BP180/Collagen XVII: A Molecular View
Abstract
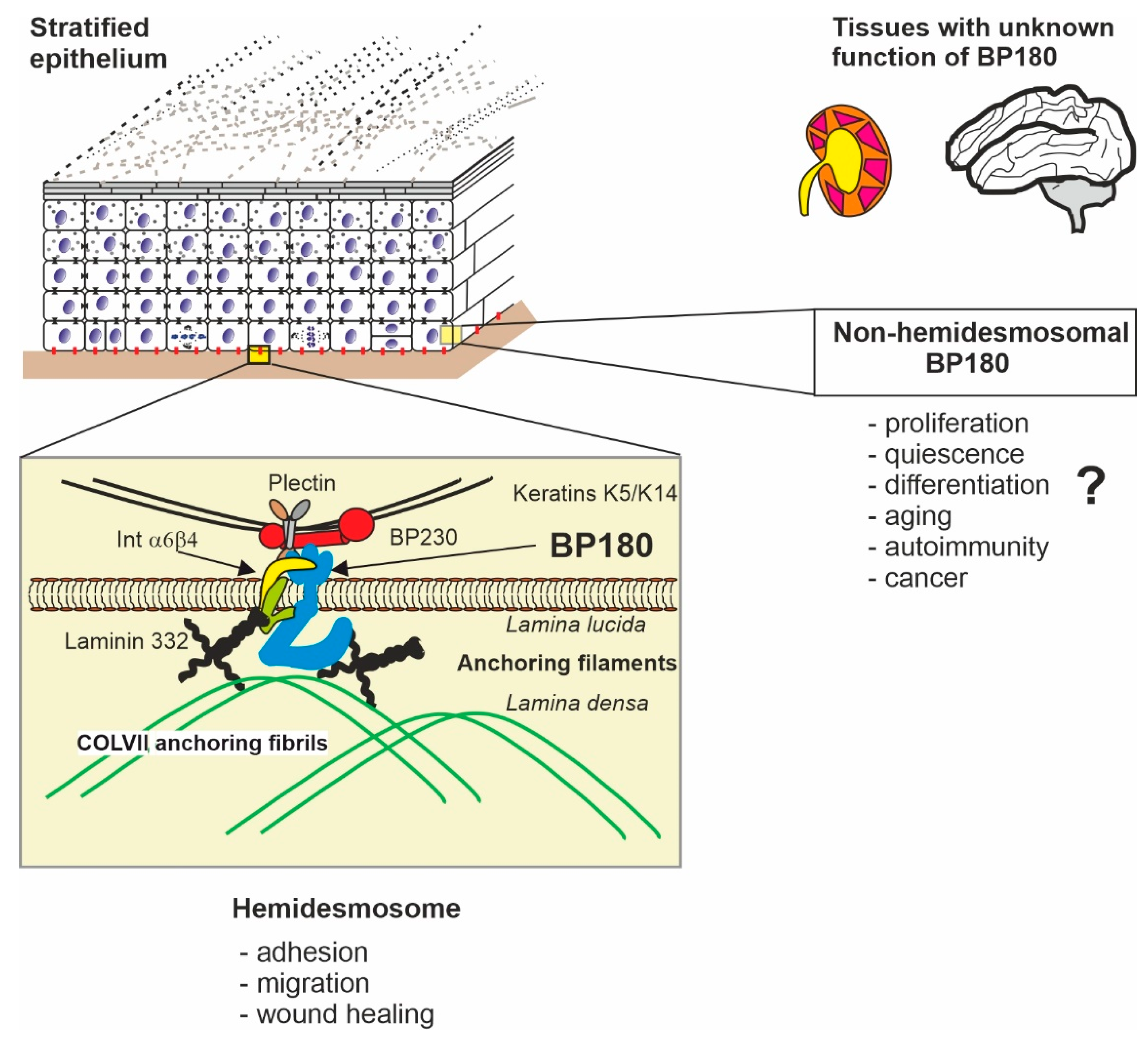
:1. Introduction
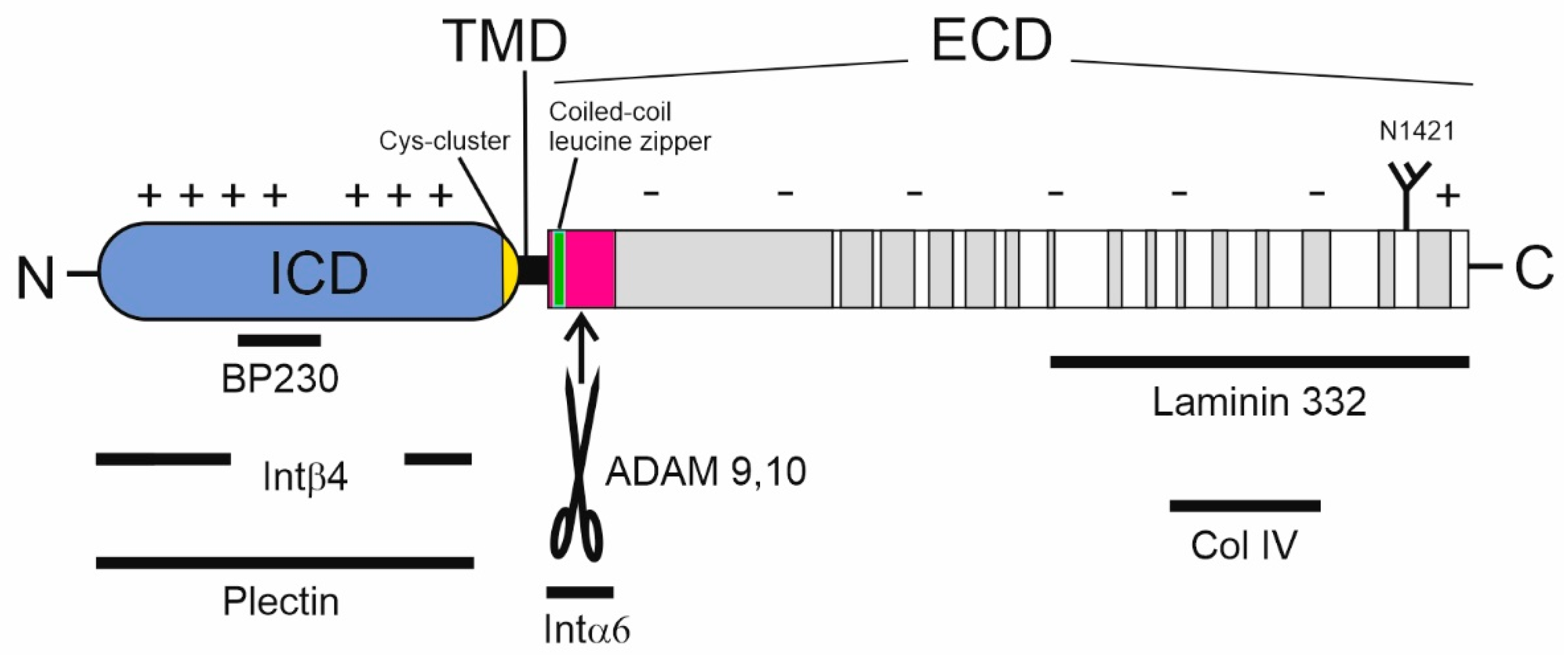
2. Structure of BP180
3. Expression of BP180
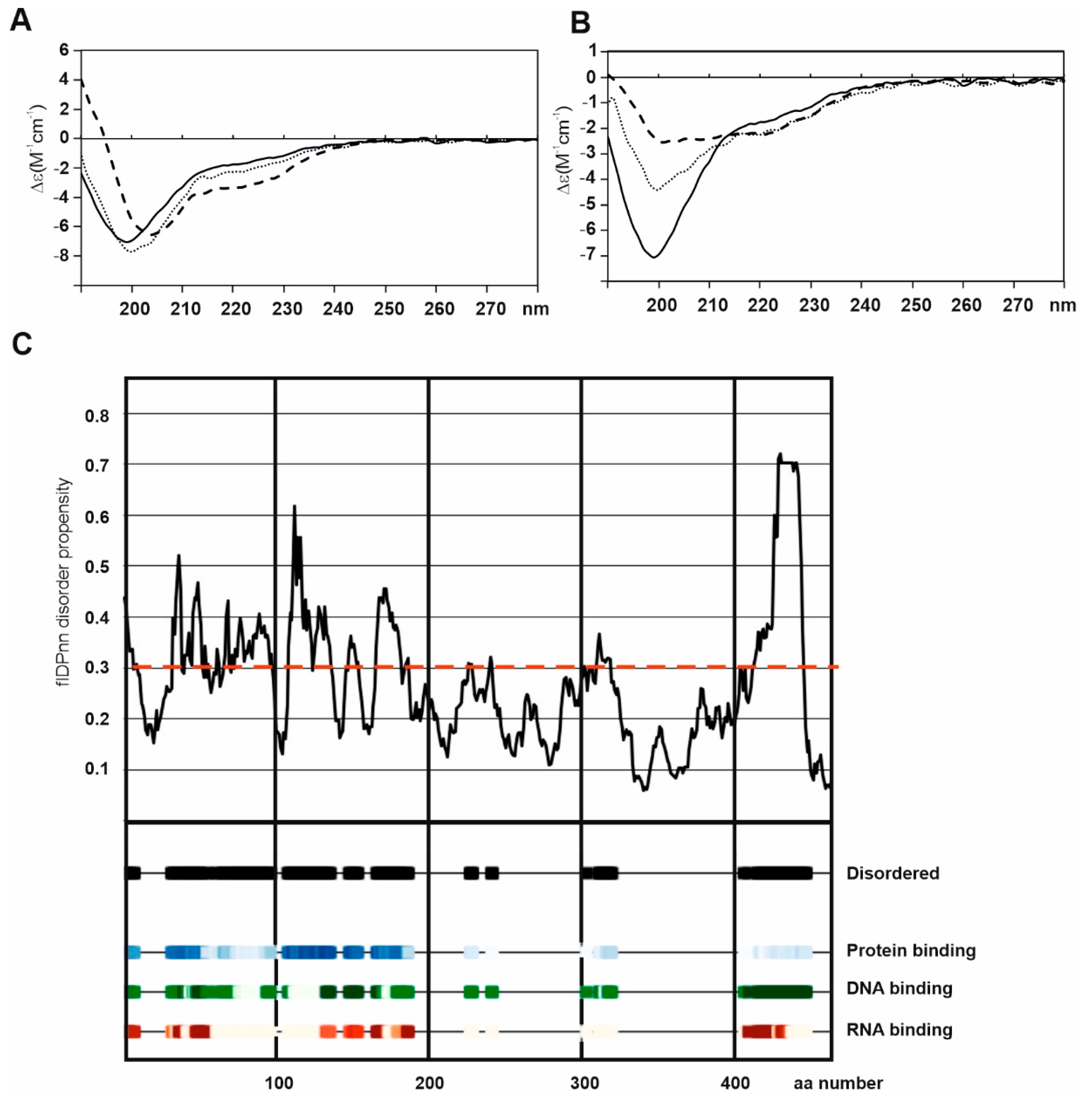
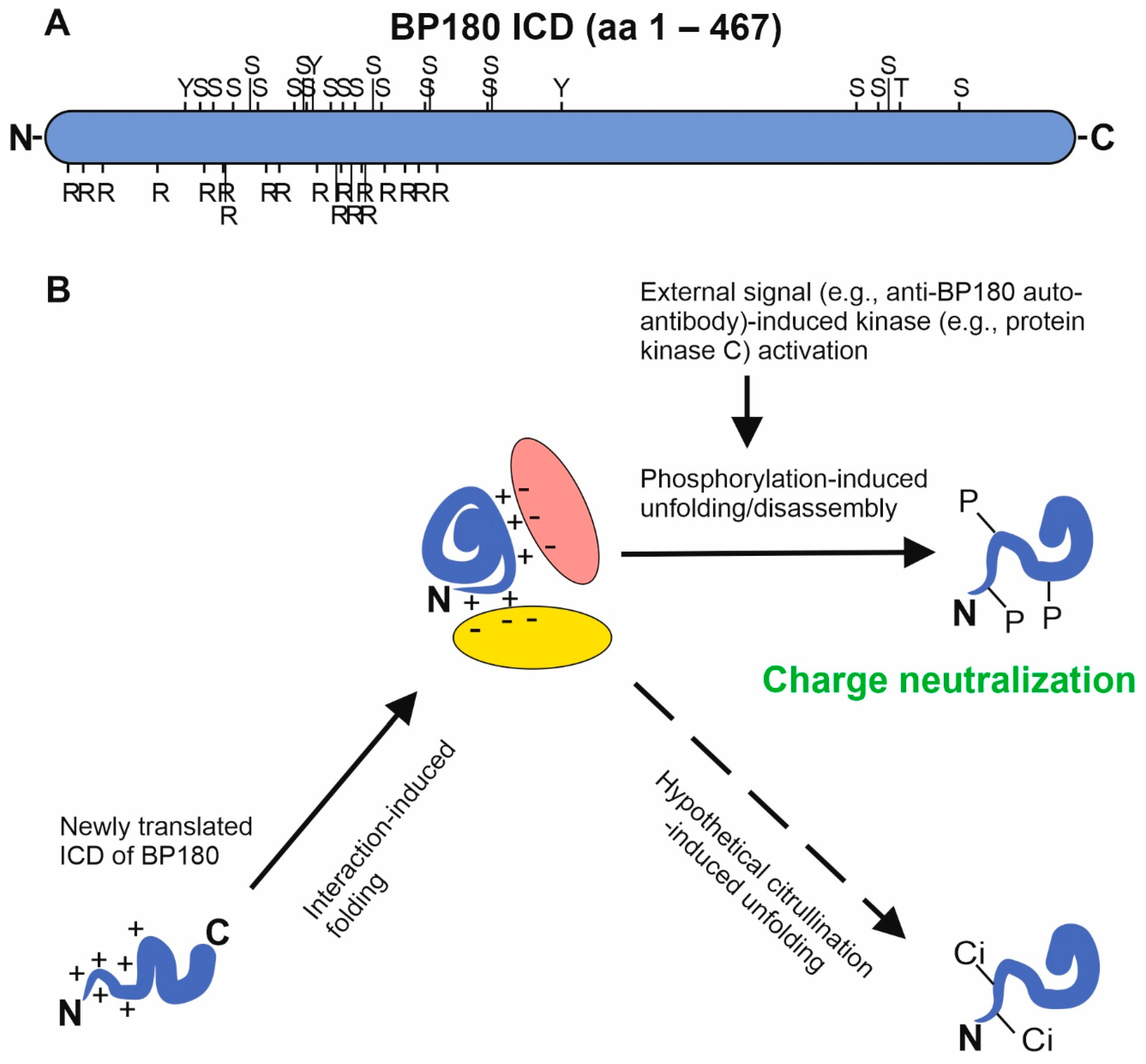
4. Post-Translational Modifications of BP180
5. BP180 Ectodomain Shedding
6. Protein–Protein Interactions of BP180
7. The Role of BP180 in Cell Migration, Proliferation, and Differentiation
8. Mechanisms and Consequences of BP180 Downregulation
| Molecular Defects | References [3,11,45,47,52,53,110,111,124,136,137,138,139,140,141,142] | |
Hereditary blistering skin diseases
| Recessive missense and nonsense mutation in several sites in COL17A1 | Bauer and Lanschuetzer, 2003 Kiritsi et al., 2011 Huber et al., 2002 Has et al., 2019 |
Autoimmune skin diseases
| IgG, IgE, IgA autoantibodies against the NC16A immune-dominant domain of BP180 and other epitopes located in the ICD, Col15 domain, and C-terminus | Bağcı et al., 2017 Xu et al., 2013 Kamaguchi and Iwata, 2019 Solano-Lopez et al., 2015 Huilaja et al., 2014 Cozzani et al., 2020 |
Cancer
| Altered expression of BP180 Altered shedding of BP180 | Moilanen et al., 2015, 2017 Parikka et al., 2001 Liu et al., 2016a,b Krenacs et al., 2012 |
9. BP180 in Health and Diseases—Future Views
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Labib, R.S.; Anhalt, G.J.; Patel, H.P.; Mutasim, D.F.; Diaz, L.A. Molecular Heterogeneity of the Bullous Pemphigoid Antigens as Detected by Immunoblotting. J. Immunol. 1986, 136, 1231–1235. [Google Scholar]
- Klatte, D.H.; Kurpakus, M.A.; Grelling, K.A.; Jones, J.C. Immunochemical Characterization of Three Components of the Hemidesmosome and their Expression in Cultured Epithelial Cells. J. Cell Biol. 1989, 109, 3377–3390. [Google Scholar] [CrossRef]
- Bağci, I.S.; Horváth, O.N.; Ruzicka, T.; Sardy, M. Bullous Pemphigoid. Autoimmun. Rev. 2017, 16, 445–455. [Google Scholar] [CrossRef]
- Schmidt, E.; Zillikens, D. Pemphigoid Diseases. Lancet 2013, 381, 320–332. [Google Scholar] [CrossRef]
- Langan, S.M.; Smeeth, L.; Hubbard, R.; Fleming, K.M.; Smith, C.J.; West, J. Bullous pemphigoid and pemphigus vulgaris–incidence and mortality in the UK: Population based cohort study. BMJ 2008, 337, a180. [Google Scholar] [CrossRef] [Green Version]
- Försti, A.K.; Huilaja, L.; Schmidt, E.; Tasanen, K. Neurological and Psychiatric Associations in Bullous Pemphigoid-More than Skin Deep? Exp. Dermatol. 2017, 26, 1228–1234. [Google Scholar] [CrossRef]
- Varpuluoma, O.; Jokelainen, J.; Försti, A.K.; Timonen, M.; Huilaja, L.; Tasanen, K. Dermatitis Herpetiformis and Celiac Disease Increase the Risk of Bullous Pemphigoid. J. Investig. Dermatol. 2019, 139, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Jedlowski, P.M.; Jedlowski, M.F.; Fazel, M.T. DPP-4 Inhibitors and Increased Reporting Odds of Bullous Pemphigoid: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS) from 2006 to 2020. Am. J. Clin. Dermatol. 2021, 22, 891–900. [Google Scholar] [CrossRef]
- Nishie, W. Update on the Pathogenesis of Bullous Pemphigoid: An Autoantibody-Mediated Blistering Disease Targeting Collagen XVII. J. Dermatol. Sci. 2014, 73, 179–186. [Google Scholar] [CrossRef]
- Christophoridis, S.; Büdinger, L.; Borradori, L.; Hunziker, T.; Merk, H.F.; Hertl, M. IgG, IgA and IgE Autoantibodies Against the Ectodomain of BP180 in Patients with Bullous and Cicatricial Pemphigoid and Linear IgA Bullous Dermatosis. Br. J. Dermatol. 2000, 143, 349–355. [Google Scholar] [CrossRef]
- Has, C.; Bauer, J.W.; Bodemer, C.; Bolling, M.C.; Bruckner-Tuderman, L.; Diem, A.; Fine, J.D.; Heagerty, A.; Hovnanian, A.; Marinkovich, M.P.; et al. Consensus Reclassification of Inherited Epidermolysis Bullosa and Other Disorders with Skin Fragility. Br. J. Dermatol. 2020, 183, 614–627. [Google Scholar] [CrossRef] [Green Version]
- Condrat, I.; He, Y.; Cosgarea, R.; Has, C. Junctional Epidermolysis Bullosa: Allelic Heterogeneity and Mutation Stratification for Precision Medicine. Front. Med. 2019, 5, 363. [Google Scholar] [CrossRef] [Green Version]
- Giudice, G.J.; Emery, D.J.; Diaz, L.A. Cloning and Primary Structural Analysis of the Bullous Pemphigoid Autoantigen BP180. J. Investig. Dermatol. 1992, 99, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Tamai, K.; Tan, E.M.; Uitto, J. Cloning of Type XVII Collagen. Complementary and Genomic DNA Sequences of Mouse 180-Kilodalton Bullous Pemphigoid Antigen (BPAG2) Predict an Interrupted Collagenous Domain, a Transmembrane Segment, and Unusual Features in the 5′-End of the Gene and the 3′-Untranslated Region of the mRNA. J. Biol. Chem. 1993, 268, 8825–8834. [Google Scholar]
