GABABR Modulation of Electrical Synapses and Plasticity in the Thalamic Reticular Nucleus
Abstract
1. Introduction
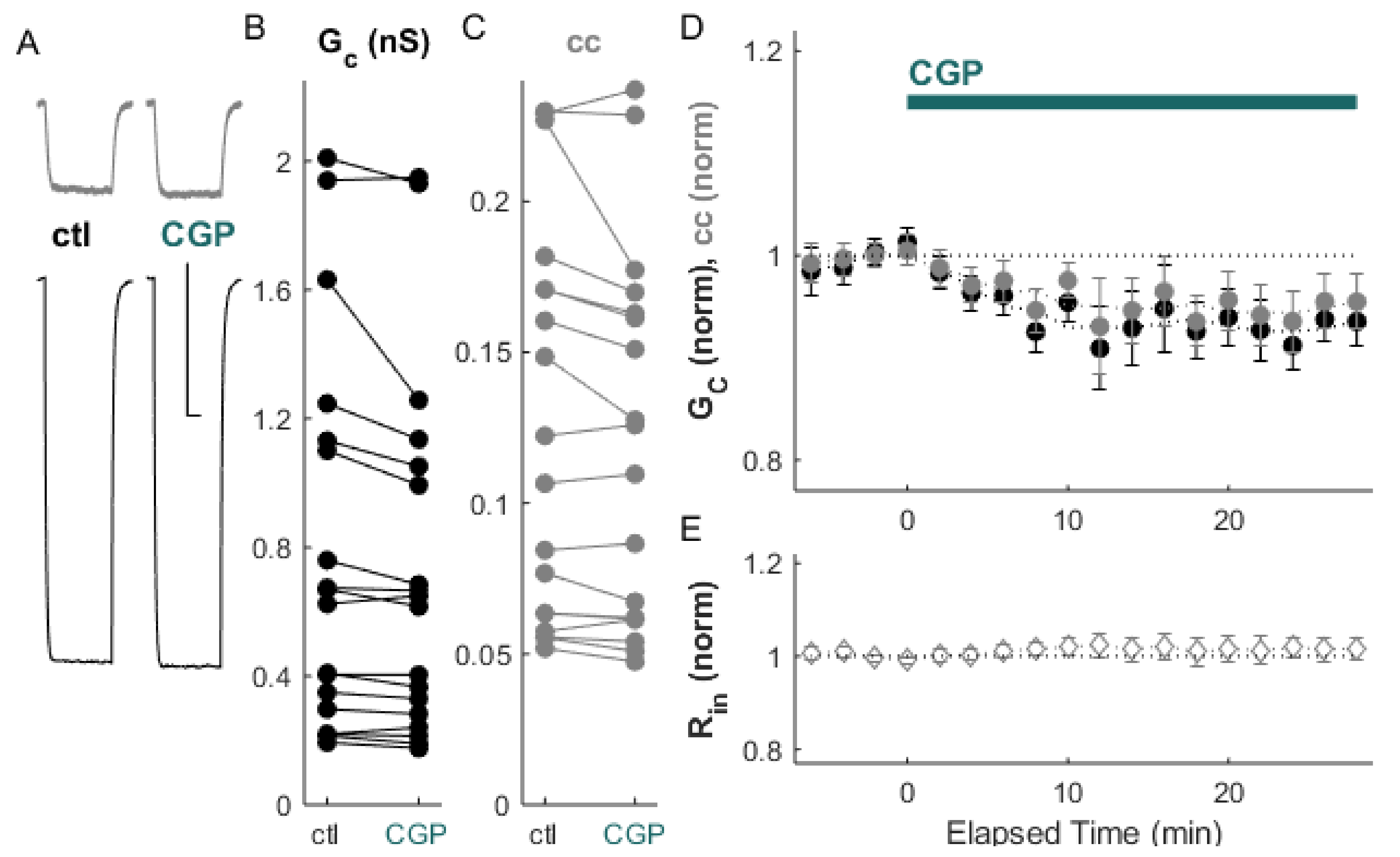
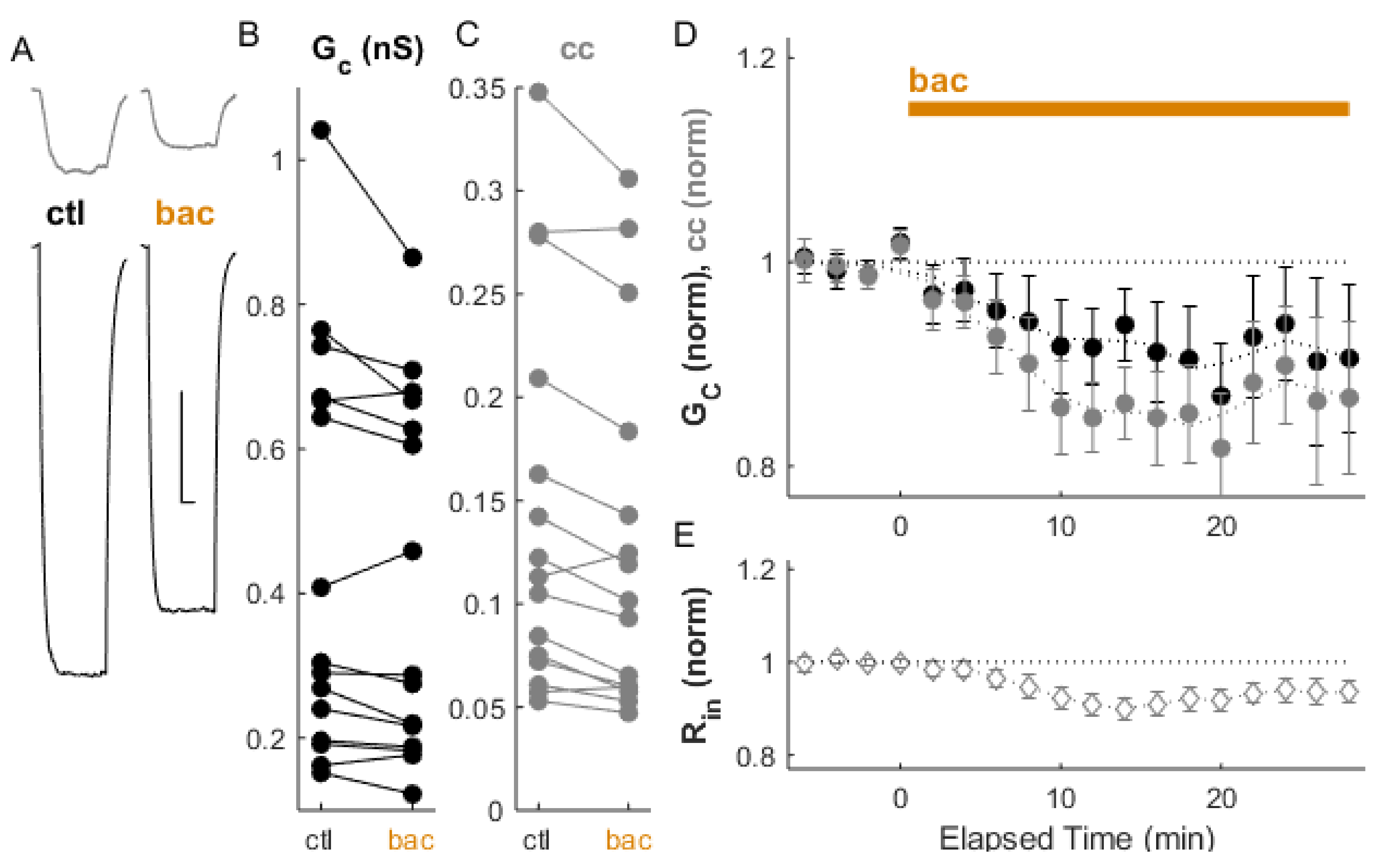
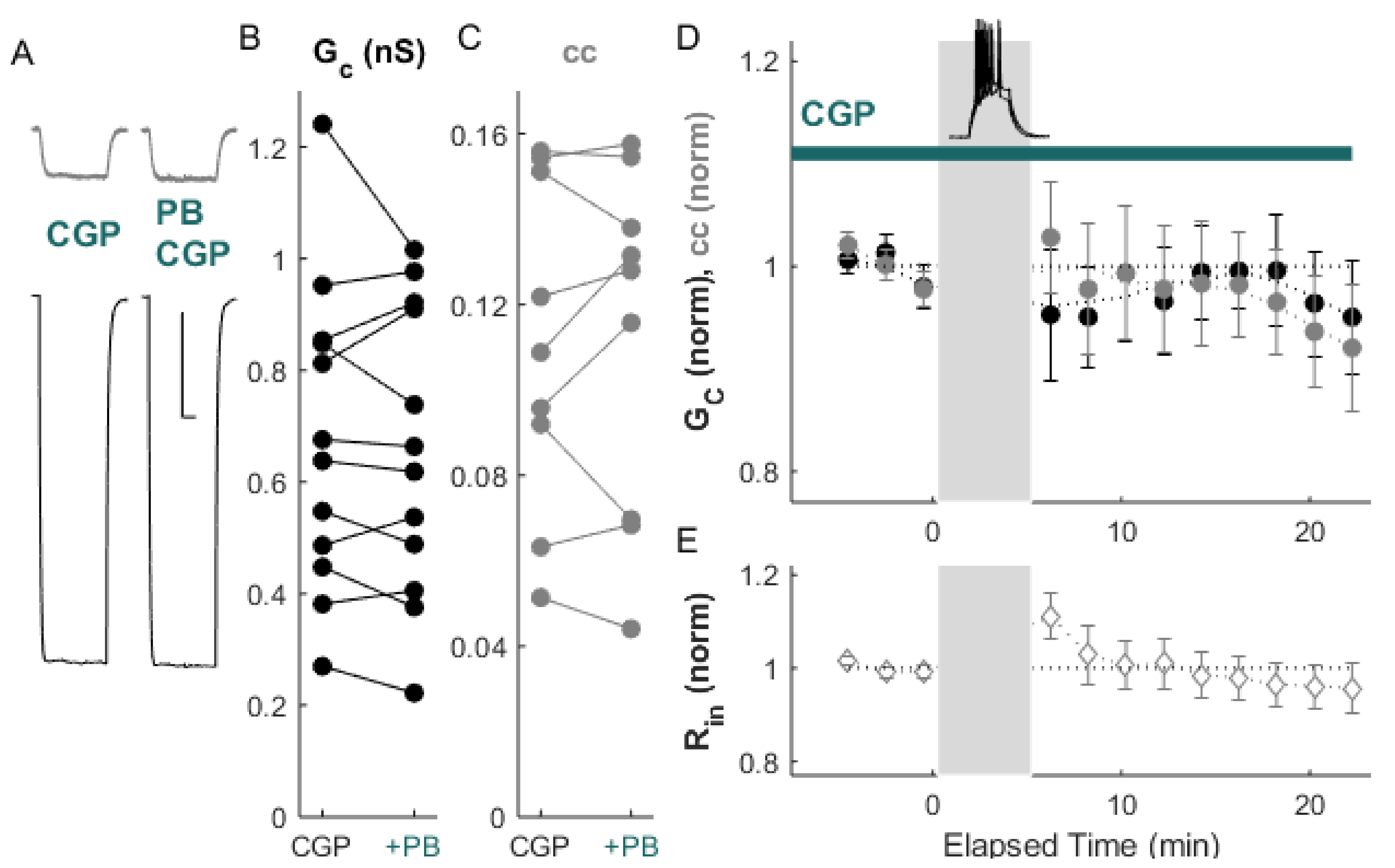
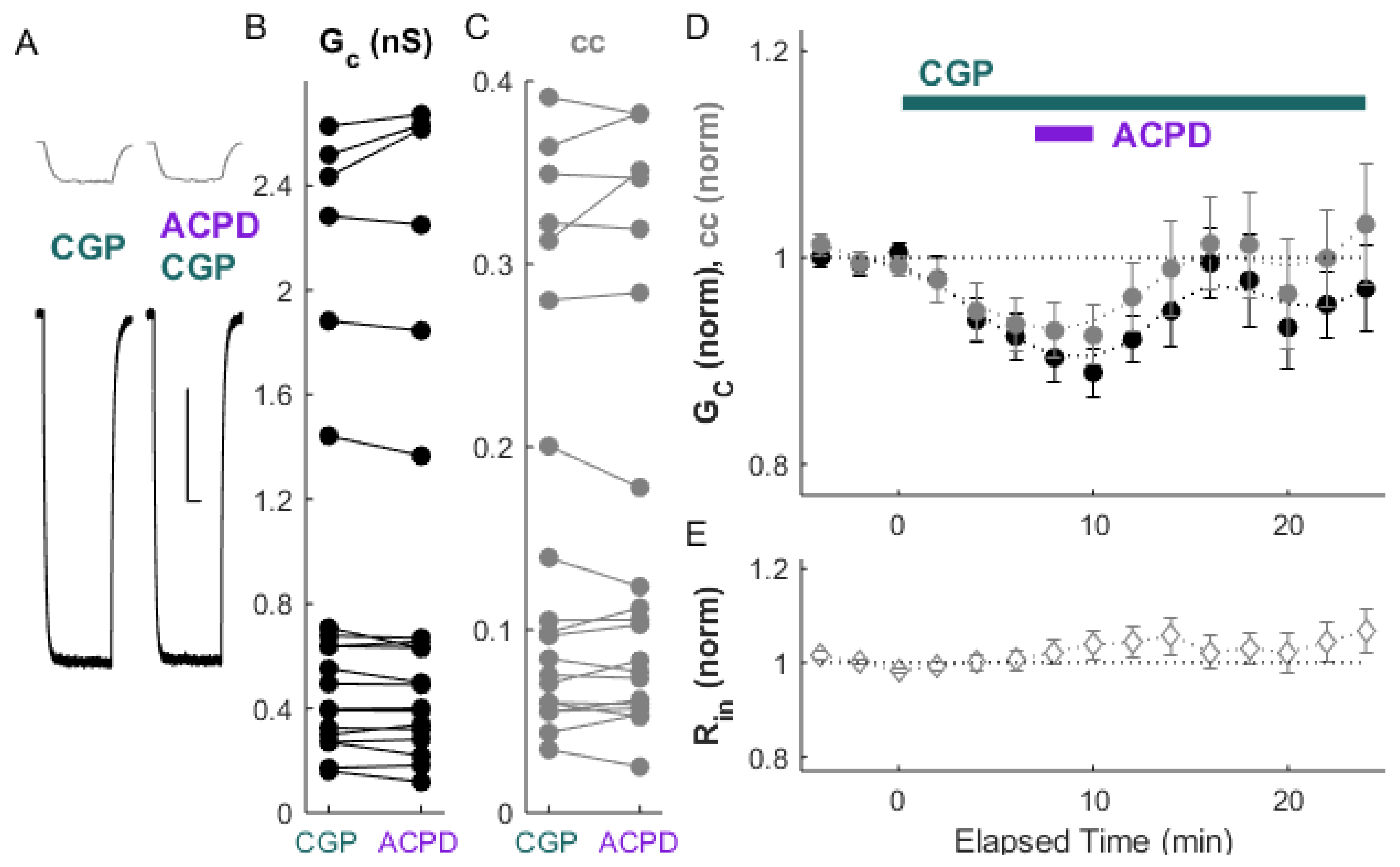
2. Results
3. Discussion
4. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steriade, M.; Domich, L.; Oakson, G.; Deschenes, M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J. Neurophysiol. 1987, 57, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Crick, F. Function of the thalamic reticular complex: The searchlight hypothesis. Proc. Natl. Acad. Sci. USA 1984, 81, 4586–4590. [Google Scholar] [CrossRef] [PubMed]
- McAlonan, K.; Cavanaugh, J.; Wurtz, R.H. Attentional Modulation of Thalamic Reticular Neurons. J. Neurosci. 2006, 26, 4444–4450. [Google Scholar] [CrossRef]
- Ohara, P.T.; Lieberman, A.R. The thalamic reticular nucleus of the adult rat: Experimental anatomical studies. J. Neurocytol. 1985, 14, 365–411. [Google Scholar] [CrossRef] [PubMed]
- Pinault, D.; Deschenes, M. Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: A three-dimensional, graphic, and morphometric analysis. J. Comp. Neurol. 1998, 391, 180–203. [Google Scholar] [CrossRef]
- Landisman, C.E.; Long, M.A.; Beierlein, M.; Deans, M.R.; Paul, D.L.; Connors, B.W. Electrical Synapses in the Thalamic Reticular Nucleus. J. Neurosci. 2002, 22, 1002–1009. [Google Scholar] [CrossRef]
- Hou, G.; Smith, A.G.; Zhang, Z.-W. Lack of Intrinsic GABAergic Connections in the Thalamic Reticular Nucleus of the Mouse. J. Neurosci. 2016, 36, 7246–7252. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 2005, 28, 317–324. [Google Scholar] [CrossRef]
- Inoue, M.; Duysens, J.; Vossen, J.M.; Coenen, A.M. Thalamic multiple-unit activity underlying spike-wave discharges in anesthetized rats. Brain Res. 1993, 612, 35–40. [Google Scholar] [CrossRef]
