Bioprospecting Antimicrobials from Lactiplantibacillus plantarum: Key Factors Underlying Its Probiotic Action
Abstract
:1. Introduction
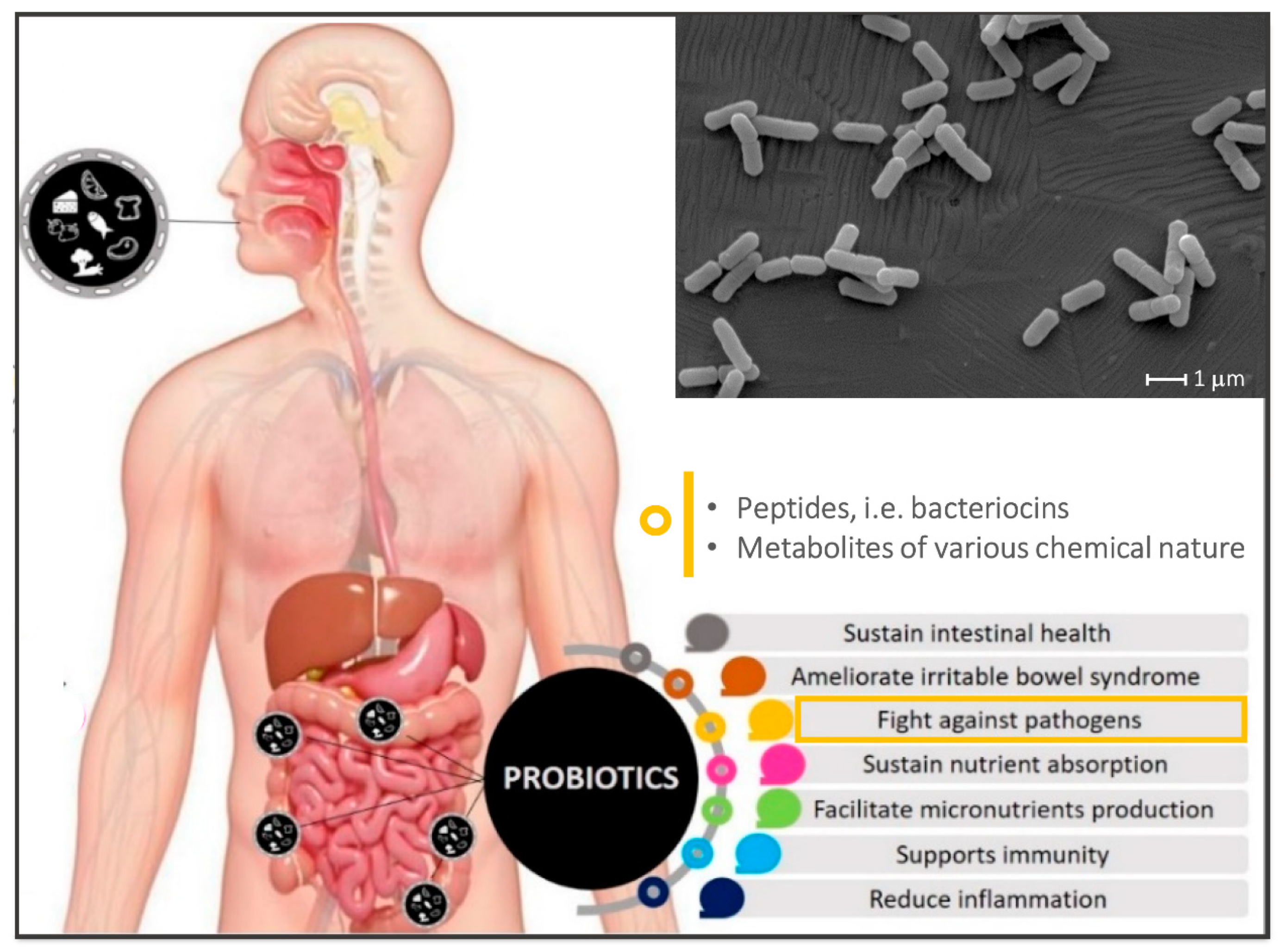
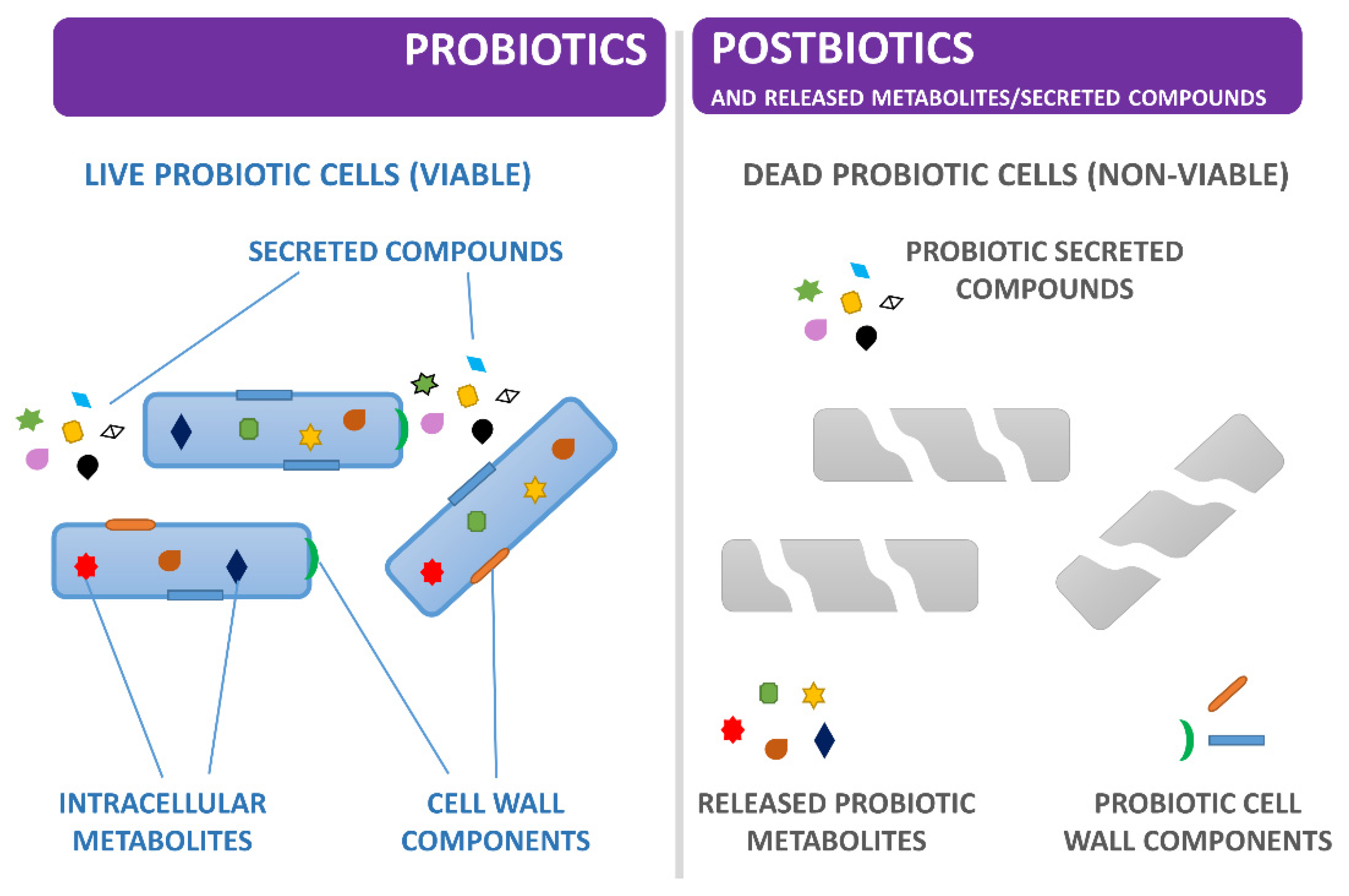
2. Emerging Trends in Probiosis, Postbiotics and Antimicrobials
3. Nature and Mechanisms of L. plantarum Antimicrobials
3.1. Bacteriocins
3.2. Organic Acids
3.3. Biosurfactants
4. Antibacterial and Antiviral Spectrum of L. plantarum Extracellular Compounds
5. In Vivo Studies on L. plantarum Strains Whose Antibacterial Activity Was Earlier Characterised In Vitro
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion of the panel on biological hazards on the request from EFSA on the maintenance of the list of QPS microorganisms intentionally added to food or feed. EFSA J. 2008, 928, 1–48. [Google Scholar]
- Gheziel, C.; Russo, P.; Arena, M.P.; Spano, G.; Ouzari, H.-I.; Kheroua, O.; Saidi, D.; Fiocco, D.; Kaddouri, H.; Capozzi, V. Evaluating the Probiotic Potential of Lactobacillus plantarum Strains from Algerian Infant Feces: Towards the Design of Probiotic Starter Cultures Tailored for Developing Countries. Probiotics Antimicrob. Proteins 2019, 11, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Fiocco, D.; Longo, A.; Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. How probiotics face food stress: They get by with a little help. Crit. Rev. Food Sci. Nutr. 2020, 60, 1552–1580. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Humaran, L.G.B.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Arena, M.P.; Russo, P.; Capozzi, V.; López, P.; Fiocco, D.; Spano, G. Probiotic abilities of riboflavin-overproducing Lactobacillus strains: A novel promising application of probiotics. Appl. Microbiol. Biotechnol. 2014, 98, 7569–7581. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. Reply to: Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 827–828. [Google Scholar] [CrossRef]
- Prabhurajeshwar, C.; Chandrakanth, K. Evaluation of antimicrobial properties and their substances against pathogenic bacteria in-vitro by probiotic Lactobacilli strains isolated from commercial yoghurt. Clin. Nutr. Exp. 2019, 23, 97–115. [Google Scholar] [CrossRef] [Green Version]
- Arena, M.P.; Capozzi, V.; Russo, P.; Drider, D.; Spano, G.; Fiocco, D. Immunobiosis and probiosis: Antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl. Microbiol. Biotechnol. 2018, 102, 9949–9958. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Fernández-Pacheco, P.; Seseña, S.; Pintado, C.; Palop, M.L. Selection of probiotic Lactobacillus strains with antimicrobial activity to be used as biocontrol agents in food industry. LWT-Food Sci. Technol. 2021, 143, 111142. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; de Chiara, M.L.V.; Amodio, M.L.; Brahimi, S.; Colelli, G.; Drider, D.; Spano, G.; Russo, P. Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms 2021, 9, 773. [Google Scholar] [CrossRef]
- Umu, Ö.C.; Bäuerl, C.; Oostindjer, M.; Pope, P.B.; Hernández, P.E.; Pérez-Martínez, G.; Diep, D. The Potential of Class II Bacteriocins to Modify Gut Microbiota to Improve Host Health. PLoS ONE 2016, 11, e0164036. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2019, 10, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yu, Y.; Garcia-Gutierrez, E.; Jin, X.; He, Y.; Wang, L.; Tian, P.; Liu, Z.; Zhao, J.; Zhang, H.; et al. Lactobacillus acidophilus JCM 1132 strain and its mutant with different bacteriocin-producing behaviour have various in situ effects on the gut microbiota of healthy mice. Microorganisms 2020, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef]
- Hernández-Aquino, S.; Miranda-Romero, L.A.; Fujikawa, H.; Maldonado-Simán, E.J.; Alarcón-Zuñiga, B. Antibacterial Activity of Lactic Acid Bacteria to Improve Shelf Life of Raw Meat. Biocontrol Sci. 2019, 24, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Fuochi, V.; Emma, R.; Furneri, P. Bacteriocins, A Natural Weapon Against Bacterial Contamination for Greater Safety and Preservation of Food: A Review. Curr. Pharm. Biotechnol. 2021, 22, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, C.; Donders, G.G.; Palmeira-de-Oliveira, R.; Queiroz, J.A.; Tomaz, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Express 2018, 8, 153. [Google Scholar] [CrossRef]
- Hertzberger, R.; Arents, J.; Dekker, H.L.; Pridmore, R.D.; Gysler, C.; Kleerebezem, M.; de Mattos, M.J.T. H2O2 production in species of the Lactobacillus acidophilus group: A central role for a novel NADH-dependent flavin reductase. Appl. Environ. Microbiol. 2014, 80, 2229–2239. [Google Scholar] [CrossRef] [Green Version]
- das Neves Selis, N.; de Oliveira, H.B.M.; Leão, H.F.; Dos Anjos, Y.B.; Sampaio, B.A.; Correia, T.M.L.; Almeida, C.F.; Pena, L.S.C.; Reis, M.M.; Brito, T.L.S.; et al. Lactiplantibacillus plantarum strains isolated from spontaneously fermented cocoa exhibit potential probiotic properties against Gardnerella vaginalis and Neisseria gonorrhoeae. BMC Microbiol. 2021, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gu, R.; Li, P.; Lu, Y.; Chen, L.; Gu, Q. Anti-Salmonella mode of action of natural l-phenyl lactic acid purified from Lactobacillus plantarum ZJ316. Appl. Microbiol. Biotechnol. 2020, 104, 5283–5292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Shi, Y.; Shen, F.; Wang, H. A new high phenyl lactic acid-yielding Lactobacillus plantarum IMAU10124 and a comparative analysis of lactate dehydrogenase gene. FEMS Microbiol. Lett. 2014, 356, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.; Ryan, A.; Hudson, S. Pre-formulation and delivery strategies for the development of bacteriocins as next generation antibiotics. Eur. J. Pharm. Biopharm. 2021, 165, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; ter Haar née Younes, J.A.; Heschl, A.; Mičetić Turk, D.; Rogelj, I. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. BioMed Res Int. 2019, 2019, 7585486. [Google Scholar] [CrossRef] [Green Version]
- Pop, O.L.; Pop, C.R.; Dufrechou, M.; Vodnar, D.C.; Socaci, S.A.; Dulf, F.V.; Minervini, F.; Suharoschi, R. Edible Films and Coatings Functionalization by Probiotic Incorporation: A Review. Polymers 2020, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar]
- Esposito, S.; Soto-Martinez, M.E.; Feleszko, W.; Jones, M.H.; Shen, K.-L.; Schaad, U.B. Nonspecific immunomodulators for recurrent respiratory tract infections, wheezing and asthma in children: A systematic review of mechanistic and clinical evidence. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 198–209. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Pankey GA, S.L. Clinical relevance of bacteriostatic versus bactericidal activity in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef]
- Maldonado, A.; Jiménez-Díaz, R.; Ruiz-Barba, J.L. Induction of plantaricin production in Lactobacillus plantarum NC8 after coculture with specific gram-positive bacteria is mediated by an autoinduction mechanism. J. Bacteriol. 2004, 186, 1556–1564. [Google Scholar] [CrossRef] [Green Version]
- Rojo-Bezares, B.; Sáenz, Y.; Navarro, L.; Zarazaga, M.; Ruiz-Larrea, F.; Torres, C. Coculture-inducible bacteriocin activity of Lactobacillus plantarum strain J23 isolated from grape must. Food Microbiol. 2007, 24, 482–491. [Google Scholar] [CrossRef]
- Man LL, X.D. LuxS-mediated quorum sensing system in Lactobacillus plantarum NMD-17 from koumiss: Induction of plantaricin MX in co-cultivation with certain lactic acid bacteria. Folia Microbiol. 2021, 66, 855–871. [Google Scholar] [CrossRef]