- Giudice, G.J.; Emery, D.J.; Zelickson, B.D.; Anhalt, G.J.; Liu, Z.; Diaz, L.A. Bullous Pemphigoid and Herpes Gestationis Autoantibodies Recognize a Common Non-Collagenous Site on the BP180 Ectodomain. J. Immunol. 1993, 151, 5742–5750. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Uematsu, J.; Owaribe, K. HD4, a 180 kDa Bullous Pemphigoid Antigen, is a Major Transmembrane Glycoprotein of the Hemidesmosome. J. Biochem. 1993, 113, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Hirako, Y.; Usukura, J.; Nishizawa, Y.; Owaribe, K. Demonstration of the Molecular Shape of BP180, a 180-kDa Bullous Pemphigoid Antigen and its Potential for Trimer Formation. J. Biol. Chem. 1996, 271, 13739–13745. [Google Scholar] [CrossRef] [Green Version]
- Nishie, W.; Jackow, J.; Hofmann, S.C.; Franzke, C.W.; Bruckner-Tuderman, L. Coiled Coils Ensure the Physiological Ectodomain Shedding of Collagen XVII. J. Biol. Chem. 2012, 287, 29940–29948. [Google Scholar] [CrossRef] [Green Version]
- Powell, A.M.; Sakuma-Oyama, Y.; Oyama, N.; Black, M.M. Collagen XVII/BP180: A Collagenous Transmembrane Protein and Component of the Dermoepidermal Anchoring Complex. Clin. Exp. Dermatol. 2005, 30, 682–687. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Bansal, M. Collagen Structure: The Madras Triple Helix and the Current Scenario. IUBMB Life 2005, 57, 161–172. [Google Scholar] [CrossRef]
- Hirose, M.; Recke, A.; Beckmann, T.; Shimizu, A.; Ishiko, A.; Bieber, K.; Westermann, J.; Zillikens, D.; Schmidt, E.; Ludwig, R.J. Repetitive Immunization Breaks Tolerance to Type XVII Collagen and Leads to Bullous Pemphigoid in Mice. J. Immunol. 2011, 187, 1176–1183. [Google Scholar] [CrossRef]
- Schäcke, H.; Schumann, H.; Hammami-Hauasli, N.; Raghunath, M.; Bruckner-Tuderman, L. Two Forms of Collagen XVII in Keratinocytes. A Full-Length Transmembrane Protein and a Soluble Ectodomain. J. Biol. Chem. 1998, 273, 25937–25943. [Google Scholar] [CrossRef] [Green Version]
- Wolf, A.M.; Nishimaki, K.; Kamimura, N.; Ohta, S. Real-Time Monitoring of Oxidative Stress in Live Mouse Skin. J. Investig. Dermatol. 2014, 134, 1701–1709. [Google Scholar] [CrossRef] [Green Version]
- Tuusa, J.; Lindgren, O.; Tertsunen, H.M.; Nishie, W.; Kokkonen, N.; Huilaja, L.; Izumi, K.; Herukka, S.K.; Miettunen, J.; Shimizu, H.; et al. BP180 Autoantibodies Target Different Epitopes in Multiple Sclerosis or Alzheimer’s Disease than in Bullous Pemphigoid. J. Investig. Dermatol. 2019, 139, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Balding, S.D.; Diaz, L.A.; Giudice, G.J. A Recombinant Form of the Human BP180 Ectodomain Forms a Collagen-Like Homotrimeric Complex. Biochemistry 1997, 36, 8821–8830. [Google Scholar] [CrossRef]
- Pas, H.H.; Kloosterhuis, G.J.; Nijenhuis, M.; de Jong, M.C.; van der Meer, J.B.; Jonkman, M.F. Type XVII Collagen (BP180) and LAD-1 are Present as Separate Trimeric Complexes. J. Investig. Dermatol. 1999, 112, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Areida, S.K.; Reinhardt, D.P.; Muller, P.K.; Fietzek, P.P.; Kowitz, J.; Marinkovich, M.P.; Notbohm, H. Properties of the Collagen Type XVII Ectodomain. Evidence for N- to C-Terminal Triple Helix Folding. J. Biol. Chem. 2001, 276, 1594–1601. [Google Scholar] [CrossRef] [Green Version]
- Van den Bergh, F.; Fu, C.L.; Olague-Marchan, M.; Giudice, G.J. The NC16A domain of collagen XVII plays a role in triple helix assembly and stability. Biochem. Biophys. Res. Commun. 2006, 350, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Latvanlehto, A.; Snellman, A.; Tu, H.; Pihlajaniemi, T. Type XIII Collagen and some Other Transmembrane Collagens Contain Two Separate Coiled-Coil Motifs, which may Function as Independent Oligomerization Domains. J. Biol. Chem. 2003, 278, 37590–37599. [Google Scholar] [CrossRef] [Green Version]
- Snellman, A.; Tuomisto, A.; Koski, A.; Latvanlehto, A.; Pihlajaniemi, T. The Role of Disulfide Bonds and Alpha-Helical Coiled-Coils in the Biosynthesis of Type XIII Collagen and Other Collagenous Transmembrane Proteins. J. Biol. Chem. 2007, 282, 14898–14905. [Google Scholar] [CrossRef] [Green Version]
- Nyström, A.; Kiritsi, D. Transmembrane Collagens-Unexplored Mediators of Epidermal-Dermal Communication and Tissue Homeostasis. Exp. Dermatol. 2021, 30, 10–16. [Google Scholar] [CrossRef]
- Masunaga, T.; Shimizu, H.; Yee, C.; Borradori, L.; Lazarova, Z.; Nishikawa, T.; Yancey, K.B. The Extracellular Domain of BPAG2 Localizes to Anchoring Filaments and its Carboxyl Terminus Extends to the Lamina Densa of Normal Human Epidermal Basement Membrane. J. Investig. Dermatol. 1997, 109, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, S.; Ishiko, A.; Masunaga, T.; Akiyama, M.; Owaribe, K.; Shimizu, H.; Nishikawa, T. The Extracellular Domain of BPAG2 has a Loop Structure in the Carboxy Terminal Flexible Tail in Vivo. J. Investig. Dermatol. 2000, 115, 889–892. [Google Scholar] [CrossRef]
- Hirako, Y.; Owaribe, K. Hemidesmosomes and their Unique Transmembrane Protein BP180. Microsc. Res. Tech. 1998, 43, 207–217. [Google Scholar] [CrossRef]
- Franzke, C.W.; Bruckner, P.; Bruckner-Tuderman, L. Collagenous Transmembrane Proteins: Recent Insights into Biology and Pathology. J. Biol. Chem. 2005, 280, 4005–4008. [Google Scholar] [CrossRef] [Green Version]
