Peek Inside the Water Mixtures of Ionic Liquids at Molecular Level: Microscopic Properties Probed by EPR Spectroscopy
Abstract
1. Introduction
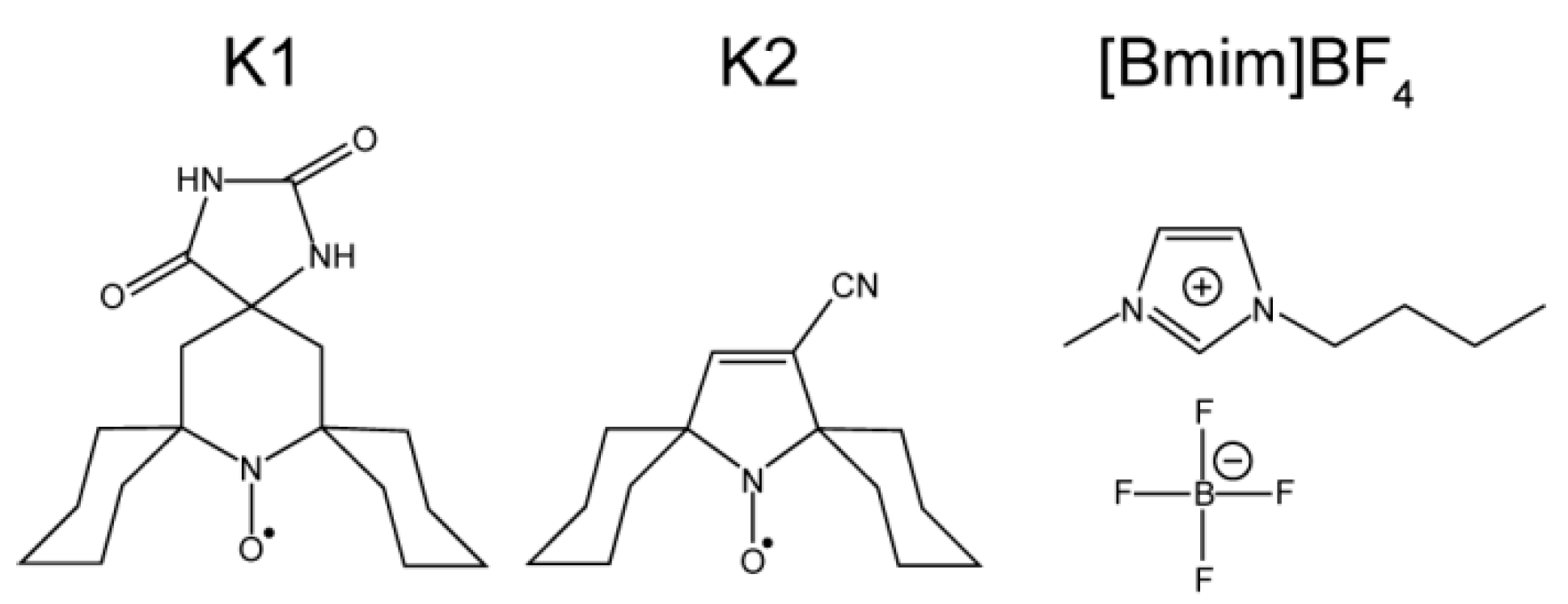
2. Experimental
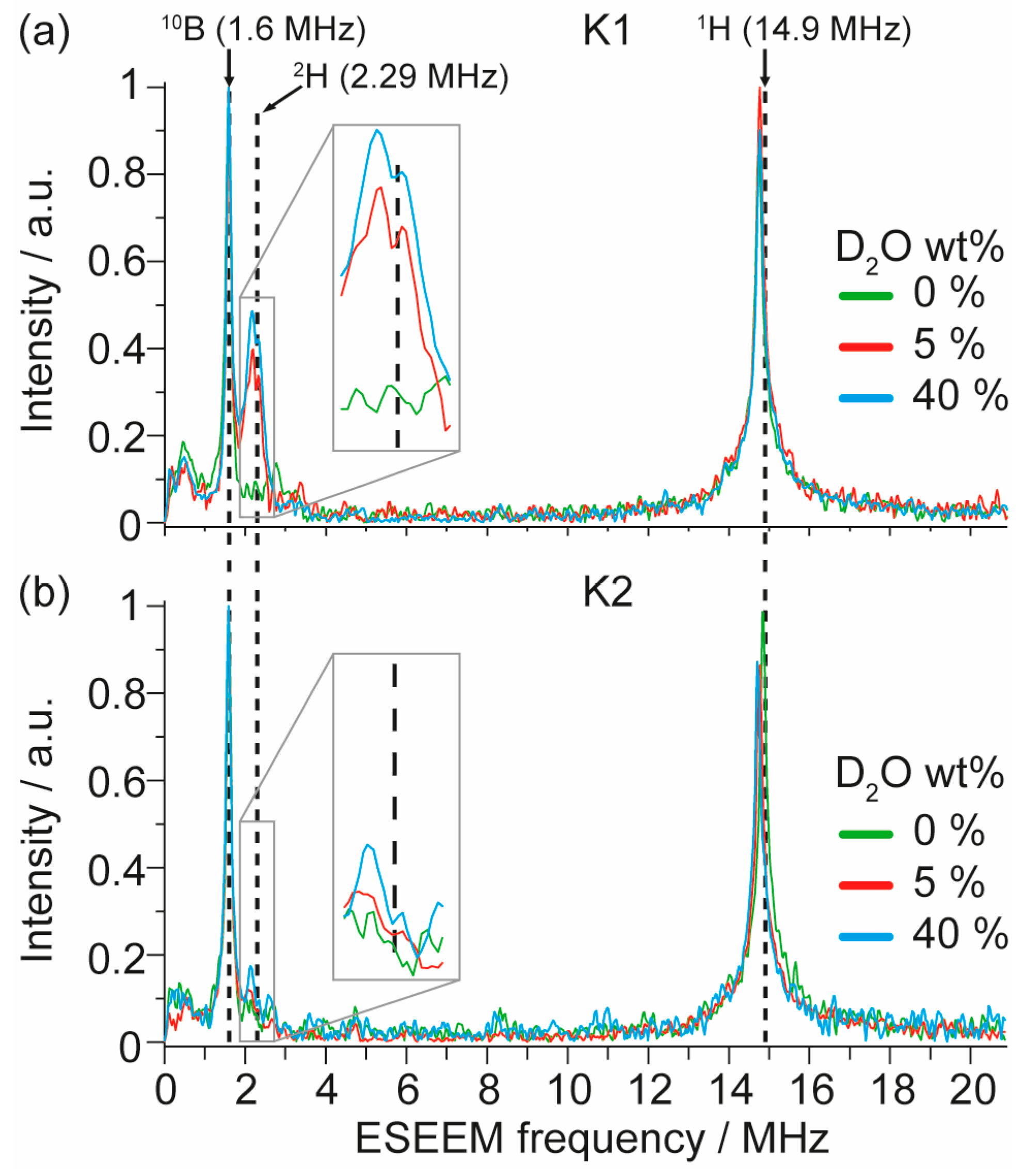
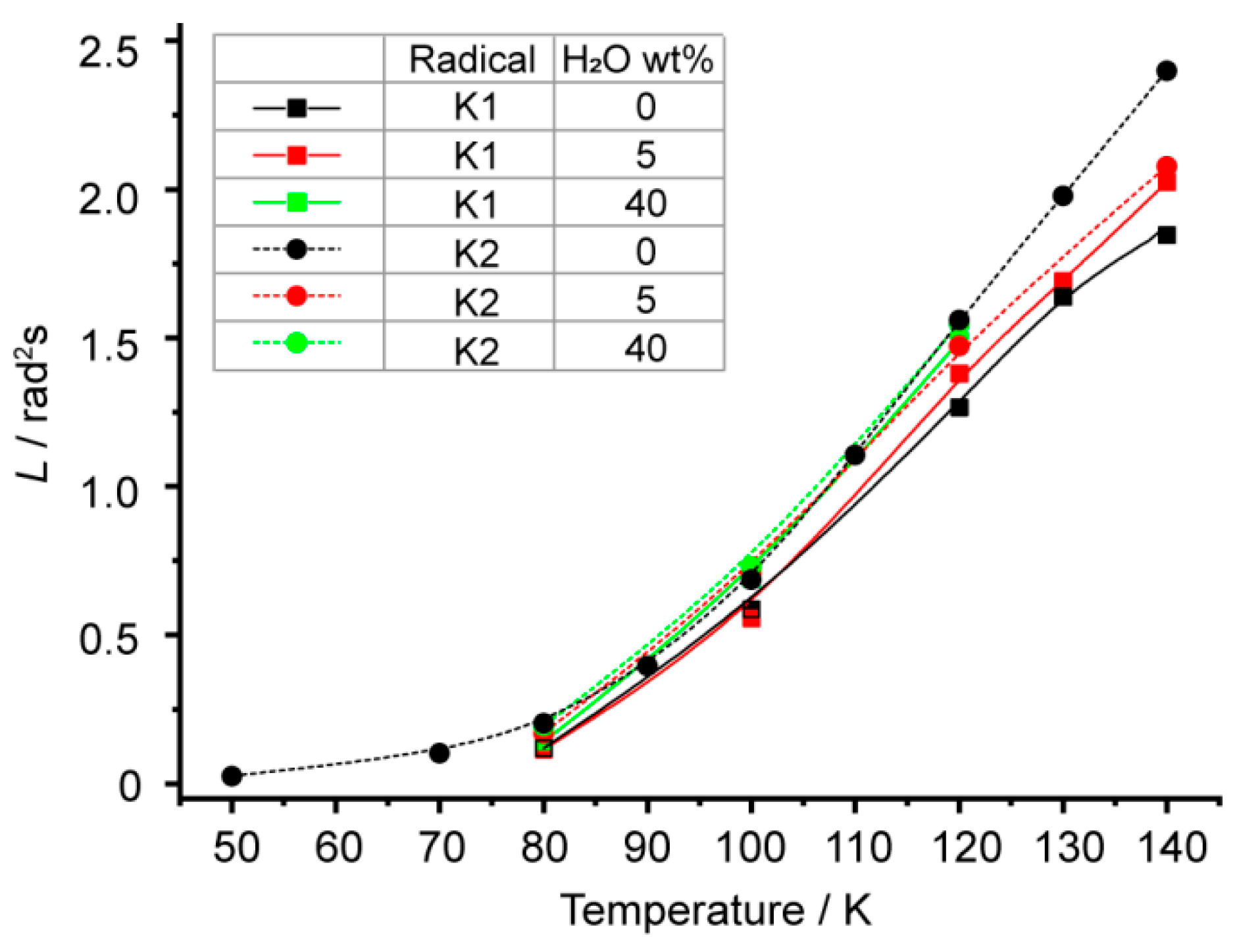
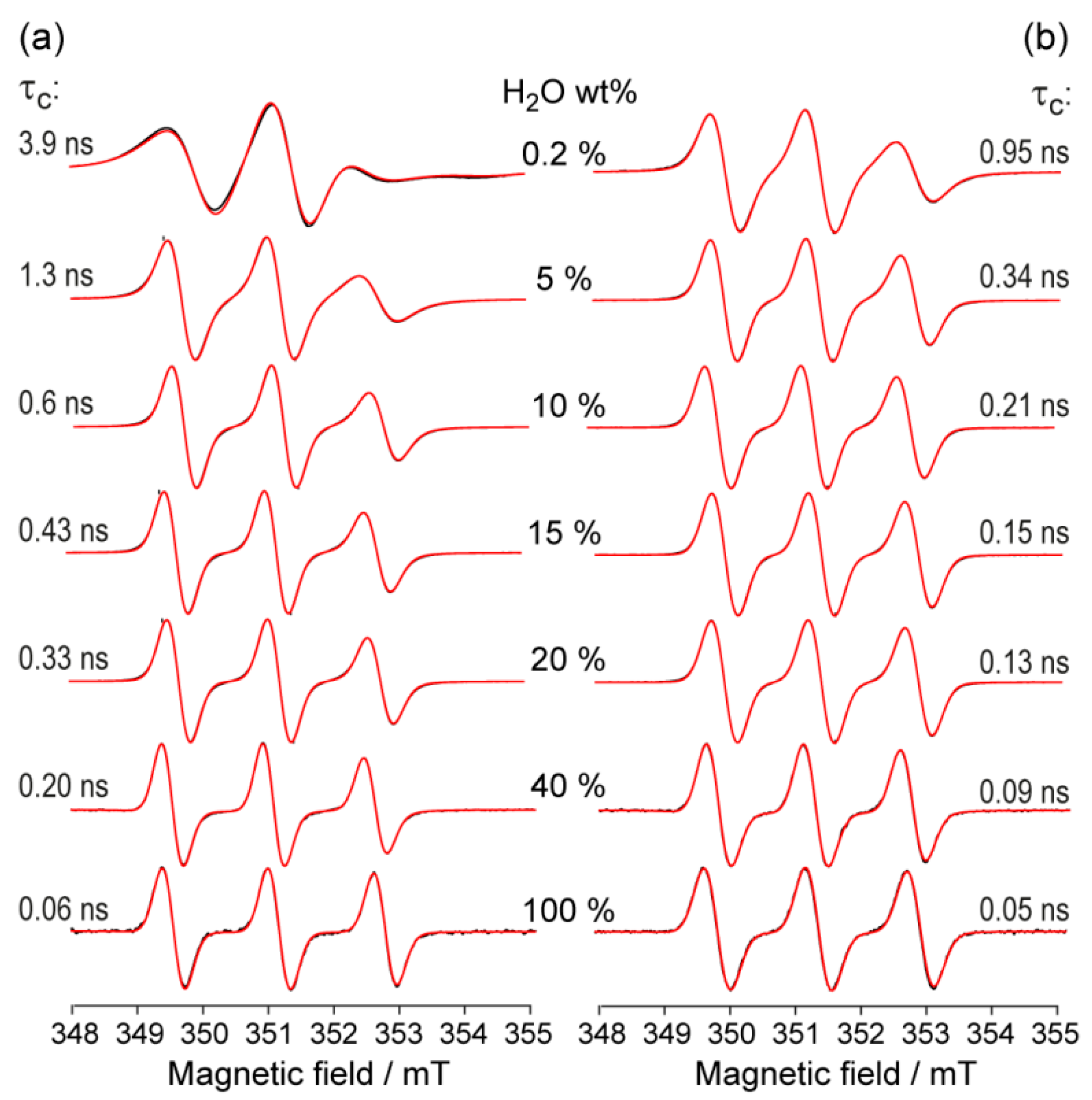
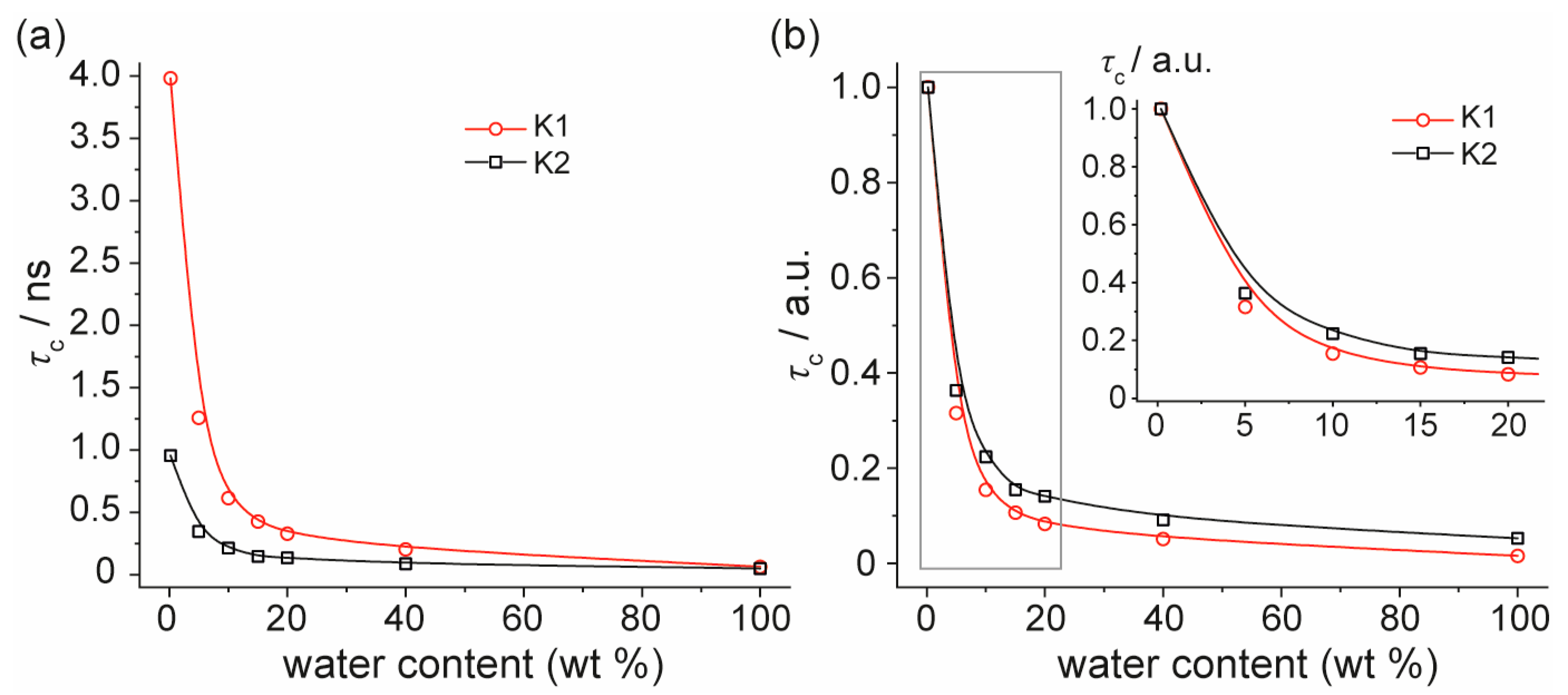
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Weingärtner, H. Understanding Ionic Liquids at the Molecular Level: Facts, Problems, and Controversies. Angew. Chem. Int. Ed. 2008, 47, 654–670. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic Liquids - New “Solutions” for Transition Metal Catalysis. Angew. Chemie Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Ionic liquids: Applications in catalysis. Catal. Today 2002, 74, 157–189. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic Liquids in Selective Oxidation: Catalysts and Solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Thomas, M.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Liu, X.; Bai, L.; Zeng, S.; Xu, Q.; Gao, H.; Zhang, X. Ionic liquids in gas separation processing. Curr. Opin. Green Sustain. Chem. 2017, 5, 74–81. [Google Scholar] [CrossRef]
- Zhang, M.; Ettelaie, R.; Yan, T.; Zhang, S.; Cheng, F.; Binks, B.P.; Yang, H. Ionic Liquid Droplet Microreactor for Catalysis Reactions Not at Equilibrium. J. Am. Chem. Soc. 2017, 139, 17387–17396. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Li, B.; Sarman, S.; Mocci, F.; Lu, Z.