- Wu, A.; Fu, Y.; Kong, L.; Shen, Q.; Liu, M.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Production of a Class IIb Bacteriocin with Broad-spectrum Antimicrobial Activity in Lactiplantibacillus plantarum RUB1. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef]
- Marco, M.L.; Bongers, R.S.; De Vos, W.M.; Kleerebezem, M. Spatial and temporal expression of Lactobacillus plantarum genes in the gastrointestinal tracts of mice. Appl. Environ. Microbiol. 2007, 73, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Bove, P.; Russo, P.; Capozzi, V.; Gallone, A.; Spano, G.; Fiocco, D. Lactobacillus plantarum passage through an oro-gastro-intestinal tract simulator: Carrier matrix effect and transcriptional analysis of genes associated to stress and probiosis. Microbiol. Res. 2013, 168, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.; van Hemert, S.; Taverne, N.; Wels, M.; De Vos, P.; Bron, P.A.; Savelkoul, H.F.; van Bilsen, J.; Kleerebezem, M.; Wells, J.M. Identification of genetic loci in Lactobacillus plantarum that modulate the immune response of dendritic cells using comparative genome hybridization. PLoS ONE 2010, 5, e10632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hemert, S.; Meijerink, M.; Molenaar, D.; Bron, P.A.; de Vos, P.; Kleerebezem, M.; Wells, J.M.; Marco, M.L. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- Golneshin, A.; Gor, M.C.; Williamson, N.; Vezina, B.; Van, T.T.H.; May, B.K.; Smith, A.T. Discovery and characterisation of circular bacteriocin plantacyclin B21AG from Lactiplantibacillus plantarum B21. Heliyon 2020, 6, e04715. [Google Scholar] [CrossRef]
- Tenea, G.N. Peptide Extracts from Native Lactic Acid Bacteria Generate Ghost Cells and Spheroplasts upon Interaction with Salmonella enterica, as Promising Food Antimicrobials. BioMed Res. Int. 2020, 2020, 6152356. [Google Scholar] [CrossRef]
- Arief, I.I.; Budiman, C.; Jenie, B.S.L.; Andreas, E.; Yuneni, A. Plantaricin IIA-1A5 from Lactobacillus plantarum IIA-1A5 displays bactericidal activity against Staphylococcus aureus. Benef. Microbes 2015, 6, 603–613. [Google Scholar] [CrossRef]
- Kim, S.W.; Kang, S.I.; Shin, D.H.; Oh, S.Y.; Lee, C.W.; Yang, Y.; Son, Y.K.; Yang, H.S.; Lee, B.H.; An, H.J.; et al. Potential of cell-free supernatant from lactobacillus plantarum nibr97, including novel bacteriocins, as a natural alternative to chemical disinfectants. Pharmaceuticals 2020, 13, 266. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, X.; Zhou, Q.; Zou, J.; Li, P.; Breukink, E.; Gu, Q. Plantaricin NC8 from Lactobacillus plantarum causes cell membrane disruption to Micrococcus luteus without targeting lipid II. Appl. Microbiol. Biotechnol. 2018, 102, 7465–7473. [Google Scholar] [CrossRef]
- Tenea, G.N.; Pozo, T.D. Antimicrobial Peptides from Lactobacillus plantarum UTNGt2 Prevent Harmful Bacteria Growth on Fresh Tomatoes. J. Microbiol. Biotechnol. 2019, 29, 1553–1560. [Google Scholar] [CrossRef]
- Tenea, G.N.; Guaña, J.M. Inhibitory substances produced by native Lactobacillus plantarum UTNCys5-4 control microbial population growth in meat. J. Food Qual. 2019, 2019, 9516981. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, R.S. Antimicrobial-induced DNA damage and genomic instability in microbial pathogens. PLoS Pathog. 2015, 11, e1004678. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.S.; Todorov, S.D.; Ivanova, I.V.; Belguesmia, Y.; Choiset, Y.; Rabesona, H.; Chobert, J.M.; Haertlé, T.; Franco, B.D.G.M. Characterization of a two-peptide plantaricin produced by Lactobacillus plantarum MBSa4 isolated from Brazilian salami. Food Control 2016, 60, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Milioni, C.; Martínez, B.; Degl’Innocenti, S.; Turchi, B.; Fratini, F.; Cerri, D.; Fischetti, R. A novel bacteriocin produced by Lactobacillus plantarum LpU4 as a valuable candidate for biopreservation in artisanal raw milk cheese. Dairy Sci. Technol. 2015, 95, 479–494. [Google Scholar] [CrossRef]
- Atrih, A.; Rekhif, N.; Moir, A.J.; Lebrihi, A.; Lefebvre, G. Mode of action, purification and amino acid sequence of plantaricin C19, an anti-Listeria bacteriocin produced by Lactobacillus plantarum C19. Int. J. Food Microbiol. 2001, 68, 93–104. [Google Scholar] [CrossRef]
- Hernández, D.; Cardell, E.; Zarate, V. Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: Initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarum TF711. J. Appl. Microbiol. 2005, 99, 77–84. [Google Scholar] [CrossRef]
- Mohapatra, A.R.; Jeevaratnam, K. Inhibiting bacterial colonization on catheters: Antibacterial and antibiofilm activities of bacteriocins from Lactobacillus plantarum SJ33. J. Glob. Antimicrob. Resist. 2019, 19, 85–92. [Google Scholar] [CrossRef]
- Rumjuankiat, K.; Perez, R.H.; Pilasombut, K.; Keawsompong, S.; Zendo, T.; Sonomoto, K.; Nitisinprasert, S. Purification and characterization of a novel plantaricin, KL-1Y, from Lactobacillus plantarum KL-1. World J. Microbiol. Biotechnol. 2015, 31, 983–994. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y.; Sun, Y.; Gu, Q. Purification and characterisation of plantaricin ZJ008, a novel bacteriocin against Staphylococcus spp. from Lactobacillus plantarum ZJ008. Food Chem. 2014, 165, 216–223. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Hao, Y.; Zhong, S.; Liu, H.; Han, T.; Xie, Y. Isolation and partial characterization of a bacteriocin produced by Lactobacillus plantarum BM-1 isolated from a traditionally fermented Chinese meat product. Microbiol. Immunol. 2013, 57, 746–755. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Zhang, H.; Jin, J.; Zhang, H. Quantitative proteomic analysis reveals the influence of plantaricin BM-1 on metabolic pathways and peptidoglycan synthesis in Escherichia coli K12. PLoS ONE 2020, 15, e0231975. [Google Scholar] [CrossRef]
- Ong, J.S.; Taylor, T.D.; Wong, C.B.; Khoo, B.Y.; Sasidharan, S.; Choi, S.B.; Ohno, H.; Liong, M.T. Extracellular transglycosylase and glyceraldehyde-3-phosphate dehydrogenase attributed to the anti-staphylococcal activity of Lactobacillus plantarum USM8613. J. Biotechnol. 2019, 300, 20–31. [Google Scholar] [CrossRef]
- de Freire Bastos, M.D.C.; Coelho, M.L.V.; da Silva Santos, O.C. Resistance to bacteriocins produced by gram-positive bacteria. Microbiology 2015, 161, 683–700. [Google Scholar] [CrossRef] [Green Version]
- Rashid, R.; Veleba, M.; Kline, K.A. Focal targeting of the bacterial envelope by antimicrobial peptides. Front. Cell Dev. Biol. 2016, 4, 55. [Google Scholar] [CrossRef]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Fighting biofilms with lantibiotics and other group of bacteriocins. NPJ Biofilms. Microbiomes 2018, 4, 9. [Google Scholar]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S.; Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; et al. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołozyn-Krajewska, D. Comparison of Antibacterial Activity of Lactobacillus plantarum Strains Isolated from Two Different Kinds of Regional Cheeses from Poland: Oscypek and Korycinski Cheese. BioMed Res. Int. 2017, 2017, 6820369. [Google Scholar] [CrossRef] [Green Version]
- Tejerosariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: Evidence for the effects of organic acids. Anaerobe 2012, 18, 530–538. [Google Scholar] [CrossRef]
- Stratford, M.; Eklund, T. Organic acids and esters. In Food Preservatives; Springer: Boston, MA, USA, 2003. [Google Scholar]
