Glycation of Host Proteins Increases Pathogenic Potential of Porphyromonas gingivalis
Abstract
1. Introduction
2. Results and Discussion
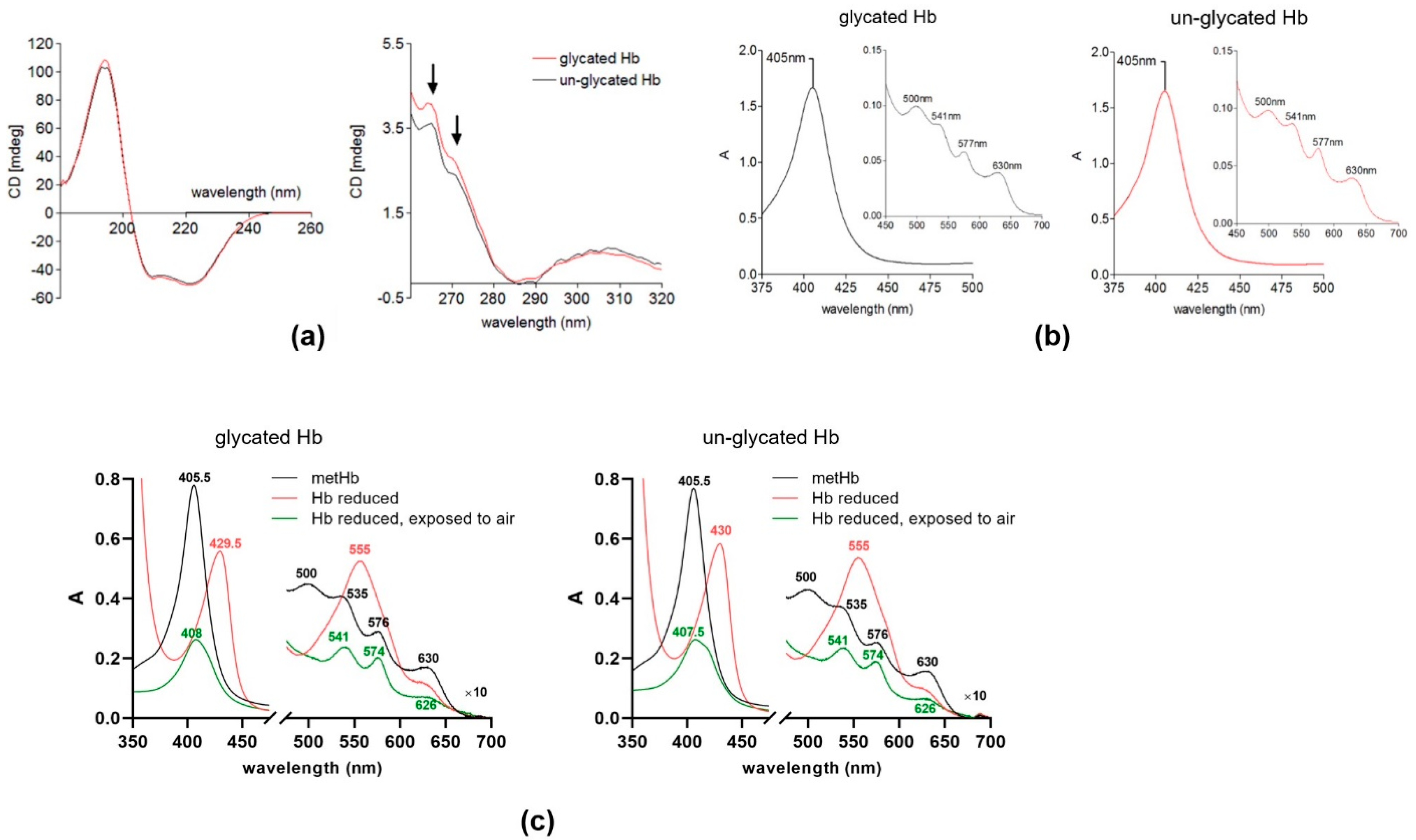
2.1. Characterization of Glycated Proteins
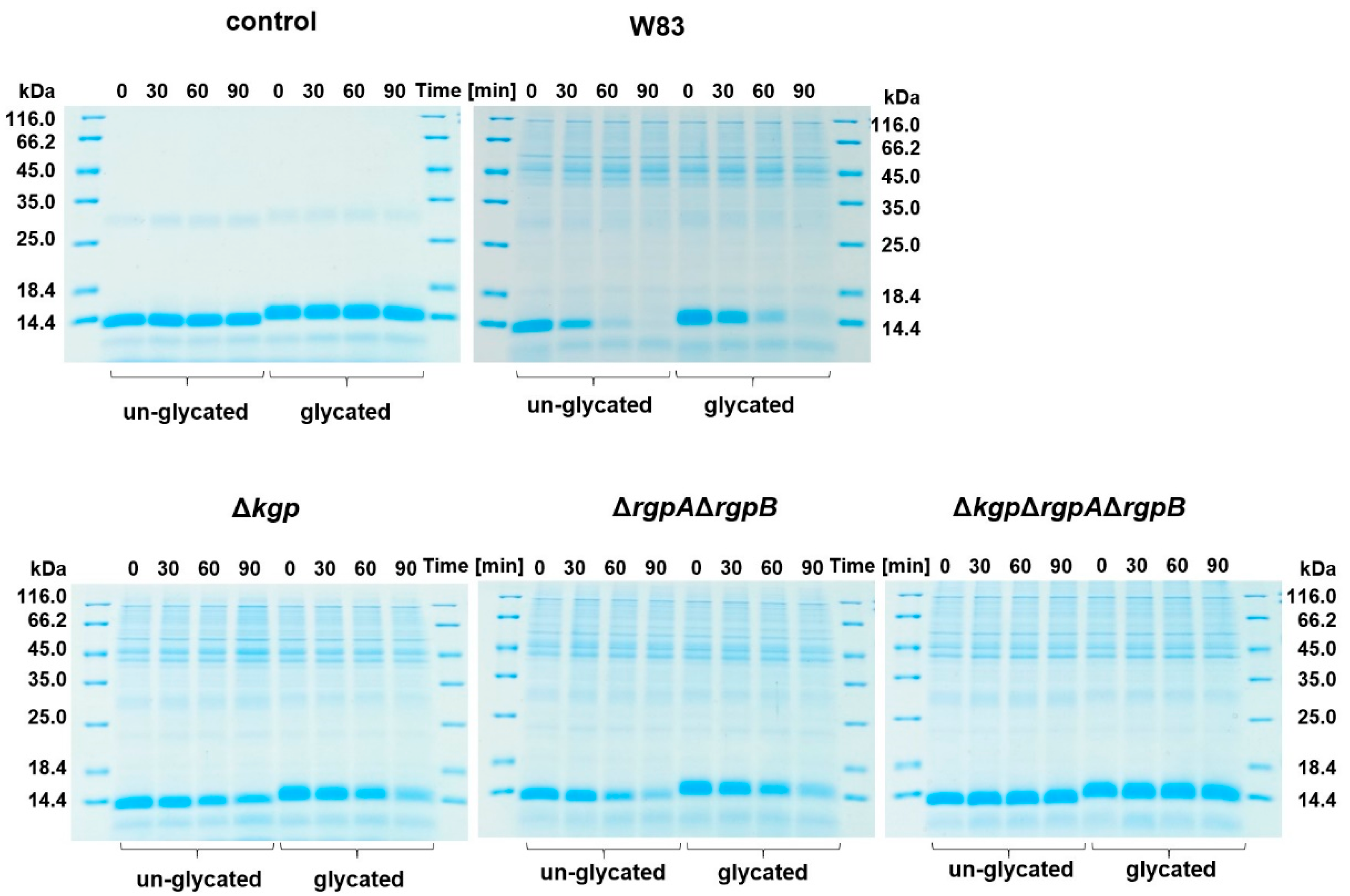
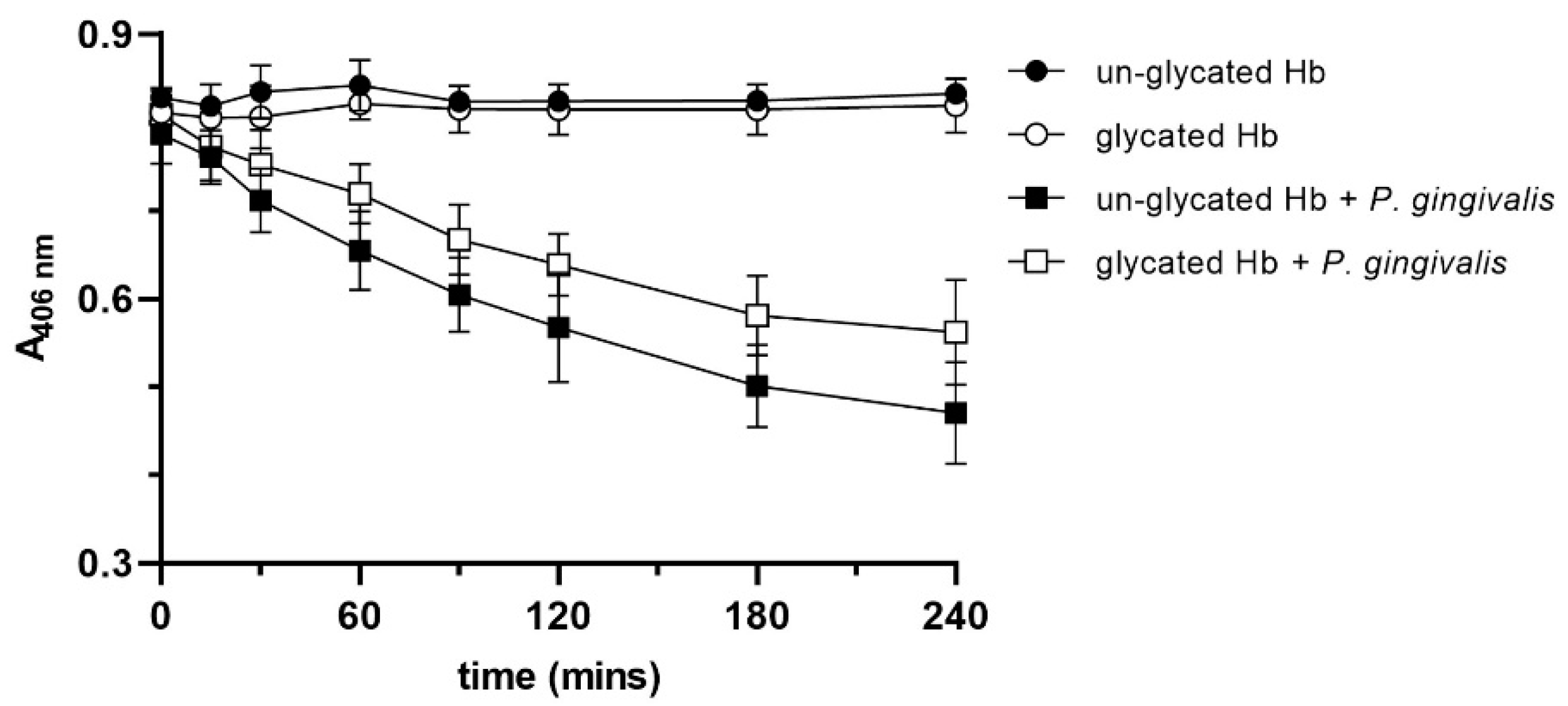
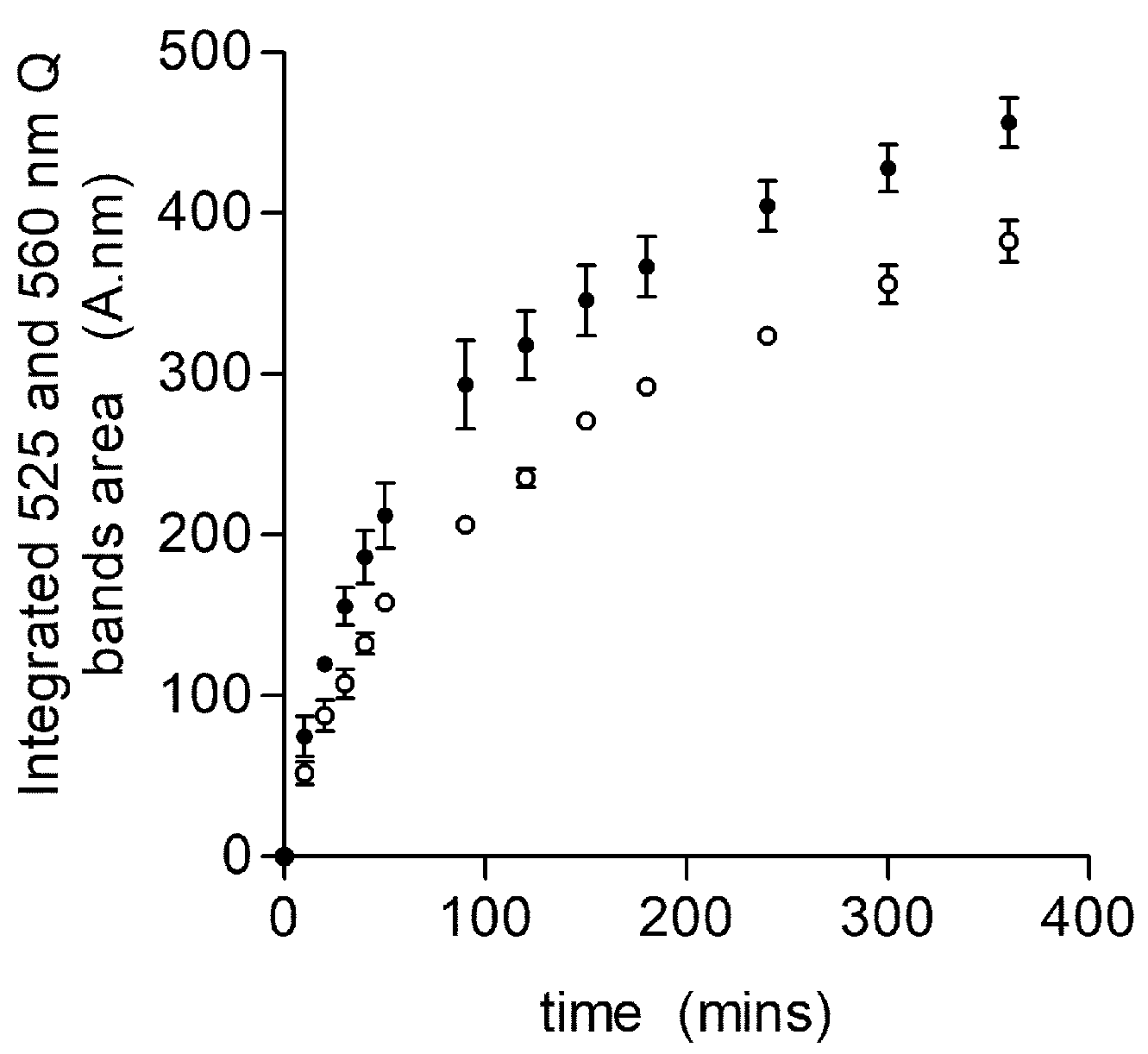
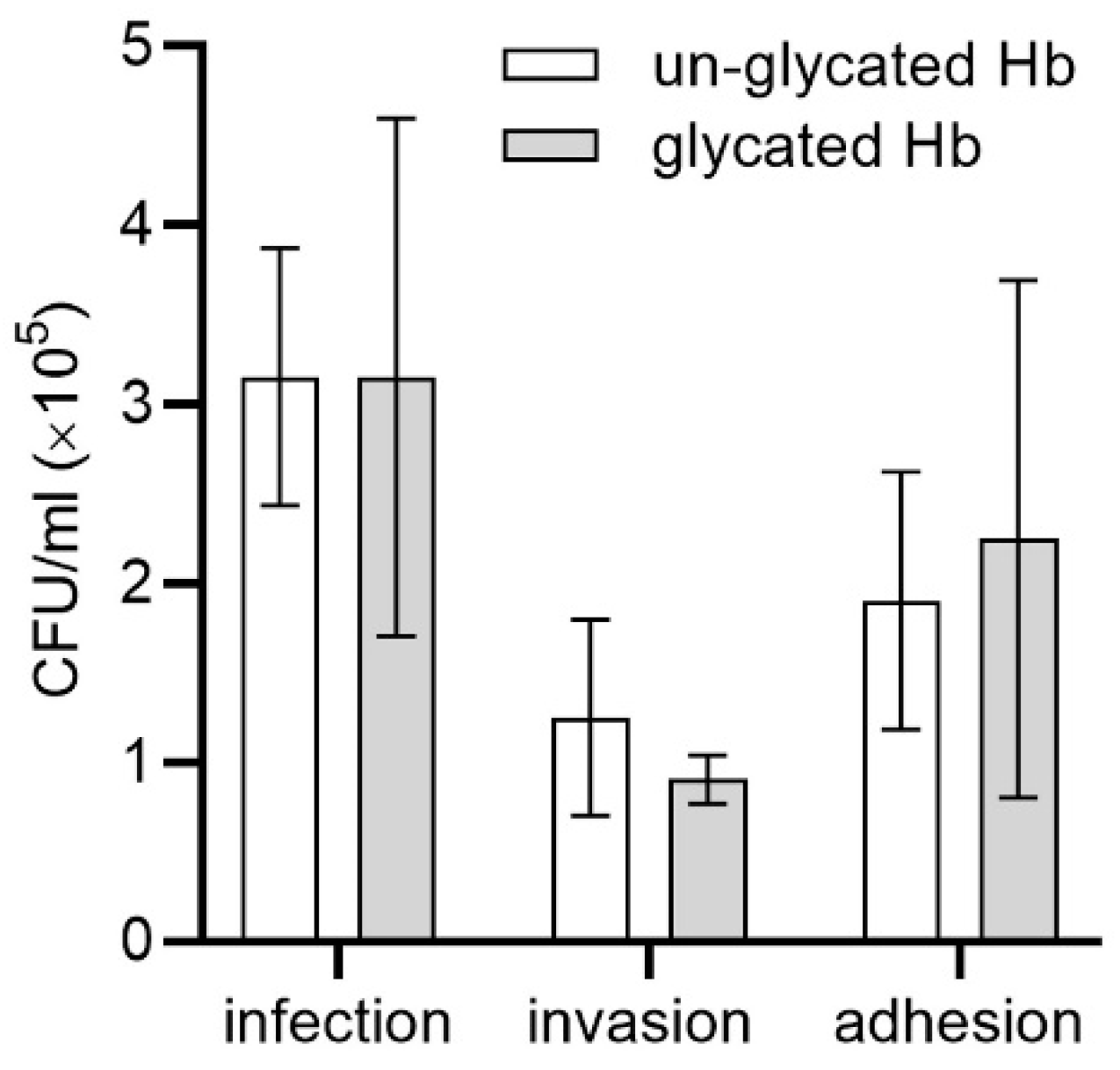
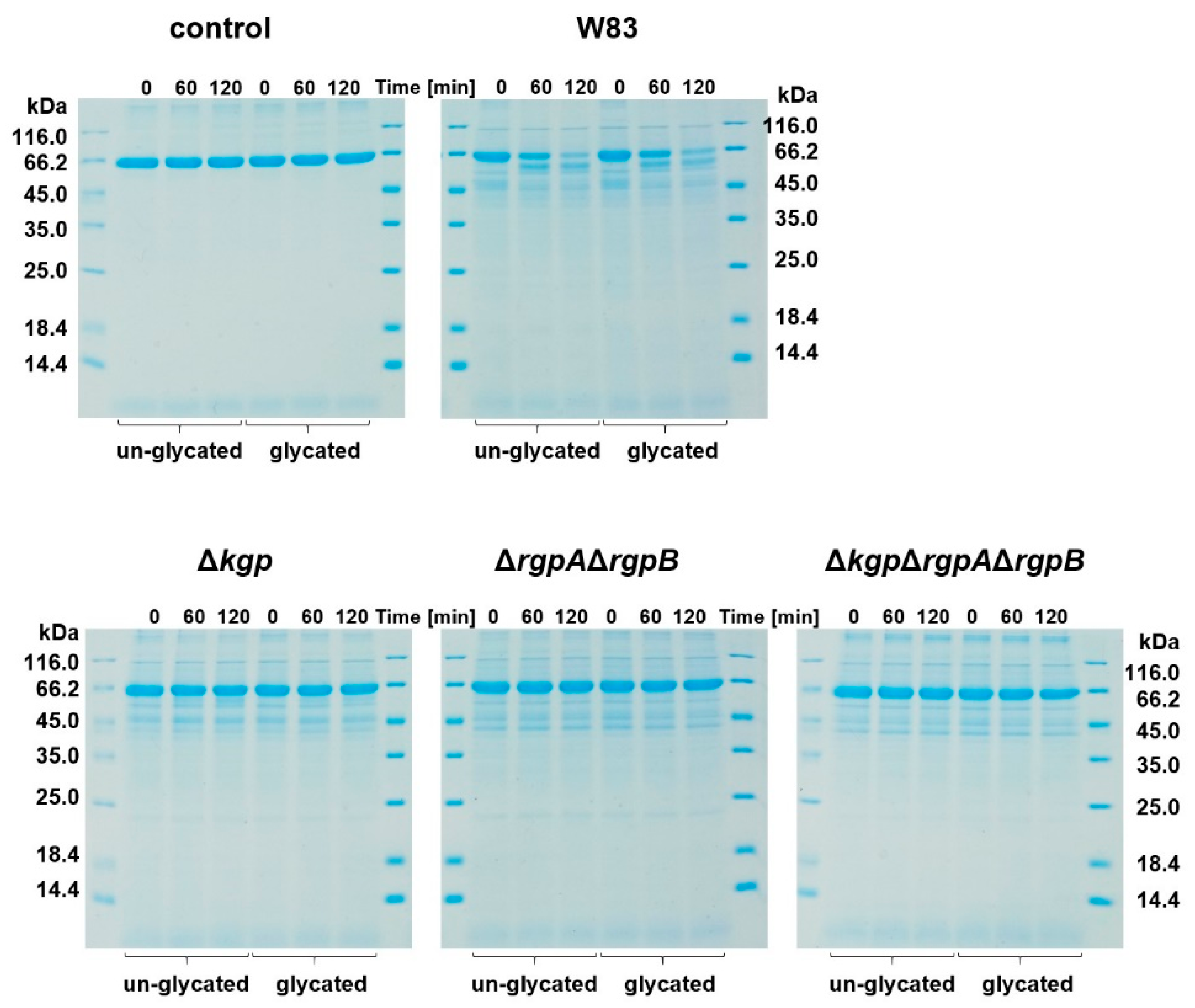
2.2. Glycated Hemoglobin as a Sole Source of Heme for P. gingivalis
2.3. Glycated Albumin as a Sole Source of Heme for P. gingivalis
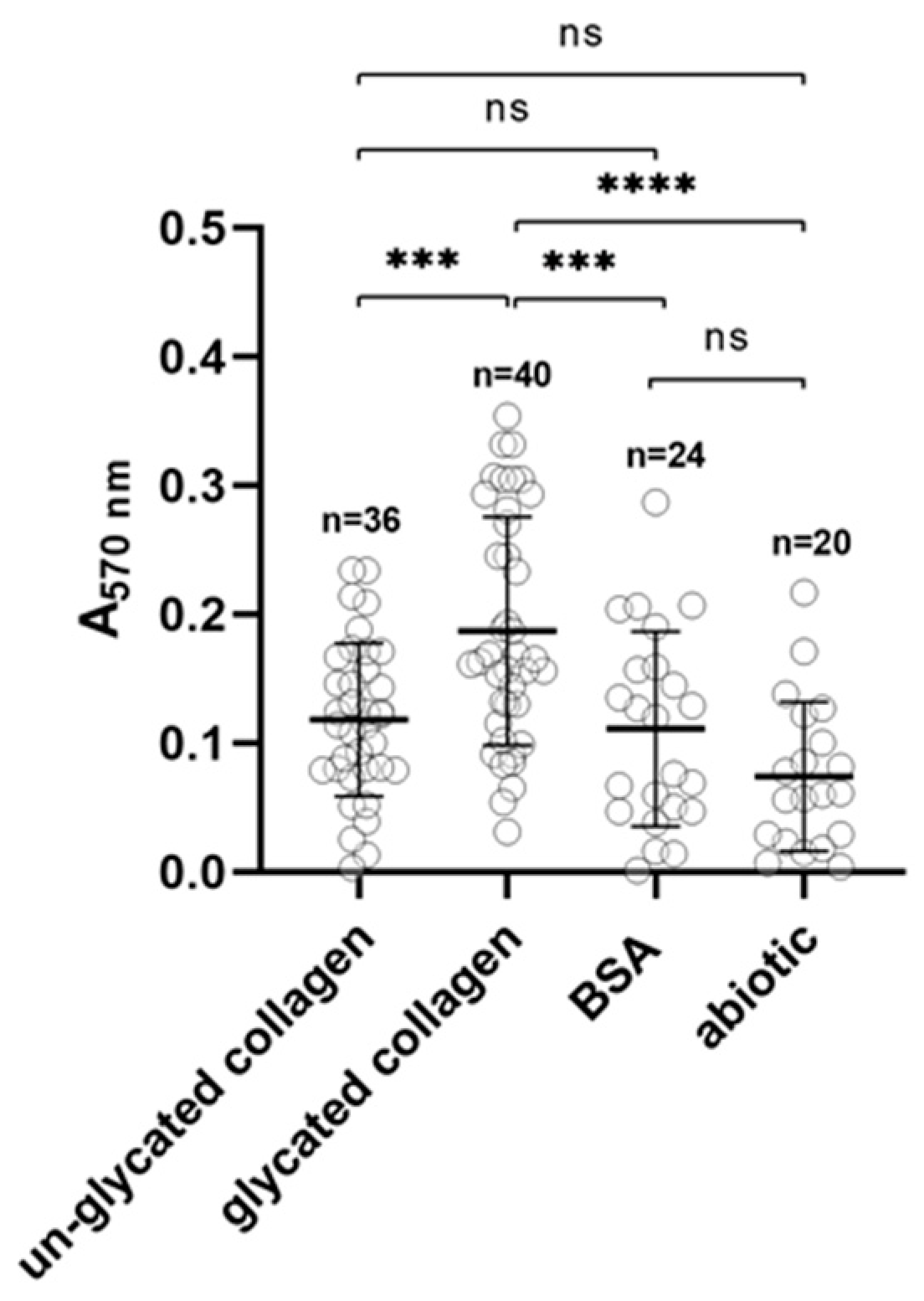
2.4. Glycated Collagen as a Substratum for P. gingivalis
3. Materials and Methods
3.1. Bacterial Stains and Growth Conditions
3.2. Preparation and Characterization of Glycated Proteins
3.3. Analysis of Heme Release from Hemoglobin
3.4. Analysis of Proteins Susceptibility to Proteolysis
3.5. Protein Overexpression and Purification
3.6. Heme-Protein Complex Formation and Analysis
3.7. Heme Sequestration Experiments
3.8. Biofilm Formation
3.9. Infection Assay
3.10. Bioinformatic and Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajishengallis, G.; Diaz, P.I. Porphyromonas gingivalis: Immune subversion activities and role in periodontal dysbiosis. Curr. Oral Health Rep. 2020, 7, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 28, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and its systemic impact: Current status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Wu, H.; Huang, S.; Chen, L.; Gui, Q.; Zhou, W.; Yang, Y.; Wu, Y.; Zhang, H.; et al. Periodontitis in elderly patients with type 2 diabetes mellitus: Impact on gut microbiota and systemic inflammation. Aging 2020, 12, 25956–25980. [Google Scholar] [CrossRef]
- Bansal, M.; Khatri, M.; Taneja, V. Potential role of periodontal infection in respiratory diseases—A review. J. Med. Life 2013, 6, 244–248. [Google Scholar]
- Benedyk, M.; Byrne, D.P.; Glowczyk, I.; Potempa, J.; Olczak, M.; Olczak, T.; Smalley, J.W. Pyocyanin, a contributory factor in haem acquisition and virulence enhancement of Porphyromonas gingivalis in the lung. PLoS ONE 2015, 10, e0118319. [Google Scholar] [CrossRef]
- Llambes, F.; Arias-Herrera, S.; Caffesse, R. Relationship between diabetes and periodontal infection. World J. Diabetes 2015, 6, 927–935. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Bissett, S.M. Periodontitis and diabetes. Br. Dent. J. 2019, 227, 577–584. [Google Scholar] [CrossRef]
- Polak, D.; Sanui, T.; Nishimura, F.; Shapira, L. Diabetes as a risk factor for periodontal disease—Plausible mechanisms. Periodontol. 2000 2020, 83, 46–58. [Google Scholar] [CrossRef]
- Graves, D.T.; Ding, Z.; Yang, Y. The impact of diabetes on periodontal diseases. Periodontol. 2000 2019, 82, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Tervonen, T.; Knuuttila, M. Relation of diabetes control to periodontal pocketing and alveolar bone level. Oral Surg. Oral Med. Oral Pathol. 1986, 61, 346–349. [Google Scholar] [CrossRef]