- Huguenard, J.; Prince, D.A. Intrathalamic rhythmicity studied in vitro: Nominal T-current modulation causes robust antioscillatory effects. J. Neurosci. 1994, 14, 5485–5502. [Google Scholar] [CrossRef]
- Von, M.K.; Bal, T.; McCormick, D.A. Cellular Mechanisms of a Synchronized Oscillation in the Thalamus. Science 1993, 261, 361–364. [Google Scholar] [CrossRef]
- Destexhe, A. Spike-and-wave oscillations based on the properties of GABAB receptors. J. Neurosci. 1998, 18, 9099–9111. [Google Scholar] [CrossRef]
- Haas, J.S.; Zavala, B.; Landisman, C.E. Activity-Dependent Long-Term Depression of Electrical Synapses. Science 2011, 334, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Landisman, C.E.; Connors, B.W. Long-Term Modulation of Electrical Synapses in the Mammalian Thalamus. Science 2005, 310, 1809–1813. [Google Scholar] [CrossRef]
- Wang, Z.; Neely, R.; Landisman, C.E. Activation of Group I and Group II Metabotropic Glutamate Receptors Causes LTD and LTP of Electrical Synapses in the Rat Thalamic Reticular Nucleus. J. Neurosci. 2015, 35, 7616–7625. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Jones, E.G. Predominance of corticothalamic synaptic inputs to thalamic reticular nucleus neurons in the rat. J. Comp. Neurol. 1999, 414, 67–79. [Google Scholar] [CrossRef]
- Williamson, A.M.; Ohara, P.T.; Ralston, D.D.; Milroy, A.M.; Ralston, H.J. Analysis of gamma-aminobutyric acidergic synaptic contacts in the thalamic reticular nucleus of the monkey. J. Comp. Neurol. 1994, 349, 182–192. [Google Scholar] [CrossRef]
- Paré, D.; Hazrati, L.-N.; Parent, A.; Steriade, M. Substantia nigra pars reticulata projects to the reticular thalamic nucleus of the cat: A morphological and electrophysiological study. Brain Res. 1990, 535, 139–146. [Google Scholar] [CrossRef]