- Li, X.; Xu, W.; Yang, J.; Zhao, H.; Pan, C.; Ding, X.; Zhang, Y. Effects of applying lactic acid bacteria to the fermentation on a mixture of corn steep liquor and air-dried rice straw. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2016, 2, 229–233. [Google Scholar] [CrossRef]
- Axelsson, L.; Salminen, S.; von Wright, A. (Eds.) Lactic Acid Bacteria: Microbiology and Functional Aspects, 2nd ed.; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Thu, T.V.; Foo, H.L.; Loh, T.C.; Bejo, M.H. Inhibitory activity and organic acid concentrations of metabolite combinations produced by various strains of Lactobacillus plantarum. Afr. J. Biotechnol. 2013, 10, 1359–1363. [Google Scholar]
- Saelim, K.; Jampaphaeng, K.; Maneerat, S. Functional properties of Lactobacillus plantarum S0/7 isolated fermented stinky bean (Sa Taw Dong) and its use as a starter culture. J. Funct. Foods 2017, 38, 370–377. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Presser, K.A.; Ratkowsky, D.A.; Ross, T. Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl. Environ. Microbiol. 1997, 63, 2355–2360. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.H.; Pan, T.M. Characterization of an antimicrobial substance produced by Lactobacillus plantarum NTU 102. J. Microbiol. Immunol. Infect. 2019, 52, 409–417. [Google Scholar] [CrossRef]
- Matsuo, T.; Nakamura, K.; Kodama, T.; Mikami, T.; Hiyoshi, H.; Tsuchiya, T.; Ogawa, W.; Kuroda, T. Characterization of all RND-type multidrug efflux transporters in Vibrio parahaemolyticus. Microbiologyopen 2013, 2, 725–742. [Google Scholar] [CrossRef]
- Wu, W.; Deng, G.; Liu, C.; Gong, X.; Ma, G.; Yuan, Q.; Yang, E.; Li, X.; Luo, Y. Optimization and Multiomic Basis of Phenyllactic Acid Overproduction by Lactobacillus plantarum. J. Agric. Food Chem. 2020, 68, 1741–1749. [Google Scholar] [CrossRef]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef]
- Dao, Y.; Zhang, K.; Lu, X.; Lu, Z.; Liu, C.; Liu, M.; Luo, Y. Role of Glucose and 2-Oxoglutarate/Malate Translocator (OMT1) in the Production of Phenyllactic Acid and p-Hydroxyphenyllactic Acid, Two Food-Borne Pathogen Inhibitors. J. Agric. Food Chem. 2019, 67, 5820–5826. [Google Scholar] [CrossRef]
- Dieuleveux, V.; Lemarinier, S.; Gueguen, M. Antimicrobial spectrum and target site of D-3-phenyllactic acid. Int. J. Food Microbiol. 1998, 40, 177–183. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.; Yan, A.; Yang, K.; Wang, Z.; Li, X.; Jia, Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef]
- Sorrentino, E.; Tremonte, P.; Succi, M.; Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Sturchio, M.; Coppola, R. Detection of antilisterial activity of 3- phenyllactic acid using Listeria innocua as amodel. Front Microbiol. 2018, 9, 1373–1381. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Surface physiological changes induced by lactic acid on pathogens in consideration of pKa and pH. Food Control 2014, 46, 525–531. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Lei, P.; Xin, X.; Liu, D.; Yi, H. Isolation, purification, identification, and discovery of the antibacterial mechanism of ld-phenyllactic acid produced by Lactiplantibacillus plantarum CXG9 isolated from a traditional Chinese fermented vegetable. Food Control 2022, 132, 108490. [Google Scholar] [CrossRef]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [CrossRef]
- Singh, S.S.; Akhtar, M.N.; Sharma, D.; Mandal, S.M.; Korpole, S. Characterization of Iturin V, a Novel Antimicrobial Lipopeptide from a Potential Probiotic Strain Lactobacillus sp. M31. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef]
- Vecino, X.; Rodríguez-López, L.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L. Bioactivity of glycolipopeptide cell-bound biosurfactants against skin pathogens. Int. J. Biol. Macromol. 2018, 109, 971–979. [Google Scholar] [CrossRef]
- Madhu, A.N.; Prapulla, S.G. Evaluation and functional characterization of a biosurfactant produced by Lactobacillus plantarum CFR 2194. Appl. Biochem. Biotechnol. 2014, 172, 1777–1789. [Google Scholar] [CrossRef]
- Sakr, E.A.E.; Ahmed, H.A.E.; Abo Saif, F.A.A. Characterization of low-cost glycolipoprotein biosurfactant produced by Lactobacillus plantarum 60 FHE isolated from cheese samples using food wastes through response surface methodology and its potential as antimicrobial, antiviral, and anticancer activi. Int. J. Biol. Macromol. 2021, 170, 94–106. [Google Scholar] [CrossRef]
- Sharma, D.; Saharan, B.S.; Chauhan, N.; Bansal, A.; Procha, S. Production and structural characterization of Lactobacillus helveticus derived biosurfactant. Sci. World J. 2014, 2014, 493548. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, A.; Moosavi-Nasab, M.; Behzadnia, A.; Rezaei, M. Enhanced biosurfactant production with low-quality date syrup by Lactobacillus rhamnosus using a fed-batch fermentation. Food Sci. Biotechnol. 2018, 27, 1137–1144. [Google Scholar] [CrossRef]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, anti-adhesive and anti-biofilm potential of biosurfactants isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Khare, P.; Sharma, S.; Rajarammohan, S.; Bishnoi, M.; Bhadada, S.K.; Sharma, S.S.; Kaur, J.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 18597. [Google Scholar] [CrossRef]
- Karina, S.; Euston, E.S. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem. 2019, 85, 143–155. [Google Scholar]
- Sambanthamoorthy, K.; Feng, X.; Patel, R.; Patel, S.; Paranavitana, C. Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiol. 2014, 14, 197. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.C.; Caggianiello, G.; van Swam, I.I.; Taverne, N.; Meijerink, M.; Bron, P.A.; Spano, G.; Kleerebezem, M. Strain-Specific Features of Extracellular Polysaccharides and Their Impact on Lactobacillus plantarum-Host Interactions. Appl. Environ. Microbiol. 2016, 82, 3959–3970. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef] [Green Version]
- Mahdhi, A.; Leban, N.; Chakroun, I.; Bayar, S.; Mahdouani, K.; Majdoub, H.; Kouidhi, B. Use of extracellular polysaccharides, secreted by Lactobacillus plantarum and Bacillus spp., as reducing indole production agents to control biofilm formation and efflux pumps inhibitor in Escherichia coli. Microb. Pathog. 2018, 125, 448–453. [Google Scholar] [CrossRef]
- Song, Y.; Sun, M.; Feng, L.; Liang, X.; Song, X.; Mu, G.; Tuo, Y.; Jiang, S.; Qian, F. Antibiofilm Activity of Lactobacillus plantarum 12 Exopolysaccharides against Shigella flexneri. Appl. Environ. Microbiol. 2020, 86, e00694-20. [Google Scholar] [CrossRef]
- Onbas, T.; Osmanagaoglu, O.; Kiran, F. Potential Properties of Lactobacillus plantarum F-10 as a Bio-control Strategy for Wound Infections. Probiotics Antimicrob. Proteins 2019, 11, 1110–1123. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Min, Z.; Xiaona, H.; Aziz, T.; Jian, Z.; Zhennai, Y. Exopolysaccharides from Lactobacillus plantarum YW11 improve immune response and ameliorate inflammatory bowel disease symptoms. Acta Biochim. Pol. 2020, 67, 485–493. [Google Scholar]
- Zhou, X.; Zhang, K.; Qi, W.; Zhou, Y.; Hong, T.; Xiong, T.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 Enhances Colonic Mucosal Homeostasis by Controlling Epithelial Cell Differentiation and c-Jun/Muc2 Signaling. J. Agric. Food Chem. 2019, 67, 9831–9839. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, L.; Jia, K.; Zhan, H.; Zhang, Z.; Shah, N.P.; Tao, X.; Wei, H. Sulfonation of Lactobacillus plantarum WLPL04 exopolysaccharide amplifies its antioxidant activities in vitro and in a Caco-2 cell model. J. Dairy Sci. 2019, 102, 5922–5932. [Google Scholar] [CrossRef]
