Estrogens Regulate Placental Angiogenesis in Horses
Abstract
:1. Introduction
2. Results
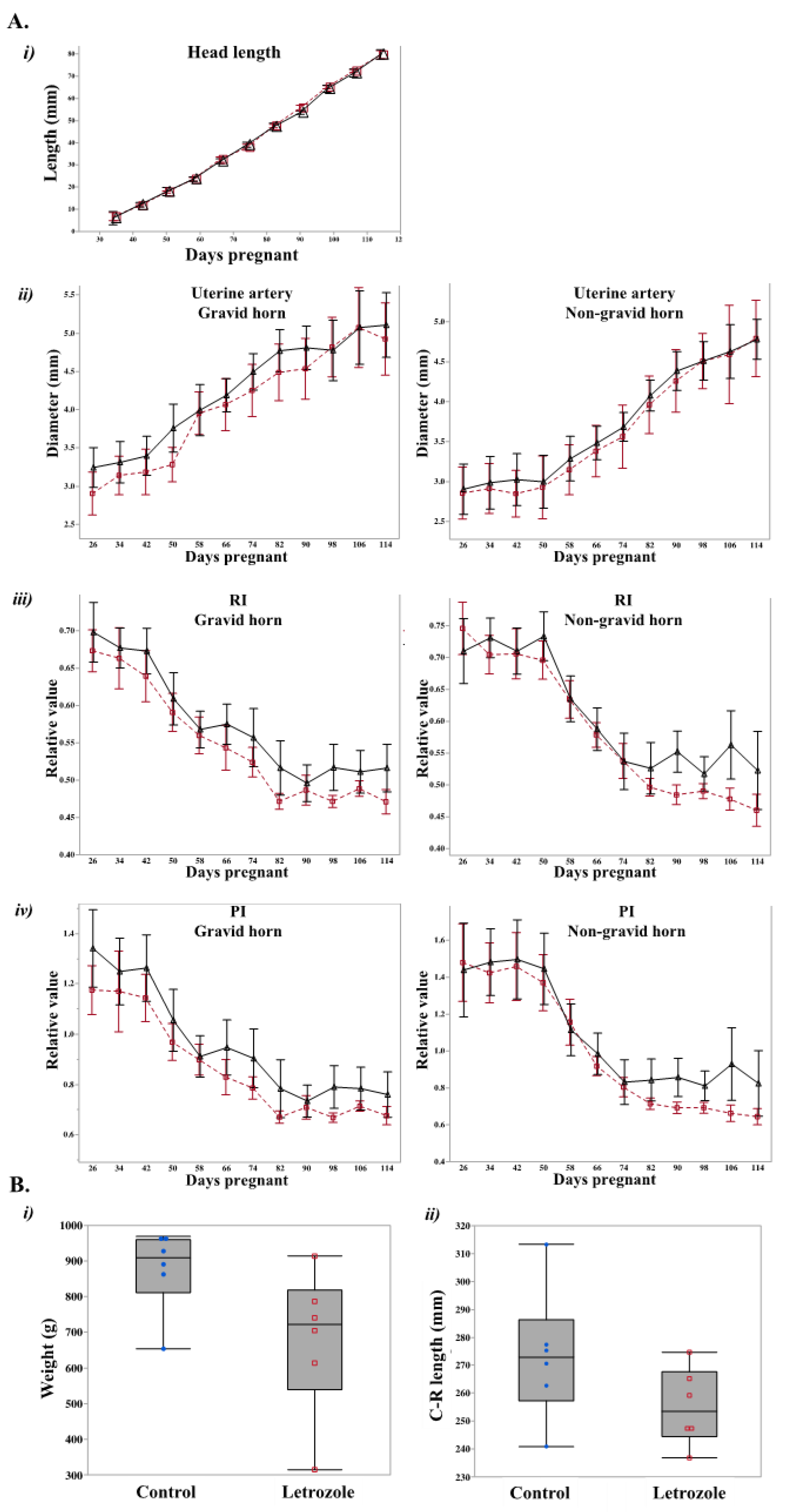
2.1. Letrozole-Treated Fetuses Tended to Weigh Less than Control Fetuses
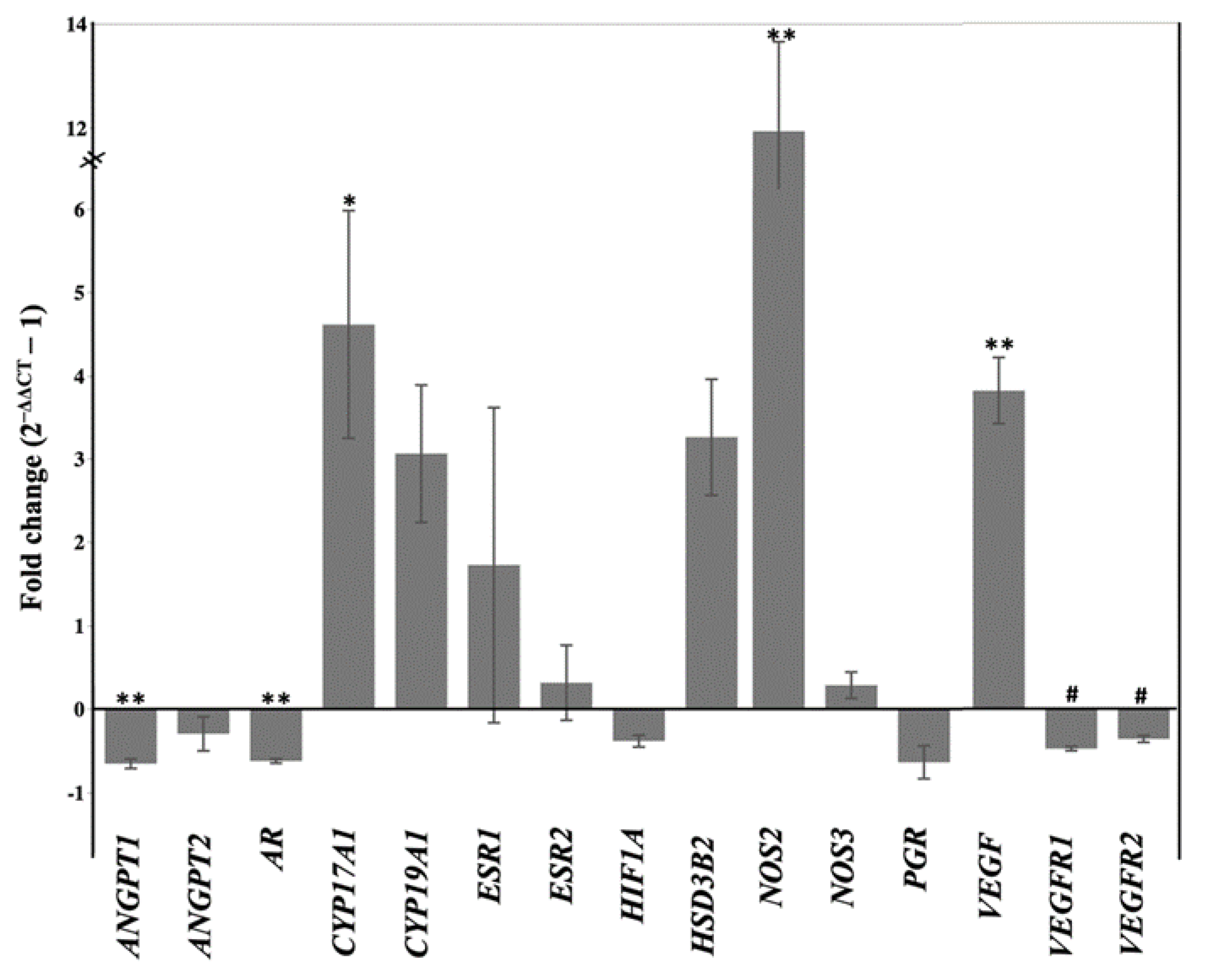
2.2. Letrozole Treatment Altered Level of Peripheral Steroid Hormones and Steroidogenic Gene Expression in Chorioallantois
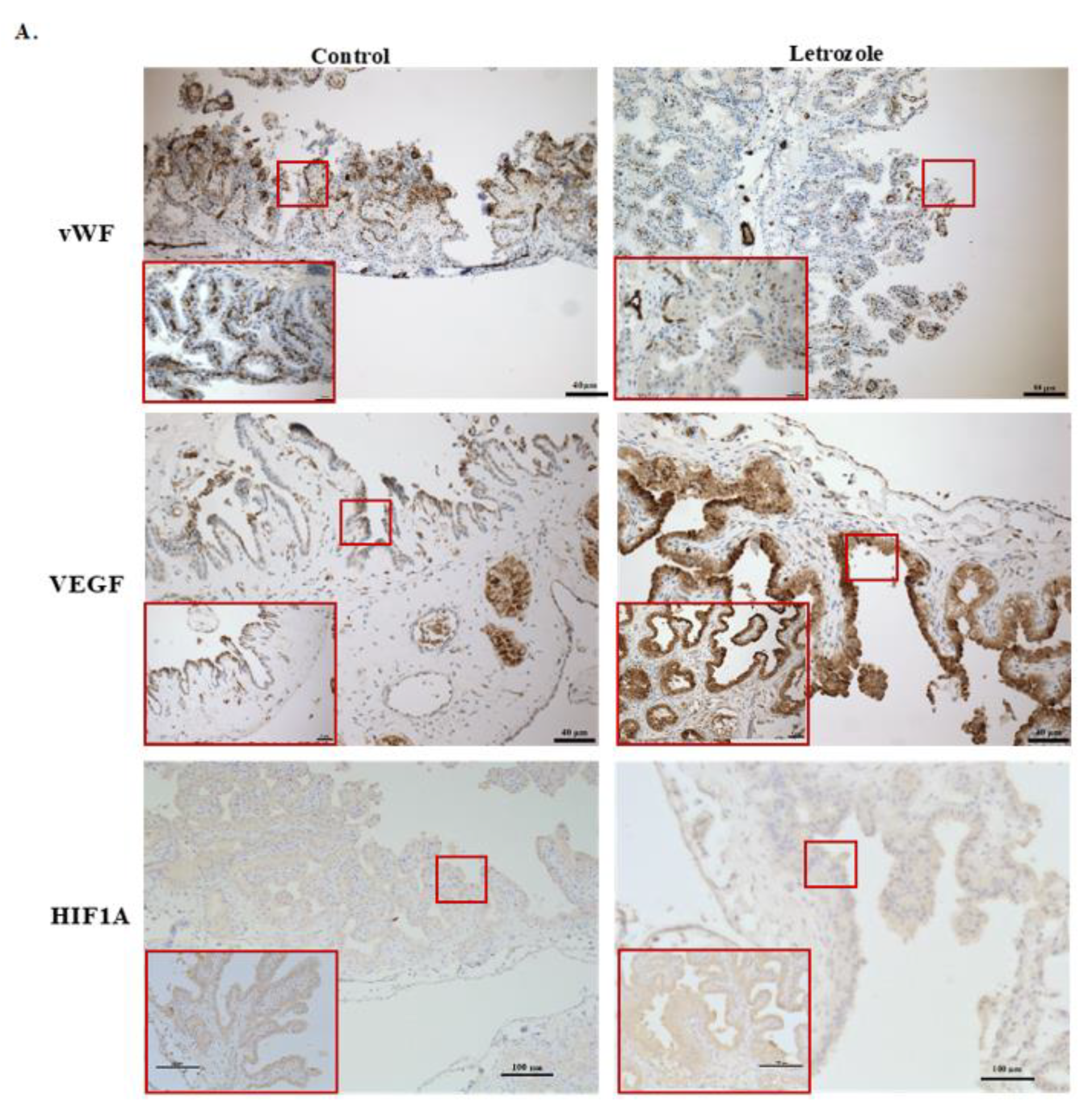
2.3. Number of Vessels Tended to Decrease in Chorioallantois with Letrozole Treatment
2.4. 17β-Estradiol Increases the Angiogenic Activity of Equine Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ultrasonographic Analysis
4.3. Recovery of the Fetus and Placenta
4.4. Endocrine Assays
4.5. Gene Expression Analysis
4.6. Histological Analysis and Capillary Density Evaluation
4.7. Evaluating the Effect of 17β-Estradiol on Equine Endothelial Cells
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambrose, C.T. The role of capillaries in the lesser ailments of old age and in Alzheimer’s disease and vascular dementia: The potential of pro-therapeutic angiogenesis. J. Alzheimer’s Dis. 2016, 54, 31–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Tao, Y.; Chen, M.; Yu, J.; Li, W.J.; Tao, L.; Li, Y.; Li, F. 17β-Estradiol promotes angiogenesis of rat cardiac microvascular endothelial cells in vitro. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Spiegelaere, W.; Cornillie, P.; Casteleyn, C.; Burvenich, C.; Van Den Broeck, W. Detection of hypoxia inducible factors and angiogenic growth factors during foetal endochondral and intramembranous ossification. Anat. Histol. Embryol. 2010, 39, 376–384. [Google Scholar] [CrossRef]
- Carmeliet, P.; Eelen, G.; Kalucka, J. Arteriogenesis versus angiogenesis. In The ESC Textbook of Vascular Biology; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Elkilani, O.A.; Soliman, M.A. Angiogenesis mediators in women with idiopathic heavy menstrual bleeding. Int. J. Gynecol. Obstet. 2017, 136, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Redmer, D.A. Angiogenesis in the placenta. Biol. Reprod. 2001, 64, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, J.A.; Winn, V.D. Vasculogenesis and Angiogenesis in the IUGR Placenta, Seminars in Perinatology, 2008; Elsevier: Amsterdam, The Netherlands, 2008; pp. 172–177. [Google Scholar]