- Tuusa, J.; Koski, M.K.; Ruskamo, S.; Tasanen, K. The Intracellular Domain of BP180/Collagen XVII is Intrinsically Disordered and Partially Folds in an Anionic Membrane Lipid-Mimicking Environment. Amino Acids 2020, 52, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Katuwawala, A.; Wang, K.; Wu, Z.; Ghadermarzi, S.; Gao, J.; Kurgan, L. flDPnn: Accurate Intrinsic Disorder Prediction with Putative Propensities of Disorder Functions. Nat. Commun. 2021, 12, 4438. [Google Scholar] [CrossRef]
- Shamilov, R.; Robinson, V.L.; Aneskievich, B.J. Seeing Keratinocyte Proteins through Looking Glass of Intrinsic Disorder. Int. J. Mol. Sci. 2021, 22, 7912. [Google Scholar] [CrossRef]
- Aho, S.; Uitto, J. 180-kD Bullous Pemphigoid Antigen/Type XVII Collagen: Tissue-Specific Expression and Molecular Interactions with Keratin 18. J. Cell. Biochem. 1999, 72, 356–367. [Google Scholar] [CrossRef]
- Parikka, M.; Kainulainen, T.; Tasanen, K.; Väänänen, A.; Bruckner-Tuderman, L.; Salo, T. Alterations of Collagen XVII Expression during Transformation of Oral Epithelium to Dysplasia and Carcinoma. J. Histochem. Cytochem. 2003, 51, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Herwig, M.C.; Müller, A.M.; Holz, F.G.; Loeffler, K.U. Immunolocalization of Different Collagens in the Cornea of Human Fetal Eyes: A Developmental Approach. Curr. Eye Res. 2013, 38, 60–69. [Google Scholar] [CrossRef]
- Thangavelu, P.U.; Krenács, T.; Dray, E.; Duijf, P.H. In Epithelial Cancers, Aberrant COL17A1 Promoter Methylation Predicts its Misexpression and Increased Invasion. eCollection 2016. Clin. Epigenet. 2016, 8, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Wen, Y.; Zhang, Z.; Guo, Y.; Warrington, J.A.; Polan, M.L. Microarray Analysis of Differentially Expressed Genes in Vaginal Tissues from Women with Stress Urinary Incontinence Compared with Asymptomatic Women. Hum. Reprod. 2006, 21, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Fairley, J.A.; Heintz, P.W.; Neuburg, M.; Diaz, L.A.; Giudice, G.J. Expression Pattern of the Bullous Pemphigoid-180 Antigen in Normal and Neoplastic Epithelia. Br. J. Dermatol. 1995, 133, 385–391. [Google Scholar] [CrossRef]
- Huilaja, L.; Hurskainen, T.; Autio-Harmainen, H.; Hofmann, S.C.; Sormunen, R.; Räsänen, J.; Ilves, M.; Franzke, C.W.; Bruckner-Tuderman, L.; Tasanen, K. Pemphigoid Gestationis Autoantigen, Transmembrane Collagen XVII, Promotes the Migration of Cytotrophoblastic Cells of Placenta and is a Structural Component of Fetal Membranes. Matrix Biol. 2008, 27, 190–200. [Google Scholar] [CrossRef]
- Asaka, T.; Akiyama, M.; Domon, T.; Nishie, W.; Natsuga, K.; Fujita, Y.; Abe, R.; Kitagawa, Y.; Shimizu, H. Type XVII Collagen is a Key Player in Tooth Enamel Formation. Am. J. Pathol. 2009, 174, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, J.M.; Kokkonen, N.; Löffek, S.; Väyrynen, J.P.; Syväniemi, E.; Hurskainen, T.; Mäkinen, M.; Klintrup, K.; Mäkelä, J.; Sormunen, R.; et al. Collagen XVII Expression Correlates with the Invasion and Metastasis of Colorectal Cancer. Hum. Pathol. 2015, 46, 434–442. [Google Scholar] [CrossRef]
- Litjens, S.H.; de Pereda, J.M.; Sonnenberg, A. Current Insights into the Formation and Breakdown of Hemidesmosomes. Trends Cell Biol. 2006, 16, 376–383. [Google Scholar] [CrossRef]
- Yamada, T.; Endo, R.; Tsukagoshi, K.; Fujita, S.; Honda, K.; Kinoshita, M.; Hasebe, T.; Hirohashi, S. Aberrant Expression of a Hemidesmosomal Protein, Bullous Pemphigoid Antigen 2, in Human Squamous Cell Carcinoma. Lab. Investig. 1996, 75, 589–600. [Google Scholar]
- Parikka, M.; Nissinen, L.; Kainulainen, T.; Bruckner-Tuderman, L.; Salo, T.; Heino, J.; Tasanen, K. Collagen XVII Promotes Integrin-Mediated Squamous Cell Carcinoma Transmigration--a Novel Role for alphaIIb Integrin and Tirofiban. Exp. Cell Res. 2006, 312, 1431–1438. [Google Scholar] [CrossRef]
- Stelkovics, E.; Korom, I.; Marczinovits, I.; Molnar, J.; Rasky, K.; Raso, E.; Ficsor, L.; Molnar, B.; Kopper, L.; Krenacs, T. Collagen XVII/BP180 Protein Expression in Squamous Cell Carcinoma of the Skin Detected with Novel Monoclonal Antibodies in Archived Tissues using Tissue Microarrays and Digital Microscopy. Appl. Immunohistochem. Mol. Morphol. 2008, 16, 433–441. [Google Scholar] [CrossRef]
- Krenacs, T.; Kiszner, G.; Stelkovics, E.; Balla, P.; Teleki, I.; Nemeth, I.; Varga, E.; Korom, I.; Barbai, T.; Plotar, V.; et al. Collagen XVII is Expressed in Malignant but Not in Benign Melanocytic Tumors and it can Mediate Antibody Induced Melanoma Apoptosis. Histochem. Cell Biol. 2012, 138, 653–667. [Google Scholar] [CrossRef]
- Parikka, M.; Kainulainen, T.; Tasanen, K.; Bruckner-Tuderman, L.; Salo, T. Altered Expression of Collagen XVII in Ameloblastomas and Basal Cell Carcinomas. J. Oral Pathol. Med. 2001, 30, 589–595. [Google Scholar] [CrossRef]
- Jones, V.A.; Patel, P.M.; Gibson, F.T.; Cordova, A.; Amber, K.T. The Role of Collagen XVII in Cancer: Squamous Cell Carcinoma and Beyond. Front. Oncol. 2020, 10, 352. [Google Scholar] [CrossRef]
