Co-Immobilization of Rhizopus oryzae and Candida rugosa Lipases onto mMWCNTs@4-arm-PEG-NH2—A Novel Magnetic Nanotube–Polyethylene Glycol Amine Composite—And Its Applications for Biodiesel Production
Abstract
:1. Introduction
2. Results and Discussion
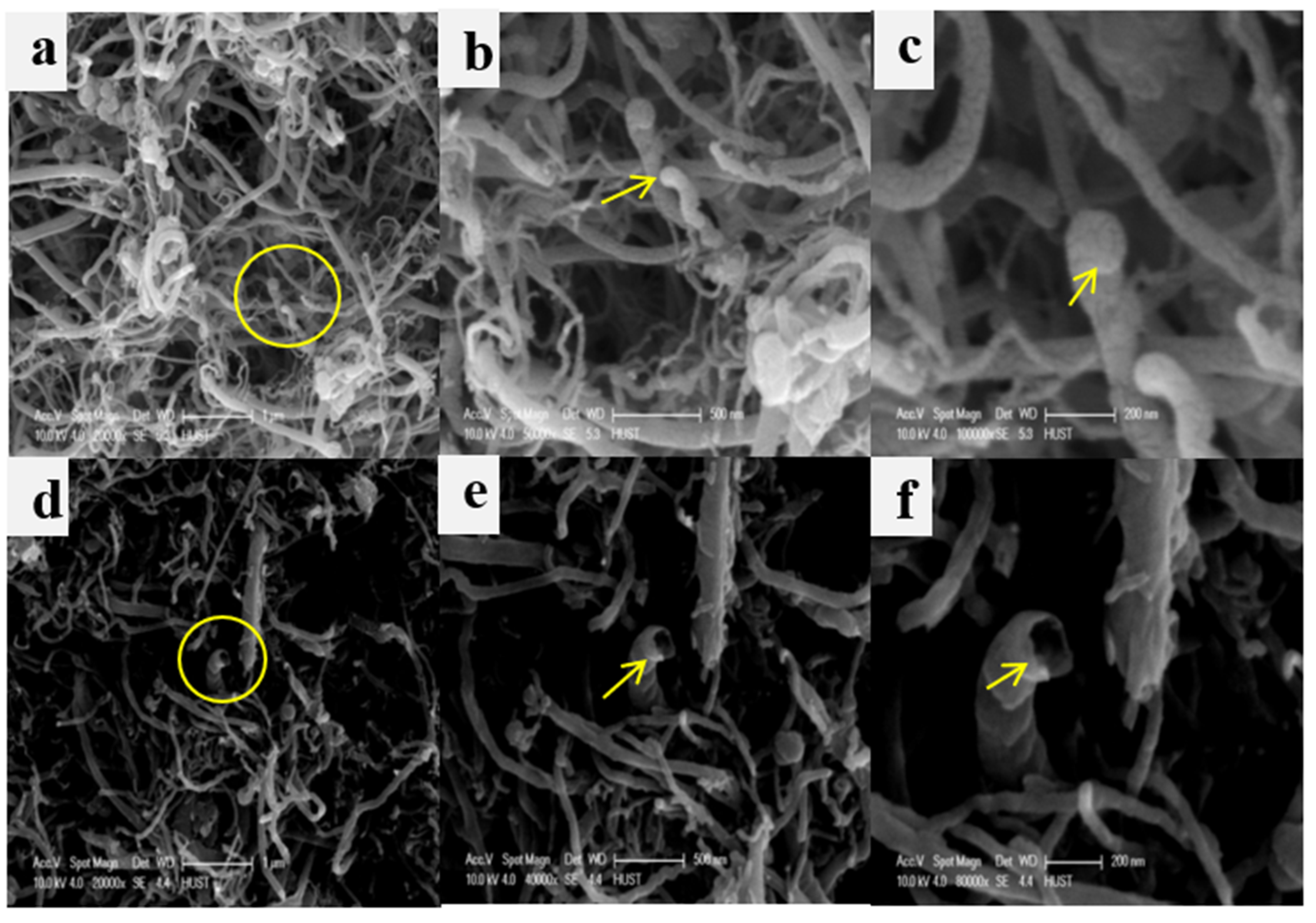
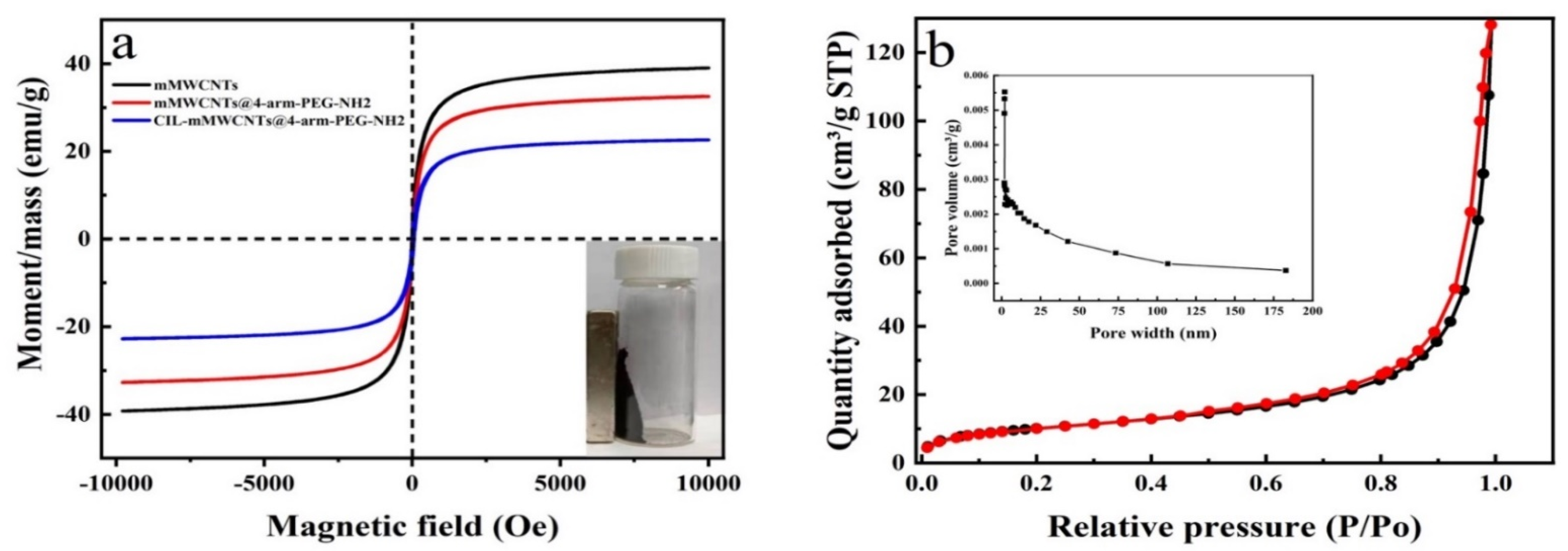
2.1. Design and Characterization of CIL-mMWCNTs@4-arm-PEG-NH2
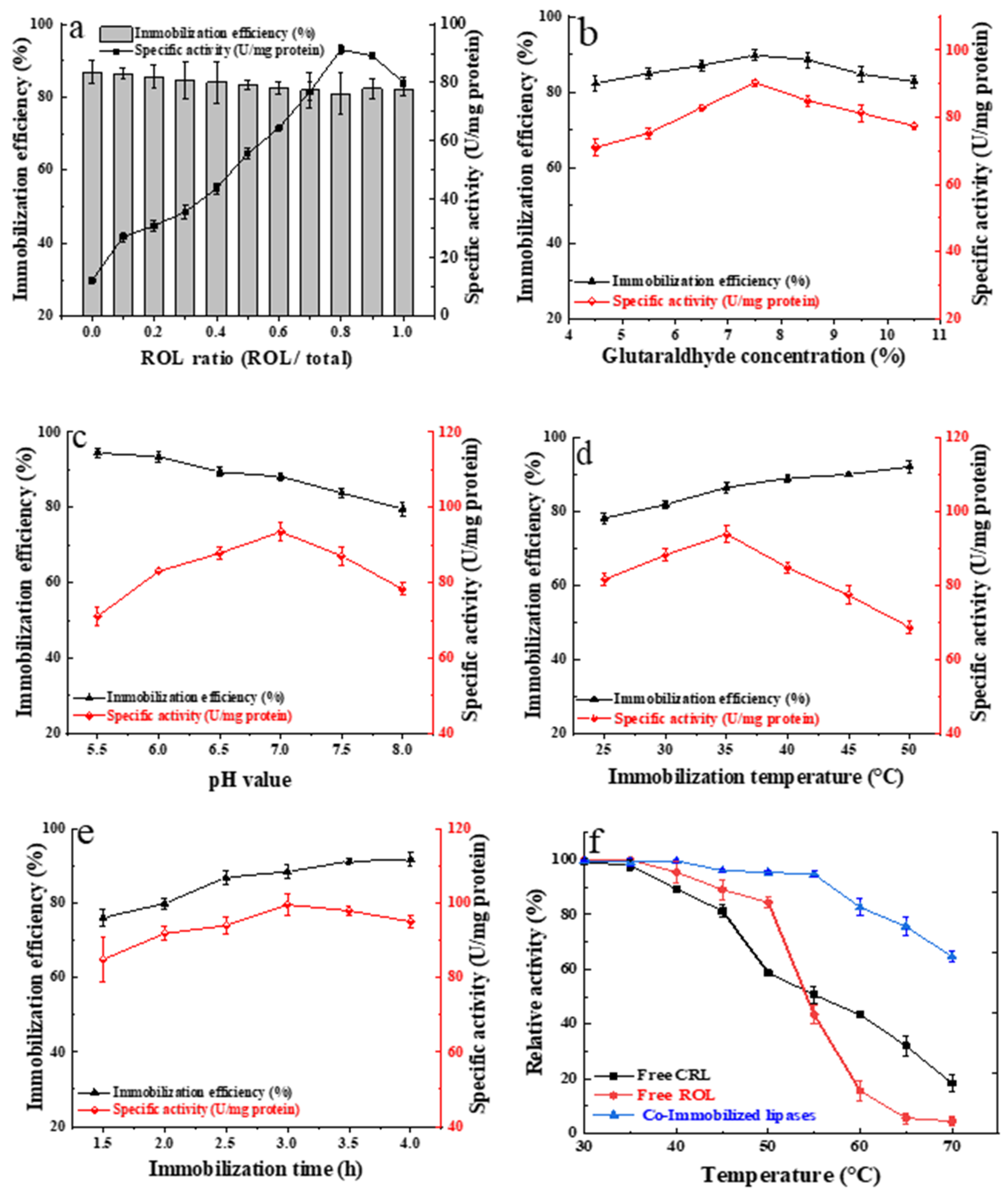
2.2. Co-Immobilization of ROL and CRL onto mMWCNTs@4-armPEG-NH2
2.3. Transesterification of WCO for Biodiesel Production Catalyzed by Single and Co-Immobilized Lipases onto mMWCNTs@4-arm-PEG-NH2
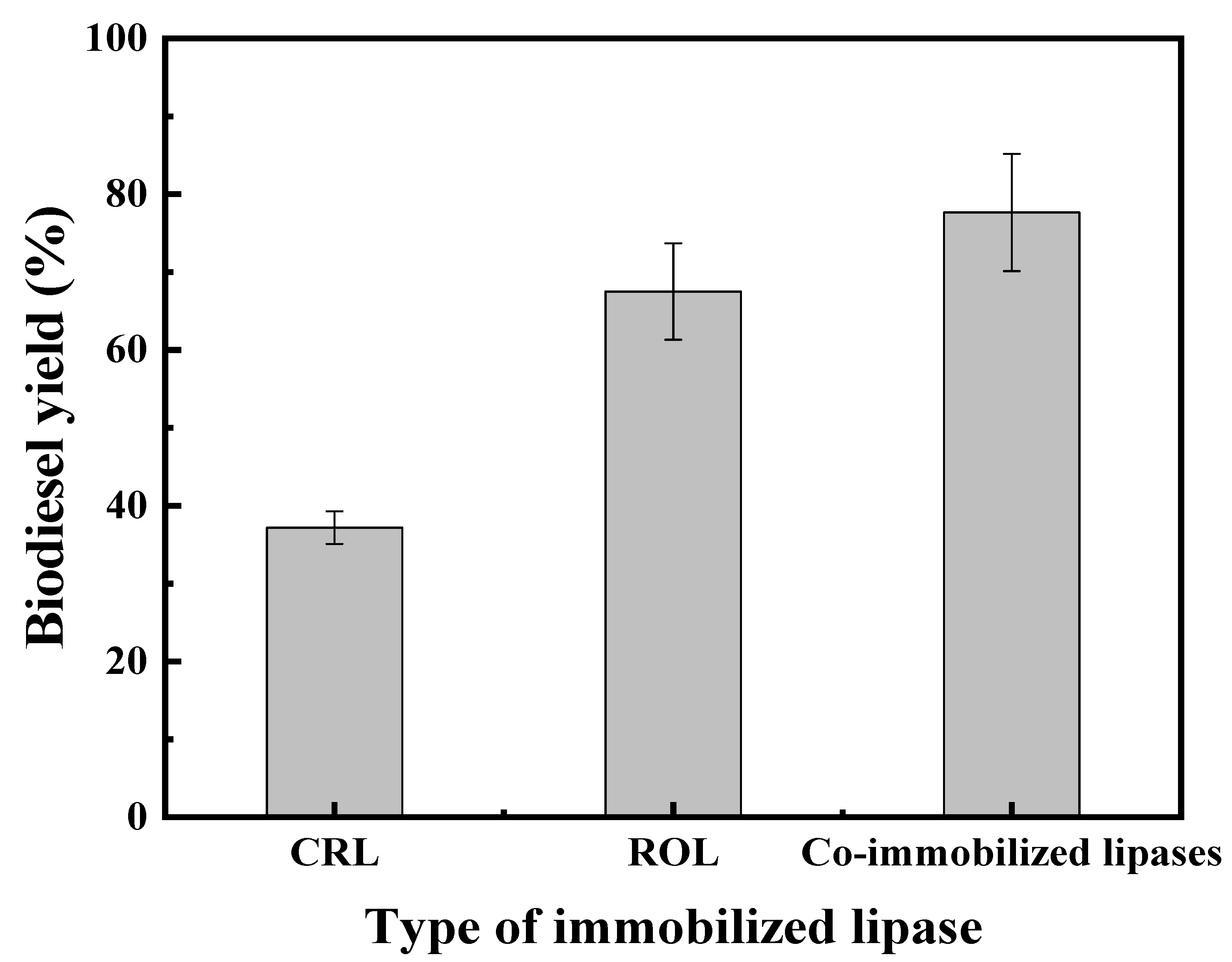
2.3.1. Effect of Single and Co-Immobilized Lipases on the Yield of Biodiesel
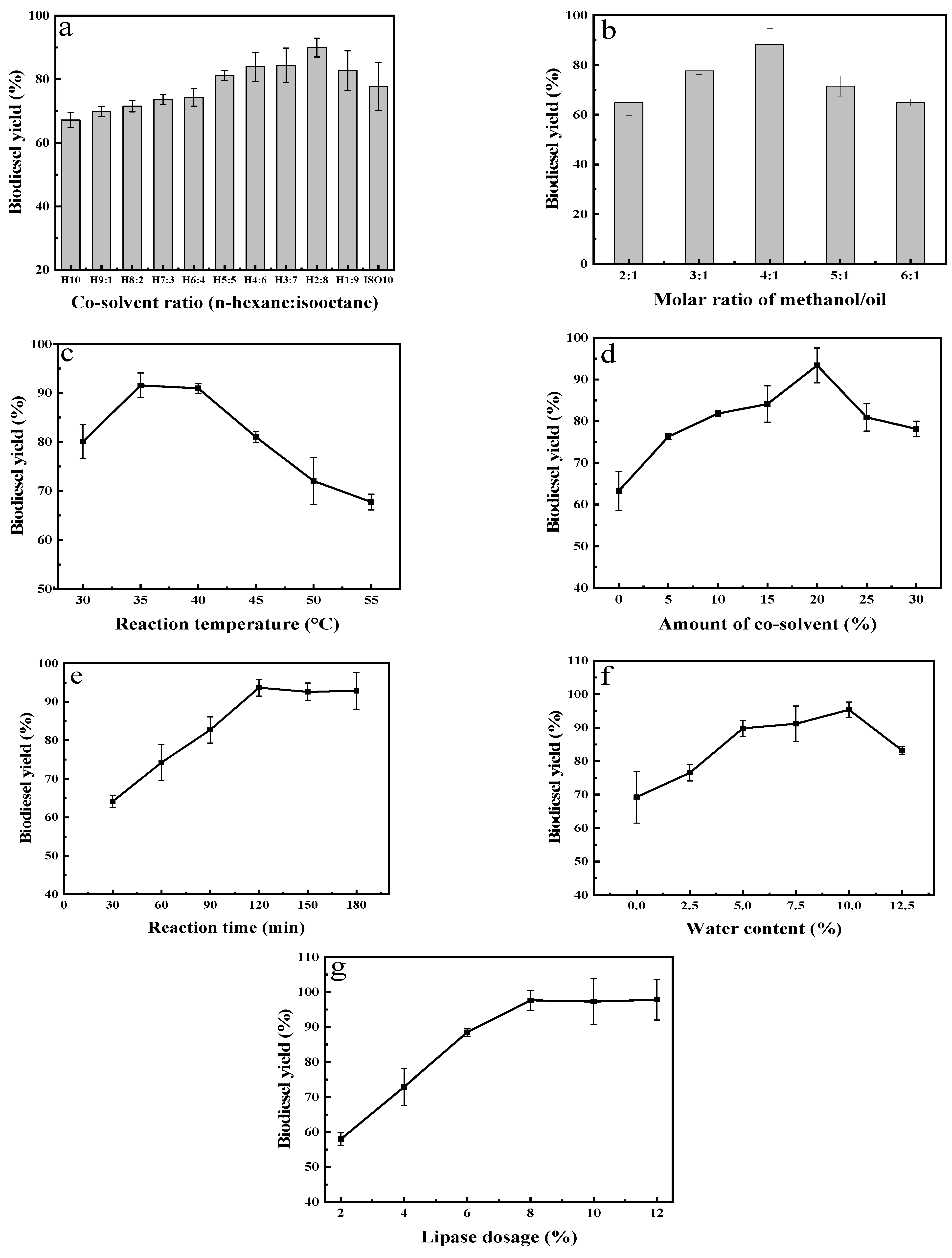
2.3.2. Effect of Different Transesterification Parameters on the Yield of Biodiesel
2.4. Reusability of Co-Immobilized Lipases–mMWCNTs@4-arm-PEG-NH2
2.5. Comparison of CIL-mMWCNTs@4-arm-PEG-NH2 and other Mixtures of Immobilized Lipases
| Lipase | Mixed Lipases Ratio | Type of Lipase | Substrate | System | Operating Conditions | Biodiesel Yield (%) | Reuse Cycles and Last Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Novozym 435: Lipozyme TL IM | 0.98:1.02 | Compound commercial immobilized lipases | Stillingia oil | Co-solvent acetonitrile: t-butanol | Biocatalyst (4.32%); methanol to oil ratio (6.4:1); co-solvent (40:60%); 40 °C; 200 rpm; 12 h | 96.38 | 30th cycle; ≥60 | [18] |
| R. oryzae: C. rugosa | 01:01 | Co-immobilized lipase onto silica gel support | Waste soybean oil | Solvent-free | Biocatalyst (20%); methanol as acyl acceptor; water content (10%); 45 °C; 250 rpm; 4 h | 97 | - | [53] |
| R. oryzae: C. rugosa | 04:01 | Combined lipase in liquid form | Rapeseed oil deodorizer distillates | Solvent-free | Biocatalyst (200U/g); methanol to oil ratio (167μL); water content (46); 34 °C; 200 rpm; 6 h | 98.16 | - | [26] |
| C. antarctica B: Pseudomonas cepacia | 01:03 | Combi-immobilized onto amino functionalized SBA-15 support | Lipids of oleagi-nous microalgae (Isochrysis galbana) | Solvent-free | Biocatalyst (15mg); ethanol as acyl acceptor; 50 °C; 300 rpm; 24 h | 97.2 | 10th cycle; 70 | [5] |
| C. rugosa: R. miehei | 01:01 | Combi-immobilized onto polyhydroxybutyrate support | WCO | Solvent-free | Biocatalyst (1%); methanol to oil ratio (6:1); water content (5%); 45 °C; 250 rpm; 24 h | 96.5 | 10th cycle; ≥30 | [16] |
| C. antarctica B: R.miehei | 2.5:01 | Co-immobilized lipase onto epoxy functionalized silica gel support | Palm oil | T-butanol | t-butanol (39.9%); methanol to oil ratio (5.9); 35.6 °C; 33.5 h | 78.3 | - | [14] |