- Regnault, T.; Galan, H.; Parker, T.; Anthony, R. Placental development in normal and compromised pregnancies—A review. Placenta 2002, 23, S119–S129. [Google Scholar] [CrossRef]
- Dini, P.; Carossino, M.; Loynachan, A.T.; Ali, H.E.S.; Wolfsdorf, K.E.; Scoggin, K.E.; Daels, P.; Ball, B.A. Equine hydrallantois is associated with impaired angiogenesis in the placenta. Placenta 2020, 93, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.C.; Seshan, S.V.; Riachi, L.E. Placental vessel morphometry in growth retardation and increased resistance of the umbilical artery Doppler flow. J. Matern.-Fetal Med. 2000, 9, 282–286. [Google Scholar] [CrossRef]
- Barcroft, J.; Barron, D.H. Observations upon the form and relations of the maternal and fetal vessels in the placenta of the sheep. Anat. Rec. 1946, 94, 569–595. [Google Scholar] [CrossRef]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the placenta: The role of reactive oxygen species signaling. BioMed Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maliqueo, M.; Echiburú, B.; Crisosto, N. Sex steroids modulate uterine-placental vasculature: Implications for obstetrics and neonatal outcomes. Front. Physiol. 2016, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xiong, W.; Xiong, Y.; Liu, H.; Liu, Y. 17 β-Estradiol promotes vascular endothelial growth factor expression via the Wnt/β-catenin pathway during the pathogenesis of endometriosis. MHR: Basic Sci. Reprod. Med. 2016, 22, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powazniak, Y.; Kempfer, A.C.; De La Paz Dominguez, M.; Farias, C.; Keller, L.; Calderazzo, J.C.; Lazzari, M.A. Effect of estradiol, progesterone and testosterone on apoptosis-and proliferation-induced MAPK signaling in human umbilical vein endothelial cells. Mol. Med. Rep. 