Immunohistochemical Evaluation of Adaptor Protein FAM159B Expression in Normal and Neoplastic Human Tissues
Abstract
:1. Introduction
2. Results
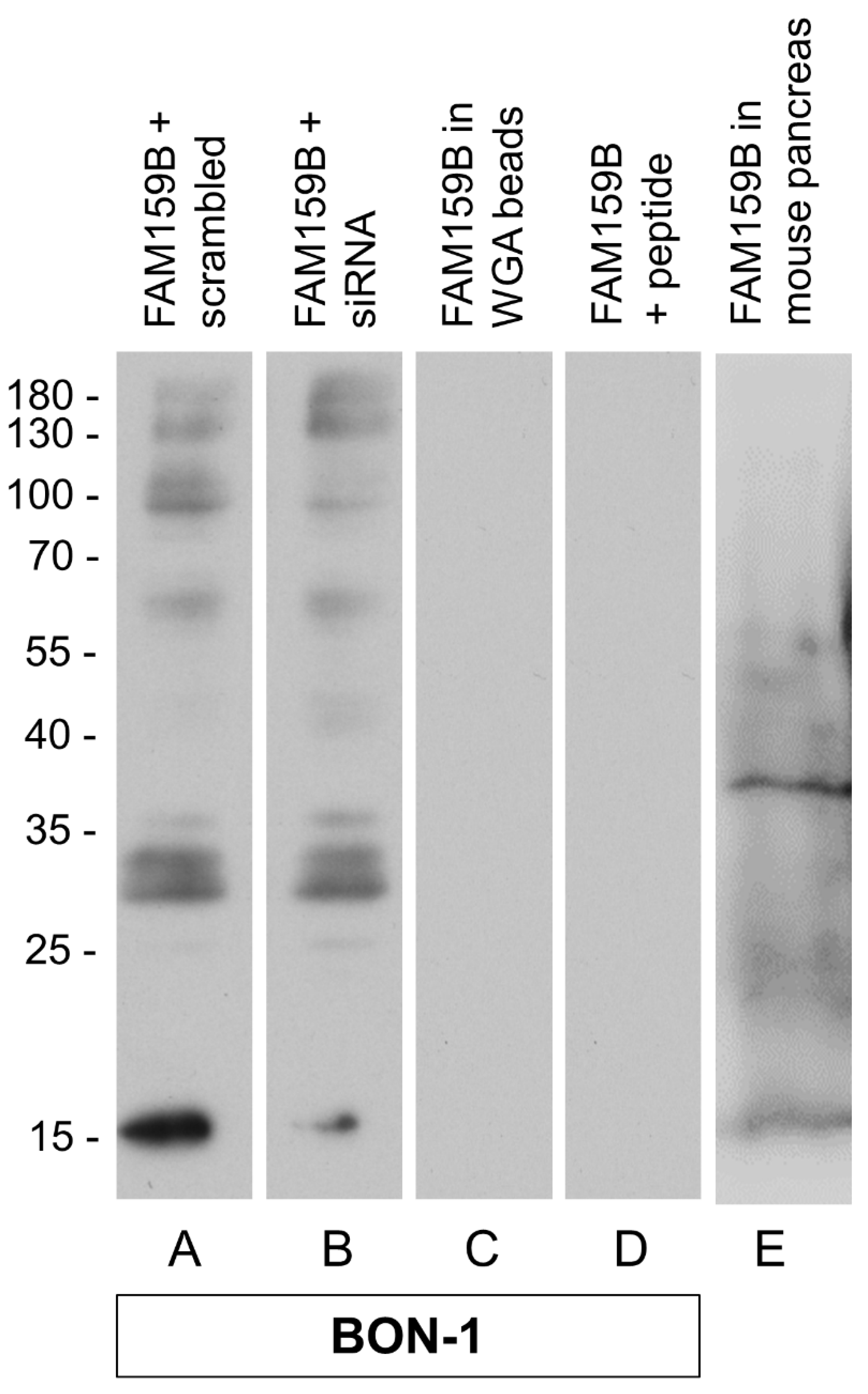
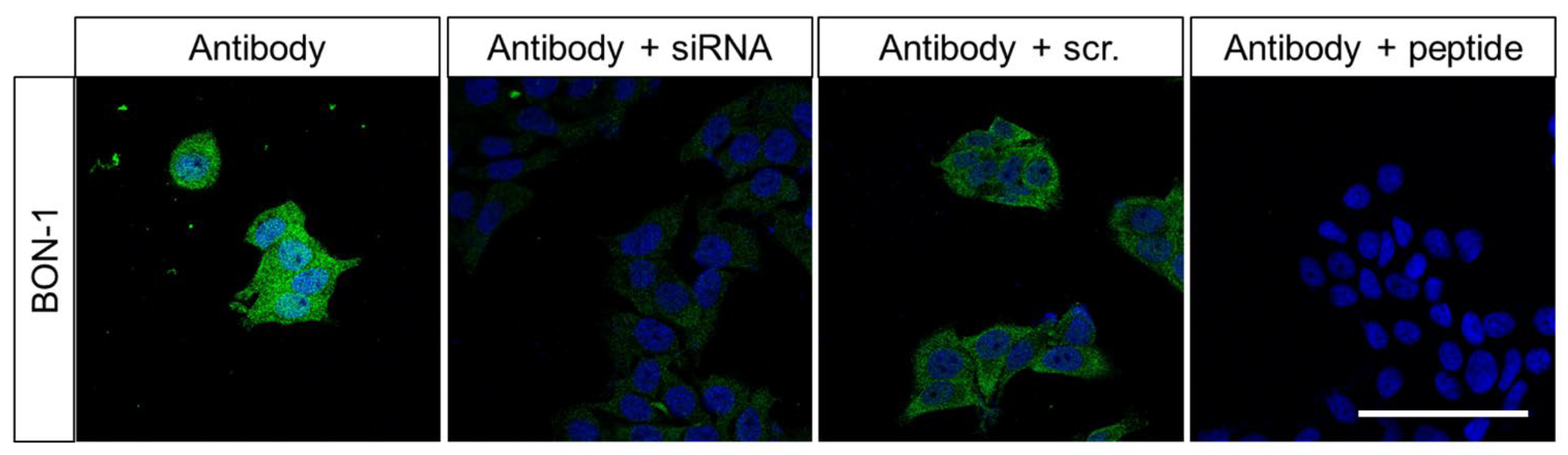
2.1. Characterisation of the Rabbit Polyclonal Anti-Human FAM159B Antibody HPA011778
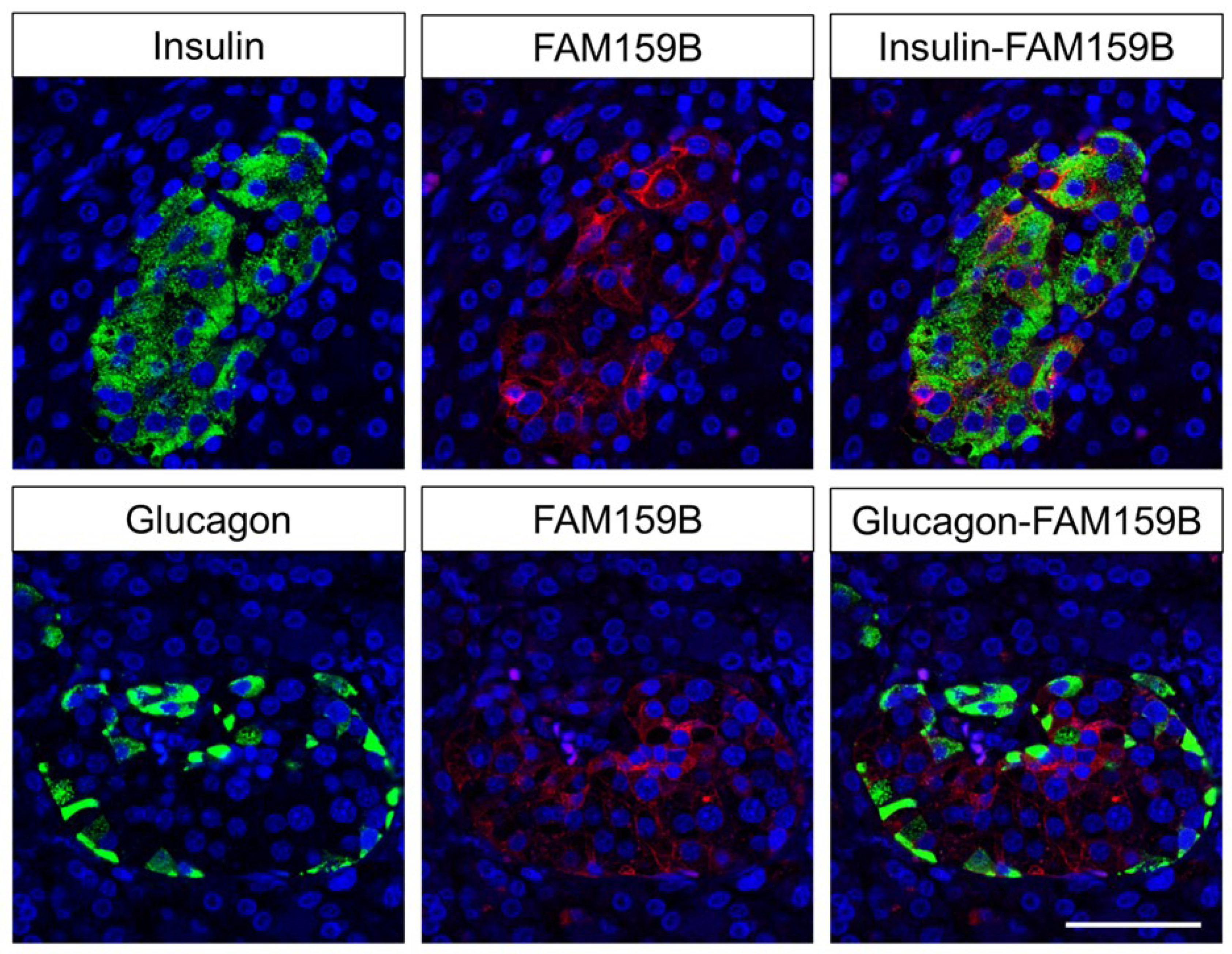
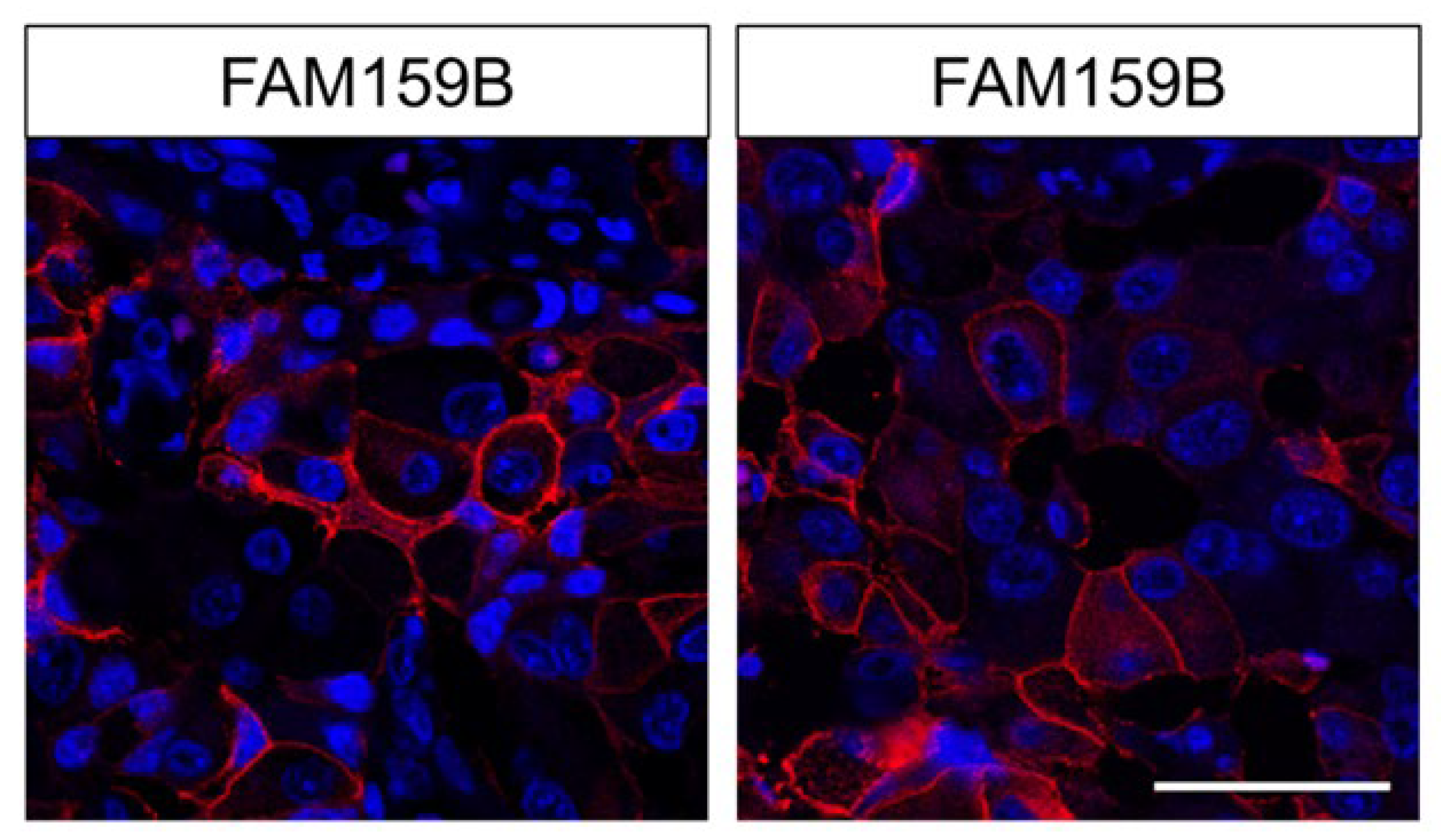
2.2. Immunohistochemical Detection of FAM159B Expression in Normal Human Tissues
2.3. Immunohistochemical Detection of FAM159B Expression in Different Human Tumours and Tumour Cell Lines
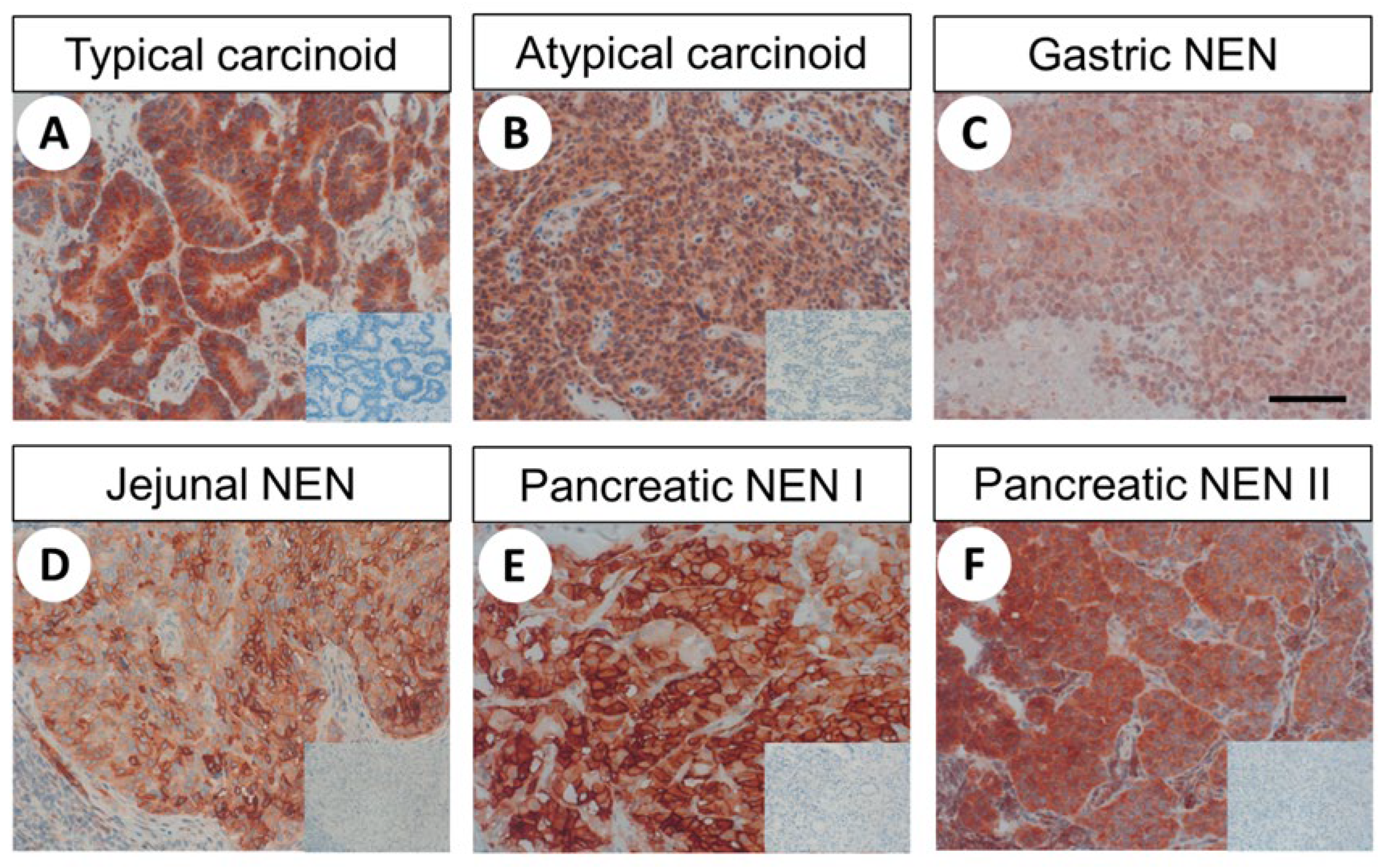
2.4. FAM159B Expression in Bronchopulmonary and Gastroenteropancreatic Neuroendocrine Neoplasms
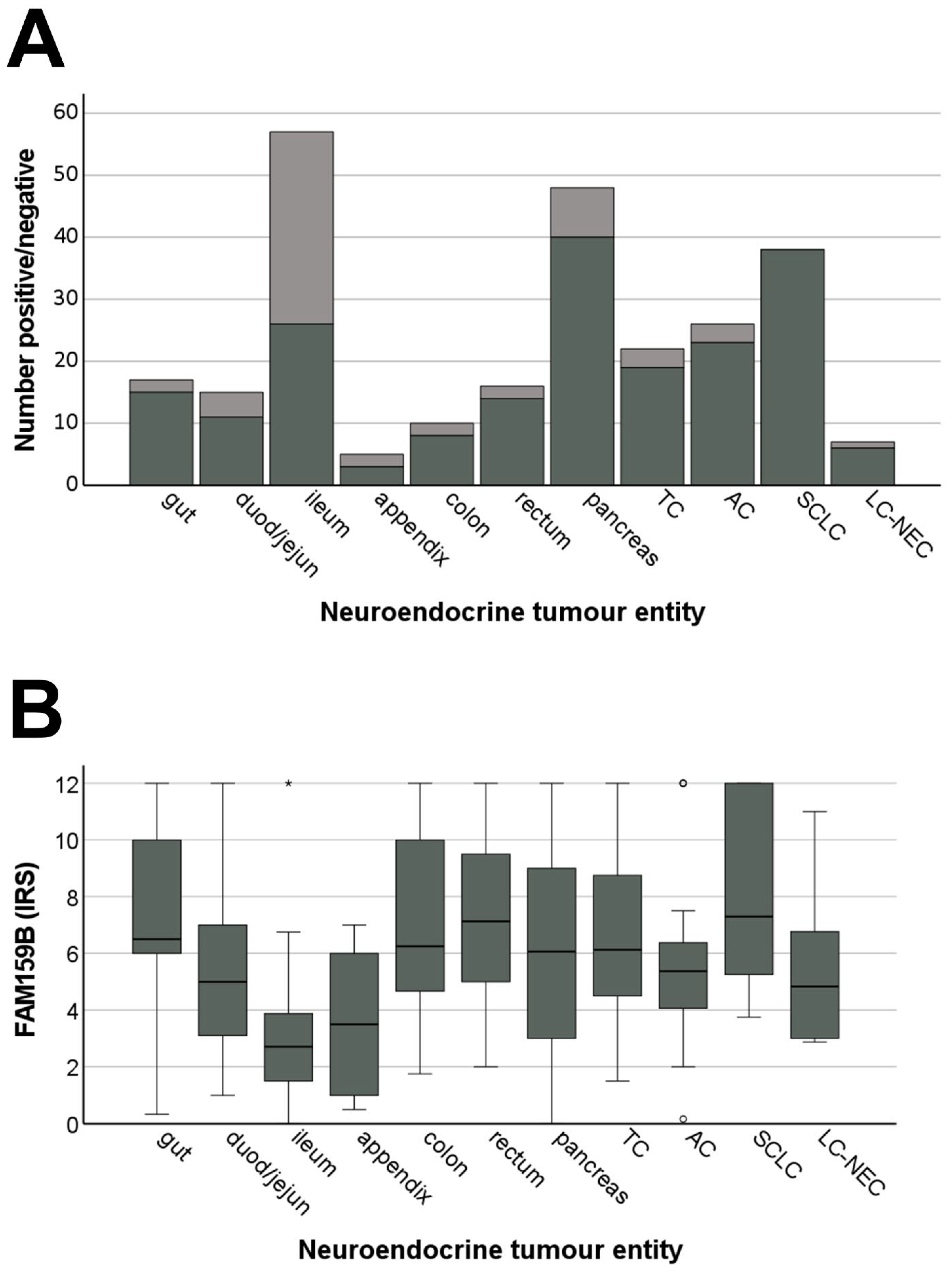
2.4.1. Patient Characteristics
2.4.2. FAM159B Expression Pattern
2.4.3. Correlations with Clinical Data
3. Discussion
3.1. Characterisation of the Rabbit Polyclonal Anti-Human FAM159B Antibody HPA011778
3.1.1. Western Blot Analysis