- Sastrowijoto, S.H.; Hillemans, P.; van Steenbergen, T.J.; Abraham-Inpijn, L.; de Graaff, J. Periodontal condition and microbiology of healthy and diseased periodontal pockets in type 1 diabetes mellitus patients. J. Clin. Periodontol. 1989, 1, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Stoicescu, M.; Calniceanu, H.; Tig, I.; Nemeth, S.; Tent, A.; Popa, A.; Brisc, C.; Ignat-Romanul, I. Significant aspects and correlation between glycemic control and generalized chronic periodontitis in type 2 diabetes mellitus patients. Exp. Ther. Med. 2021, 22, 671. [Google Scholar] [CrossRef] [PubMed]
- Stohr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef]
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontal. 2005, 76 (Suppl. 1), 2075–2084. [Google Scholar] [CrossRef]
- Seyama, M.; Yoshida, K.; Yohida, K.; Fujiwara, N.; Ono, K.; Eguchi, T.; Kawai, H.; Guo, J.; Weng, Y.; Haoze, Y.; et al. Outer membrane vesicles of Porphyromonas gingivalis attenuate insulin sensitivity by delivering gingipains to the liver. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165731. [Google Scholar] [CrossRef]
- Finot, P.A. Historical perspective of the Maillard reaction in food science. Ann. N. Y. Acad. Sci. 2005, 1043, 390–395. [Google Scholar] [CrossRef]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef]
- Ledesma-Osuna, A.I.; Ramos-Clamont, G.; Vazquez-Moreno, L. Characterization of bovine serum albumin glycated with glucose, galactose and lactose. Acta Biochim. Pol. 2008, 55, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Shaklai, N.; Garlick, R.L.; Bunn, H.F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J. Biol. Chem. 1984, 259, 3812–3817. [Google Scholar] [CrossRef]
- Schleicher, E.D.; Olgemoller, B.; Wiedenmann, E.; Gerbitz, K.D. Specific glycation of albumin depends on its half-life. Clin. Chem. 1993, 39, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Garlick, R.L.; Mazer, J.S. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J. Biol. Chem. 1983, 258, 6142–6146. [Google Scholar] [CrossRef]
- McDonald, M.J.; Bleichman, M.; Bunn, H.F.; Noble, R.W. Functional properties of the glycosylated minor components of human adult hemoglobin. J. Biol. Chem. 1979, 254, 702–707. [Google Scholar] [CrossRef]
- Sen, S.; Kar, M.; Roy, A.; Chakraborti, A.S. Effect of nonenzymatic glycation on functional and structural properties of hemoglobin. Biophys. Chem. 2005, 113, 289–298. [Google Scholar] [CrossRef]
- De Rosa, M.C.; Sanna, M.T.; Messana, I.; Castagnola, M.; Galtieri, A.; Tellone, E.; Scatena, R.; Bolta, M.; Giardina, B. Glycated human hemoglobin (HbA1c): Functional characteristics and molecular modeling studies. Biophys. Chem. 1998, 72, 323–335. [Google Scholar] [CrossRef]
- Costa, K.L.; Taboza, Z.A.; Angelino, G.B.; Silveira, V.R.; Montenegro, R., Jr.; Haas, A.N.; Rego, R.O. The influence of periodontal disease on changes of glycated hemoglobin levels in type 2 diabetics: A retrospective cohort study. J. Periodontol. 2017, 88, 17–25. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; Bynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef]