- Matsumura, H.; Mohri, Y.; Binh, N.T.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Hair Follicle Aging is Driven by Transepidermal Elimination of Stem Cells Via COL17A1 Proteolysis. Science 2016, 351, aad4395. [Google Scholar] [CrossRef]
- Watanabe, M.; Natsuga, K.; Nishie, W.; Kobayashi, Y.; Donati, G.; Suzuki, S.; Fujimura, Y.; Tsukiyama, T.; Ujiie, H.; Shinkuma, S.; et al. Type XVII Collagen Coordinates Proliferation in the Interfollicular Epidermis. Elife 2017, 6, e26635. [Google Scholar] [CrossRef]
- Natsuga, K.; Watanabe, M.; Nishie, W.; Shimizu, H. Life before and Beyond Blistering: The Role of Collagen XVII in Epidermal Physiology. Exp. Dermatol. 2019, 28, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Hurskainen, T.; Moilanen, J.; Sormunen, R.; Franzke, C.W.; Soininen, R.; Löffek, S.; Huilaja, L.; Nuutinen, M.; Bruckner-Tuderman, L.; Autio-Harmainen, H.; et al. Transmembrane Collagen XVII is a Novel Component of the Glomerular Filtration Barrier. Cell Tissue Res. 2012, 348, 579–588. [Google Scholar] [CrossRef]
- Claudepierre, T.; Manglapus, M.K.; Marengi, N.; Radner, S.; Champliaud, M.F.; Tasanen, K.; Bruckner-Tuderman, L.; Hunter, D.D.; Brunken, W.J. Collagen XVII and BPAG1 Expression in the Retina: Evidence for an Anchoring Complex in the Central Nervous System. J. Comp. Neurol. 2005, 487, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Seppänen, A.; Autio-Harmainen, H.; Alafuzoff, I.; Särkioja, T.; Veijola, J.; Hurskainen, T.; Bruckner-Tuderman, L.; Tasanen, K.; Majamaa, K. Collagen XVII is Expressed in Human CNS Neurons. Matrix Biol. 2006, 25, 185–188. [Google Scholar] [CrossRef]
- Bastian, F.; Parmentier, G.; Roux, J.; Moretti, S.; Laudet, V.; Robinson-Rechavi, M. Bgee: Integrating and Comparing Heterogeneous Transcriptome Data among Species. In DILS: Data Integration in Life Sciences. Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5109, pp. 124–131. [Google Scholar]
- Petryszak, R.; Keays, M.; Tang, Y.A.; Fonseca, N.A.; Barrera, E.; Burdett, T.; Füllgrabe, A.; Fuentes, A.M.; Jupp, S.; Koskinen, S.; et al. Expression Atlas Update—An Integrated Database of Gene and Protein Expression in Humans, Animals and Plants. Nucleic Acids Res. 2016, 44, 746. [Google Scholar] [CrossRef]
- Seppänen, A.; Suuronen, T.; Hofmann, S.C.; Majamaa, K.; Alafuzoff, I. Distribution of Collagen XVII in the Human Brain. Brain Res. 2007, 1158, 50–56. [Google Scholar] [CrossRef]
- Seppänen, A.; Miettinen, R.; Alafuzoff, I. Neuronal Collagen XVII is Localized to Lipofuscin Granules. Neuroreport 2010, 21, 1090–1094. [Google Scholar] [CrossRef]
- Messingham, K.A.; Aust, S.; Helfenberger, J.; Parker, K.L.; Schultz, S.; McKillip, J.; Narayanan, N.S.; Fairley, J.A. Autoantibodies to Collagen XVII are Present in Parkinson’s Disease and Localize to Tyrosine-Hydroxylase Positive Neurons. J. Investig. Dermatol. 2016, 136, 721–723. [Google Scholar] [CrossRef] [Green Version]
- Barrick, B.J.; Ida, C.M.; Laniosz, V.; Jentoft, M.E.; Sominidi-Damodaran, S.; Wieland, C.N.; Meves, A.; Lehman, J.S. Bullous Pemphigoid, Neurodegenerative Disease, and Hippocampal BP180 Expression: A Retrospective Postmortem Neuropathologic Study. J. Investig. Dermatol. 2016, 136, 2090–2092. [Google Scholar] [CrossRef] [Green Version]
- Nishie, W.; Sawamura, D.; Goto, M.; Ito, K.; Shibaki, A.; McMillan, J.R.; Sakai, K.; Nakamura, H.; Olasz, E.; Yancey, K.B.; et al. Humanization of Autoantigen. Nat. Med. 2007, 13, 378–383. [Google Scholar] [CrossRef]
- Lin, L.; Hwang, B.J.; Li, N.; Googe, P.; Diaz, L.A.; Miao, E.; Vilen, B.; Thomas, N.E.; Ting, J.; Liu, Z. Non-Cell-Autonomous Activity of the Hemidesmosomal Protein BP180/Collagen XVII in Granulopoiesis in Humanized NC16A Mice. J. Immunol. 2020, 205, 2786–2794. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Davda, D.; Majmudar, J.D.; Won, S.J.; Prakash, A.; Choi, A.I.; Martin, B.R. Correlated S-Palmitoylation Profiling of Snail-Induced Epithelial to Mesenchymal Transition. Mol. Biosyst. 2016, 12, 1799–1808. [Google Scholar] [CrossRef] [Green Version]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and Structure-Based Prediction of Eukaryotic Protein Phosphorylation Sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Kitajima, Y.; Owaribe, K.; Nishizawa, Y.; Jokura, Y.; Yaoita, H. Phorbol Ester- and Calcium-Induced Reorganization of 180-kDa Bullous Pemphigoid Antigen on the Ventral Surface of Cultured Human Keratinocytes as Studied by Immunofluorescence and Immunoelectron Microscopy. Exp. Cell Res. 1992, 203, 17–24. [Google Scholar] [CrossRef]
- Kitajima, Y.; Owada, M.K.; Fujisawa, Y.; Seishima, M.; Yaoita, H.; Hirako, Y.; Owaribe, K. A Hemidesmosomal Transmembrane Collagenous Molecule, the 180-kDa Bullous Pemphigoid Antigen (BPA II), is Phosphorylated with 12-O-Tetradecanoylphorbol-13-Acetate in a Human Squamous Cell Carcinoma Cell Line (DJM-1). Epithelial. Cell Biol. 1995, 4, 70–75. [Google Scholar]