2009, 2, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oviedo, P.J.; Sobrino, A.; Laguna-Fernandez, A.; Novella, S.; Tarín, J.J.; García-Pérez, M.A.; Sanchís, J.; Cano, A.; Hermenegildo, C. Estradiol induces endothelial cell migration and proliferation through estrogen receptor-enhanced RhoA/ROCK pathway. Mol. Cell. Endocrinol. 2011, 335, 96–103. [Google Scholar] [CrossRef]
- Maniyar, R.; Chakraborty, S.; Suriano, R. Ethanol Enhances Estrogen Mediated Angiogenesis in Breast Cancer. J. Cancer 2018, 9, 3874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Healy, C.; Nothnick, W.B. Estrogen suppresses expression of the matrix metalloproteinase inhibitor reversion-inducing cysteine-rich protein with Kazal motifs (RECK) within the mouse uterus. Endocrine 2012, 42, 97–106. [Google Scholar] [CrossRef]
- Hervé, M.; Meduri, G.; Petit, F.; Domet, T.; Lazennec, G.; Mourah, S.; Perrot-Applanat, M. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J. Endocrinol. 2006, 188, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvin, S.; Nilsson, U.W.; Huss, F.R.; Kratz, G.; Dabrosin, C. Estradiol increases VEGF in human breast studied by whole-tissue culture. Cell Tissue Res. 2006, 325, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Suzuma, I.; Mandai, M.; Takagi, H.; Suzuma, K.; Otani, A.; Oh, H.; Kobayashi, K.; Honda, Y. 17 β-estradiol increases VEGF receptor-2 and promotes DNA synthesis in retinal microvascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2122–2129. [Google Scholar]
- Arnal, J.-F.; Fontaine, C.; Billon-Galés, A.; Favre, J.; Laurell, H.; Lenfant, F.; Gourdy, P. Estrogen receptors and endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.; Han, Z.; Gao, L.; Cui, J.; Sun, Y.; Niu, Y.; You, B.; Huang, C.-P.; Chang, C. Targeting the ERβ/Angiopoietin-2/Tie-2 signaling-mediated angiogenesis with the FDA-approved anti-estrogen Faslodex to increase the Sunitinib sensitivity in RCC. Cell Death Dis. 2020, 11, 367. [Google Scholar] [CrossRef]