- Makiura, N.; Ojima, M.; Kou, Y.; Furuta, N.; Okahashi, N.; Shizukushi, S.; Amano, A. Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Mol. Microbiol. 2008, 23, 348–351. [Google Scholar] [CrossRef]
- Kajiura, Y.; Bando, M.; Inagaki, Y.; Nagata, T.; Kido, J. Glycated albumin and calprotectin levels in gingival crevicular fluid from patients with periodontitis and type 2 diabetes. J. Periodontol. 2014, 85, 1667–1675. [Google Scholar] [CrossRef]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Turk, Z.; Misur, N.; Turk, B.; Benko, B. Rat tissue collagen modified by advanced glycation: Correlation with duration of diabetes and glycemic control. Clin. Chem. Lab. Med. 1999, 37, 813–820. [Google Scholar] [CrossRef]
- Nass, N.; Bartling, B.; Santos, A.N.; Scheubel, R.J.; Borgermann, J.; Silber, R.E.; Simm, A. Advanced glycation end products, diabetes, and aging. Z. Gerontol. Geriatr. 2007, 40, 349–356. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Gautieri, A. The role of collagen crosslinks in aging and diabetes—The good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014, 4, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.M.A.P.; Benso, B.; Naulin, P.A.; Barrera, N.P.; Bozec, L.; Aguayo, S. Modulatory effect of glycated collagen on oral streptococcal nanoadhesion. J. Dent. Res. 2021, 100, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, T.; Knuuttila, M.; Pohjamo, L.; Haukipuro, K.J. Relation between control and diabetes and gingival bleeding. J. Periodontol. 1985, 56, 154–157. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Marlow, N.M.; Fernandes, J.K.; Leite, R.S.J. Periodontal disease progression and glycaemic control among Gullah African Americans with type-2 diabetes. J. Clin. Periodontol. 2010, 37, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Olczak, T. Haem acquisition mechanisms of Porphyromonas gingivalis—Strategies used in polymicrobial community in a haem-limited host environment. Mol. Oral Microbiol. 2017, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Simpson, W.; Olczak, T.; Genco, C.A. Characterization and expression of HmuR, a TonB-dependent hemoglobin receptor of Porphyromonas gingivalis. J. Bacteriol. 2000, 182, 5737–5748. [Google Scholar] [CrossRef] [PubMed]
- Olczak, T.; Dixon, D.W.; Genco, C.A. Binding specificity of the Porphyromonas gingivalis heme and hemoglobin receptor HmuR, gingipain K, and gingipain R1 for heme, porphyrins, and metalloporphyrins. J. Bacteriol. 2001, 183, 5599–5608. [Google Scholar] [CrossRef]
- Olczak, T.; Sroka, A.; Potempa, J.; Olczak, M. Porphyromonas gingivalis HmuY and HmuR—Further characterization of a novel mechanism of heme utilization. Arch. Microbiol. 2008, 183, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Olczak, T. Analysis of conserved glutamate residues in Porphyromonas gingivalis outer membrane receptor HmuR: Toward a further understanding of heme uptake. Arch. Microbiol. 2006, 186, 393–402. [Google Scholar] [CrossRef]