- Hiroyasu, S.; Ozawa, T.; Kobayashi, H.; Ishii, M.; Aoyama, Y.; Kitajima, Y.; Hashimoto, T.; Jones, J.C.; Tsuruta, D. Bullous Pemphigoid IgG Induces BP180 Internalization Via a Macropinocytic Pathway. Am. J. Pathol. 2013, 182, 828–840. [Google Scholar] [CrossRef] [Green Version]
- Zimina, E.P.; Fritsch, A.; Schermer, B.; Bakulina, A.Y.; Bashkurov, M.; Benzing, T.; Bruckner-Tuderman, L. Extracellular Phosphorylation of Collagen XVII by Ecto-Casein Kinase 2 Inhibits Ectodomain Shedding. J. Biol. Chem. 2007, 282, 22737–22746. [Google Scholar] [CrossRef] [Green Version]
- Zimina, E.P.; Hofmann, S.C.; Fritsch, A.; Kern, J.S.; Sitaru, C.; Bruckner-Tuderman, L. Bullous Pemphigoid Autoantibodies Preferentially Recognize Phosphoepitopes in Collagen XVII. J. Investig. Dermatol. 2008, 128, 2736–2739. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, M.; Alasmari, D.; Assiri, A.; Mattar, E.; Aljaddawi, A.A.; Alattas, S.G.; Redwan, E.M. An Overview of the Intrinsic Role of Citrullination in Autoimmune Disorders. J. Immunol. Res. 2019, 2019, 7592851. [Google Scholar] [CrossRef] [Green Version]
- Sipilä, K.H.; Ranga, V.; Rappu, P.; Torittu, A.; Pirilä, L.; Käpylä, J.; Johnson, M.S.; Larjava, H.; Heino, J. Extracellular Citrullination Inhibits the Function of Matrix Associated TGF-B. Matrix Biol. 2016, 55, 77–89. [Google Scholar] [CrossRef]
- Sipilä, K.H.; Ranga, V.; Rappu, P.; Mali, M.; Pirilä, L.; Heino, I.; Jokinen, J.; Käpylä, J.; Johnson, M.S.; Heino, J. Joint Inflammation Related Citrullination of Functional Arginines in Extracellular Proteins. Sci. Rep. 2017, 7, 8246. [Google Scholar] [CrossRef]
- Ujiie, H.; Sasaoka, T.; Izumi, K.; Nishie, W.; Shinkuma, S.; Natsuga, K.; Nakamura, H.; Shibaki, A.; Shimizu, H. Bullous Pemphigoid Autoantibodies Directly Induce Blister Formation without Complement Activation. J. Immunol. 2014, 193, 4415–4428. [Google Scholar] [CrossRef] [Green Version]
- Tasanen, K.; Eble, J.A.; Aumailley, M.; Schumann, H.; Baetge, J.; Tu, H.; Bruckner, P.; Bruckner-Tuderman, L. Collagen XVII is Destabilized by a Glycine Substitution Mutation in the Cell Adhesion Domain Col15. J. Biol. Chem. 2000, 275, 3093–3099. [Google Scholar] [CrossRef] [Green Version]
- Franzke, C.W.; Has, C.; Schulte, C.; Huilaja, L.; Tasanen, K.; Aumailley, M.; Bruckner-Tuderman, L. C-Terminal Truncation Impairs Glycosylation of Transmembrane Collagen XVII and Leads to Intracellular Accumulation. J. Biol. Chem. 2006, 281, 30260–30268. [Google Scholar] [CrossRef] [Green Version]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision Mapping of the Human O-GalNAc Glycoproteome through SimpleCell Technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [Green Version]
- Hirako, Y.; Usukura, J.; Uematsu, J.; Hashimoto, T.; Kitajima, Y.; Owaribe, K. Cleavage of BP180, a 180-kDa Bullous Pemphigoid Antigen, Yields a 120-kDa Collagenous Extracellular Polypeptide. J. Biol. Chem. 1998, 273, 9711–9717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzke, C.W.; Tasanen, K.; Borradori, L.; Huotari, V.; Bruckner-Tuderman, L. Shedding of Collagen XVII/BP180: Structural Motifs Influence Cleavage from Cell Surface. J. Biol. Chem. 2004, 279, 24521–24529. [Google Scholar] [CrossRef] [Green Version]
- Franzke, C.W.; Tasanen, K.; Schacke, H.; Zhou, Z.; Tryggvason, K.; Mauch, C.; Zigrino, P.; Sunnarborg, S.; Lee, D.C.; Fahrenholz, F.; et al. Transmembrane Collagen XVII, an Epithelial Adhesion Protein, is Shed from the Cell Surface by ADAMs. EMBO J. 2002, 21, 5026–5035. [Google Scholar] [CrossRef] [Green Version]
- Franzke, C.W.; Bruckner-Tuderman, L.; Blobel, C.P. Shedding of Collagen XVII/BP180 in Skin Depends on both ADAM10 and ADAM9. J. Biol. Chem. 2009, 284, 23386–23396. [Google Scholar] [CrossRef] [Green Version]
- Nishie, W.; Natsuga, K.; Iwata, H.; Izumi, K.; Ujiie, H.; Toyonaga, E.; Hata, H.; Nakamura, H.; Shimizu, H. Context-Dependent Regulation of Collagen XVII Ectodomain Shedding in Skin. Am. J. Pathol. 2015, 185, 1361–1371. [Google Scholar] [CrossRef]
- Nishie, W.; Lamer, S.; Schlosser, A.; Licarete, E.; Franzke, C.W.; Hofmann, S.C.; Jackow, J.; Sitaru, C.; Bruckner-Tuderman, L. Ectodomain Shedding Generates Neoepitopes on Collagen XVII, the Major Autoantigen for Bullous Pemphigoid. J. Immunol. 2010, 185, 4938–4947. [Google Scholar] [CrossRef] [Green Version]
- Hurskainen, T.; Kokkonen, N.; Sormunen, R.; Jackow, J.; Löffek, S.; Soininen, R.; Franzke, C.W.; Bruckner-Tuderman, L.; Tasanen, K. Deletion of the Major Bullous Pemphigoid Epitope Region of Collagen XVII Induces Blistering, Autoimmunization, and Itching in Mice. J. Investig. Dermatol. 2015, 135, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Jackow, J.; Schlosser, A.; Sormunen, R.; Nystrom, A.; Sitaru, C.; Tasanen, K.; Bruckner-Tuderman, L.; Franzke, C.W. Generation of a Functional Non-Shedding Collagen XVII Mouse Model: Relevance of Collagen XVII Shedding in Wound Healing. J. Investig. Dermatol. 2016, 136, 516–525. [Google Scholar] [CrossRef]