-Y.; Yuan, J.; Laaksonen, A.; Fayer, M.D. Microstructural and Dynamical Heterogeneities in Ionic Liquids. Chem. Rev. 2020, 120, 5798–5877. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Y.; Zhao, Y.; Zhuo, K. A volumetric and viscosity study for the mixtures of 1-n-butyl-3-methylimidazolium tetrafluoroborate ionic liquid with acetonitrile, dichloromethane, 2-butanone and N, N ? dimethylformamide. Green Chem. 2003, 5, 618–622. [Google Scholar] [CrossRef]
- Widegren, J.; Saurer, E.M.; Marsh, K.N.; Magee, J.W. Electrolytic conductivity of four imidazolium-based room-temperature ionic liquids and the effect of a water impurity. J. Chem. Thermodyn. 2005, 37, 569–575. [Google Scholar] [CrossRef]
- Najdanovic-Visak, V.; Esperança, J.M.S.S.; Rebelo, L.P.N.; da Ponte, M.N.; Guedes, H.J.R.; Seddon, K.R.; de Sousa, H.C.; Szydlowski, J. Pressure, Isotope, and Water Co-solvent Effects in Liquid−Liquid Equilibria of (Ionic Liquid + Alcohol) Systems. J. Phys. Chem. B 2003, 107, 12797–12807. [Google Scholar] [CrossRef]
- Rebelo, L.P.N.; Najdanovic-Visak, V.; Visak, Z.P.; da Ponte, M.N.; Szydlowski, J.; Cerdeiriña, C.A.; Troncoso, J.; Romaní, L.; Esperança, J.M.S.S.; Guedes, H.J.R.; et al. A detailed thermodynamic analysis of [C4mim][BF4] + water as a case study to model ionic liquid aqueous solutions. Green Chem. 2004, 6, 369–381. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Wang, Y.; Li, H. Prediction of the Solvation and Structural Properties of Ionic Liquids in Water by Two-Dimensional Correlation Spectroscopy. J. Phys. Chem. B 2008, 112, 6411–6419. [Google Scholar] [CrossRef]