- Sun, M.; Liu, W.; Song, Y.; Tuo, Y.; Mu, G.; Ma, F. The Effects of Lactobacillus plantarum-12 Crude Exopolysaccharides on the Cell Proliferation and Apoptosis of Human Colon Cancer (HT-29) Cells. Probiotics Antimicrob. Proteins 2021, 13, 413–421. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Du, H.; Yang, J.; Lu, X.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lu, F. Purification, Characterization, and Mode of Action of Plantaricin GZ1-27, a Novel Bacteriocin against Bacillus cereus. J. Agric. Food Chem. 2018, 66, 4716–4724. [Google Scholar] [CrossRef]
- Schlech, W.F., III. Foodborne listeriosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 31, 770–775. [Google Scholar] [CrossRef]
- Arrioja-Bretón, D.; Mani-López, E.; Bach, H.; López-Malo, A. Antimicrobial activity of protein-containing fractions isolated from Lactobacillus plantarum NRRL B-4496 culture. Braz. J. Microbiol. 2020, 51, 1289–1296. [Google Scholar] [CrossRef]
- Twomey, E.; Hill, C.; Field, D.; Begley, M. Recipe for Success: Suggestions and Recommendations for the Isolation and Characterisation of Bacteriocins. Int. J. Microbiol. 2021, 2021, 9990635. [Google Scholar] [CrossRef]
- Park, D.M.; Bae, J.H.; Kim, M.S.; Kim, H.; Kang, S.D.; Shim, S.; Lee, D.; Seo, J.H.; Kang, H.; Han, N.S. Suitability of Lactobacillus plantarum SPC-SNU 72-2 as a Probiotic Starter for Sourdough Fermentation. J. Microbiol. Biotechnol. 2019, 29, 1729–1738. [Google Scholar] [CrossRef]
- Milillo, S.R.; Friedly, E.C.; Saldivar, J.C.; Muthaiyan, A.; O’Bryan, C.; Crandall, P.G.; Johnson, M.G.; Ricke, S.C. A review of the ecology, genomics, and stress response of Listeria innocua and Listeria monocytogenes. Crit. Rev. Food Sci. Nutr. 2012, 52, 712–725. [Google Scholar] [CrossRef]
- Dhanasekara, C.S.; Marschke, B.; Morris, E.; Kahathuduwa, C.N.; Dissanaike, S. Global patterns of necrotizing soft tissue infections: A systematic review and meta-analysis. Surgery 2021. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, D.; Xia, X.; Zhang, K.; Adil, R.M.; Batool, Z.; Wang, J. Five major two components systems of Staphylococcus aureus for adaptation in diverse hostile environment. Microb. Pathog. 2021, 159, 105119. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Jesser, K.J.; Levy, K. Updates on defining and detecting diarrheagenic Escherichia coli pathotypes. Curr. Opin. Infect. Dis. 2020, 33, 372–380. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yi, H.; Han, X.; Chi, C. Identification and characterization of plantaricin Q7, a novel plantaricin produced by Lactobacillus plantarum Q7. LWT-Food Sci. Technol. 2016, 71, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Li, P.; Gu, Q. Heterologous expression and purification of plantaricin NC8, a two-peptide bacteriocin against Salmonella spp. from Lactobacillus plantarum ZJ316. Protein Expr. Purif. 2016, 127, 28–34. [Google Scholar] [CrossRef]
- Sihombing, D.E.; Arief, I.I.; Budiarti, S. Application of antimicrobial agents produced by Lactobacillus plantarum IIA-1A5 as natural preservative on beef during room temperature storage. Adv. J. Food Sci. Technol. 2015, 8, 251–255. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Q.; Li, P.; Li, Y.; Song, D.; Yang, J. Purification and characterization of plantaricin ZJ316, a novel bacteriocin against listeria monocytogenes from lactobacillus plantarum ZJ316. J. Food Prot. 2018, 81, 1929–1935. [Google Scholar] [CrossRef]
- de Jong, H.K.; Parry, C.M.; van der Poll, T.; Wiersinga, W.J. Host-pathogen interaction in invasive Salmonellosis. PLoS Pathog. 2012, 8, e1002933. [Google Scholar] [CrossRef] [Green Version]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; Zavistanaviciute, P.; Starkute, V.; Zokaityte, E.; Lele, V.; Dauksiene, A.; Grashorn, M.; et al. Study of the antibiotic residues in poultry meat in some of the EU countries and selection of the best compositions of lactic acid bacteria and essential oils against Salmonella enterica. Poult. Sci. 2020, 99, 4065–4076. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.K. Enterobacter sakazakii infections among neonates, infants, children, and adults. Case reports and a review of the literature. Medicine 2001, 80, 113–122. [Google Scholar] [CrossRef]
- Bowen, A.B.; Braden, C. Invasive Enterobacter sakazakii disease in infants. Emerg. Infect. Dis. 2006, 12, 1185–1189. [Google Scholar] [CrossRef] [Green Version]
- Gales, A.C.; Jones, R.N.; Turnidge, J.; Rennie, R.; Ramphal, R. Characterization of Pseudomonas aeruginosa isolates: Occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32, S146–S155. [Google Scholar] [CrossRef] [Green Version]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef]
- Lei, S.; Zhao, R.; Sun, J.; Ran, J.; Ruan, X.; Zhu, Y. Partial purification and characterization of a broad-spectrum bacteriocin produced by a Lactobacillus plantarum zrx03 isolated from infant’s feces. Food Sci. Nutr. 2020, 8, 2214–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, J.; Li, X.; Han, H.; Tao, Y. Purification and characterization of plantaricin SLG1, a novel bacteriocin produced by Lb. plantarum isolated from yak cheese. Food Control 2018, 84, 111–117. [Google Scholar] [CrossRef]
- Zhao, S.; Han, J.; Bie, X.; Lu, Z.; Zhang, C.; Lv, F. Purification and Characterization of Plantaricin JLA-9: A Novel Bacteriocin against Bacillus spp. Produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a Traditional Chinese Fermented Cabbage. J. Agric. Food Chem. 2016, 64, 2754–2764. [Google Scholar] [CrossRef]
- Song, D.F.; Zhu, M.Y.; Gu, Q. Purification and characterization of plantaricin ZJ5, a new bacteriocin produced by Lactobacillus plantarum ZJ5. PLoS ONE 2014, 9, e105549. [Google Scholar] [CrossRef]
- Lin, C.J.; Pan, C.F.; Chuang, C.K.; Sun, F.J.; Wang, D.J.; Chen, H.H.; Liu, H.L.; Wu, C.J. P-cresyl sulfate is a valuable predictor of clinical outcomes in pre-ESRD patients. BioMed Res. Int. 2014, 2014, 526932. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sheoran, P.; Gupta, A.; Yadav, J.P.; Tiwari, S.K. Antibacterial property of bacteriocin produced by Lactobacillus plantarum LD4 isolated from a fermented food. Ann. Microbiol. 2016, 66, 1431–1440. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, H.; Zhang, C.; Yu, J.; Lu, Z. Purification and characterization of plantaricin 163, a novel bacteriocin produced by Lactobacillus plantarum 163 isolated from traditional Chinese fermented vegetables. J. Agric. Food Chem. 2013, 61, 11676–11682. [Google Scholar] [CrossRef]
- Choi, E.A.; Chang, H.C. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT-Food Sci. Technol. 2015, 62, 210–217. [Google Scholar] [CrossRef]
- Qian, Z.; Zhu, H.; Zhao, D.; Yang, P.; Gao, F.; Lu, C.; Yin, Y.; Kan, S.; Chen, D. Probiotic lactobacillus Sp. Strains inhibit growth, adhesion, biofilm formation, and gene expression of bacterial vaginosis-inducing gardnerella vaginalis. Microorganisms 2021, 9, 728. [Google Scholar] [CrossRef]