- Zimina, E.P.; Bruckner-Tuderman, L.; Franzke, C.W. Shedding of Collagen XVII Ectodomain Depends on Plasma Membrane Microenvironment. J. Biol. Chem. 2005, 280, 34019–34024. [Google Scholar] [CrossRef] [Green Version]
- Hirako, Y.; Nishizawa, Y.; Sitaru, C.; Opitz, A.; Marcus, K.; Meyer, H.E.; Butt, E.; Owaribe, K.; Zillikens, D. The 97-kDa (LABD97) and 120-kDa (LAD-1) Fragments of Bullous Pemphigoid Antigen 180/Type XVII Collagen have Different N-Termini. J. Investig. Dermatol. 2003, 121, 1554–1556. [Google Scholar]
- Hofmann, S.C.; Voith, U.; Schönau, V.; Sorokin, L.; Bruckner-Tuderman, L.; Franzke, C.W. Plasmin Plays a Role in the in Vitro Generation of the Linear IgA Dermatosis Antigen LADB97. J. Investig. Dermatol. 2009, 129, 1730–1739. [Google Scholar] [CrossRef] [Green Version]
- Toyonaga, E.; Nishie, W.; Izumi, K.; Natsuga, K.; Ujiie, H.; Iwata, H.; Yamagami, J.; Hirako, Y.; Sawamura, D.; Fujimoto, W.; et al. C-Terminal Processing of Collagen XVII Induces Neoepitopes for Linear IgA Dermatosis Autoantibodies. J. Investig. Dermatol. 2017, 137, 2552–2559. [Google Scholar] [CrossRef] [Green Version]
- Borradori, L.; Sonnenberg, A. Structure and Function of Hemidesmosomes: More than Simple Adhesion Complexes. J. Investig. Dermatol. 1999, 112, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Walko, G.; Castanon, M.J.; Wiche, G. Molecular Architecture and Function of the Hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef] [Green Version]
- Nahidiazar, L.; Kreft, M.; van den Broek, B.; Secades, P.; Manders, E.M.; Sonnenberg, A.; Jalink, K. The Molecular Architecture of Hemidesmosomes, as Revealed with Super-Resolution Microscopy. J. Cell. Sci. 2015, 128, 3714–3719. [Google Scholar] [CrossRef] [Green Version]
- Hamill, K.J.; Hopkinson, S.B.; Jonkman, M.F.; Jones, J.C. Type XVII Collagen Regulates Lamellipod Stability, Cell Motility, and Signaling to Rac1 by Targeting Bullous Pemphigoid Antigen 1e to a6b4 Integrin. J. Biol. Chem. 2011, 286, 26768–26780. [Google Scholar] [CrossRef] [Green Version]
- Fontao, L.; Tasanen, K.; Huber, M.; Hohl, D.; Koster, J.; Bruckner-Tuderman, L.; Sonnenberg, A.; Borradori, L. Molecular Consequences of Deletion of the Cytoplasmic Domain of Bullous Pemphigoid 180 in a Patient with Predominant Features of Epidermolysis Bullosa Simplex. J. Investig. Dermatol. 2004, 122, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Koster, J.; Geerts, D.; Favre, B.; Borradori, L.; Sonnenberg, A. Analysis of the Interactions between BP180, BP230, Plectin and the Integrin alpha6beta4 Important for Hemidesmosome Assembly. J. Cell Sci. 2003, 116, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Schaapveld, R.Q.; Borradori, L.; Geerts, D.; van Leusden, M.R.; Kuikman, I.; Nievers, M.G.; Niessen, C.M.; Steenbergen, R.D.; Snijders, P.J.; Sonnenberg, A. Hemidesmosome Formation is Initiated by the Beta4 Integrin Subunit, Requires Complex Formation of Beta4 and HD1/Plectin, and Involves a Direct Interaction between Beta4 and the Bullous Pemphigoid Antigen 180. J. Cell Biol. 1998, 142, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lin, X.; Kilani, R.T.; Jones, J.C.; Ghahary, A. 14-3-3 Sigma Isoform Interacts with the Cytoplasmic Domain of the Transmembrane BP180 in Keratinocytes. J. Cell. Physiol. 2007, 212, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.M.; Otey, C.; Edlund, M.; Jones, J.C. Interactions of a Hemidesmosome Component and Actinin Family Members. J. Cell. Sci. 2001, 114, 4197–4206. [Google Scholar] [CrossRef]
- Aho, S.; Rothenberger, K.; Uitto, J. Human p120ctn Catenin: Tissue-Specific Expression of Isoforms and Molecular Interactions with BP180/Type XVII Collagen. J. Cell. Biochem. 1999, 73, 390–399. [Google Scholar] [CrossRef]
- Tasanen, K.; Tunggal, L.; Chometon, G.; Bruckner-Tuderman, L.; Aumailley, M. Keratinocytes from Patients Lacking Collagen XVII Display a Migratory Phenotype. Am. J. Pathol. 2004, 164, 2027–2038. [Google Scholar] [CrossRef] [Green Version]
- Jackow, J.; Laffek, S.; Nyström, A.; Bruckner-Tuderman, L.; Franzke, C.W. Collagen XVII Shedding Suppresses Re-Epithelialization by Directing Keratinocyte Migration and Dampening mTOR Signaling. J. Investig. Dermatol. 2016, 136, 1031–1041. [Google Scholar] [CrossRef]
- Löffek, S.; Hurskainen, T.; Jackow, J.; Sigloch, F.C.; Schilling, O.; Tasanen, K.; Bruckner-Tuderman, L.; Franzke, C.W. Transmembrane Collagen XVII Modulates Integrin Dependent Keratinocyte Migration Via PI3K/Rac1 Signaling. PLoS ONE 2014, 9, e87263. [Google Scholar] [CrossRef]
- Hopkinson, S.B.; Baker, S.E.; Jones, J.C. Molecular Genetic Studies of a Human Epidermal Autoantigen (the 180-kD Bullous Pemphigoid Antigen/BP180): Identification of Functionally Important Sequences within the BP180 Molecule and Evidence for an Interaction between BP180 And a6 Integrin. J. Cell Biol. 1995, 130, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishie, W.; Kiritsi, D.; Nyström, A.; Hofmann, S.C.; Bruckner-Tuderman, L. Dynamic Interactions of Epidermal Collagen XVII with the Extracellular Matrix: Laminin 332 as a Major Binding Partner. Am. J. Pathol. 2011, 179, 829–837. [Google Scholar] [CrossRef]
