application contexts. Adv. Drug Deliv. Rev. 2007, 59, 1504–1520. [Google Scholar] [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design Strategies for Functionalized Poly(2-oxazoline)s and Derived Materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef] [Green Version]
- Dworak, A.; Trzebicka, B.; Kowalczuk, A.; Tsvetanov, C.; Rangelov, S. Polyoxazolines—Mechanism of synthesis and solution properties. Polimery 2014, 59, 88–94. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef] [Green Version]
- Zahoranova, A.; Luxenhofer, R. Poly(2-oxazoline)- and poly(2-oxazine)-based self-assemblies, polyplexes, and drug nanoformulations–An update. Adv. Healthc. Mater. 2021, 10, 2001382. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Tsuchiizu, A.; Qiu, X.; Winnik, F.M. Dissecting the Mechanism of the Heat-Induced Phase Separation and Crystallization of Poly(2-isopropyl-2-oxazoline) in Water through Vibrational Spectroscopy and Molecular Orbital Calculations. Macromolecues 2012, 45, 3531–3541. [Google Scholar] [CrossRef]
- Kudryavtseva, A.A.; Kurlykin, M.P.; Tarabukina, E.B.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of thermosensitive graft copolymer with aromatic polyester backbone and poly-2-ethyl-2-oxazoline side chains in aqueous solutions. Int. J. Polym. Anal. Charact. 2017, 22, 526–533. [Google Scholar] [CrossRef]