- Strehmel, V.; Rexhausen, H.; Strauch, P.; Strehmel, B. Temperature Dependence of Interactions Between Stable Piperidine-1-yloxyl Derivatives and a Semicrystalline Ionic Liquid. ChemPhysChem 2010, 11, 2182–2190. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Akdoğan, Y.; Lieberwirth, I.; Hinderberger, D. Spin probing of supramolecular structures in 1-butyl-3-methyl-imidazolium tetrafluoroborate/water mixtures. Mol. Phys. 2013, 111, 2723–2737. [Google Scholar] [CrossRef]
- Frade, R.F.; Rosatella, A.A.; Marques, C.S.; Branco, L.C.; Kulkarni, P.S.; Mateus, N.M.M.; Afonso, C.A.M.; Duarte, C.M. Toxicological evaluation on human colon carcinoma cell line (CaCo-2) of ionic liquids based on imidazolium, guanidinium, ammonium, phosphonium, pyridinium and pyrrolidinium cations. Green Chem. 2009, 11, 1660–1665. [Google Scholar] [CrossRef]
- Viciosa, M.T.; Santos, G.; Costa, A.; Danède, F.; Branco, L.C.; Jordão, N.; Correia, N.T.; Dionísio, M. Dipolar motions and ionic conduction in an ibuprofen derived ionic liquid. Phys. Chem. Chem. Phys. 2015, 17, 24108–24120. [Google Scholar] [CrossRef][Green Version]
- Takekiyo, T.; Yamazaki, K.; Yamaguchi, E.; Abe, H.; Yoshimura, Y. High Ionic Liquid Concentration-Induced Structural Change of Protein in Aqueous Solution: A Case Study of Lysozyme. J. Phys. Chem. B 2012, 116, 11092–11097. [Google Scholar] [CrossRef]