| C. rugosa: R. miehei | 1.5:01 | Combi-immobilized onto polyhydroxybutyrate support | Chicken waste oil | Solvent-free | Biocatalyst (2.5%); methanol to oil ratio (6:1); water content (5%); 40 °C; 200 rpm; 12 h | 97.1 | 15th cycle; 10 | [50] |
| Lipozyme TL-IM: Lipozyme RM-IM: Novozym 435 | 1.6:1:1.4 | Combi-commercial immobilized lipases | Waste oil | Solvent-free | Biocatalyst (25%); ethanol/oil ratio (9:1); 40 °C; 18 h under ultrasound-assisted reaction | 70 | 2nd cycle; lost 50% of its initial activity | [52] |
| C. rugosa | Individual immobilized lipase on dendrimer -coated mMWCNTs | Soybean oil | Isooctane | Biocatalyst (10%); methanol to oil ratio (4:1); water content (7.5%); 40 °C; 200 rpm; 40 h | 85.1 | 10th cycle; 58% | [36] | |
| R. oryzae | Individual immobilized lipase in microcapsules | Soybean oil | - | Biocatalyst (1g of lipase loading; ethanol/oil ratio (4:1); water content (30%); 45°C; 200 rpm; 48 h | 82.86 | 10th cycle; 42.98% | [1] | |
| R. oryzae: C. rugosa | 04:01 | Co-immobilized lipases onto mMWCNTs@4-arm-PEG-NH2 | WCO | Co-solvent n-hexane: isooctane | Biocatalyst (8%); methanol to oil ratio (4:1); co-solvent (20%); water content (10%); 35 °C; 120 min; under ultrasound-assisted reaction | 97.64 | 10th cycle; 78.55 | This study |
3. Materials and Methodology
3.1. Materials
3.2. Methodology
3.2.1. Synthesis of mMWCNTs@4-arm-PEG-NH2 Nanocomposites
3.2.2. Co-Immobilization of ROL and CRL onto mMWCNTs@4-arm-PEG-NH2
3.2.3. Assay of Lipase Activity and Thermal Stability
3.2.4. Characterization
3.2.5. Enzymatic Transesterification and GC Analysis for Biodiesel Production
3.2.6. Reusability of Co-Immobilized Lipases–mMWCNTs@4-arm-PEG-NH2
4. Limitation of the Study and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, F.; Li, G.; Fan, Y.; Yan, Y. Enhanced performance of lipase via microcapsulation and its application in biodiesel preparation. Sci. Rep. 2016, 6, 29670. [Google Scholar] [CrossRef] [Green Version]
- Aarthy, M.; Saravanan, P.; Gowthaman, M.K.; Rose, C.; Kamini, N.R. Enzymatic transesterification for production of biodiesel using yeast lipases: An overview. Chem. Eng. Res. Des. 2014, 92, 1591–1601. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Erick da Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Bhan, C.; Singh, J. Role of microbial lipases in transesterification process for biodiesel production. Environ. Sustain. 2020, 3, 257–266. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L. Biodiesel production (FAEEs) by heterogeneous combi-lipase biocatalysts using wet extracted lipids from Microalgae. Catalysts 2019, 9, 296. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, K.; He, Y.; Wang, Y.; Han, X.; Yan, Y. Enhanced performance of lipase immobilized onto Co2+-chelated magnetic nanoparticles and its application in biodiesel production. Fuel 2019, 255, 115794. [Google Scholar] [CrossRef]