- Wojtowicz, H.; Wojaczynski, J.; Olczak, M.; Kroliczewski, J.; Latos-Grazynski, L.; Olczak, T. Heme environment in HmuY, the heme-binding protein of Porphyromonas gingivalis. Biochem. Biophys. Res. Commun. 2009, 383, 178–182. [Google Scholar] [CrossRef]
- Wojtowicz, H.; Guevara, T.; Tallant, C.; Olczak, M.; Sroka, A.; Potempa, J.; Sola, M.; Olczak, T.; Gomis-Ruth, F.X. Unique structure and stability of HmuY, a novel heme-binding protein of Porphyromonas gingivalis. PLoS Pathog. 2009, 5, e100041. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Olczak, T.; Guo, H.C.; Dixon, D.W.; Genco, C.A. Identification of amino acid residues involved in heme binding and hemoprotein utilization in the Porphyromonas gingivalis heme receptor HmuR. Infect. Immun. 2006, 74, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, M.; Antonyuk, S.; Strange, R.W.; Smalley, J.W.; Mackiewicz, P.; Smiga, M.; Stepien, P.; Olczak, M.; Olczak, T. Tannerella forsythia Tfo belongs to Porphyromonas gingivalis HmuY-like family of proteins but differs in heme-binding properties. Biosci. Rep. 2018, 38, BSR20181325. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Byrne, D.P.; Birss, A.J.; Wojtowicz, H.; Sroka, A.; Potempa, J.; Olczak, T. HmuY haemophore and gingipain proteases constitute a unique syntrophic system of haem acquisition by Porphyromonas gingivalis. PLoS ONE 2011, 6, e17182. [Google Scholar] [CrossRef]
- Benson, D.R.; Rivera, M. Heme uptake and metabolism in bacteria. Met. Ions Life Sci. 2013, 12, 279–332. [Google Scholar] [CrossRef] [PubMed]
- Olczak, T.; Wojtowicz, H.; Ciuraszkiewicz, J.; Olczak, M. Species specificity, surface exposure, protein expression, immunogenicity, and participation in biofilm formation of Porphyromonas gingivalis HmuY. BMC Microbiol. 2010, 10, 134. [Google Scholar] [CrossRef]
- Veith, P.D.; Chen, Y.Y.; Gorasia, D.G.; Chen, D.; Glew, M.D.; O’Brien-Simpson, N.M.; Cecil, J.D.; Holden, J.A.; Reynolds, E.C. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 2014, 13, 2420–2432. [Google Scholar] [CrossRef]
- Veith, P.D.; Luong, C.; Tan, K.H.; Dashper, S.G.; Reynolds, E.C. Outer membrane vesicle proteome of Porphyromonas gingivalis is differentially modulated relative to the outer membrane in response to heme availability. Proteome Res. 2018, 17, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Olczak, T.; Sosicka, P.; Olczak, M. HmuY is an important virulence factor for Porphyromonas gingivalis growth in the heme-limited host environment and infection of macrophages. Biochem. Biophys. Res. Commun. 2015, 467, 48–753. [Google Scholar] [CrossRef]