- Kamaguchi, M.; Iwata, H.; Nishie, W.; Toyonaga, E.; Ujiie, H.; Natsuga, K.; Kitagawa, Y.; Shimizu, H. The Direct Binding of Collagen XVII and Collagen IV is Disrupted by Pemphigoid Autoantibodies. Lab. Investig. 2019, 99, 48–57. [Google Scholar] [CrossRef]
- Kiritsi, D.; Kern, J.S.; Schumann, H.; Kohlhase, J.; Has, C.; Bruckner-Tuderman, L. Molecular Mechanisms of Phenotypic Variability in Junctional Epidermolysis Bullosa. J. Med. Genet. 2011, 48, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Sproule, T.J.; Bubier, J.A.; Grandi, F.C.; Sun, V.Z.; Philip, V.M.; McPhee, C.G.; Adkins, E.B.; Sundberg, J.P.; Roopenian, D.C. Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice. PLoS Genet. 2014, 10, e1004068. [Google Scholar] [CrossRef] [Green Version]
- Kroeger, J.; Hoppe, E.; Galiger, C.; Has, C.; Franzke, C.W. Amino Acid Substitution in the C-Terminal Domain of Collagen XVII Reduces Laminin-332 Interaction Causing Mild Skin Fragility with Atrophic Scarring. Matrix Biol. 2019, 80, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Stark, C.; Breitkreutz, B.J.; Rust, J.; Boucher, L.; Chang, C.; Kolas, N.; O’Donnell, L.; Leung, G.; McAdam, R.; et al. The BioGRID Interaction Database: 2019 Update. Nucleic Acids Res. 2019, 47, D529–D541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, B.U.; DeBiase, P.J.; Matzno, S.; Chew, T.L.; Claiborne, J.N.; Hopkinson, S.B.; Russell, A.; Marinkovich, M.P.; Jones, J.C. Integrin Beta4 Regulates Migratory Behavior of Keratinocytes by Determining Laminin-332 Organization. J. Biol. Chem. 2006, 281, 35487–35498. [Google Scholar] [CrossRef] [Green Version]
- Rabinovitz, I.; Tsomo, L.; Mercurio, A.M. Protein Kinase C-Alpha Phosphorylation of Specific Serines in the Connecting Segment of the Beta 4 Integrin Regulates the Dynamics of Type II Hemidesmosomes. Mol. Cell. Biol. 2004, 24, 4351–4360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiger, C.; Löffek, S.; Stemmler, M.P.; Kroeger, J.K.; Mittapalli, V.R.; Fauth, L.; Esser, P.R.; Kern, J.S.; Meiss, F.; Laßmann, S.; et al. Targeting of Cell Surface Proteolysis of Collagen XVII Impedes Squamous Cell Carcinoma Progression. Mol. Ther. 2018, 26, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekoninck, S.; Blanpain, C. Stem Cell Dynamics, Migration and Plasticity during Wound Healing. Nat. Cell Biol. 2019, 21, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Schumann, H.; Baetge, J.; Tasanen, K.; Wojnarowska, F.; Schäcke, H.; Zillikens, D.; Bruckner-Tuderman, L. The Shed Ectodomain of Collagen XVII/BP180 is Targeted by Autoantibodies in Different Blistering Skin Diseases. Am. J. Pathol. 2000, 156, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Lothong, M.; Sakares, W.; Rojsitthisak, P.; Tanikawa, C.; Matsuda, K.; Yodsurang, V. Collagen XVII Inhibits Breast Cancer Cell Proliferation and Growth through Deactivation of the AKT/mTOR Signaling Pathway. PLoS ONE 2021, 16, e0255179. [Google Scholar] [CrossRef]
- Tanimura, S.; Tadokoro, Y.; Inomata, K.; Binh, N.T.; Nishie, W.; Yamazaki, S.; Nakauchi, H.; Tanaka, Y.; McMillan, J.R.; Sawamura, D.; et al. Hair Follicle Stem Cells Provide a Functional Niche for Melanocyte Stem Cells. Cell Stem Cell 2011, 8, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Shirai, K.; Obara, K.; Tohgi, N.; Yamazaki, A.; Aki, R.; Hamada, Y.; Arakawa, N.; Singh, S.R.; Hoffman, R.M.; Amoh, Y. Expression of Anti-Aging Type-XVII Collagen (COL17A1/BP180) in Hair Follicle-Associated Pluripotent (HAP) Stem Cells during Differentiation. Tissue Cell 2019, 59, 33–38. [Google Scholar] [CrossRef]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem Cell Competition Orchestrates Skin Homeostasis and Ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Lin, J.H.; Hsu, T.W.; Hsu, J.W.; Chang, J.W.; Su, K.; Hsu, H.S.; Hung, S.C. Collagen XVII/Laminin-5 Activates Epithelial-to-Mesenchymal Transition and is Associated with Poor Prognosis in Lung Cancer. Oncotarget 2016, 9, 1656–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.S.; Liu, C.C.; Lin, J.H.; Hsu, T.W.; Hsu, J.W.; Li, A.F.; Hung, S.C. Involvement of Collagen XVII in Pluripotency Gene Expression and Metabolic Reprogramming of Lung Cancer Stem Cells. J. Biomed. Sci. 2020, 27, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozawa, K.; Sekai, M.; Ohba, K.; Ito, S.; Sako, H.; Maruyama, T.; Kakeno, M.; Shirai, T.; Kuromiya, K.; Kamasaki, T.; et al. The CD44/COL17A1 Pathway Promotes the Formation of Multilayered, Transformed Epithelia. Curr. Biol. 2021, 31, 3086–3097. [Google Scholar] [CrossRef]
- Frijns, E.; Sachs, N.; Kreft, M.; Wilhelmsen, K.; Sonnenberg, A. EGF-Induced MAPK Signaling Inhibits Hemidesmosome Formation through Phosphorylation of the Integrin ß4. J. Biol. Chem. 2010, 285, 37650–37662. [Google Scholar] [CrossRef] [Green Version]