- Takekiyo, T.; Koyama, Y.; Yamazaki, K.; Abe, H.; Yoshimura, Y. Ionic Liquid-Induced Formation of the α-Helical Structure of β-Lactoglobulin. J. Phys. Chem. B 2013, 117, 10142–10148. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.; Rogers, R.D. Controlling the Aqueous Miscibility of Ionic Liquids: Aqueous Biphasic Systems of Water-Miscible Ionic Liquids and Water-Structuring Salts for Recycle, Metathesis, and Separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, A. Aggregation Behavior of Ionic Liquids in Aqueous Solutions: Effect of Alkyl Chain Length, Cations, and Anions. J. Phys. Chem. B 2007, 111, 7843–7851. [Google Scholar] [CrossRef]
- Sturlaugson, A.L.; Fruchey, K.S.; Fayer, M.D. Orientational Dynamics of Room Temperature Ionic Liquid/Water Mixtures: Water-Induced Structure. J. Phys. Chem. B 2012, 116, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Hunger, J.; Stoppa, A.; Buchner, R.; Steinhauser, O. On the collective network of ionic liquid/water mixtures. II. Decomposition and interpretation of dielectric spectra. J. Chem. Phys. 2008, 129, 184501. [Google Scholar] [CrossRef] [PubMed]
- Kashin, A.; Galkin, K.I.; Khokhlova, E.A.; Ananikov, V.P. Direct Observation of Self-Organized Water-Containing Structures in the Liquid Phase and Their Influence on 5-(Hydroxymethyl)furfural Formation in Ionic Liquids. Angew. Chem. Int. Ed. 2016, 55, 2161–2166. [Google Scholar] [CrossRef]
- Akdoğan, Y.; Heller, J.; Zimmermann, H.; Hinderberger, D. The solvation of nitroxide radicals in ionic liquids studied by high-field EPR spectroscopy. Phys. Chem. Chem. Phys. 2010, 12, 7874–7882. [Google Scholar] [CrossRef] [PubMed]