- Furuta, N.; Tsuda, K.; Omori, H.; Yoshimori, T.; Amano, A. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect. Immun. 2009, 77, 4187–4196. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Furuta, N.; Inaba, H.; Kawai, S.; Hanada, K.; Yoshimori, T.; Amano, A. Functional analysis of alpha5beta1 integrin and lipid rafts in invasion of epithelial cells by Porphyromonas gingivalis using fluorescent beads coated with bacterial membrane vesicles. Cell Struct. Funct. 2008, 33, 123–132. [Google Scholar] [CrossRef]
- Gui, M.J.; Dashper, S.G.; Slakeski, N.; Chen, Y.Y.; Reynolds, E.C. Spheres of influence: Porphyromonas gingivalis outer membrane vesicles. Mol. Oral Microbiol. 2016, 31, 365–378. [Google Scholar] [CrossRef]
- Smalley, J.W.; Birss, A.J.; Shuttleworth, C.A. The degradation of type I collagen and human plasma fibronectin by trypsin-like enzyme and extracellular membrane vesicles of Bacteroides gingivalis W50. Arch. Oral Biol. 1988, 33, 323–329. [Google Scholar] [CrossRef]
- Smalley, J.W.; Shuttleworth, C.A.; Birss, A.J. Collagenolytic activity of the extracellular vesicles of Bacteroides gingivalis W50 and an avirulent variant W50/BE1. Arch. Oral Biol. 1989, 34, 579–583. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yoshida, K.; Okamura, H.; Ochiai, K.; Takamura, H.; Fujiwara, N.; Ozaki, K. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3beta signaling pathway. Biochim. Biophys. Acta 2013, 1832, 2035–2043. [Google Scholar] [CrossRef]
- Ter Steeg, P.F.; Van der Hoeven, J.S.; de Jong, M.H.; van Munster, P.J.; Jansen, M.J. Enrichment of subgingival microflora on human serum leading to accumulation of Bacteroides species, Peptostreptococci and Fusobacteria. Antonie Van Leeuwenhoek 1987, 53, 261–272. [Google Scholar] [CrossRef]
- Wei, G.X.; van der Hoeven, J.S.; Smalley, J.W.; Mikx, F.H.; Fan, M.W. Proteolysis and utilization of albumin by enrichment cultures of subgingival microbiota. Oral Microbiol. Immunol. 1999, 14, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, S.L.; Marinah, T.A. In vitro glycation of dried serum. Clin. Chim. Acta 1988, 173, 165–171. [Google Scholar] [CrossRef]
- Fluckiger, R.; Winterhalter, K.H. In vitro synthesis of hemoglobin A1c. FEBS Lett. 1976, 71, 356–360. [Google Scholar] [CrossRef]
- Bunn, H.F.; Haney, D.N.; Kamin, S.; Gabbay, K.H.; Gallop, P.M. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J. Clin. Investig. 1976, 57, 1652–1659. [Google Scholar] [CrossRef]
- Shapiro, R.; McManus, M.J.; Zalut, C.; Bunn, H.F. Sites of nonenzymatic glycosylation of human hemoglobin A. J. Biol. Chem. 1980, 255, 3120–3127. [Google Scholar] [CrossRef]
- Zhang, X.; Medzihradszky, K.F.; Cunningham, J.; Lee, P.D.; Rognerud, C.L.; Ou, C.N.; Harmatz, P.; Witkowska, H.E. Characterization of glycated hemoglobin in diabetic patients: Usefulness of electrospray mass spectrometry in monitoring the extent and distribution of glycation. J. Chromatogr. B Biomed. Sci. Appl. 2001, 759, 1–15. [Google Scholar] [CrossRef]