- Filippov, A.; Tarabukina, E.; Kudryavtseva, A.; Fatullaev, E.; Kurlykin, M.; Tenkovtsev, A. Molecular brushes with poly-2-ethyl-2-oxazoline side chains and aromatic polyester backbone manifesting double stimuli responsiveness. Colloid Polym. Sci. 2019, 297, 1445–1454. [Google Scholar] [CrossRef]
- Tarabukina, E.; Fatullaev, E.; Krasova, A.; Kurlykin, M.; Tenkovtsev, A.; Sheiko, S.S.; Filippov, A. Synthesis, Structure, Hydrodynamics and Thermoresponsiveness of Graft Copolymer with Aromatic Polyester Backbone at Poly(2-isopropyl-2-oxazoline) Side Chains. Polymers 2020, 12, 2643. [Google Scholar] [CrossRef] [PubMed]
- Kurlykin, M.; Bursian, A.; Filippov, A.; Dudkina, M.; Tenkovtsev, A. Multicenter Polyester Initiators for the Preparation of Graft Copolymers with Oligo(2-Oxazoline) Side Chains. Macromol. Symp. 2017, 375, 1600162. [Google Scholar] [CrossRef]
- Spinner, I.H.; Yannopoulos, J.; Metanomski, W. OXIDATION–REDUCTION POLYMERS: I. SYNTHESIS OF MONOMERS. Can. J. Chem. 1961, 39, 2529–2535. [Google Scholar] [CrossRef]