- Darenius, K.; Kindahl, H.; Madej, A. Clinical and endocrine aspects of early fetal death in the mare. J. Reprod. Fertil. 1987, 35, 497–498. [Google Scholar]
- Douglas, R. Endocrine diagnostics in the broodmare: What you need to know about progestins and estrogens. Proc. Soc. 2004, 106–115. [Google Scholar]
- Shikichi, M.; Iwata, K.; Ito, K.; Miyakoshi, D.; Murase, H.; Sato, F.; Korosue, K.; Nagata, S.; Nambo, Y. Abnormal pregnancies associated with deviation in progestin and estrogen profiles in late pregnant mares: A diagnostic aid. Theriogenology 2017, 98, 75–81. [Google Scholar] [CrossRef]
- Raeside, J.I.; Christie, H.L.; Renaud, R.L.; Waelchli, R.O.; Betteridge, K.J. Estrogen metabolism in the equine conceptus and endometrium during early pregnancy in relation to estrogen concentrations in yolk-sac fluid. Biol. Reprod. 2004, 71, 1120–1127. [Google Scholar] [CrossRef] [Green Version]
- Daels, P.; Shideler, S.; Lasley, B.; Hughes, J.; Stabenfeldt, G. Source of oestrogen in early pregnancy in the mare. Reproduction 1990, 90, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daels, P.; DeMoraes, J.; Stabenfeldt, G.; Hughes, J.; Lasley, B. The corpus luteum: Source of oestrogen during early pregnancy in the mare. J. Reprod. Fertil. Suppl. 1991, 44, 501–508. [Google Scholar]
- Albrecht, B.; Daels, P. Immunolocalization of 3β-hydroxysteroid dehydrogenase, cytochrome P450 17α-hydroxylase/17, 20-lyase and cytochrome P450 aromatase in the equine corpus luteum of dioestrus and early pregnancy. Reproduction 1997, 111, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terqui, M.; Palmer, E. Oestrogen pattern during early pregnancy in the mare. J. Reprod. Fertil. Suppl. 1979, 441–446. [Google Scholar]
- Nett, T.M.; Holtan, D.W.; Estergreen, V.L. Plasma estrogens in pregnant and postpartum mares. J. Anim. Sci. 1973, 37, 962–970. [Google Scholar] [CrossRef]
- Pashen, R.; Sheldrick, E.; Allen, W.; Flint, A. Dehydroepiandrosterone synthesis by the fetal foal and its importance as an oestrogen precursor. J. Reprod. Fertil. Suppl. 1982, 32, 389–397. [Google Scholar]