- Qian, Z.; Zhao, D.; Yin, Y.; Zhu, H.; Chen, D. Antibacterial Activity of Lactobacillus Strains Isolated from Mongolian Yogurt against Gardnerella vaginalis. BioMed Res. Int. 2020, 2020, 3548618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, A.; Halami, P.M.; Tamang, J.P. Genome Analysis of Lactobacillus plantarum Isolated From Some Indian Fermented Foods for Bacteriocin Production and Probiotic Marker Genes. Front. Microbiol. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Rutsch, F.; Uekötter, A.; Kipp, F.; König, J.; Marquardt, T.; Peters, G.; von Eiff, C. Kocuria rhizophila adds to the emerging spectrum of micrococcal species involved in human infections. J. Clin. Microbiol. 2008, 46, 3537–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Jung, H.H.; Kim, Y.H.; Kim, T.H. Antiobesity Effects of Lactobacillus plantarum LMT1-48 Accompanied by Inhibition of Enterobacter cloacae in the Intestine of Diet-Induced Obese Mice. J. Med. Food 2019, 22, 560–566. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.K.; Liu, R.; Kwon, J.O.; Kim, M.K.; Kim, A.H.J.; Kang, S.O. Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza a virus. J. Microbiol. 2013, 51, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Sunmola, A.A.; Ogbole, O.O.; Faleye, T.O.C.; Adetoye, A.; Adeniji, J.A.; Ayeni, F.A. Antiviral potentials of Lactobacillus plantarum, Lactobacillus amylovorus, and Enterococcus hirae against selected Enterovirus. Folia Microbiol. 2019, 64, 257–264. [Google Scholar] [CrossRef]
- Wang, K.; Ran, L.; Yan, T.; Niu, Z.; Kan, Z.; Zhang, Y.; Yang, Y.; Xie, L.; Huang, S.; Yu, Q.; et al. Anti-TGEV Miller Strain Infection Effect of Lactobacillus plantarum Supernatant Based on the JAK-STAT1 Signaling Pathway. Front. Microbiol. 2019, 10, 2540. [Google Scholar] [CrossRef]
- Arena, M.P.; Elmastour, F.; Sane, F.; Drider, D.; Fiocco, D.; Spano, G.; Hober, D. Inhibition of coxsackievirus B4 by Lactobacillus plantarum. Microbiol. Res. 2018, 210, 59–64. [Google Scholar] [CrossRef]
- Sirichokchatchawan, W.; Temeeyasen, G.; Nilubol, D.; Prapasarakul, N. Protective Effects of Cell-Free Supernatant and Live Lactic Acid Bacteria Isolated from Thai Pigs Against a Pandemic Strain of Porcine Epidemic Diarrhea Virus. Probiotics Antimicrob. Proteins 2018, 10, 383–390. [Google Scholar] [CrossRef]
- Kim, K.; Lee, G.; Thanh, H.D.; Kim, J.H.; Konkit, M.; Yoon, S.; Park, M.; Yang, S.; Park, E.; Kim, W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. J. Dairy Sci. 2018, 101, 5702–5712. [Google Scholar] [CrossRef]
- Cantú-Bernal, S.; Domínguez-Gámez, M.; Medina-Peraza, I.; Aros-Uzarraga, E.; Ontiveros, N.; Flores-Mendoza, L.; Gomez-Flores, R.; Tamez-Guerra, P.; González-Ochoa, G. Enhanced Viability and Anti-rotavirus Effect of Bifidobacterium longum and Lactobacillus plantarum in Combination With Chlorella sorokiniana in a Dairy Product. Front. Microbiol. 2020, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Yi, D.Y.; Jo, S.; Lee, Y.M.; Kim, J.H.; Kim, W.; Park, M.R.; Yoon, S.M.; Kim, Y.; Yang, S.; et al. Effect of a new Lactobacillus plantarum product, LRCC5310, on clinical symptoms and virus reduction in children with rotaviral enteritis. Medicine 2020, 99, e22192. [Google Scholar] [CrossRef] [PubMed]
- Cave, R.; Cole, J.; Mkrtchyan, H.V. Surveillance and prevalence of antimicrobial resistant bacteria from public settings within urban built environments: Challenges and opportunities for hygiene and infection control. Environ. Int. 2021, 157, 106836. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Huang, H.C.; Lee, I.J.; Huang, C.; Chang, T.M. Lactic Acid Bacteria and Lactic Acid for Skin Health and Melanogenesis Inhibition. Curr. Pharm. Biotechnol. 2020, 21, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.N.; Sesto Cabral, M.E.; Noseda, D.; Bosch, A.; Yantorno, O.M.; Valdez, J.C. Antipathogenic properties of Lactobacillus plantarum on Pseudomonas aeruginosa: The potential use of its supernatants in the treatment of infected chronic wounds. Wound Repair Regen. 2012, 20, 552–562. [Google Scholar] [CrossRef]
- Beck, B.R.; Park, G.-S.; Lee, Y.H.; Im, S.; Jeong, D.Y.; Kang, J. Whole genome analysis of Lactobacillus plantarum strains isolated from kimchi and determination of probiotic properties to treat mucosal infections by Candida albicans and Gardnerella vaginalis. Front. Microbiol. 2019, 10, 433. [Google Scholar] [CrossRef]
- Vicariotto, F.; Mogna, L.; Del Piano, M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: A pilot study. J. Clin. Gastroenterol. 2014, 48, 106–112. [Google Scholar] [CrossRef]
- Zhai, Q.; Yu, L.; Li, T.; Zhu, J.; Zhang, C.; Zhao, J.; Zhang, H.; Chen, W. Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie VanLeeuwenhoek 2017, 110, 501–513. [Google Scholar] [CrossRef]
- Zang, L.; Ma, Y.; Huang, W.; Ling, Y.; Sun, L.; Wang, X.; Zeng, A.; Dahlgren, R.A.; Wang, C.; Wang, H. Dietary Lactobacillus plantarum ST-III alleviates the toxic effects of triclosan on zebrafish (Danio rerio) via gut microbiota modulation. Fish Shellfish. 2019, 84, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; Molina-Infante, J.; Lucendo, A.J.; Calleja, J.L.; Pérez-Aisa, Á.; Modolell, I.; Aldeguer, X.; Calafat, M.; Comino, L.; Ramas, M.; et al. Probiotic supplementation with Lactobacillus plantarum and Pediococcus acidilactici for Helicobacter pylori therapy: A randomized, double-blind, placebo-controlled trial. Helicobacter 2018, 23, e12529. [Google Scholar] [CrossRef]
- Kujawa-Szewieczek, A.; Adamczak, M.; Kwiecień, K.; Dudzicz, S.; Gazda, M.; Więcek, A. The effect of Lactobacillus plantarum 299v on the incidence of Clostridium difficile infection in high risk patients treated with antibiotics. Nutrients 2015, 7, 10179–10188. [Google Scholar] [CrossRef]
- Vicariotto, V. Effectiveness of an association of a cranberry dry extract, D-mannose, and the two microorganisms Lactobacillus plantarum LP01 and Lactobacillus paracasei LPC09 in women affected by cystitis: A pilot study. J. Clin. Gastroenterol. 2014, 48, S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Fuentes, M.C.; Audivert, S.; Bonachera, M.A.; Peiró, S.; Cuñé, J. Lactobacillus plantarum CECT 7527, 7528 and 7529: Probiotic candidates to reduce cholesterol levels. J. Sci. Food Agric. 2014, 94, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014, 39, 1276–1285. [Google Scholar]
- Miraghajani, M.; Dehsoukhteh, S.S.; Rafie, N.; Hamedani, S.G.; Sabihi, S.; Ghiasvand, R. Potential mechanisms linking probiotics to diabetes: A narrative review of the literature. Sao Paulo Med. J. 2017, 135, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Folwarski, M.; Witkowski, J.M.; Bryl, E.; Makarewicz, W. The role of Lactobacillus plantarum 299v in supporting treatment of selected diseases. Cent. Eur. J. Immunol. 2020, 45, 488–493. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Siezen, R.J.; van Hylckama Vlieg, J.E.T. Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb. Cell Fact. 2011, 10, S3. [Google Scholar] [CrossRef] [Green Version]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in vivo Models. Front. Microbiol. 2021, 12, 630695. [Google Scholar] [CrossRef]
- Fu, T.; Liu, Y.M. Antibacterial effect of bacteriocin isolated from lactobacillus plantarum ATCC 8014 on postoperative infection of mandibular fracture in vivo. J. Craniofac. Surg. 2017, 28, 679–682. [Google Scholar] [CrossRef]
- Xu, Z.; Li, J.; Zhou, X.; Dai, J.; Zhang, J.; Huang, Y.; Xu, N. The combined use of tea polyphenols and lactobacillus plantarum ST8SH bacteriocin in a rabbit model of infection following femoral fracture with internal fixation. Med. Sci. Monit. 2019, 25, 312–317. [Google Scholar] [CrossRef]
- Fu, T.; Yu, M.; Yan, Q.; Liu, Y.M. Bacteriocin Isolated from Lactobacillus Rhamnosus L34 Has Antibacterial Effects in a Rabbit Model of Infection After Mandible Fracture Fixation. Med. Sci. Monit. 2018, 24, 8009–8014. [Google Scholar] [CrossRef] [PubMed]