- Kattnig, D.R.; Hinderberger, D. Temperature-Dependent Formation and Transformation of Mesostructures in Water-Ionic Liquid Mixtures. Chem. – Asian J. 2012, 7, 1000–1008. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Akdoğan, Y.; Bauer, C.; Hinderberger, D. High-Field EPR Spectroscopic Characterization of Spin Probes in Aqueous Ionic Liquid Mixtures. Z. Phys. Chem. 2012, 226, 1363–1378. [Google Scholar] [CrossRef]
- Mladenova, B.Y.; Chumakova, N.A.; Pergushov, V.I.; Kokorin, A.; Grampp, G.; Kattnig, D.R. Rotational and Translational Diffusion of Spin Probes in Room-Temperature Ionic Liquids. J. Phys. Chem. B 2012, 116, 12295–12305. [Google Scholar] [CrossRef]
- Mladenova, B.Y.; Kattnig, D.R.; Grampp, G. Room-Temperature Ionic Liquids Discerned Via Nitroxyl Spin Probe Dynamics. J. Phys. Chem. B 2011, 115, 8183–8198. [Google Scholar] [CrossRef]
- Strehmel, V.; Laschewsky, A.; Stoesser, R.; Zehl, A.; Herrmann, W. Mobility of spin probes in ionic liquids. J. Phys. Org. Chem. 2006, 19, 318–325. [Google Scholar] [CrossRef]
- Stoesser, R.; Herrmann, W.; Zehl, A.; Strehmel, V.; Laschewsky, A. ESR Spin Probes in Ionic Liquids. ChemPhysChem 2006, 7, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Strehmel, V. Radicals in Ionic Liquids. ChemPhysChem 2012, 13, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Prikhod’Ko, S.A.; Adonin, N.Y.; Fedin, M.V. Structural Anomalies in Binary Mixtures of Ionic Liquid [Bmim]BF4 with Water Studied by EPR. J. Phys. Chem. B 2019, 123, 9956–9962. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Veber, S.; Prikhod’Ko, S.A.; Adonin, N.Y.; Bagryanskaya, E.; Fedin, M. Probing Microenvironment in Ionic Liquids by Time-Resolved EPR of Photoexcited Triplets. J. Phys. Chem. B 2015, 119, 13440–13449. [Google Scholar] [CrossRef]