- Pashen, R.; Allen, W. The role of the fetal gonads and placenta in steroid production, maintenance of pregnancy and parturition in the mare. J. Reprod. Fertil. Suppl. 1979, 499–509. [Google Scholar]
- Albrecht, E.D.; Robb, V.A.; Pepe, G.J. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J. Clin. Endocrinol. Metab. 2004, 89, 5803–5809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, E.D.; Pepe, G.J. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int. J. Dev. Biol. 2010, 54, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteller-Vico, A.; Ball, B.A.; Troedsson, M.H.; Squires, E.L. Endocrine changes, fetal growth, and uterine artery hemodynamics after chronic estrogen suppression during the last trimester of equine pregnancy. Biol. Reprod. 2017, 96, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salafia, C.M.; Minior, V.K.; Pezzullo, J.C.; Popek, E.J.; Rosenkrantz, T.S.; Vintzileos, A.M. Intrauterine growth restriction in infants of less than thirty-two weeks’ gestation: Associated placental pathologic features. Am. J. Obstet. Gynecol. 1995, 173, 1049–1057. [Google Scholar] [CrossRef]
- Bailey, C.S.; Heitzman, J.M.; Buchanan, C.N.; Bare, C.; Sper, R.; Borst, L.; Macpherson, M.; Archibald, K.; Whitacre, M. B-mode and D oppler ultrasonography in pony mares with experimentally induced ascending placentitis. Equine Vet. J. 2012, 44, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, D.; De Cramer, K. Prostaglandin E (2) as an adjunct to the induction of abortion in mares. J. Reprod. Fertil. 1991, 722–723. [Google Scholar]
- Weidner, N. Measuring intratumoral microvessel density. Methods Enzymol. 2008, 444, 305–323. [Google Scholar] [PubMed]
- Jadeski, L.C.; Lala, P.K. Nitric oxide synthase inhibition by NG-nitro-L-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am. J. Pathol. 1999, 155, 1381–1390. [Google Scholar] [CrossRef] [Green Version]
- Hedges, J.F.; Demaula, C.D.; Moore, B.D.; Mclaughlin, B.E.; Simon, S.I.; Maclachlan, N.J. Characterization of equine E-selectin. Immunology 2001, 103, 498–504. [Google Scholar] [CrossRef]