- Valdéz, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigon, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, E.G.; Moreira, D.A.; Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Tavaria, F.K. Topical application of probiotics in skin: Adhesion, antimicrobial and antibiofilm in vitro assays. J. Appl. Microbiol. 2017, 122, 450–461. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, J.; Baek, J.; Kim, W. Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus chungangensis CAU 1447 on Diabetic Mice. Nutrients 2021, 13, 2666. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, W.F.; Deane, S.M.; Dicks, L.M.T. Bacteriocin production and adhesion properties as mechanisms for the anti-listerial activity of Lactobacillus plantarum 423 and Enterococcus mundtii ST4SA. Benef. Microbes 2019, 10, 329–349. [Google Scholar] [CrossRef]
- Sharafi, H.; Maleki, H.; Ahmadian, G.; Zahiri, H.S.; Sajedinejad, N.; Houshmand, B.; Vali, H.; Noghabi, K.A. Antibacterial activity and probiotic potential of Lactobacillus plantarum HKN01: A new insight into the morphological changes of antibacterial compound-treated Escherichia coli by electron microscopy. J. Microbiol. Biotechnol. 2013, 23, 225–236. [Google Scholar] [CrossRef]
- Sunanliganon, C.; Thong-Ngam, D.; Tumwasorn, S.; Klaikeaw, N. Lactobacillus plantarum B7 inhibits Helicobacter pylori growth and attenuates gastric inflammation. World J. Gastroenterol. 2012, 18, 2472–2480. [Google Scholar] [CrossRef]
- Chomwong, S.; Charoensapsri, W.; Amparyup, P.; Tassanakajon, A. Two host gut-derived lactic acid bacteria activate the proPO system and increase resistance to an AHPND-causing strain of Vibrio parahaemolyticus in the shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2018, 89, 54–65. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; de Vos, W.M. Next-generation therapeutic bacteria for treatment of obesity, diabetes, and other endocrine diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101504. [Google Scholar] [CrossRef] [PubMed]

| Isolation Niche | Strain Name | Type of Antimicrobial | Investigated Action Mechanism | Strong Antimicrobial Activity/ Inhibited Bacterial Species | Reference |
|---|---|---|---|---|---|
| Fermented cocoa | Lp 1 03, Lp 289, Lp 291 | Organic acid (lactic acid) | n.i. 2 | Gardnerella vaginalis, Neisseria gonorrhoeae | das Neves Selis N, 2021 |
| Yoghurt fermented by koumiss | Lp RUB1 | Class II bacteriocin | n.i. | Bacillus cereus ATCC 14579 | Wu A, 2021 |
| Cheese | Lp 60FHE | Biosurfactant: glycoprotein | Cell membrane lysis | Staphylococcus epidermidis ATCC 12228, Microcccus luteus ATCC 10240, Escherichia coli ATCC10536, Pseudomonas aeruginosa ATCC 9027, Salmonella typhimurium, Enterobacter aerogenes 9805, Serratia marcescens 98027, Staphylococcus aureus ATCC 29737, Bacillus. pumilis ATCC 14884, Bacillus subtilis | Sakr AE, 2021 |
| Ghanaian traditionally fermented cow milk | Lp NL27 Lp PA27 | CFS 3 | n.i. | E. coli, S. Typhimurium | Motey GA, 2021 |
| Indonesian traditional fermented meat | Lp S34 | Plantaricin S34 | n.i. | Enteropathogenic E. coli (EPEC) K1.1., S. aureus, Salmonella typhosa, S typhimurium, Proteus sp. | Ahaddin AY, 2021 |
| Nem ‘chua’ (vietnamese sausage) | Lp B21 | Plantacyclin B21AG | Deduced by comparisons with other circular bacteriocins using multiple sequence alignment: insertion into the phospholipid bilayer of the target cell membrane | Clostridium perfringens 52/6-1, Listeria monocytogenes 192/1-2 ACM 3173 | Golneshin A, 2020 |
| Kimchi | Lp NIBR97 | Plantaricin 3, 5 | Cellular lysis via pore formation in bacterial membranes by cellular penetrating peptides | Salmonella enterica Serovar Enteritidis | Kim SW, 2020 |
| Sauerkraut | Lp SF9C | Plantaricin | n.i. | L. monocytogenes ATCC® 19111™, S. aureus 3048, S. enterica serovar Typhimurium FP1, E. coli 3014 | Butorac K, 020 |
| Kimchi | Lp EM | Plantaricin and bovicin | n.i. | Vibrio parahaemolyticus ATCC 17802, P. aeruginosa, S. enterica serovar Typhi, B. cereus | Kim E, 2020 Choi EA, 2015 |
| Yoghurt | Lp ZX27 | Plantaricin CFS | n.i. Reduction in G. vaginalis biofilm formation and preformed biofilm; suppressing the expression of genes related to G. vaginalis pathogenicity | E. coli, G. vaginalis | Qian Z, 2020 Qian Z, 2021 |
| Intestines of a turbot | Lp-12 | EPSs 4 | Inhibition of biofilm formation | Shigella flexneri | Song Y, 2020 |
| ‘Dahi’, a fermented milk product | Lp DHCU70, Lp DKP1 | NC8 type of bacteriocin | Inhibition of cell wall biosynthesis | Kocuria rhizophila | Goel A, 2020 |
| Infant’s feces | Lp zrx03 | Bacteriocin | n.i. | S. aureus ATCC 25923, E. coli JM109 ATCC 67387, B. subtilis CICC 10002, Bacillus anthracis CICC 20443, Salmonella CMCC 541 | Lei S, 2020 |
| Human oral cavities | Lp 108 | CFS | Inhibited growth and biofilm formation by preventing microbial coaggregation; inhibit the adhesion of Streptococcus mutans and Candida albicans to solid surfaces | Streptococcus mutans UA159 | Srivastava N, 2020 |
| Slovak raw sheep milk cheese | Lp L5, L19, L20, and L22 | Partially purified bacteriocins | n.i. | L. monocytogenes, S. aureus | Vataščinová T, 2020 |
| Weaned piglet faeces | Lp ZA3 | lactic acid and acetic acid | n.i. | Enterotoxigenic E. coli (ETEC) K88 | Wang W, 2020 |
| Stool human samples | Lp 69.1 | CFS | n.i. | ETEC and Enteroaggregative E. coli (EAEC) | Pazhoohan M, 2020 |
| Faeces of healthy infants | Lp 34-5 | CFS (pH acid) | n.i | S. flexneri ATCC 12022, ETEC H10407 enteropathogenic bacteria | Pazhoohan M, 2020 |
| Wild-type fruits of Theobroma grandiflorum (white coffee), and Malus sp. | Lp UTNGt2, Lp UTNCys5-4 | Gt2 peptides, Cys5-4 peptide | Cell membrane disruption and leaking of cytoplasmic β-galactosidase, RNA and DNA molecules. Binding and interacting with pathogen genomic DNA | S. enterica subsp. enterica ATCC 51741, E. coli ATCC 25922, Shigella sonnei ATCC 25931 | Tenea GN, 2020, 2019a, 2019b |
| Faeces of infants | Lp N20 | Organic acid | n.i. | Yersinia enterocolitica ATCC 23715, S. flexneri ATCC 12022, S.enterica ATCC 9270, enteropathogenic E. coli (EPEC) ATCC 43887 | Jomehzadeh N, 2020 |
| Kimchi | Lp KU200656 | CFS | Downregulation of the expression of pathogen’s biofilm-related genes | S. aureus ATCC 6538, L. monocytogenes ATCC 15313, E. coli ATCC 25922 | Lee JE, 2020 |
| Honey | Lp H46, H47, and H59 | CFS | n.i | S. flexneri ATCC 12022, S. aureus ATCC 25923, S. enteritidis F17, EPEC E2348/69, E. coli O157 H7 EDL 933, B. cereus D14 | Lashani E, 2020 |
| Faeces of healthy infants | Lp ZJ316 | L-PLA 5 Plantaricin ZJ316 Plantaricin NC8 | Membrane destruction and DNA binding n.i. Cell membrane permeabilization and disruption | S.enterica subsp. enterica ATCC 14028. L. monocytogenes, Listeria welshimeri, E. coli JM109, Pseudomonas putida ATCC 23288, S. enterica ZJJK18. S. enterica, S. typhimurium, Salmonella paratyphi-A, S. paratyphi-B, Micrococcus luteus CGMCC 1.193, V. parahaemolyticus, Staphylococcus epidermidis | Zhou Q, 2020 Chen L, 2018 Jiang H, 2018 Jiang H, 2016 |
| Sauerkraut | Lp NRRL B-4496 | Proteinaceous compound CFS (acid) | n.i | L. monocytogenes Methicillin resistant S. aureus (MRSA), L. monocytogenes, E. coli | Arrioja-Bretón D, 2020 |
| Pork minced meat | Lp USM8613 | Transglycosylase and glyceraldehyde-3-phosphate dehydrogenase (GADPH) | Cell wall-mediated killing mechanism; GADPH penetrates into S. aureus cells, inducing the overexpression of autolysis regulators | S. aureus | Ong JS, 2019 |