- Bilibin, A.Y.; Tenkovtsev, A.V.; Piraner, O.N.; Skorokhodov, S.S. Investigation of the possibility of transesterification in the polycondensation of dihydroxyl compounds with acid dichlorides containing an ester bond. Makromol. Chem. Rapid Commun. 1989, 10, 249–254. [Google Scholar] [CrossRef]
- Kurlykin, M.P.; Golub, O.V.; Filippov, A.P.; Tenkovtsev, A.V.; Bursian, A.E. Multicenter polyester initiators for the synthesis of graft copolymers with oligo(2-ethyl-2-oxazoline) side chains. Polym. Sci. Ser. B 2016, 58, 421–427. [Google Scholar] [CrossRef]
- Allen, B.J.; Elsea, G.M.; Keller, K.P.; Kinder, H.D. Quantitative hydrolysis-gas chromatographic methods for the determination of selected acids and glycols in polyesters. Anal. Chem. 1977, 49, 741–743. [Google Scholar] [CrossRef]
- Yamakawa, H.; Fujii, M. Translational Friction Coefficient of Wormlike Chains. Macromolecues 1973, 6, 407–415. [Google Scholar] [CrossRef]
- Yamakawa, H.; Fujii, M. Intrinsic Viscosity of Wormlike Chains. Determination of the Shift Factor. Macromolecues 1974, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Yoshizaki, T. Transport Coefficients of Helical Wormlike Chains. 3. Intrinsic Viscosity. Macromolecues 1980, 13, 633–643. [Google Scholar] [CrossRef]