3.1.2. Immunocytochemistry
3.1.3. Immunohistochemistry
3.2. FAM159B Expression in Normal Human Tissues
3.3. FAM159B Expression in Human Neoplastic Tissues
4. Materials and Methods
4.1. Antibody
4.2. Western Blot Analysis
4.3. Immuncytochemistry
4.4. Immunohistochemical Evaluation of FAM159B Expression on Normal and Neoplastic Human Tissues and Human Tumour Cell Lines
4.4.1. Tumour Specimens
4.4.2. Cell Lines and Cytoblocks
4.4.3. Immunohistochemistry
4.4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pei, J.; Grishin, N.V. Unexpected diversity in Shisa-like proteins suggests the importance of their roles as transmembrane adaptors. Cell. Signal. 2012, 24, 758–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielsson, A.; Pontén, F.; Fagerberg, L.; Hallström, B.M.; Schwenk, J.M.; Uhlén, M.; Korsgren, O.; Lindskog, C. The human pancreas proteome defined by transcriptomics and antibody-based profiling. PLoS ONE 2014, 9, e115421. [Google Scholar] [CrossRef] [PubMed]
- Camunas-Soler, J.; Dai, X.Q.; Hang, Y.; Bautista, A.; Lyon, J.; Suzuki, K.; Kim, S.K.; Quake, S.R.; MacDonald, P.E. Patch-seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab. 2020, 31, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Augsornworawat, P.; Maxwell, K.G.; Velazco-Cruz, L.; Millman, J.R. Single-cell transcriptome profiling reveals beta cell maturation in stem cell-derived islets after transplantation. Cell Rep. 2020, 32, 108067. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Herzig, M.; Dasgupta, P.; Kaemmerer, D.; Sänger, J.; Evert, K.; Schulz, S.; Lupp, A. Comprehensive assessment of GPR68 expression in normal and neoplastic human tissues using a novel rabbit monoclonal antibody. Int. J. Mol. Sci. 2019, 20, 5261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, R.; Kaemmerer, D.; Träger, T.; Neubauer, E.; Sänger, J.; Baum, R.P.; Schulz, S.; Lupp, A. Different somatostatin and CXCR4 chemokine receptor expression in gastroenteropancreatic neuroendocrine neoplasms depending on their origin. Sci. Rep. 2019, 9, 4339. [Google Scholar] [CrossRef] [PubMed]