- Delpierre, G.; Vertommen, D.; Communi, D.; Rider, M.H.; Van Schaftingen, E. Identification of fructosamine residues deglycated by fructosamine-3-kinase in human hemoglobin. J. Biol. Chem. 2004, 279, 27613–27620. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakahari, T.; Yamamoto, D. The structural feature surrounding glycated lysine residues in human hemoglobin. Biomed. Res. 2011, 32, 217–223. [Google Scholar] [CrossRef]
- Kamps, J.J.A.G.; Hopkinson, R.J.; Schofield, C.J.; Claridge, T.D.W. How formaldehyde reacts with amino acids. Nat. Commun. Chem. 2019, 2, 126. [Google Scholar] [CrossRef]
- Kar, M.; Chakraborti, A.S. Release of iron from hemoglobin—A possible source of free radicals in diabetes mellitus. Indian J. Exp. Biol. 1999, 37, 190–192. [Google Scholar] [PubMed]
- Miksik, I.; Deyl, Z. Change in the amount of epsilon-hexosyllysine, UV absorbance, and fluorescence of collagen with age in different animal species. J. Gerontol. 1991, 46, B111–B116. [Google Scholar] [CrossRef]
- Johansen, M.; Kiemer, L.; Brunak, S. Analysis and prediction of mammalian protein glycation. Glycobiology 2006, 16, 844–853. [Google Scholar] [CrossRef]
- Smalley, J.W.; Thomas, M.F.; Birss, A.J.; Withnall, R.; Silver, J. A combination of both arginine- and lysine-specific gingipain activity of Porphyromonas gingivalis is necessary for the generation of the micro-oxo bishaem-containing pigment from haemoglobin. Biochem. J. 2004, 379, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Birss, A.J.; Szmigielski, B.; Potempa, J. Sequential action of R-and K-specific gingipains of Porphyromonas gingivalis in the generation of the haem-containing pigment from oxyhaemoglobin. Arch. Biochem. Biophys. 2007, 465, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Birss, A.J.; Szmigielski, B.; Potempa, J. Mechanism of methemoglobin breakdown by the lysine-specific gingipain of the periodontal pathogen Porphyromonas gingivalis. Biol. Chem. 2008, 389, 1235–1238. [Google Scholar] [CrossRef]
- Ladner, R.C.; Heidner, E.J.; Perutz, M.F. The structure of horse methemoglobin at 2.0 Å resolution. J. Mol. Biol. 1977, 114, 385–414. [Google Scholar] [CrossRef]
- Roy, A.; Sen, S.; Chakraborti, A.S. In vitro nonenzymatic glycation enhances the role of myoglobin as a source of oxidative stress. Free Radic. Res. 2004, 38, 139–146. [Google Scholar] [CrossRef]
- Iwamoto, H.; Motomiya, Y.; Miura, K.; Morisawa, M.; Yoshimura, Y.; Maruyama, I. Immunochemical assay of hemoglobin with N(epsilon)-(carboxymethyl)lysine at lysine 66 of the beta chain. Clin. Chem. 2001, 47, 1249–1255. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, T.F.; Wu, C.H.; Chen, S.H. In-depth comparative characterization of hemoglobin glycation in normal and diabetic bloods by LC-MSMS. J. Am. Soc. Mass Spectrom. 2014, 25, 758–766. [Google Scholar] [CrossRef]
- Friess, U.; Kohne, E.; Lehmann, R.; Koch, S.; Haring, H.U.; Schmuelling, R.M.; Schleicher, E. Novel hemoglobin variant [beta66(E10) Lys→Asn], with decreased oxygen affinity, causes falsely low hemoglobin A1c values by HPLC. Clin. Chem. 2003, 49, 1412–1415. [Google Scholar] [CrossRef][Green Version]