- Munro, C.; Stabenfeldt, G. Development of a microtitre plate enzyme immunoassay for the determination of progesterone. J. Endocrinol. 1984, 101, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabenfeldt, G.; Daels, P.; Munro, C.; Kindahl, H.; Hughes, J.; Lasley, B. An oestrogen conjugate enzyme immunoassay for monitoring pregnancy in the mare: Limitations of the assay between days 40 and 70 of gestation. J. Reprod. Fertil. Suppl. 1991, 44, 37–44. [Google Scholar]
- Peng, B.; Robert, K.Y.; DeHoff, K.L.; Amos, C.I. Normalizing a Large Number of Quantitative Traits Using Empirical Normal Quantile Transformation. In BMC Proceedings, 2007; BioMed Central: London, UK, 2007. [Google Scholar]
- Klein, C.; Rutllant, J.; Troedsson, M.H. Expression stability of putative reference genes in equine endometrial, testicular, and conceptus tissues. BMC Res. Notes 2011, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Dini, P.; Esteller-Vico, A.; Scoggin, K.E.; Daels, P.; Ball, B.A. Extraction of RNA from formalin-fixed, paraffin-embedded equine placenta. Reprod. Domest. Anim. 2019, 54, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis—Correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dini, P.; Carossino, M.; Balasuriya, U.B.; El-Sheikh Ali, H.; Loux, S.C.; Esteller-Vico, A.; Scoggin, K.E.; Loynachan, A.T.; Kalbfleisch, T.; De Spiegelaere, W. Paternally expressed retrotransposon Gag-like 1 gene, RTL1, is one of the crucial elements for placental angiogenesis in horses. Biol. Reprod. 2021, 104, 1386–1399. [Google Scholar] [CrossRef] [PubMed]




| Transcript | NCBI ID | Sequence of Primer (5′-3′) | Product Size (bp) | |
|---|---|---|---|---|
| ACTB | 100033878 | F: | CGACATCCGTAAGGACCTGT | 100 |
| R: | CAGGGCTGTGATCTCCTTCT | |||
| ANGPT1 | 100056482 | F: | GGACAGCAGGAAAACAGAGC | 93 |
| R: | GGGCACATTTGCACATACAG | |||
| ANGPT2 | 100051890 | F: | GACGCAGACAACGACAAATG | 76 |
| R: | GACCACATGCATCAAACCAC | |||
| AR | 100033980 | F: | AGCTGCCATCCACTCTGTCT | 61 |
| R: | TGATAAACTGCTGCCTCGTC | |||