- Faizollahzadeh Ardabili, S.; Najafi, B.; Alizamir, M.; Mosavi, A.; Shamshirband, S.; Rabczuk, T. Using SVM-RSM and ELM-RSM approaches for optimizing the production process of methyl and ethyl esters. Energies 2018, 11, 2889. [Google Scholar] [CrossRef] [Green Version]
- Najafi, B.; Faizollahzadeh Ardabili, S.; Shamshirband, S.; Chau, K.-W.; Rabczuk, T. Application of ANNs, ANFIS and RSM to estimating and optimizing the parameters that affect the yield and cost of biodiesel production. Eng. Appl. Comput. Fluid Mech. 2018, 12, 611–624. [Google Scholar] [CrossRef]
- Adnan, M.; Li, K.; Wang, J.; Xu, L.; Yan, Y. Hierarchical ZIF-8 toward immobilizing Burkholderia cepacia lipase for application in biodiesel preparation. Int. J. Mol. Sci. 2018, 19, 1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quayson, E.; Amoah, J.; Hama, S.; Kondo, A.; Ogino, C. Immobilized lipases for biodiesel production: Current and future greening opportunities. Renew. Sustain. Energy Rev. 2020, 134, 110355. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; de Castro, A.M.; Coelho, M.A.; Freire, D.M. Production and use of lipases in bioenergy: A review from the feedstocks to biodiesel production. Enzym. Res. 2011, 2011, 615803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, F.; Li, G.-L.; Fan, Y.-L.; Yan, Y.-J. Enhancing biodiesel production via a synergic effect between immobilized Rhizopus oryzae lipase and Novozym 435. Fuel Process. Technol. 2015, 137, 298–304. [Google Scholar] [CrossRef]
- Shahedi, M.; Habibi, Z.; Yousefi, M.; Brask, J.; Mohammadi, M. Improvement of biodiesel production from palm oil by co-immobilization of Thermomyces lanuginosa lipase and Candida antarctica lipase B: Optimization using response surface methodology. Int. J. Biol. Macromol. 2021, 170, 490–502. [Google Scholar] [CrossRef]
- Shahedi, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; As’habi, M.A. Co-immobilization of Rhizomucor miehei lipase and Candida antarctica lipase B and optimization of biocatalytic biodiesel production from palm oil using response surface methodology. Renew. Energy 2019, 141, 847–857. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Kang, S.W.; Song, Y.S.; Park, C.; Han, S.O.; Kim, S.W. Biodiesel production by a mixture of Candida rugosa and Rhizopus oryzae lipases using a supercritical carbon dioxide process. Bioresour. Technol. 2011, 102, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Binhayeeding, N.