- Rösner, E.; Kaemmerer, D.; Neubauer, E.; Sänger, J.; Lupp, A. Prognostic value of PD-L1 expression in bronchopulmonary neuroendocrine tumours. Endocr. Connect. 2021, 10, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, D.; Trager, T.; Hoffmeister, M.; Sipos, B.; Hommann, M.; Sänger, J.; Schulz, S.; Lupp, A. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget 2015, 6, 27566–27579. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, D.; Sänger, J.; Arsenic, R.; D’Haese, J.G.; Neumann, J.; Schmitt-Graeff, A.; Wirtz, R.M.; Schulz, S.; Lupp, A. Evaluation of somatostatin, CXCR4 chemokine and endothelin A receptor expression in a large set of paragangliomas. Oncotarget 2017, 8, 89958–89969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]


| Tumour Type (Total Number of Cases) | FAM159B-Positive Tumours | Immunoreactive Score (IRS) | |||
|---|---|---|---|---|---|
| n | % | Mean | Min | Max | |
| Glioblastoma (10) | 1 | 10 | 1.11 | 0.0 | 3.0 |
| Pituitary adenoma (2) | 1 | 50 | 7.00 | 2.0 | 12.0 |
| Thyroid carcinoma (37) | 29 | 78.4 | 4.90 | 0.0 | 12.0 |
| - papillary (10) | 9 | 90 | 4.40 | 2.0 | 6.0 |
| - follicular (10) | 4 | 40 | 2.40 | 0.0 | 6.0 |
| - medullary (9) | 9 | 100 | 6.90 | 3.0 | 12.0 |
| - anaplastic (8) | 7 | 87.5 | 6.40 | 1.5 | 12.0 |
| Parathyroid adenoma (10) | 9 | 90 | 5.10 | 2.0 | 8.0 |
| Lung cancer (30) | 26 | 86.7 | 5.18 | 1.0 | 12.0 |
| - Adenocarcinoma (10) | 10 | 100 | 5.60 | 3.0 | 12.0 |
| - Squamous cell carcinoma (10) | 6 | 60 | 4.30 | 1.0 | 9.0 |
| - Small-cell lung cancer (10) | 10 | 100 | 6.25 | 3.0 | 10.0 |
| Gastric adenocarcinoma (10) | 6 | 60 | 4.40 | 0.0 | 9.0 |
| Colon carcinoma (9) | 6 | 66.7 | 3.50 | 0.0 | 6.0 |
| Gastrointestinal stromal tumour (10) | 2 | 20 | 1.95 | 0.0 | 9.0 |
| Hepatocellular carcinoma (11) | 3 | 27.3 | 1.36 | 0.0 | 4.0 |
| Cholangiocellular carcinoma (8) | 3 | 37.5 | 2.63 | 0.0 | 9.0 |