- Asanuma, C. Axonal arborizations of a magnocellular basal nucleus input and their relation to the neurons in the thalamic reticular nucleus of rats. Proc. Natl. Acad. Sci. USA 1989, 86, 4746–4750. [Google Scholar] [CrossRef]
- Jourdain, A.; Semba, K.; Fibiger, H.C. Basal forebrain and mesopontine tegmental projections to the reticular thalamic nucleus: An axonal collateralization and immunohistochemical study in the rat. Brain Res. 1989, 505, 55–65. [Google Scholar] [CrossRef]
- Bickford, M.E.; Günlük, A.E.; van Horn, S.C.; Sherman, S.M. GABAergic projection from the basal forebrain to the visual sector of the thalamic reticular nucleus in the cat. J. Comp. Neurol. 1994, 348, 481–510. [Google Scholar] [CrossRef] [PubMed]
- Gandia, J.; Heras, S.D.L.; García, M.; Giménez-Amaya, J.M. Afferent projections to the reticular thalamic nucleus from the globus pallidus and the substantia nigra in the rat. Brain Res. Bull. 1993, 32, 351–358. [Google Scholar] [CrossRef]
- Halassa, M.M.; Acsády, L. Thalamic Inhibition: Diverse Sources, Diverse Scales. Trends Neurosci. 2016, 39, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, J.; Cooper, J.; Phillipson, O.T. Projections to the rostral reticular thalamic nucleus in the rat. Exp. Brain Res. 1990, 80, 157–171. [Google Scholar] [CrossRef]
- Herrera, C.G.; Cadavieco, M.C.; Jego, S.; Ponomarenko, A.; Korotkova, T.M.; Adamantidis, A.R. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 2016, 19, 290–298. [Google Scholar] [CrossRef]
- Munoz, A.; Huntsman, M.M.; Jones, E.G. GABA(B) receptor gene expression in monkey thalamus. J. Comp. Neurol. 1998, 394, 118–126. [Google Scholar] [CrossRef]
- Margeta-Mitrovic, M.; Mitrovic, I.; Riley, R.C.; Jan, L.Y.; Basbaum, A.I. Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J. Comp. Neurol. 1999, 405, 299–321. [Google Scholar] [CrossRef]
- Cain, S.M.; Garcia, E.; Waheed, Z.; Jones, K.L.; Bushell, T.J.; Snutch, T.P. GABAB receptors suppress burst-firing in reticular thalamic neurons. Channels 2017, 11, 574–586. [Google Scholar] [CrossRef]