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of biodiesel production using waste cooking oil and applying single and mixed immobilised lipases on polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Záchia Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combi-lipase for heterogeneous substrates: A new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC. Adv. 2014, 4, 6863–6868. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zheng, J.; Yan, Y. Biodiesel preparation catalyzed by compound-lipase in co-solvent. Fuel Process. Technol. 2010, 91, 1229–1234. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Botelho, C.; Ribeiro, A.; Castro, R.; Pereira, M.A.; Gogate, P.R.; Cavaco-Paulo, A. Ultrasound intensification suppresses the need of methanol excess during the biodiesel production with Lipozyme TL-IM. Ultrason. Sonochem. 2015, 27, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Tupufia, S.C.; Jeon, Y.J.; Marquis, C.; Adesina, A.A.; Rogers, P.L. Enzymatic conversion of coconut oil for biodiesel production. Fuel Process. Technol. 2013, 106, 721–726. [Google Scholar] [CrossRef]
- Freitas, V.O.D.; Matte, C.R.; Poppe, J.K.; Rodrigues, R.C.; Ayub, M.A.Z. Ultrasound-assisted transesterification of soybean oil using combi-lipase biocatalysts. Braz. J. Chem. Eng. 2019, 36, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Lee, J.H.; Han, S.O.; Park, C.; Kim, S.W. Co-immobilization of Candida rugosa and Rhyzopus oryzae lipases and biodiesel production. Korean J. Chem. Eng. 2013, 30, 1335–1338. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Kim, D.S.; Yoo, H.Y.; Park, C.; Kim, S.W. Biodiesel production by lipases co-immobilized on the functionalized activated carbon. Bioresour. Technol. Rep. 2019, 7, 100248. [Google Scholar] [CrossRef]
- Arana-Pena, S.; Rios, N.S.; Mendez-Sanchez, C.; Lokha, Y.; Carballares, D.; Goncalves, L.R.B.; Fernandez-Lafuente, R. Coimmobilization of different lipases: Simple layer by layer enzyme spatial ordering. Int. J. Biol. Macromol. 2020, 145, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Toro, E.C.; Rodríguez, D.F.; Morales, N.; García, L.M.; Godoy, C.A. Novel combi-lipase systems for fatty acid ethyl esters production. Catalysts 2019, 9, 546. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; He, Y.; Jiao, L.; Li, K.; Yan, Y. Preparation of biodiesel with liquid synergetic lipases from rapeseed oil deodorizer distillate. Appl. Biochem. Biotechnol. 2017, 183, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Pang, H.; Yuan, S.; Wang, X.; Hu, Z.; Zhou, Q.; He, Y.; Yan, Y.; Xu, L. Codisplay of Rhizopus oryzae and Candida rugosa lipases for biodiesel production. Catalysts 2021, 11, 421. [Google Scholar] [CrossRef]