- Rosa, J.; Labie, D.; Wajcman, H.; Boigne, J.M.; Cabannes, R.; Bierme, R.; Ruffie, J. Haemoglobin I Toulouse: Beta-66 (E 10) lys glu: A new abnormal haemoglobin with a mutation localized on the E 10 porphyrin surrounding zone. Nature 1969, 223, 190–191. [Google Scholar] [CrossRef]
- Gmiterek, A.; Klopot, A.; Wojtowicz, H.; Trindade, S.C.; Olczak, M.; Olczak, T. Immune response of macrophages induced by Porphyromonas gingivalis requires HmuY protein. Immunobiology 2016, 221, 1382–1394. [Google Scholar] [CrossRef]
- Sasaki, N.; Takeuchi, H.; Kitano, S.; Amano, A.; Matsusaki, M. Dynamic analysis of Porphyromonas gingivalis invasion into blood capillaries during the infection process in host tissues using a vascularized three-dimensional human gingival model. Biomater. Sci. 2021, 9, 6574–6583. [Google Scholar] [CrossRef]
- Iberg, N.; Fluckiger, R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J. Biol. Chem. 1986, 261, 13542–13545. [Google Scholar] [CrossRef]
- Hulmes, D.J.S. Building collagen molecules. J. Struct. Biol. 2002, 137, 2–10. [Google Scholar] [CrossRef]
- Alvarez, S.; Leiva-Sabadini, C.; Schuh, C.M.A.P.; Aguayo, S. Bacterial adhesion to collagens: Implications for biofilm formation and disease progression in the oral cavity. Crit. Rev. Microbiol. 2021, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Yagishita, H.; Yajima, A.; Okamoto, T.; Konishi, K. Molecular mechanism for connective tissue destruction by dipeptidyl aminopeptidase IV produced by the periodontal pathogen Porphyromonas gingivalis. Infect. Immun. 2005, 73, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Fleury, C.; Jalalvand, F.; Riesbeck, K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol. Rev. 2012, 36, 1122–1180. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, M.; Karunakaran, T.; Duncan, M.; Hamada, N.; Kuramitsu, H. Role of Arg-gingipain A in virulence of Porphyromonas gingivalis. Infect. Immun. 1998, 66, 1159–1166. [Google Scholar] [CrossRef]
- Sztukowska, M.; Sroka, A.; Bugno, M.; Banbula, A.; Takahashi, Y.; Pike, R.N.; Genco, C.A.; Travis, J.; Potempa, J. The C-terminal domains of the gingipain K polyprotein are necessary for assembly of the active enzyme and expression of associated activities. Mol. Microbiol. 2004, 54, 1393–1408. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Travis, J.; Potempa, J. Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-negative bacteria? J. Bacteriol. 2007, 189, 833–843. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Bras, G.; Chruscicka, B.; Karkowska-Kuleta, J.; Sroka, A.; Herwald, H.; Nguyen, K.A.; Eick, S.; Potempa, J.; Kozik, A. Adsorption of components of the plasma kinin-forming system on the surface of Porphyromonas gingivalis involves gingipains as the major docking platforms. Infect. Immun. 2011, 79, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Birss, A.J.; Withnall, R.; Silver, J. Interactions of Porphyromonas gingivalis with oxyhaemoglobin and deoxyhaemoglobin. Biochem. J. 2002, 362, 239–245. [Google Scholar] [CrossRef]