- Coulon, P.; Herr, D.; Kanyshkova, T.; Meuth, P.; Budde, T.; Pape, H.-C. Burst discharges in neurons of the thalamic reticular nucleus are shaped by calcium-induced calcium release. Cell Calcium 2009, 46, 333–346. [Google Scholar] [CrossRef]
- Ulrich, D.; Bettler, B. GABAB receptors: Synaptic functions and mechanisms of diversity. Curr. Opin. Neurobiol. 2007, 17, 298–303. [Google Scholar] [CrossRef]
- Sevetson, J.; Fittro, S.; Heckman, E.; Haas, J.S. A calcium-dependent pathway underlies activity-dependent plasticity of electrical synapses in the thalamic reticular nucleus. J. Physiol. 2017, 595, 4417–4430. [Google Scholar] [CrossRef] [PubMed]
- Karschin, C.; Dißmann, E.; Stühmer, W.; Karschin, A. IRK(1–3) and GIRK(1–4) Inwardly Rectifying K+Channel mRNAs Are Differentially Expressed in the Adult Rat Brain. J. Neurosci. 1996, 16, 3559–3570. [Google Scholar] [CrossRef]
- Iftinca, M.C.; Zamponi, G.W. Regulation of neuronal T-type calcium channels. Trends Pharmacol. Sci. 2009, 30, 32–40. [Google Scholar] [CrossRef]
- Smith, M.; Pereda, A.E. Chemical synaptic activity modulates nearby electrical synapses. Proc. Natl. Acad. Sci. USA 2003, 100, 4849–4854. [Google Scholar] [CrossRef]
- Pereda, A.E. Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 2014, 15, 250–263. [Google Scholar] [CrossRef]
- Zsiros, V.; Maccaferri, G. Noradrenergic Modulation of Electrical Coupling in GABAergic Networks of the Hippocampus. J. Neurosci. 2008, 28, 1804–1815. [Google Scholar] [CrossRef]
- McHahon, D.G.; Knapp, A.G.; Dowling, J.E. Horizontal cell gap junctions: Single-channel conductance and modulation by dopamine. Proc. Natl. Acad. Sci. USA 1989, 86, 7639–7643. [Google Scholar] [CrossRef] [PubMed]