- Ivanov, M.; Prikhod’Ko, S.A.; Adonin, N.Y.; Bagryanskaya, E.; Fedin, M.V. Influence of C2-Methylation of Imidazolium Based Ionic Liquids on Photoinduced Spin Dynamics of the Dissolved ZnTPP Studied by Time-Resolved EPR. Z. Phys. Chem. 2017, 231, 391–404. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Krumkacheva, O.A.; Dzuba, S.A.; Fedin, M.V. Microscopic rigidity and heterogeneity of ionic liquids probed by stochastic molecular librations of the dissolved nitroxides. Phys. Chem. Chem. Phys. 2017, 19, 26158–26163. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Prikhod’Ko, S.A.; Adonin, N.Y.; Kirilyuk, I.A.; Adichtchev, S.V.; Surovtsev, N.V.; Dzuba, S.A.; Fedin, M.V. Structural Anomalies in Ionic Liquids near the Glass Transition Revealed by Pulse EPR. J. Phys. Chem. Lett. 2018, 9, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Fedin, M.V. Nanoscale heterogeneities in ionic liquids: Insights from EPR of spin probes. Mendeleev Commun. 2018, 28, 565–573. [Google Scholar] [CrossRef]
- Bakulina, O.D.; Ivanov, M.Y.; Prikhod’Ko, S.A.; Pylaeva, S.; Zaytseva, I.V.; Surovtsev, N.V.; Adonin, N.Y.; Fedin, M.V. Nanocage formation and structural anomalies in imidazolium ionic liquid glasses governed by alkyl chains of cations. Nanoscale 2020, 12, 19982–19991. [Google Scholar] [CrossRef]