- Rafiee, F.; Rezaee, M. Different strategies for the lipase immobilization on the chitosan based supports and their applications. Int. J. Biol. Macromol. 2021, 179, 170–195. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, G.; Su, F.; Li, K.; Xu, L.; Han, X.; Yan, Y. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel 2016, 178, 172–178. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; He, Y.; Wang, Y.; Yan, J.; Xu, L.; Han, X.; Yan, Y. Lipase immobilized on a novel rigid-flexible dendrimer-grafted hierarchically porous magnetic microspheres for effective resolution of (R,S)-1-phenylethanol. ACS Appl. Mater. Interfaces 2020, 12, 4906–4916. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, Z.; Wang, X.; Liu, X.; Xia, J.; Xiong, P.; He, B. Enhancement in ionic liquid tolerance of cellulase immobilized on PEGylated graphene oxide nanosheets: Application in saccharification of lignocellulose. Bioresour. Technol. 2016, 200, 1060–1064. [Google Scholar] [CrossRef]
- Xie, X.; Luo, P.; Han, J.; Chen, T.; Wang, Y.; Cai, Y.; Liu, Q. Horseradish peroxidase immobilized on the magnetic composite microspheres for high catalytic ability and operational stability. Enzym. Microb. Technol. 2019, 122, 26–35. [Google Scholar] [CrossRef]
- Rong, J.; Zhou, Z.; Wang, Y.; Han, J.; Li, C.; Zhang, W.; Ni, L. Immobilization of horseradish peroxidase on multi-armed magnetic graphene oxide composite: Improvement of loading amount and catalytic activity. Food Technol. Biotechnol. 2019, 57, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, L.; Wang, Y.; Dong, J.; Tang, X.; Ni, L.; Wang, L. Preparation and characterization of Fe3O4-NH2@4-arm-PEG-NH2, a novel magnetic four-arm polymer-nanoparticle composite for cellulase immobilization. Biochem. Eng. J. 2018, 130, 90–98. [Google Scholar] [CrossRef]
- Zhong, K.; Du, X.; Li, J. Progress in polyethylene glycol modified chitosan as drug delivery carriers. Chemistery 2014, 77, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Ke, C.; Su, F.; Li, K.; Yan, Y. Various types of lipases immobilized on dendrimer-functionalized magnetic nanocomposite and application in biodiesel preparation. Energy Fuels 2017, 31, 4372–4381. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Wong, J.R.; Tan, K.W.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J. Mol. Catal. B Enzym. 2014, 107, 124–131. [Google Scholar] [CrossRef]
- Li, K.; Fan, Y.L.; He, Y.J.; Zeng, L.P.; Han, X.T.; Yan, Y.J. Burkholderia cepacia lipase immobilized on heterofunctional magnetic nanoparticles and its application in biodiesel synthesis. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Anwar, M.Z.; Kim, D.J.; Kumar, A.; Patel, S.K.S.; Otari, S.; Mardina, P.; Jeong, J.H.; Sohn, J.H.; Kim, J.H.; Park, J.T.; et al. SnO2 hollow nanotubes: A novel and efficient support matrix for enzyme immobilization. Sci. Rep. 2017, 7, 15333. [Google Scholar] [CrossRef] [Green Version]