- Fricker, B.; Heckman, E.; Cunningham, P.C.; Wang, H.; Haas, J.S. Activity-dependent long-term potentiation of electrical synapses in the mammalian thalamus. J. Neurophysiol. 2021, 125, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.W.; Lodge, D.; Bashir, Z.I.; Isaac, J.T.R. GABAA, NMDA and mGlu2 receptors tonically regulate inhibition and excitation in the thalamic reticular nucleus. Eur. J. Neurosci. 2013, 37, 850–859. [Google Scholar] [CrossRef]
- Haas, J.S.; Landisman, C.E. State-Dependent Modulation of Gap Junction Signaling by the Persistent Sodium Current. Front. Cell. Neurosci. 2012, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Kohmann, D.; Lüttjohann, A.; Seidenbecher, T.; Coulon, P.; Pape, H.-C. Short-term depression of gap junctional coupling in reticular thalamic neurons of absence epileptic rats. J. Physiol. 2016, 594, 5695–5710. [Google Scholar] [CrossRef]
- Lee, S.-C.; Patrick, S.L.; Richardson, K.A.; Connors, B.W. Two functionally distinct networks of gap junction-coupled inhibitory neurons in the thalamic reticular nucleus. J. Neurosci. 2014, 34, 13170–13182. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Chapuis, S.; Panjkovich, A.; Fregeac, J.; Nagy, J.I.; Bukauskas, F.F. Molecular determinants of magnesium-dependent synaptic plasticity at electrical synapses formed by connexin36. Nat. Commun. 2014, 5, 5667. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parker, P.R.L.; Cruikshank, S.J.; Connors, B. Stability of Electrical Coupling despite Massive Developmental Changes of Intrinsic Neuronal Physiology. J. Neurosci. 2009, 29, 9761–9770. [Google Scholar] [CrossRef] [PubMed]
- Sevetson, J.; Haas, J.S. Asymmetry and modulation of spike timing in electrically coupled neurons. J. Neurophysiol. 2015, 113, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, T.A.; Connors, B.W. Electrical synapses and the development of inhibitory circuits in the thalamus. J. Physiol. 2016, 594, 2579–2592. [Google Scholar] [CrossRef]