- Ivanov, M.Y.; Poryvaev, A.S.; Polyukhov, D.M.; Prikhod’Ko, S.A.; Adonin, N.Y.; Fedin, M.V. Nanoconfinement effects on structural anomalies in imidazolium ionic liquids. Nanoscale 2020, 12, 23480–23487. [Google Scholar] [CrossRef] [PubMed]
- PREPARATION OF 1-BUTYL-3-METHYL IMIDAZOLIUM-BASED ROOM TEMPERATURE IONIC LIQUIDS. Org. Synth. 2002, 79, 236. [CrossRef]
- Rajca, A.; Kathirvelu, V.; Roy, S.K.; Pink, M.; Rajca, S.; Sarkar, S.; Eaton, S.S.; Eaton, G.R. A Spirocyclohexyl Nitroxide Amino Acid Spin Label for Pulsed EPR Spectroscopy Distance Measurements. Chem.—A Eur. J. 2010, 16, 5778–5782. [Google Scholar] [CrossRef] [PubMed]
- Kirilyuk, I.A.; Polienko, Y.F.; Krumkacheva, O.A.; Strizhakov, R.K.; Gatilov, Y.V.; Grigor’Ev, I.A.; Bagryanskaya, E.G. Synthesis of 2,5-Bis(spirocyclohexane)-Substituted Nitroxides of Pyrroline and Pyrrolidine Series, Including Thiol-Specific Spin Label: An Analogue of MTSSL with Long Relaxation Time. J. Org. Chem. 2012, 77, 8016–8027. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Kathirvelu, V.; Smith, C.; Parks, C.; Mannan, A.; Miura, Y.; Takeshita, K.; Eaton, S.S.; Eaton, G.R. Relaxation rates for spirocyclohexyl nitroxyl radicals are suitable for interspin distance measurements at temperatures up to about 125 K. Chem. Commun. 2008, 454–456. [Google Scholar] [CrossRef]
- Milov, A.D.; Samoilova, R.I.; Shubin, A.A.; Grishin, Y.A.; Dzuba, S.A. ESEEM Measurements of Local Water Concentration in D2O-Containing Spin-Labeled Systems. Appl. Magn. Reson. 2008, 35, 73–94. [Google Scholar] [CrossRef]