| Vaginal microbiota | Lp GF011 | CFS (acid pH) | n.i. | Uropathogens: S. aureus sp. GF01, P. aeruginosa GF01, Klebsiella sp. GF01 | ADEOSHUN FG, 2019 |
| Yoghurt, Fermentation of millet and urum | Lp P1, S11, and M7 | Organic acid (lactic, acetic, tartaric and malic acids) | n.i. | E. coli and S. typhimurium | Hu CH, 2019 |
| Kimchi | Lp SPC-SNU 72-2 | Organic acid | n.i | E. coli O157, L. monocytogenes, S. typhimurium, H. pylori | Park DM, 2019 |
| Tarkhineh human faeces Lighvan cheese | Lp PT10 Lp PF11 Lp PL4 | Bacteriocins | n.i | E. coli O157:H7, S. typhimurium | Joghataei M, 2019 |
| Kimchi | Lp LMT1-48 | SCFA 6 (hypothesised) | n.i | E. cloacae | Choi WJ, 2019 |
| Sorghum beer Fruits and vegetables from Pakistan | Lp 423 Lp AS-4, AS-14 | Plantaricin 423 | n.i n.i. | L. monocytogenes Listeria innocua, E. coli EC10, L. monocytogenes DPC 6179 | van Zyl WF, 2019 Manzoor A, 2019 |
| NIQCH (Brazil) | Lp ATCC 8014 | CFS (pH acid) Bacteriocin Bacteriocin | n.i n.i Growth inhibitory activity against planktonic cells; inhibition of biofilm formation | Clostridium butyricum, Clostridium difficile, C. perfringens S. aureus, S. marcenses | Monteiro CRM, 2019 Fu T, 2017 Shahandashti RV, 2016 |
| Artisanal milk cheese | Lp 27172 | Biosurfactants | Inhibits adhesion and biofilm formation by interfering with AI-2 signalling molecules and reducing expression of biofilm-related genes | S. aureus CMCC 26003 | Yan X, 2019 |
| Pineapple | Lp NRIC 149 | Plantaricin 149 | Carpet-like model of interaction with Gram + membrane | Listeria and Staphylococcus genera | Kumagai PS, 2019 |
| Faeces of healthy humans | Lp PBS067 | Plantaricin P1053 | n.i. | S. aureus, E. coli | De Giani A, 2019 |
| Koumiss | Lp MXG-68 | Plantaricin MXG-68 | Bactericidal mode of action | L. monocytogenes ATCC 15313, B. cereus ATCC 11788, E. coli ATCC 25922, and S. typhimurium ATCC 14028. | Man L, 2019 |
| MTCC | Lp subsp. argentoratensis SJ33 | Bacteriocin F1 and F2 | Bactericidal activity on S. aureus by membrane pore formation and leakage of cellular contents; antibiofilm activity for P. aeruginosa | P. aeruginosa and S. aureus, Aeromonas hydrophila, Clostridium sporogenes, C. perfringens, E. coli, Klebsiella pneumoniae | Mohapatra AR, 2019 |
| Faeces of breastfed infant | Lp F-10 | CFS (acid pH), EPSs | Reduced quorum-sensing signals needed for biofilm formation, CFS might modify the target surface, causing a reduction or inhibition of irreversible attachment of the biofilm-forming bacteria that prevent biofilm formation | P. aeruginosa PAO1/ATCC 27853, MRSA ATCC 43300 | Onbas T, 2019 |
| Papaya | Lp ST16Pa | Bacteriocin ST16Pa | Cell lysis and enzymes leakage | L. innocua, Latilactobacillus sakei, Enterococcus faecalis | Sabo SS, 2019; Todorov SV, 2011 |
| Cabbage pickles | Lp NTU 102 | LPB102 7 | Suppression of resistance nodulation cell division (RND)-type efflux transporter genes | V. parahaemolyticus, Cronobacter sakazakii | Lin T, 2019 |
| Yoghurt | Lp DM 69 | Protein (MW 12.0 kDa) Proteinaceous compound | Inhibited adhesion and invasion of S. enterica into colon cells | S. enterica subsp. enterica ATCC 35640 B. cereus ATCC 10702, S. aureus subsp. aureus ATCC 29213, S. aureus MTCC 902, P. aeruginosa MTCC 741, Klebsiella pneumonia MTCC 109 | Mohanty DP, 2019 Mohanty DP, 2016 |
| Fish | Lp LPL-1 | Bacteriocin LPL-1 | Increases membrane permeability, induces collapse of proton motive force, inhibits expression of genes related to virulence factors, biofilm formation factors, and RNA polymerase sigma factor | L. monocytogenes 54002 | Wang Y, 2019 and 2018 |
| Ricotta cheese | Lp L899 | EPSs | Inhibition of biofilm and efflux pumps | E. coli ATCC 35218 | Mahdhi A, 2018 |
| Salted and fermented shrimp | Lp FB003 | CFS | n.i. | L. monocytogenes, S. aureus, Salmonella enterica serotype Choleraesuis, V. parahaemolyticus | Le B, 2018 |
| Shrimp gut | Lp SGLAB01 | CFS | Modulation of the host proPO 8 system | Aerococcus viridans, Vibrio harveyi, S. aureus, Bacillus megaterium, Bacillus subtilis, E. coli, V. parahaemolyticus | Chomwong S, 2018 |
| Yak cheese | LP SLG1 | Plantaricin SLG1 | Bactericidal mode of action, it damages cell membrane and induces the release of cytoplasmic components | B. subtilis, B. cereus, B. megaterium, M. luteus, Brochothrix thermosphacta, C. butyricum, S. aureus, L. innocua, L. monocytogenes, E. coli, P. aeruginosa, Enterobacter cloacae and Salmonella paratyphi b | Pei J, 2018 |
| Fermented chinese milk | Lp J23 | Bacteriocin Lac-B23 | n.i. | L. monocytogenes | Zhang J, 2018 |
| Dong-nationality kipper | Lp GZ1-27 | Plantaricin GZ1-27 | Increased cell membrane permeability, triggered K+ leakage and pore formation, damaged cell membrane integrity, reduced expression of genes related to cytotoxin production, peptidoglycan synthesis, and cell division | B. cereus | Du H, 2018 |
| Sai krok e-san mu | Lp SKI19 | BLIS | n.i. | L. monocytogenes DMST 17303, B. cereus DMST 5040, C. perfringens DMST 1663, S. aureus DMST 8840, E. coli DMST 4212, S. Typhimurium DMST 15674, S. enteritidis DMST 15676 | Botthoulath V, 2018 |
| Cabbage | Lp DL3 | Plantaricin DL3 | Disruption of pathogen cell wall and leakage of proteins | P. aeruginosa, L. monocytogenes, Shewanella putrefaciens, Psychrobacter sp., S. aureus, B. cereus, Bacillus licheniformis, P. fuorescens | Lv X, 2018 |
| Olive | Lp NI326 | Plantaricyclin A (PlcA) | n.i. | Alicyclobacillus acidoterrestris, Lactococcus lactis spp., Lactobacillus bulgaricus UCC, Pediococcus inopinatus 1011 | Borrero J, 2018 |
| Fermented stinky bean | Lp S0/7 | Organic acids | Lowering cytoplasmic pH of target pathogens | E. coli DMST4212, S. aureus DMST8840, B. cereus DMST5040, L. monocytogenes DMST17303 | Saelim K, 2017 |
| Human breast milk | Lp WLPL04 | EPSs | Inhibition of the biofilm formation or modification of the bacterial cell surfaces | P. aeruginosa CMCC10104, E. coli O157:H7, S. Typhimurium ATCC 13311, and S. aureus CMCC26003 | Liu Z, 2017 |
| Shpek, bulgarian salami | Lp ST8Sh | Bacteriocin ST8SH (pediocin PA-1 family) | Pathogen’s cell lysis and intracellular material leakage | L. monocytogenes Scott A, Enterococcus faecalis ATCC 19433 S. aureus | Todorov SD, 2016 and 2017 |
| Salami | Lp MBSa4 | Plantaricin W | Bacteriostatic: electrostatic interactions with cytoplasmic membranes of bacteria, binds to the cell surface, but not killing effect | L. monocytogenes, S. aureus ATCC 25923, Enterococcus hirae, Enterococcus faecium, L. innocua, L. welshimeri | Barbosa MS, 2016 |
| Yak yogurt | Lp Q7 | Plantaricin Q7 | n.i. | Pseudomonas fluorescens AS1.1802, P. putida AS1.1819, P, aeruginosa CICC 21636, L. monocytogenes ATCC 19111, S. aureus, E. coli ATCC 25922, S. flexneri ATCC 12022, Shigella sonnei ATCC 25931, S. enterica serovar typhimurium ATCC 14028 | Liu H, 2016 |
| Wine | Lp 105 Lp 106, Lp 107 Lp 119, Lp 32, Lp108 | CFS (pH acid) | n.i. | L. monocytogenes CECT 4032, E. coli O157:H7, S. Enteritidis CECT 409, S. aureus R1070, R1208, S1209, and S1220 | Arena MP, 2016 |
| Suan-Tsai: chinese fermented cabbage | Lp JLA-9 | Plantaricin JLA-9 | Inhibited growth by preventing the establishment of oxidative metabolism and disrupting membrane integrity in germinating spores of B. cereus | B. cereus, B. pumilus, B. megaterium, Bacillus coagulans, B. subtilis, Geobacillus stearothermophilus, Alicyclobacillus acidoterrestris, Paenibacillus polymyxa, C. difficile, C. perfringens, C. sporogenes, S. aureus, M. luteus, P. fluorescens, S. marcescens, E. coli, S. enteritidis, S. typhimurium, S. paratyphi A, S. paratyphi B, S. flexneri, Proteus mirabilis | Zhao S, 2016 |