- Arana-Peña, S.; Carballares, D.; Berenguer-Murcia, Á.; Alcántara, A.; Rodrigues, R.; Fernandez-Lafuente, R. One pot use of combilipases for full modification of oils and Fats: Multifunctional and heterogeneous substrates. Catalysts 2020, 10, 605. [Google Scholar] [CrossRef]
- Fan, Y.; Su, F.; Li, K.; Ke, C.; Yan, Y. Carbon nanotube filled with magnetic iron oxide and modified with polyamidoamine dendrimers for immobilizing lipase toward application in biodiesel production. Sci. Rep. 2017, 7, 45643. [Google Scholar] [CrossRef] [Green Version]
- Rehm, S.; Trodler, P.; Pleiss, J. Solvent-induced lid opening in lipases: A molecular dynamics study. Protein Sci. 2010, 19, 2122–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The lid domain in lipases: Structural and functional determinant of enzymatic properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subhedar, P.B.; Gogate, P.R. Ultrasound assisted intensification of biodiesel production using enzymatic interesterification. Ultrason. Sonochem. 2016, 29, 67–75. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.M.; Shin, H.Y.; Kang, S.W.; Kim, S.W. Biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. Biotechnol. Bioprocess Eng. 2006, 11, 522–525. [Google Scholar] [CrossRef]
- Fu, B.; Vasudevan, P.T. Effect of solvent−co-solvent mixtures on lipase-catalyzed transesterification of canola oil. Energy Fuels 2010, 24, 4646–4651. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; Brask, J. Effect of water, organic solvent and adsorbent contents on production of biodiesel fuel from canola oil catalyzed by various lipases immobilized on epoxy-functionalized silica as low cost biocatalyst. J. Mol. Catal. B Enzym. 2015, 120, 93–99. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Influence of the reaction conditions on the enzyme catalyzed transesterification of castor oil: A possible step in biodiesel production. Bioresour. Technol. 2017, 243, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Li, K.; Xu, L.; Yan, Y. X-Shaped ZIF-8 for immobilization Rhizomucor miehei lipase via encapsulation and its application toward biodiesel production. Catalyst 2018, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Sangkharak, K.; Mhaisawat, S.; Rakkan, T.; Paichid, N.; Yunu, T. Utilization of mixed chicken waste for biodiesel production using single and combination of immobilized lipase as a catalyst. Biomass Convers. Biorefin. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Wang, X.; Li, Q.; Wang, J.; Yan, Y. Improving catalytic performance of Burkholderia cepacia lipase immobilized on macroporous resin NKA.J. Mol. Catal. B Enzym. 2011, 71, 45–50. [Google Scholar] [CrossRef]