- Belluardo, N.; Mudò, G.; Salinaro, A.T.; Le Gurun, S.; Charollais, A.; Serre-Beinier, V.; Amato, G.; Haefliger, J.-A.; Meda, P.; Condorelli, D.F. Expression of Connexin36 in the adult and developing rat brain. Brain Res. 2000, 865, 121–138. [Google Scholar] [CrossRef]
- Condorelli, D.F.; Belluardo, N.; Salinaro, A.T.; Mudò, G. Expression of Cx36 in mammalian neurons. Brain Res. Rev. 2000, 32, 72–85. [Google Scholar] [CrossRef]
- Degen, J.; Meier, C.; Van Der Giessen, R.S.; Söhl, G.; Petrasch-Parwez, E.; Urschel, S.; Dermietzel, R.; Schilling, K.; De Zeeuw, C.I.; Willecke, K. Expression pattern of lacZ reporter gene representing connexin36 in transgenic mice. J. Comp. Neurol. 2004, 473, 511–525. [Google Scholar] [CrossRef]
- Warren, R.A.; Jones, E.G. Maturation of Neuronal Form and Function in a Mouse Thalamo-Cortical Circuit. J. Neurosci. 1997, 17, 277–295. [Google Scholar] [CrossRef]
- Blethyn, K.L.; Hughes, S.W.; Crunelli, V. Evidence for electrical synapses between neurons of the nucleus reticularis thalami in the adult brain in vitro. Thalamus Relat. Syst. 2008, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Zhang, L.; Zhou, W.; Yu, S.; Yi, R.; Luo, D.; Fu, X. Effects of Propofol on Electrical Synaptic Strength in Coupling Reticular Thalamic GABAergic Parvalbumin-Expressing Neurons. Front. Neurosci. 2020, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Deleuze, C.; Huguenard, J.R. Distinct Electrical and Chemical Connectivity Maps in the Thalamic Reticular Nucleus: Potential Roles in Synchronization and Sensation. J. Neurosci. 2006, 26, 8633–8645. [Google Scholar] [CrossRef] [PubMed]
- Lozsádi, D.A. Organization of cortical afferents to the rostral, limbic sector of the rat thalamic reticular nucleus. J. Comp. Neurol. 1994, 341, 520–533. [Google Scholar] [CrossRef]
- Jones, E.G. Some aspects of the organization of the thalamic reticular complex. J. Comp. Neurol. 1975, 162, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.-W.; Sherman, S.M. Functional Organization of the Thalamic Input to the Thalamic Reticular Nucleus. J. Neurosci. 2011, 31, 6791–6799. [Google Scholar] [CrossRef]