- Middle, F.A.; Bannister, A.; Bellingham, A.J.; Dean, P.D. Separation of glycosylated haemoglobins using immobilized phenylboronic acid. Effect of ligand concentration, column operating conditions, and comparison with ion-exchange and isoelectric focusing. Biochem. J. 1983, 209, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Glimcher, M.J.; Katz, E.P.; Travis, D.F. The solubilization and reconstitution of bone collagen. J. Ultrastruct. Res. 1965, 13, 163–171. [Google Scholar] [CrossRef]
- Bielecki, M.; Antonyuk, S.; Strange, R.W.; Sieminska, K.; Smalley, J.W.; Mackiewicz, P.; Smiga, M.; Cowan, M.; Capper, M.J.; Slezak, P.; et al. Prevotella intermedia produces two proteins homologous to Porphyromonas gingivalis HmuY but with different heme coordination mode. Biochem. J. 2020, 477, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Davies, K.J. A novel antioxidant role for hemoglobin. The comproportionation of ferrylhemoglobin with oxyhemoglobin. J. Biol. Chem. 1990, 265, 19453–19460. [Google Scholar] [CrossRef]
- Brown, J.L.; Yates, E.A.; Bielecki, M.; Olczak, T.; Smalley, J.W. Potential role for Streptococcus gordonii-derived hydrogen peroxide in heme acquisition by Porphyromonas gingivalis. Mol. Oral Microbiol. 2018, 33, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Ceriotti, F.; Ceriotti, G. Improved direct specific determination of serum iron and total iron-binding capacity. Clin. Chem. 1980, 26, 327–331. [Google Scholar] [CrossRef]
- Gao, J.L.; Nguyen, K.A.; Hunter, N. Characterization of a hemophore-like protein from Porphyromonas gingivalis. J. Biol. Chem. 2010, 285, 40028–40038. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997, 8, 2714–2723. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmiga, M.; Smalley, J.W.; Ślęzak, P.; Brown, J.L.; Siemińska, K.; Jenkins, R.E.; Yates, E.A.; Olczak, T. Glycation of Host Proteins Increases Pathogenic Potential of Porphyromonas gingivalis. Int. J. Mol. Sci. 2021, 22, 12084. https://doi.org/10.3390/ijms222112084
Śmiga M, Smalley JW, Ślęzak P, Brown JL, Siemińska K, Jenkins RE, Yates EA, Olczak T. Glycation of Host Proteins Increases Pathogenic Potential of Porphyromonas gingivalis. International Journal of Molecular Sciences. 2021; 22(21):12084. https://doi.org/10.3390/ijms222112084
Chicago/Turabian StyleŚmiga, Michał, John W. Smalley, Paulina Ślęzak, Jason L. Brown, Klaudia Siemińska, Rosalind E. Jenkins, Edwin A. Yates, and Teresa Olczak. 2021. "Glycation of Host Proteins Increases Pathogenic Potential of Porphyromonas gingivalis" International Journal of Molecular Sciences 22, no. 21: 12084. https://doi.org/10.3390/ijms222112084
APA StyleŚmiga, M., Smalley, J. W., Ślęzak, P., Brown, J. L., Siemińska, K., Jenkins, R. E., Yates, E. A., & Olczak, T. (2021). Glycation of Host Proteins Increases Pathogenic Potential of Porphyromonas gingivalis. International Journal of Molecular Sciences, 22(21), 12084. https://doi.org/10.3390/ijms222112084