- Frijns, E.; Kuikman, I.; Litjens, S.; Raspe, M.; Jalink, K.; Ports, M.; Wilhelmsen, K.; Sonnenberg, A. Phosphorylation of Threonine 1736 in the C-Terminal Tail of Integrin ß4 Contributes to Hemidesmosome Disassembly. Mol. Biol. Cell 2012, 23, 1475–1485. [Google Scholar] [CrossRef]
- Te Molder, L.; Sonnenberg, A. PKD2 and RSK1 Regulate Integrin b4 Phosphorylation at Threonine 1736. PLoS ONE 2015, 10, e0143357. [Google Scholar]
- Iwata, H.; Kamaguchi, M.; Ujiie, H.; Nishimura, M.; Izumi, K.; Natsuga, K.; Shinkuma, S.; Nishie, W.; Shimizu, H. Macropinocytosis of Type XVII Collagen Induced by Bullous Pemphigoid IgG is Regulated Via Protein Kinase C. Lab. Investig. 2016, 96, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bosch, M.L.; Salgaller, M.L. Current Methods for Loading Dendritic Cells with Tumor Antigen for the Induction of Antitumor Immunity. J. Immunother. 2002, 25, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Hiroyasu, S.; Zeglinski, M.R.; Zhao, H.; Pawluk, M.A.; Turner, C.T.; Kasprick, A.; Tateishi, C.; Nishie, W.; Burleigh, A.; Lennox, P.A.; et al. Granzyme B Inhibition Reduces Disease Severity in Autoimmune Blistering Diseases. Nat. Commun. 2021, 12, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Betsuyaku, T.; Heimbach, L.; Li, N.; Rubenstein, D.; Shapiro, S.D.; An, L.; Giudice, G.J.; Diaz, L.A.; Senior, R.M.; et al. Neutrophil Elastase Cleaves the Murine Hemidesmosomal Protein BP180/Type XVII Collagen and Generates Degradation Products that Modulate Experimental Bullous Pemphigoid. Matrix Biol. 2012, 31, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laval, S.; Laklai, H.; Fanjul, M.; Pucelle, M.; Laurell, H.; Billon-Galés, A.; Le Guellec, S.; Delisle, M.B.; Sonnenberg, A.; Susini, C.; et al. Dual Roles of Hemidesmosomal Proteins in the Pancreatic Epithelium: The Phosphoinositide 3-Kinase Decides. Oncogene 2014, 33, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kosumi, H.; Osada, S.I.; Takashima, S.; Wang, Y.; Nishie, W.; Oikawa, T.; Hirose, T.; Shimizu, H.; Natsuga, K. Type XVII Collagen Interacts with the aPKC-PAR Complex and Maintains Epidermal Cell Polarity. Exp. Dermatol. 2021, 30, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.W.; Lanschuetzer, C. Type XVII Collagen Gene Mutations in Junctional Epidermolysis Bullosa and Prospects for Gene Therapy. Clin. Exp. Dermatol. 2003, 28, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, M.; Floeth, M.; Borradori, L.; Schäcke, H.; Rugg, E.L.; Lane, E.B.; Frenk, E.; Hohl, D.; Bruckner-Tuderman, L. Deletion of the Cytoplasmatic Domain of BP180/Collagen XVII Causes a Phenotype with Predominant Features of Epidermolysis Bullosa Simplex. J. Investig. Dermatol. 2002, 118, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.H.; Werth, V.P.; Parisi, E.; Sollecito, T.P. Mucous Membrane Pemphigoid. Dent. Clin. N. Am. 2013, 57, 611–630. [Google Scholar] [CrossRef] [Green Version]
- Solano-López, G.; Concha-Garzón, M.J.; Sánchez-Pérez, J.; Hirako, Y.; Li, X.; Ishii, N.; Hashimoto, T.; Daudén, E. Pure Ocular Mucous Membrane Pemphigoid Reactive with Both b4 Integrin and the BP180 C-Terminal Domain. Br. J. Dermatol. 2015, 172, 542–544. [Google Scholar] [CrossRef]
- Cozzani, E.; Di Zenzo, G.; Gasparini, G.; Salemme, A.; Agnoletti, A.F.; Vassallo, C.; Caproni, M.; Antiga, E.; Marzano, A.V.; Cavalli, R.; et al. Autoantibody Profile of a Cohort of 54 Italian Patients with Linear IgA Bullous Dermatosis: LAD-1 Denoted as a Major Auto-Antigen of the Lamina Lucida Subtype. Acta Derm. Venereol. 2020, 100, adv00070-3415. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, J.M.; Löffek, S.; Kokkonen, N.; Salo, S.; Väyrynen, J.P.; Hurskainen, T.; Manninen, A.; Riihilä, P.; Heljasvaara, R.; Franzke, C.W.; et al. Significant Role of Collagen XVII and Integrin b4 in Migration and Invasion of the Less Aggressive Squamous Cell Carcinoma Cells. Sci. Rep. 2017, 7, 45057. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Lin, S.P.; Hsu, H.S.; Yang, S.H.; Lin, C.H.; Yang, M.H.; Hung, M.C.; Hung, S.C. Suspension Survival Mediated by PP2A-STAT3-Col XVII Determines Tumour Initiation and Metastasis in Cancer Stem Cells. Nat. Commun. 2016, 7, 11798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuusa, J.; Kokkonen, N.; Tasanen, K. BP180/Collagen XVII: A Molecular View. Int. J. Mol. Sci. 2021, 22, 12233. https://doi.org/10.3390/ijms222212233
Tuusa J, Kokkonen N, Tasanen K. BP180/Collagen XVII: A Molecular View. International Journal of Molecular Sciences. 2021; 22(22):12233. https://doi.org/10.3390/ijms222212233
Chicago/Turabian StyleTuusa, Jussi, Nina Kokkonen, and Kaisa Tasanen. 2021. "BP180/Collagen XVII: A Molecular View" International Journal of Molecular Sciences 22, no. 22: 12233. https://doi.org/10.3390/ijms222212233
APA StyleTuusa, J., Kokkonen, N., & Tasanen, K. (2021). BP180/Collagen XVII: A Molecular View. International Journal of Molecular Sciences, 22(22), 12233. https://doi.org/10.3390/ijms222212233