| Pancreatic adenocarcinoma (10) | 6 | 60 | 3.25 | 0.0 | 6.0 |
| Renal clear cell carcinoma (10) | 1 | 10 | 1.50 | 0.0 | 3.0 |
| Urinary bladder carcinoma (7) | 2 | 28.6 | 2.00 | 0.0 | 4.0 |
| Pheochromocytoma (7) | 3 | 42.9 | 2.29 | 0.0 | 4.0 |
| Breast carcinoma (9) | 5 | 55.6 | 3.17 | 0.0 | 6.0 |
| Ovarian cancer (9) | 8 | 88.9 | 5.39 | 2.0 | 9.0 |
| Endometrial cancer (10) | 5 | 50 | 2.50 | 0.0 | 9.0 |
| Cervical cancer (9) | 5 | 55.6 | 4.00 | 0.0 | 12.0 |
| Prostate adenocarcinoma (12) | 4 | 33.3 | 2.17 | 0.0 | 6.0 |
| Testicular cancer (10) | 2 | 20 | 2.10 | 0.0 | 6.0 |
| Lymphoma (10) | 9 | 90 | 6.40 | 0.0 | 10.0 |
| Melanoma (5) | 3 | 60 | 4.00 | 2.0 | 6.0 |
| Sarcoma (14) | 1 | 7.1 | 0.71 | 0.0 | 6.0 |
| - Liposarcoma (4) | 0 | 0 | 0.00 | 0.0 | 0.0 |
| - Rhabdomyosarcoma (4) | 1 | 25 | 2.50 | 0.0 | 6.0 |
| - Leiomyosarcoma (4) | 0 | 0 | 0.00 | 0.0 | 0.0 |
| - Pleomorphic sarcoma (2) | 0 | 0 | 0.00 | 0.0 | 0.0 |
| Correlation between FAM159B Expression and: | Correlation-Coefficient (rsp) | Level of Significance (p) | |