- Bushin, S.; Smirnov, K.; Andreeva, L.; Belyaeva, Y.; Bilibin, A.; Stepanova, A.; Tsvetkov, V. Hydrodynamic and conformational properties of a phenyl-substituted para-aromatic polyester in dioxane and dichloroacetic acid. Polym. Sci. USSR 1990, 32, 1015–1021. [Google Scholar] [CrossRef]
- Tsvetkov, V.; Andreyeva, L.; Filippov, A.; Belyneva, Y.; Bilibin, A.; Stepanova, A. Dynamo-optical and electro-optical properties of solutions of diphenyl-substituted poly-para-phenylene terephthalate in polar and non-polar solvents. Polym. Sci. USSR 1991, 33, 316–324. [Google Scholar] [CrossRef]
- Porod, G. Zusammenhang zwischen mittlerem Endpunktsabstand und Kettenlänge bei Fadenmolekülen. Mon. Für Chem.—Chem. Mon. 1949, 80, 251–255. [Google Scholar] [CrossRef]
- Kratky, O.; Porod, G. Röntgenuntersuchung gelöster Fadenmoleküle. Recl. Trav. Chim. B 1949, 68, 1106–1122. [Google Scholar] [CrossRef]
- Gubarev, A.S.; Monnery, B.D.; Lezov, A.A.; Sedlacek, O.; Tsvetkov, N.V.; Hoogenboom, R.; Filippov, S.K. Conformational properties of biocompatible poly(2-ethyl-2-oxazoline)s in phosphate buffered saline. Polym. Chem. 2018, 9, 2232–2237. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, W. Äußere Abmessungen von Fadenmolekülen in Lösung. Experentia 1945, 1, 28–29. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly(2-oxazoline)s: A Polymer Class with Numerous Potential Applications. Angew. Chem. Int. Ed. 2009, 48, 7978–7994. [Google Scholar] [CrossRef] [PubMed]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-ionic Thermoresponsive Polymers in Water. In Self Organized Nanostructures of Amphiphilic Block Copolymers II. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 242, pp. 29–89. [Google Scholar] [CrossRef] [Green Version]
- Uyama, H.; Kobayashi, S. A Novel Thermo-Sensitive Polymer. Poly(2-iso-propyl-2-oxazoline). Chem. Lett. 1992, 21, 1643–1646. [Google Scholar] [CrossRef]
- Diab, C.; Akiyama, Y.; Kataoka, K.; Winnik, F.M. Microcalorimetric Study of the Temperature-Induced Phase Separation in Aqueous Solutions of Poly(2-isopropyl-2-oxazolines). Macromolecues 2004, 37, 2556–2562. [Google Scholar] [CrossRef]
- Amirova, A.; Rodchenko, S.; Filippov, A. Time dependence of the aggregation of star-shaped poly(2-isopropyl-2-oxazolines) in aqueous solutions. J. Polym. Res. 2016, 23, 221. [Google Scholar] [CrossRef]
- Ye, J.; Xu, J.; Hu, J.; Wang, X.; Zhang, G.; Liu, S.; Wu, C. Comparative Study of Temperature-Induced Association of Cyclic and Linear Poly(N-isopropylacrylamide) Chains in Dilute Solutions by Laser Light Scattering and Stopped-Flow Temperature Jump. Macromolecues 2008, 41, 4416–4422. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhu, H.; Yin, Q.; Liu, H.; Hu, Y. Effect of Composition of PDMAEMA-b-PAA Block Copolymers on Their pH- and Temperature-Responsive Behaviors. Langmuir 2013, 29, 1024–1034. [Google Scholar] [CrossRef]
- Adelsberger, J.; Grillo, I.; Kulkarni, A.; Sharp, M.; Bivigou-Koumba, A.M.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Kinetics of aggregation in micellar solutions of thermoresponsive triblock copolymers—Influence of concentration, start and target temperatures. Soft Matter 2013, 9, 1685–1699. [Google Scholar] [CrossRef]
- Tarabukina, E.; Harabagiu, V.; Fundueanu, G.; Constantin, M.; Filippov, A. Thermo- and pH-responsive copolymer of N-isopropylacrylamide with acryloylvaline: Synthesis and properties in aqueous solutions. J. Polym. Res. 2021, 28, 155. [Google Scholar] [CrossRef]
- Witte, H.; Seeliger, W. Cyclische imidsäreester aus nitrilen und aminoalkoholen. Liebigs Ann. Chem. 1974, 996–1009. [Google Scholar] [CrossRef]
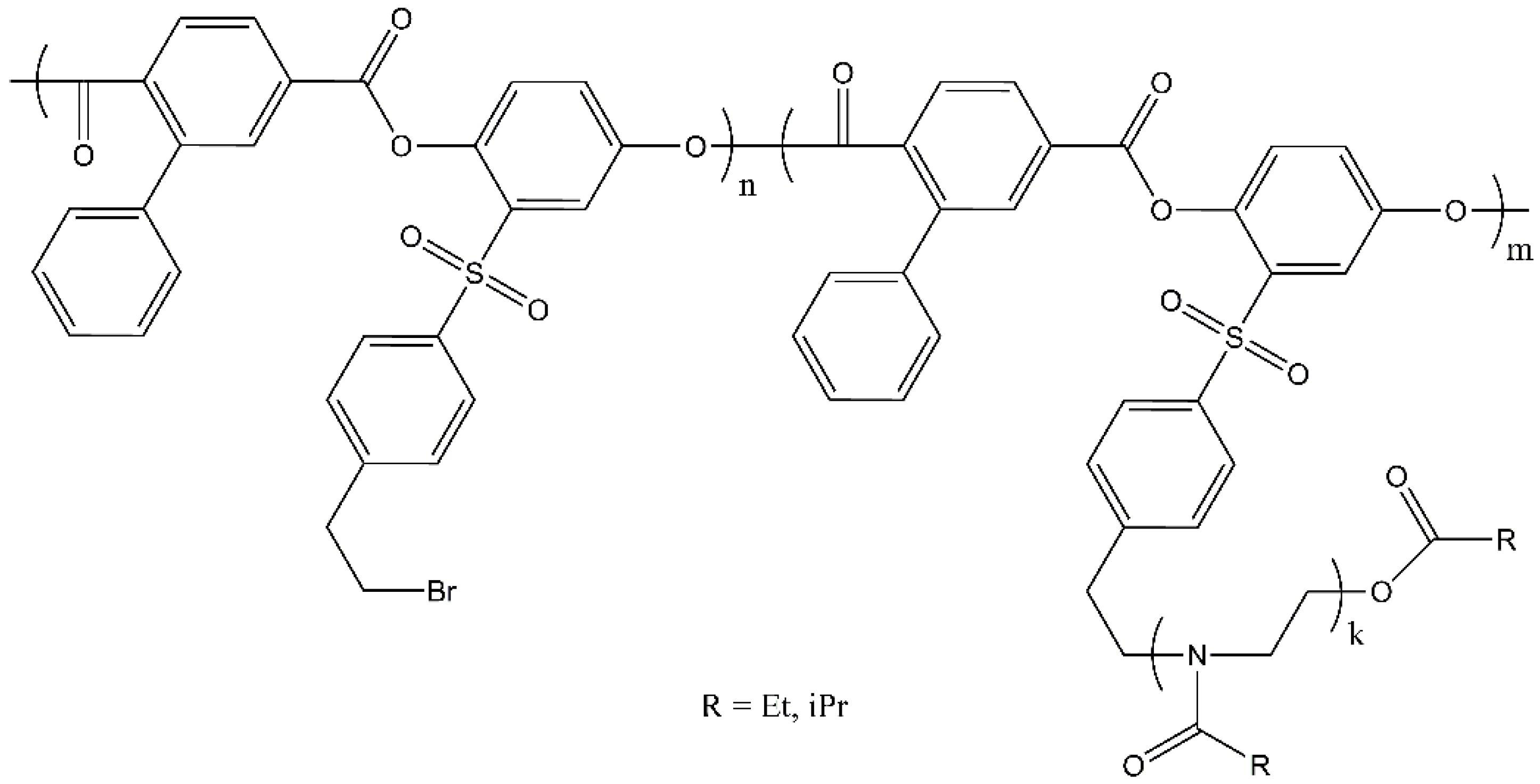


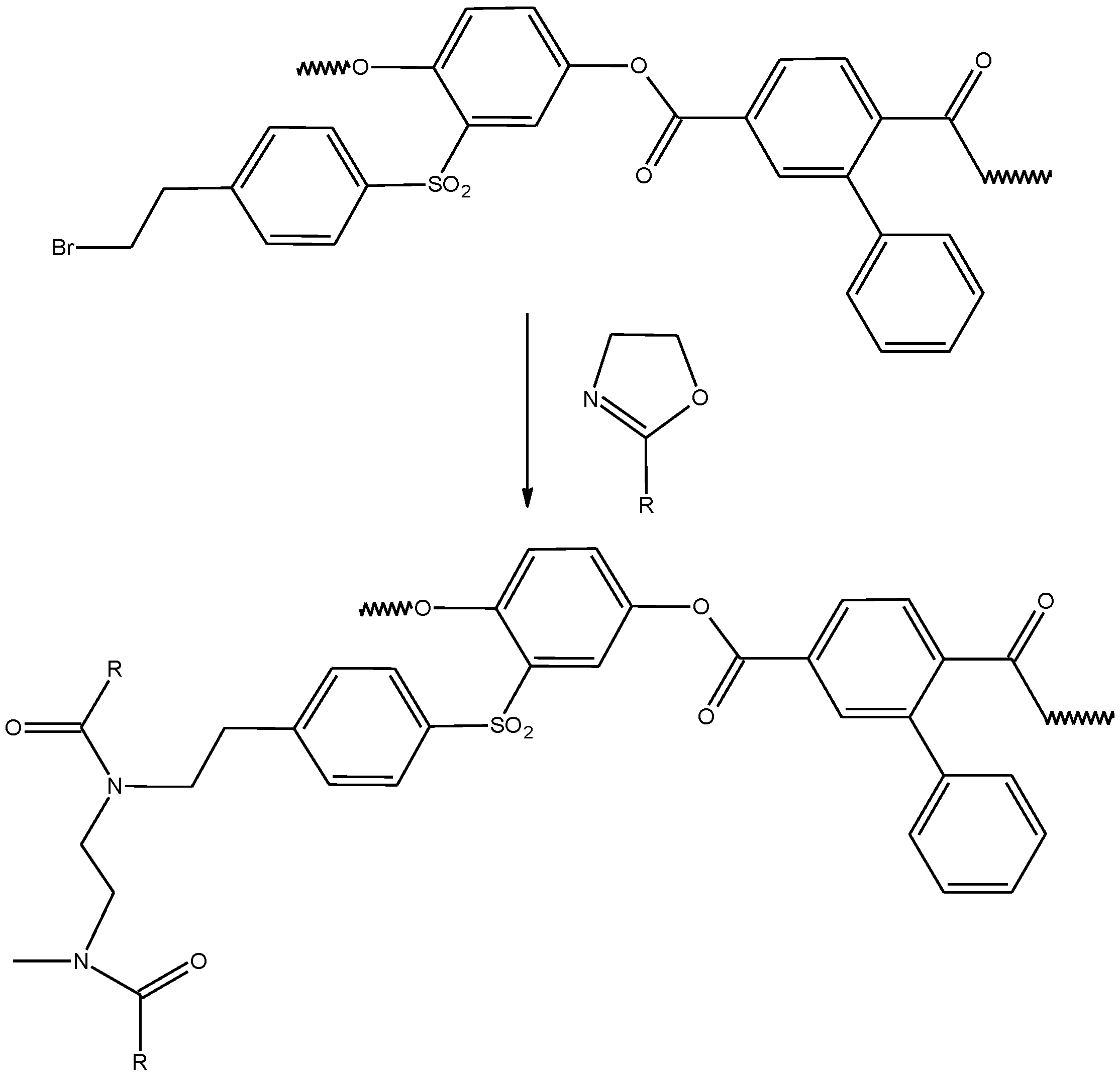
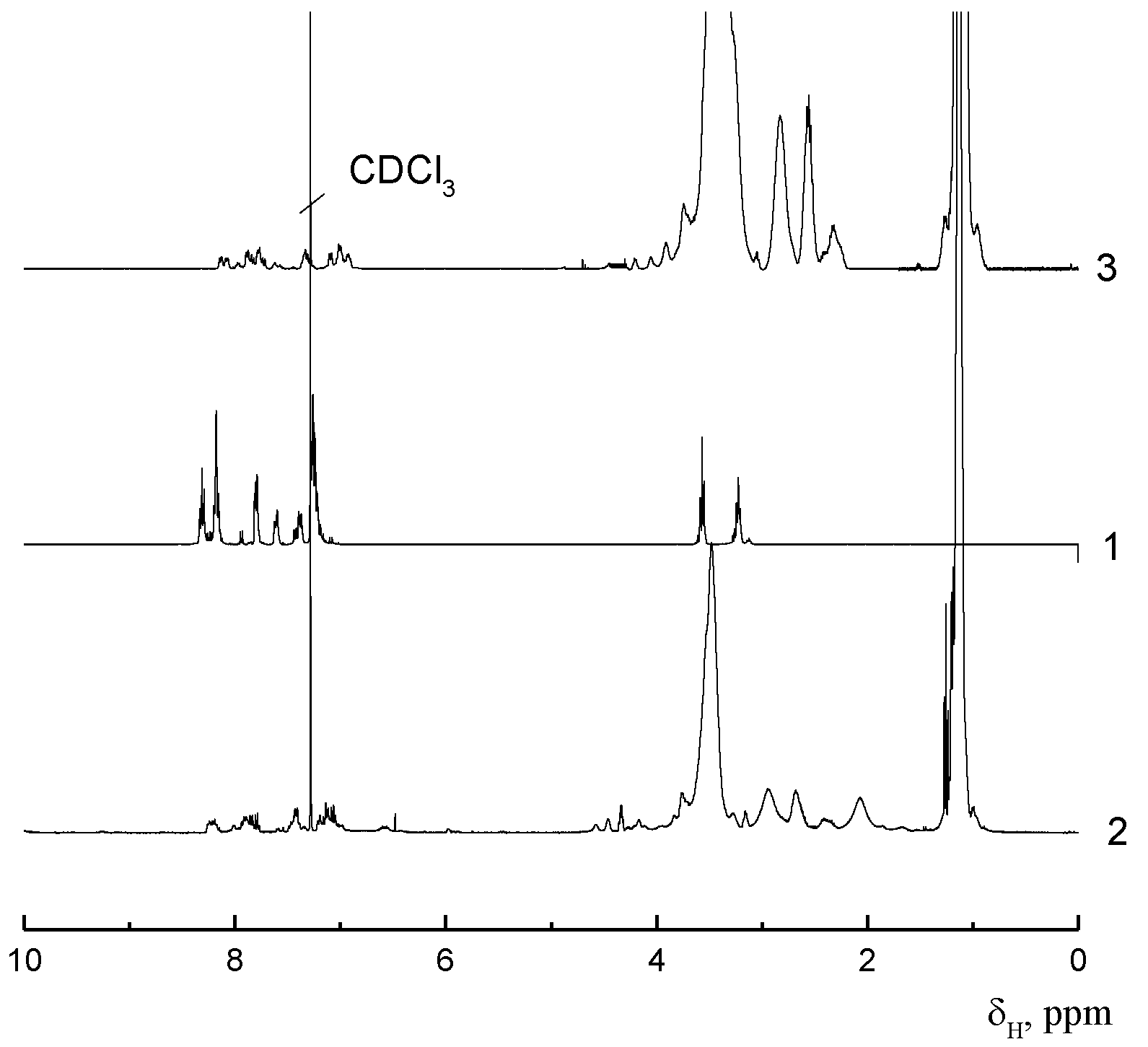