- Poppe, J.K.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Transesterification of waste frying oil and soybean oil by combi-lipases under ultrasound-assisted reactions. Appl. Biochem. Biotechnol. 2018, 186, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Suh, Y.J.; Kang, G.B.; Jang, W.I.; Kang, J.; Park, C.; Kim, S.W. Biodiesel production by enzymatic process using Jatropha oil and waste soybean oil. Biotechnol. Bioprocess Eng. 2013, 18, 703–708. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification–Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Ji, Q.; Xiao, S.; He, B.; Liu, X. Purification and characterization of an organic solvent-tolerant lipase from Pseudomonas aeruginosa LX1 and its application for biodiesel production. J. Mol. Catal. B Enzym. 2010, 66, 264–269. [Google Scholar] [CrossRef]
- Lopez, E.N.; Medina, A.R.; Moreno, P.A.G.; Cerdan, L.E.; Valverde, L.M.; Grima, E.M. Biodiesel production from Nannochloropsis gaditana lipids through transesterification catalyzed by Rhizopus oryzae lipase. Bioresour. Technolo. 2016, 203, 236–244. [Google Scholar] [CrossRef]
- Abahazi, E.; Boros, Z.; Poppe, L. Additives enhancing the catalytic properties of lipase from Burkholderia cepacia immobilized on mixed-function-grafted mesoporous silica gel. Molecules 2014, 19, 9818–9837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, D.; Yan, Y.; Peng, C.; Xu, L. Biodiesel synthesis and conformation of lipase from Burkholderia cepacia in room temperature ionic liquids and organic solvents. Bioresour. Technol. 2011, 102, 10414–10418. [Google Scholar] [CrossRef]
- Gusniah, A.; Veny, H.; Hamzah, F. Ultrasonic assisted enzymatic transesterification for biodiesel production. Ind. Eng. Chem. Res. 2018, 58, 581–589. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Zhao, Y.; Zhang, Y. Biodiesel production from Jatropha oil catalyzed by immobilized Burkholderia cepacia lipase on modified attapulgite. Bioresour. Technol. 2013, 148, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Garmroodi, M.; Mohammadi, M.; Ramazani, A.; Ashjari, M.; Mohammadi, J.; Sabour, B.; Yousefi, M. Covalent binding of hyper-activated Rhizomucor miehei lipase (RML) on hetero-functionalized siliceous supports. Int. J. Biol. Macromol. 2016, 86, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Tamalampudy, S.; Li, C.J.; Yanglin, L.; Wu, J.; Kondo, A.; Fukuda, H. An improved method of lipase preparation incorporating both solvent treatment and immobilization onto matrix. Biochem. Eng. J. 2007, 33, 60–65. [Google Scholar] [CrossRef]