- Clemente-Perez, A.; Makinson, S.R.; Higashikubo, B.; Brovarney, S.; Cho, F.S.; Urry, A.; Holden, S.S.; Wimer, M.; Dávid, C.; Fenno, L.E.; et al. Distinct Thalamic Reticular Cell Types Differentially Modulate Normal and Pathological Cortical Rhythms. Cell Rep. 2017, 19, 2130–2142. [Google Scholar] [CrossRef]
- Li, Y.; Lopez-Huerta, V.G.; Adiconis, X.; Levandowski, K.; Choi, S.; Simmons, S.K.; Arias-Garcia, M.A.; Guo, B.; Yao, A.Y.; Blosser, T.R.; et al. Distinct subnetworks of the thalamic reticular nucleus. Nat. Cell Biol. 2020, 583, 819–824. [Google Scholar] [CrossRef]
- Martinez-Garcia, R.I.; Voelcker, B.; Zaltsman, J.B.; Patrick, S.L.; Stevens, T.R.; Connors, B.; Cruikshank, S.J. Two dynamically distinct circuits drive inhibition in the sensory thalamus. Nat. Cell Biol. 2020, 583, 813–818. [Google Scholar] [CrossRef]
- Mathy, A.; Clark, B.A.; Häusser, M. Synaptically Induced Long-Term Modulation of Electrical Coupling in the Inferior Olive. Neuron 2014, 81, 1290–1296. [Google Scholar] [CrossRef]
- Lefler, Y.; Yarom, Y.; Uusisaari, M.Y. Cerebellar Inhibitory Input to the Inferior Olive Decreases Electrical Coupling and Blocks Subthreshold Oscillations. Neuron 2014, 81, 1389–1400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turecek, J.; Yuen, G.S.; Han, V.Z.; Zeng, X.-H.; Bayer, K.U.; Welsh, J.P. NMDA Receptor Activation Strengthens Weak Electrical Coupling in Mammalian Brain. Neuron 2014, 81, 1375–1388. [Google Scholar] [CrossRef][Green Version]
- Haas, J.S. A new measure for the strength of electrical synapses. Front. Cell. Neurosci. 2015, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Haas, J.S. Electrical synapses between inhibitory neurons shape the responses of principal neurons to transient inputs in the thalamus: A modeling study. Sci. Rep. 2018, 8, 7763. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Haas, J.S. Electrical synapses regulate both subthreshold integration and population activity of principal cells in response to transient inputs within canonical feedforward circuits. PLoS Comput. Biol. 2019, 15, e1006440. [Google Scholar] [CrossRef]
- Curti, S.; Pereda, A.E. Voltage-dependent enhancement of electrical coupling by a subthreshold sodium current. J. Neurosci. 2004, 24, 3999–4010. [Google Scholar] [CrossRef]
- Curti, S.; Hoge, G.; Nagy, J.I.; Pereda, A.E. Synergy between Electrical Coupling and Membrane Properties Promotes Strong Synchronization of Neurons of the Mesencephalic Trigeminal Nucleus. J. Neurosci. 2012, 32, 4341–4359. [Google Scholar] [CrossRef]
- Fortier, P.A. Detecting and estimating rectification of gap junction conductance based on simulations of dual-cell recordings from a pair and a network of coupled cells. J. Theor. Biol. 2010, 265, 104–114. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Haas, J.S. GABABR Modulation of Electrical Synapses and Plasticity in the Thalamic Reticular Nucleus. Int. J. Mol. Sci. 2021, 22, 12138. https://doi.org/10.3390/ijms222212138
Wang H, Haas JS. GABABR Modulation of Electrical Synapses and Plasticity in the Thalamic Reticular Nucleus. International Journal of Molecular Sciences. 2021; 22(22):12138. https://doi.org/10.3390/ijms222212138
Chicago/Turabian StyleWang, Huaixing, and Julie S. Haas. 2021. "GABABR Modulation of Electrical Synapses and Plasticity in the Thalamic Reticular Nucleus" International Journal of Molecular Sciences 22, no. 22: 12138. https://doi.org/10.3390/ijms222212138
APA StyleWang, H., & Haas, J. S. (2021). GABABR Modulation of Electrical Synapses and Plasticity in the Thalamic Reticular Nucleus. International Journal of Molecular Sciences, 22(22), 12138. https://doi.org/10.3390/ijms222212138