| Kimchi | Lp K25 | Plantaricin K25 | Membrane surface disruption of the B. cereus cells, leakage and release of cellular contents | B. cereus, L. monocytogenes NCTC 10890 | Wen LS, 2016 |
| Dosa batter | Lp LD4 | bacteriocin LD4 | K+ ion efflux and pore-forming on membrane of M. luteus and E. coli cells | M. luteus, S. aureus, E. coli (urogenic), P. aeruginosa, S. typhi, Vibrio sp., E. cloacae, E. faecium | Kumar V, 2016 |
| Meat | Lp KL-1 | Plantaricin KL-1Y | Bactericidal activity without cell lysis | B. cereus JCM 2152T, S. enterica serovar Enteritidis DMST 17368, P. aeruginosa ATCC 15442, P. aeruginosa ATCC 9027, E. coli O157:H7, E. coli ATCC 8739, B. coagulans JCM 2257T, L. innocua ATCC 33090T, S. aureus TISTR 118 | Rumjuankiat K, 2015 |
| Indonesian beef | Lp IIA-IA5 | Plantaricin IIA-1A5 | Loss of membrane integrity, release of proteinaceous and genetic materials | S. aureus, Enteropathogenic E. coli K1, Shigella A33, Salmonella 38 | Sihombing DE, 2015 Arief II, 2015 |
| Kefir grains | Lp YW32 | EPSs | Concentration-dependent inhibitory effect on the biofilms’ formation | E. coli O157, S. flexneri CMCC, S. aureus AC1, S. typhimurium S50333 | Wang J, 2015 |
| Sheep-milk cheese | Lp U4 | Plantaricin LpU4 | Bacteriostatic mode of action and an enhanced activity at acidic pHs | E. faecalis JH2-2, MRSA | Milioni C, 2015 |
| Koshu vineyard | Lp 510 | Plantaricin Y | n.i. | L. monocytogenes BCRC 14845 | Chen Y, 2014 |
| Vaginal microbiota | Lp CMUL140 | bacteriocin-like inhibitory substances (BLIS) | n.i. | G. vaginalis CIP7074T, E. coli CIP103982, S. aureus ATCC 33862 | Al Kassaa I, 2014 |
| ‘Kanjika’ (ayurvedic rice-based fermented product) | Lp CFR 2194 | Biosurfactants | Cell membrane lysis; antiadhesive activity | E. coli ATCC 31705, E. coli MTCC 108, S. aureus F 722, Y. enterocolitica MTCC 859 | Madhu AN, 2014 |
| mustard | Lp ZJ5 | Plantaricin ZJ5 | n.i. | S. aureus CGMCC 1.128, L. plantarum, L. monocytogenes, B. subtilis, M. luteus, P. putida, E. coli, Shigella dysenteriae | Song DF, 2014 |
| Breast milk | Lp R315 | EPSs | n.i. | L. monocytogenes CMCC54007, S. aureus CGMCC26003, B. cereus ATCC 14579, S. typhimurium ATCC 1331, C. sakazakii ATCC 29544, S. sonnei ATCC 25931 | Li S, 2014 |
| Fresh milk | Lp ZJ008 | Plantaricin ZJ008 | Bactericidal mode of action, pores formation in the surface of cell membrane but not cell lysis | S. citreus LC5, S. carnosus LTH1502 MRSA D48, S. epidermidis Z80, Micrococcus luteus 10209, L. monocytogenes LM1, E. coli DH5α, S. flexneri DSM4782 | Zhu X, 2014 |
| Dairy | Lp HKN01 | bacteriocin-like | n.i. | E. coli (PTCC 1338), S. Typhimurium (ATCC 13311), K. pneumoniae (PTCC 1290) | Sharafi H, 2013 |
| Vegetable | Lp 163 | Plantaricin 163 | n.i. | S. aureus, B. cereus, L. monocytogenes, B. pumilus, E. coli, P. aeruginosa, and P. fluorescens, M. luteus, L. thermophilus, L. rhamnosus | Hu M, 2013 |
| Meat | Lp BM-1 | bacteriocin BM-1 | Bactericidal mode of action without cell membrane lysis | L. monocytogenes ATCC 54003, E. facealis AS 1.2984, L. pentosus ATCC 8041, L. plantarum F1, S. aureus ATCC6535, E. coli CDC85933, S. dysenteriae CMCC 51105 and S. enteritidis CMCC 50041 | Zhang H, 2013 |
| - | Lp ATCC 10241 | CFS | Prevents P. aeruginosa quorum-sensing; inhibition of biofilm formation; inhibited production of virulence factors (elastase, pyocyanin, rhamnolipids) | P. aeruginosa | Ramos AN, 2012 |
| Papaya | Lp ST16Pa | bacteriocin ST16Pa | Bactericidal mode of action, cell lysis and enzyme-leakage | L. innocua 2030C, L. sakei ATCC 15521, E. faecalis ATCC 19433 | Todorov, 2011 |
| Thai dyspeptic patient | Lp B7 | CFS (pH acid) | Inhibition of the pathogen’s urease activity and viability | Helicobacter pylori ATCC 43504 | Sunanliganon C, 2012 |
| Koumiss | Lp LB-B1 | pediocin LB-B1 | n.i. | L. monocytogenes, Lactobacillus spp, Streptococcus spp, Enterococcus spp, Pediococcus spp, E. coli | Xie Y, 2011 |
| Isolation Niche | Strain Name | Type of Antiviral | Mechanism | Strong Antiviral Activity/ Virus Inhibited | Reference |
|---|---|---|---|---|---|
| Kimchi | Lp 1 NIBR97 | Plantaricin 3 and 5 | Lysis through envelope collapse | HIV-based lentivirus, Influenza virus A/H3N2 | Kim SW, 2020 |
| - | Lp ATCC LP299v | Metabolites | n.i. | Rotavirus Wa | Bernal SC, 2020 |
| Animals faeces | Lp AA09a | CFS 2 | n.i. | Echovirus 7 (E7), E19 | Sunmola AA, 2019 |
| Piglet faeces | Lp-1s | CFS | n.i. | Transmissible gastroenteritis virus (TGEV) | Wang K, 2019 |
| Kimchi | Lp LRCC5310 | EPSs 3 | n.i. | Human rotavirus (HRV) | Kim K, 2018 |
| Wine | Lp UNIFG30 Lp UNIFG121 | CFS | n.i. | Enterovirus Coxsackievirus B4 | Arena MP, 2018 |
| Pig faeces | Lp 22F, 25F, 31F | CFS | n.i. | Porcine epidemic diarrhoea virus (PEDV) | Sirichokchatchawan W, 2018 |
| Kimchi | Lp LBP-K10 | Cyclic dipeptides | Conformational structures of cyclic dipeptides influence genes that cause viral infections | Influenza A (H3N2) virus | Kwak MK, 2013 |
| Strain Name | Nature of Antimicrobial Postbiotic | Some Pathogens Inhibited | Application | Reference |
|---|---|---|---|---|
| Lp 1 423 | plantaricin 423 | L. monocytogenes EGDe | Competitive exclusion of L. monocytogenes EGDe from the GIT of mice by plantaricin 423 | van Zyl WF, 2019 |
| Lp LMT1-48 | SCFA 2 (hypothesised) | E. cloacae | Antiobesity effects in an E. cloacae-induced high-fat diet (HFD)-fed animal obesity model | Choi WJ, 2019 |
| Lp ST8SH | Bacteriocin | S. aureus | Antibacterial activity in a rabbit model of femoral fracture with internal fixation | Xu Z, 2019 |
| Lp SGLAB01 | CFS 3 | V. parahaemolyticus | Modulation of the immune system and increase shrimp resistance to V. parahaemolyticus infection | Chomwong S, 2018 |
| Lp ATCC 8014 | Bacteriocin | S. aureus | Control post-operative infection of mandibular fracture in mice model | Fu T, 2017 |
| Lp HKN01 | Bacteriocin-like | E. coli (PTCC 1338), S. Typhimurium (ATCC 13311), K. pneumoniae (PTCC 1290) | Recovery of S. typhimurium- infected BALB/c mice | Sharafi H, 2013 |
| Lp B7 | CFS (pH acid) | H. pylori | Attenuate H. pylori-induced gastric inflammation in rat | Sunanliganon C, 2012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchetti, M.T.; Russo, P.; Capozzi, V.; Drider, D.; Spano, G.; Fiocco, D. Bioprospecting Antimicrobials from Lactiplantibacillus plantarum: Key Factors Underlying Its Probiotic Action. Int. J. Mol. Sci. 2021, 22, 12076. https://doi.org/10.3390/ijms222112076
Rocchetti MT, Russo P, Capozzi V, Drider D, Spano G, Fiocco D. Bioprospecting Antimicrobials from Lactiplantibacillus plantarum: Key Factors Underlying Its Probiotic Action. International Journal of Molecular Sciences. 2021; 22(21):12076. https://doi.org/10.3390/ijms222112076
Chicago/Turabian StyleRocchetti, Maria Teresa, Pasquale Russo, Vittorio Capozzi, Djamel Drider, Giuseppe Spano, and Daniela Fiocco. 2021. "Bioprospecting Antimicrobials from Lactiplantibacillus plantarum: Key Factors Underlying Its Probiotic Action" International Journal of Molecular Sciences 22, no. 21: 12076. https://doi.org/10.3390/ijms222112076
APA StyleRocchetti, M. T., Russo, P., Capozzi, V., Drider, D., Spano, G., & Fiocco, D. (2021). Bioprospecting Antimicrobials from Lactiplantibacillus plantarum: Key Factors Underlying Its Probiotic Action. International Journal of Molecular Sciences, 22(21), 12076. https://doi.org/10.3390/ijms222112076