- Otari, S.V.; Patel, S.K.S.; Kalia, V.C.; Lee, J.K. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour. Technol. 2020, 302, 122887. [Google Scholar] [CrossRef] [PubMed]






| Items | Peak | Position BE (eV) | Atomic Mass % | Atomic Content % | Mass Content % |
|---|---|---|---|---|---|
| MWCNTs | O1s | 532.500 | 15.999 | 0.75 | 1.00 |
| C1s | 284.300 | 12.011 | 99.25 | 99.00 | |
| MWCNTs-COOH | O1s | 531.750 | 15.999 | 11.03 | 14.17 |
| C1s | 284.300 | 12.011 | 88.97 | 85.83 | |
| mMWCNTs | Fe2p | 711.050 | 55.846 | 3.80 | 14.95 |
| O1s | 531.550 | 15.999 | 12.65 | 14.27 | |
| C1s | 284.350 | 12.011 | 83.55 | 70.78 | |
| mMWCNTs@4-arm-PEG-NH2 | Fe2p | 711.40 | 55.846 | 2.77 | 11.02 |
| O1s | 531.25 | 15.999 | 19.71 | 22.46 | |
| N1s | 399.70 | 14.007 | 1.4 | 1.4 | |
| C1s | 284.350 | 12.011 | 76.12 | 65.12 | |
| Lipase-mMWCNTs@4-arm-PEG-NH2 | Fe2p | 711.450 | 55.846 | 0.85 | 3.65 |
| O1s | 532.550 | 15.999 | 16.00 | 19.61 | |
| N1s | 400.300 | 14.007 | 1.44 | 1.54 | |
| C1s | 284.400 | 12.011 | 81.70 | 75.17 | |
| S2p | 165.150 | 32.065 | 0.010 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulmalek, S.A.; Li, K.; Wang, J.; Ghide, M.K.; Yan, Y. Co-Immobilization of Rhizopus oryzae and Candida rugosa Lipases onto mMWCNTs@4-arm-PEG-NH2—A Novel Magnetic Nanotube–Polyethylene Glycol Amine Composite—And Its Applications for Biodiesel Production. Int. J. Mol. Sci. 2021, 22, 11956. https://doi.org/10.3390/ijms222111956
Abdulmalek SA, Li K, Wang J, Ghide MK, Yan Y. Co-Immobilization of Rhizopus oryzae and Candida rugosa Lipases onto mMWCNTs@4-arm-PEG-NH2—A Novel Magnetic Nanotube–Polyethylene Glycol Amine Composite—And Its Applications for Biodiesel Production. International Journal of Molecular Sciences. 2021; 22(21):11956. https://doi.org/10.3390/ijms222111956
Chicago/Turabian StyleAbdulmalek, Saadiah A., Kai Li, Jianhua Wang, Michael Kidane Ghide, and Yunjun Yan. 2021. "Co-Immobilization of Rhizopus oryzae and Candida rugosa Lipases onto mMWCNTs@4-arm-PEG-NH2—A Novel Magnetic Nanotube–Polyethylene Glycol Amine Composite—And Its Applications for Biodiesel Production" International Journal of Molecular Sciences 22, no. 21: 11956. https://doi.org/10.3390/ijms222111956
APA StyleAbdulmalek, S. A., Li, K., Wang, J., Ghide, M. K., & Yan, Y. (2021). Co-Immobilization of Rhizopus oryzae and Candida rugosa Lipases onto mMWCNTs@4-arm-PEG-NH2—A Novel Magnetic Nanotube–Polyethylene Glycol Amine Composite—And Its Applications for Biodiesel Production. International Journal of Molecular Sciences, 22(21), 11956. https://doi.org/10.3390/ijms222111956