| CYP17A1 | 100034232 | F: | GCATGCTGGACTTACTGATCC | 60 |
| R: | CTGGGCCAGTGTTGTTATTG | |||
| CYP19A1 | 100009712 | F: | CCACATCATGAAACACGATCA | 60 |
| R: | TACTGCAACCCAAATGTGCT | |||
| ESR1 | 791249 | F: | TCCATGGAGCACCCAGGAAAGC | 125 |
| R: | CGGAGCCGAGATGACGTAGCC | |||
| ESR2 | 100033964 | F: | TCCTGAATGCTGTGACCGAC | 116 |
| R: | GTGCCTGACGTGAGAAAGGA | |||
| GAPDH | 100033897 | F: | AGAAGGAGAAAGGCCCTCAG | 88 |
| R: | GGAAACTGTGGAGGTCAGGA | |||
| HIF1A | 100061166 | F: | CACCAGAGCCTAACAGTCCC | 141 |
| R: | AGTCCGTGTCCTGAGTGGAA | |||
| HSD3B2 | 100034078 | F: | AGCAAATACCATGAGCACGA | 62 |
| R: | TAACGTGGGCATCTTGTGAA | |||
| NOS2 | 791246 | F: | GCCAAGGTCTGAGCTACCTG | 200 |
| R: | GAGTGCCTGGCTGAGTGAG | |||
| NOS3 | 100063339 | F: | GAAGCACCTGGAGAATGAGC | 150 |
| R: | TCTGGCTGGTAGCGGAAG | |||
| PGR | 100033883 | F: | GTGAGAAGGGAAGTGGAAC | 226 |
| R: | GGAGGGCAGGAGAAGGTAGT | |||
| VEGF | 100033839 | F: | CTACCTCCACCATGCCAAGT | 88 |
| R: | GACGTCCATGAACTTCACCA | |||
| VEGFR1 | 100033957 | F: | GGCACAAAGACCCAAAAGAA | 88 |
| R: | CCGTCCTGTTGTACATTTGC | |||
| VEGFR2 | 100033959 | F: | AGATGCTGTAACCCGGAGTG | 79 |
| R: | CGTGCTGTTCTTCTTGGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haneda, S.; Dini, P.; Esteller-Vico, A.; Scoggin, K.E.; Squires, E.L.; Troedsson, M.H.; Daels, P.; Nambo, Y.; Ball, B.A. Estrogens Regulate Placental Angiogenesis in Horses. Int. J. Mol. Sci. 2021, 22, 12116. https://doi.org/10.3390/ijms222212116
Haneda S, Dini P, Esteller-Vico A, Scoggin KE, Squires EL, Troedsson MH, Daels P, Nambo Y, Ball BA. Estrogens Regulate Placental Angiogenesis in Horses. International Journal of Molecular Sciences. 2021; 22(22):12116. https://doi.org/10.3390/ijms222212116
Chicago/Turabian StyleHaneda, Shingo, Pouya Dini, Alejandro Esteller-Vico, Kirsten E. Scoggin, Edward L. Squires, Mats H. Troedsson, Peter Daels, Yasuo Nambo, and Barry A. Ball. 2021. "Estrogens Regulate Placental Angiogenesis in Horses" International Journal of Molecular Sciences 22, no. 22: 12116. https://doi.org/10.3390/ijms222212116