|---|---|---|---|
| Ki-67 index | All tumours | 0.306 | <0.001 |
| GEP-NEN | 0.279 | <0.001 | |
| BP-NEN | 0.218 | <0.001 | |
| IRS CgA | All tumours | 0.037 | 0.337 |
| GEP-NEN | 0.001 | 0.983 | |
| BP-NEN | 0.096 | 0.122 | |
| IRS D2-receptor | All tumours | 0.215 | <0.001 |
| GEP-NEN | 0.281 | <0.001 | |
| BP-NEN | 0.041 | 0.542 | |
| IRS SST1 | All tumours | 0.156 | <0.001 |
| GEP-NEN | 0.166 | 0.001 | |
| BP-NEN | −0.117 | 0.056 | |
| IRS SST2 | All tumours | 0.042 | 0.267 |
| GEP-NEN | 0.177 | <0.001 | |
| BP-NEN | −0.049 | 0.421 | |
| IRS SST3 | All tumours | 0.154 | <0.001 |
| GEP-NEN | 0.216 | <0.001 | |
| BP-NEN | −0.024 | 0.699 | |
| IRS SST4 | All tumours | 0.155 | <0.001 |
| GEP-NEN | 0.076 | 0.117 | |
| BP-NEN | 0.151 | 0.013 | |
| IRS SST5 | All tumours | 0.234 | <0.001 |
| GEP-NEN | 0.313 | <0.001 | |
| BP-NEN | −0.048 | 0.438 | |
| IRS CXCR4 | All tumours | 0.253 | <0.001 |
| GEP-NEN | 0.234 | <0.001 | |
| BP-NEN | 0.173 | 0.006 | |
| IRS PD-L1 | All tumours | 0.440 | <0.001 |
| GEP-NEN | 0.467 | <0.001 | |
| BP-NEN | 0.201 | 0.056 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyer, A.-S.L.; Kaemmerer, D.; Sänger, J.; Evert, K.; Lupp, A. Immunohistochemical Evaluation of Adaptor Protein FAM159B Expression in Normal and Neoplastic Human Tissues. Int. J. Mol. Sci. 2021, 22, 12250. https://doi.org/10.3390/ijms222212250
Beyer A-SL, Kaemmerer D, Sänger J, Evert K, Lupp A. Immunohistochemical Evaluation of Adaptor Protein FAM159B Expression in Normal and Neoplastic Human Tissues. International Journal of Molecular Sciences. 2021; 22(22):12250. https://doi.org/10.3390/ijms222212250
Chicago/Turabian StyleBeyer, Anna-Sophia Lieselott, Daniel Kaemmerer, Jörg Sänger, Katja Evert, and Amelie Lupp. 2021. "Immunohistochemical Evaluation of Adaptor Protein FAM159B Expression in Normal and Neoplastic Human Tissues" International Journal of Molecular Sciences 22, no. 22: 12250. https://doi.org/10.3390/ijms222212250