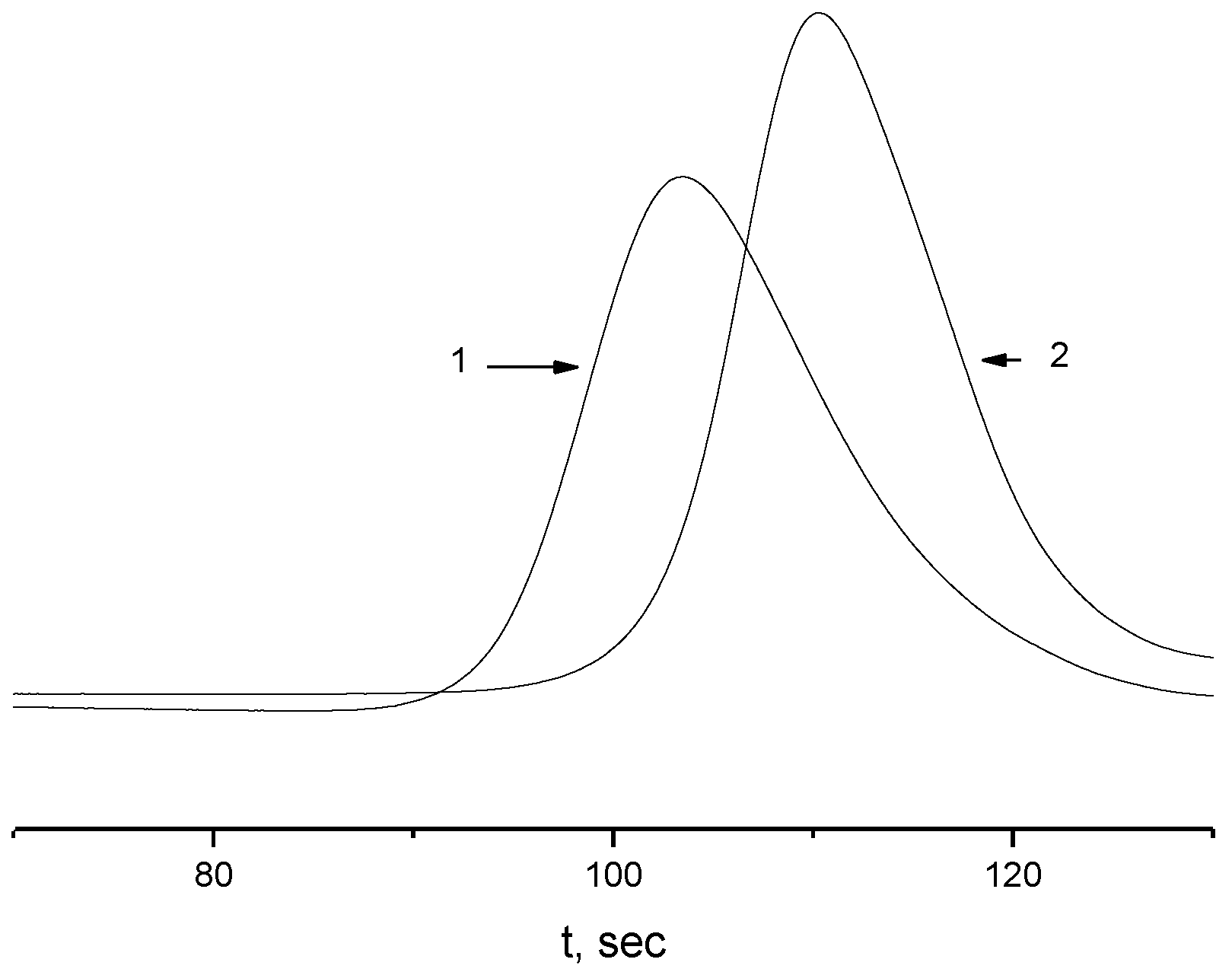



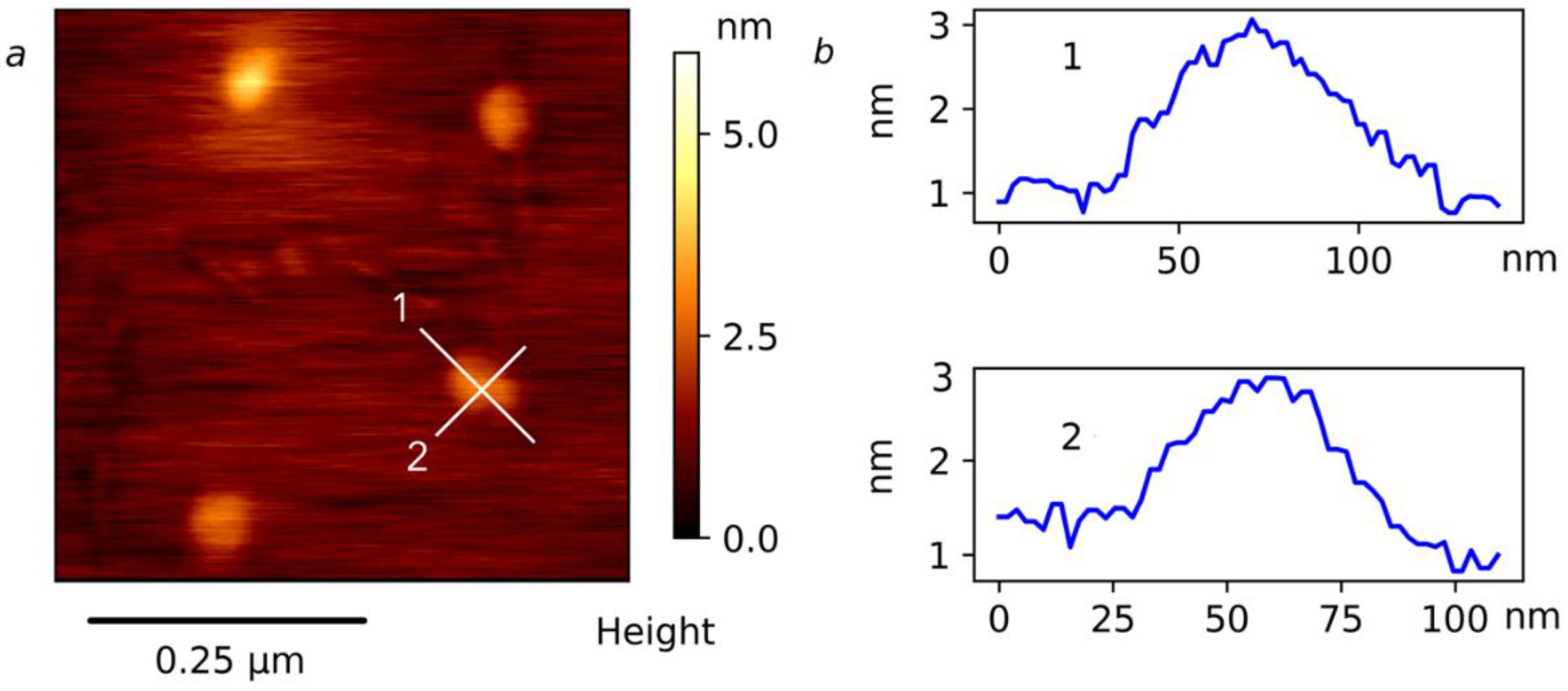


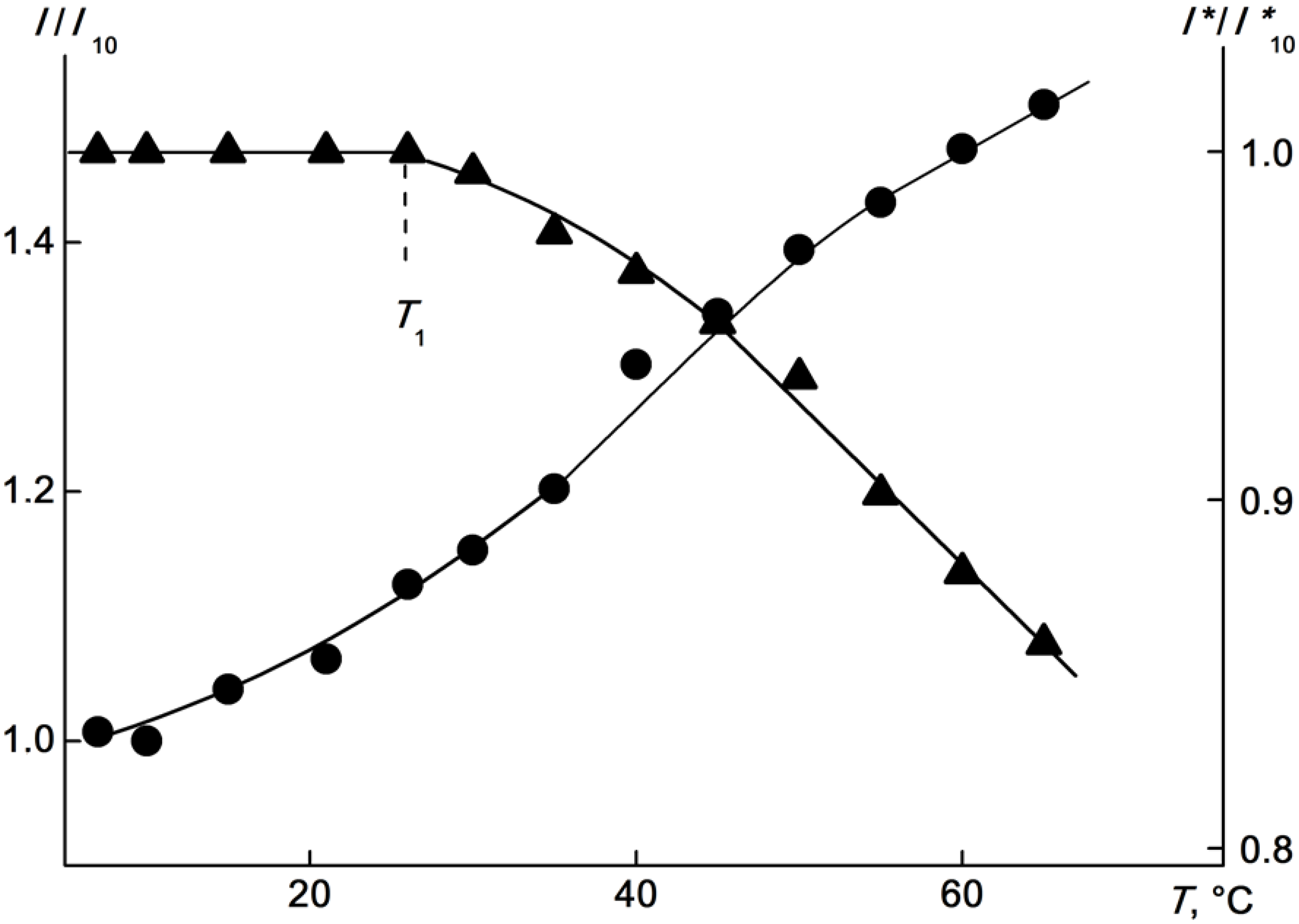
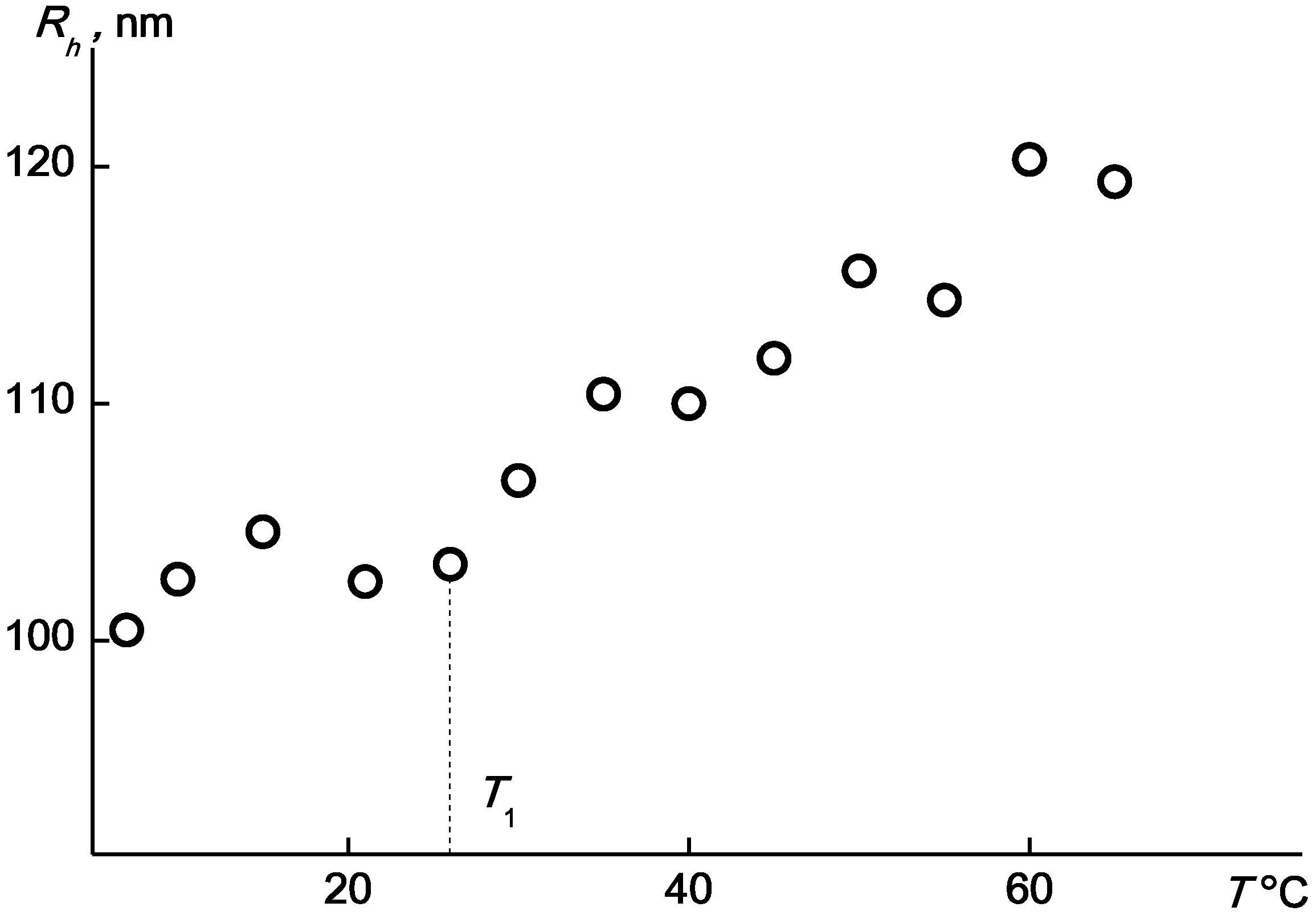
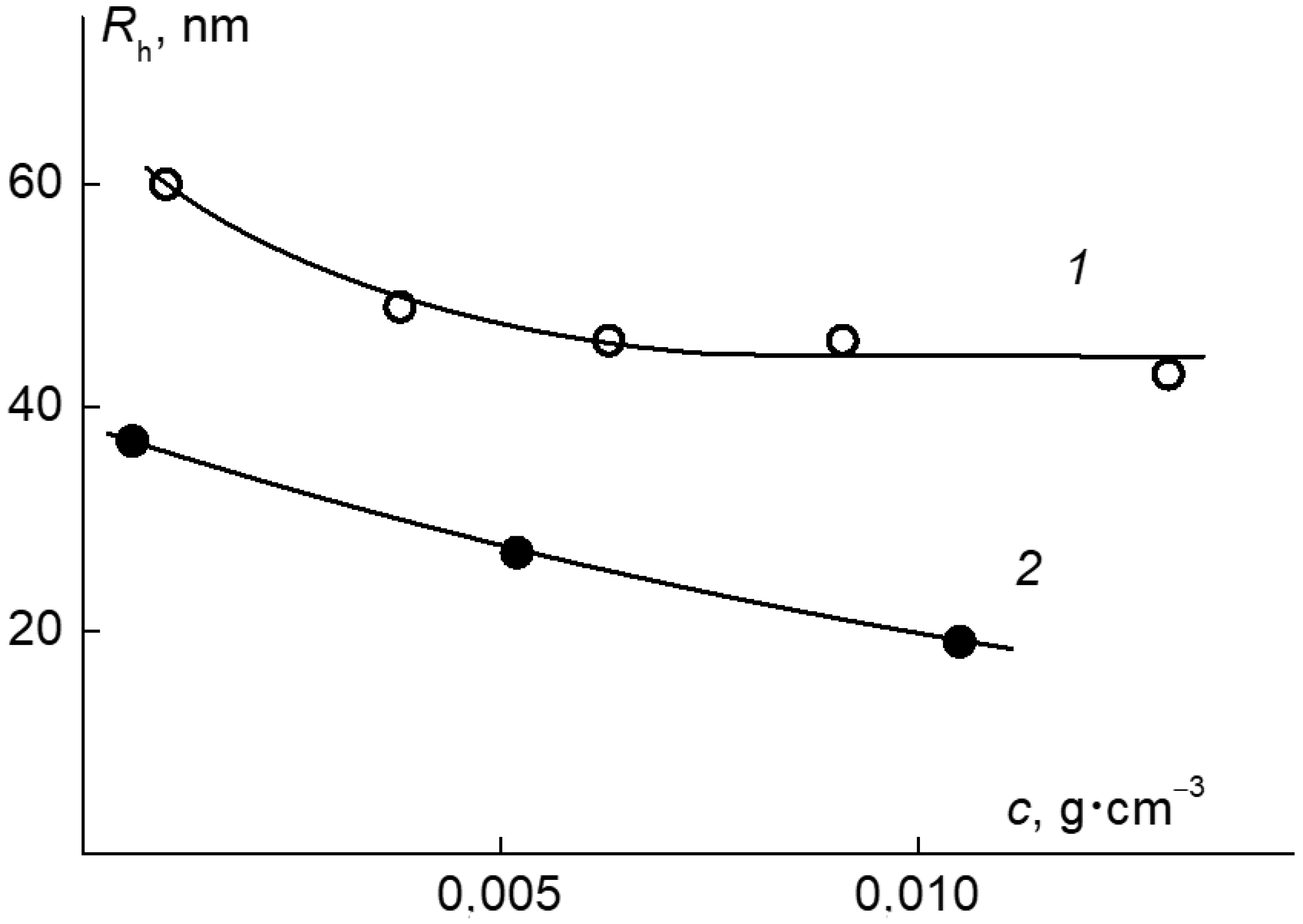

| Polymer | Mw, g·mol−1 | A2 × 104, cm3mol/g2 | Rh, nm |
|---|---|---|---|
| APEr.ch. | 26,500 | 0.4 | 12 |
| APEr.ch.-graft-PEtOx | 208,000 | −0.6 | 31 |
| APEr.ch.-graft-PiPrOx | 68,000 | 5.9 | 18 |
| Polymer | Ms, g·mol−1 | z | Nsc | Lsc, nm | ΔL, nm | fsc |
|---|---|---|---|---|---|---|
| APEr.ch.-graft-PEtOx | 7400 | 0.53 | 75 | 28 | 2.9 | 24 |
| APEr.ch.-graft-PiPrOx | 3400 | 0.27 | 30 | 11 | 5.6 | 13 |
| Polymer | Mw, g·mol−1 | Lsc/ΔL | ω, mol% | LCST, °C | Reference |
|---|---|---|---|---|---|
| APEr.ch.-graft-PEtOx | 208,000 | 10 | 13 | 45 | this work |
| APE6-graft-PEtOx 1 | 59,000 | 1.7 | 33 | 50 | [70] |
| APE6-graft-PEtOx 1 | 75,000 | 2.3 | 24 | 55 | [70] |
| APEr.ch.-graft-PiPrOx | 68,000 | 2.0 | 50 | <20 | this work |
| APE8-graft-PiPrOx 2 | 74,000 | 2.0 | 26 | 20 | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarabukina, E.; Fatullaev, E.; Krasova, A.; Sokolova, M.; Kurlykin, M.; Neelov, I.; Tenkovtsev, A.; Filippov, A. Thermoresponsive Molecular Brushes with a Rigid-Chain Aromatic Polyester Backbone and Poly-2-alkyl-2-oxazoline Side Chains. Int. J. Mol. Sci. 2021, 22, 12265. https://doi.org/10.3390/ijms222212265
Tarabukina E, Fatullaev E, Krasova A, Sokolova M, Kurlykin M, Neelov I, Tenkovtsev A, Filippov A. Thermoresponsive Molecular Brushes with a Rigid-Chain Aromatic Polyester Backbone and Poly-2-alkyl-2-oxazoline Side Chains. International Journal of Molecular Sciences. 2021; 22(22):12265. https://doi.org/10.3390/ijms222212265
Chicago/Turabian StyleTarabukina, Elena, Emil Fatullaev, Anna Krasova, Maria Sokolova, Mikhail Kurlykin, Igor Neelov, Andrey Tenkovtsev, and Alexander Filippov. 2021. "Thermoresponsive Molecular Brushes with a Rigid-Chain Aromatic Polyester Backbone and Poly-2-alkyl-2-oxazoline Side Chains" International Journal of Molecular Sciences 22, no. 22: 12265. https://doi.org/10.3390/ijms222212265
APA StyleTarabukina, E., Fatullaev, E., Krasova, A., Sokolova, M., Kurlykin, M., Neelov, I., Tenkovtsev, A., & Filippov, A. (2021). Thermoresponsive Molecular Brushes with a Rigid-Chain Aromatic Polyester Backbone and Poly-2-alkyl-2-oxazoline Side Chains. International Journal of Molecular Sciences, 22(22), 12265. https://doi.org/10.3390/ijms222212265