- Syryamina, V.; Maryasov, A.; Bowman, M.; Dzuba, S. Electron spin echo envelope modulation of molecular motions of deuterium nuclei. J. Magn. Reson. 2015, 261, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kirilina, E.P.; Grigoriev, I.A.; Dzuba, S.A. Orientational motion of nitroxides in molecular glasses: Dependence on the chemical structure, on the molecular size of the probe, and on the type of the matrix. J. Chem. Phys. 2004, 121, 12465. [Google Scholar] [CrossRef]
- Paschenko, S.V.; Toropov, Y.V.; Dzuba, S.A.; Tsvetkov, Y.D.; Vorobiev, A.K. Temperature dependence of amplitudes of libration motion of guest spin-probe molecules in organic glasses. J. Chem. Phys. 1999, 110, 8150–8154. [Google Scholar] [CrossRef]
- Dzuba, S. Libration motion of guest spin probe molecules in organic glasses: CW EPR and electron spin echo study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 227–234. [Google Scholar] [CrossRef]
- Kuzhelev, A.A.; Strizhakov, R.K.; Krumkacheva, O.A.; Polienko, Y.F.; Morozov, D.A.; Shevelev, G.Y.; Pyshnyi, D.V.; Kirilyuk, I.A.; Fedin, M.V.; Bagryanskaya, E.G. Room-Temperature Electron Spin Relaxation of Nitroxides Immobilized in Trehalose: Effect of Substituents Adjacent to NO-Group. J. Magn. Reson. 2016, 266, 1–7. [Google Scholar] [CrossRef]
- Isaev, N.P.; Dzuba, S.A. Fast Stochastic Librations and Slow Rotations of Spin Labeled Stearic Acids in a Model Phospholipid Bilayer at Cryogenic Temperatures. J. Phys. Chem. B 2008, 112, 13285–13291. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, M.Y.; Polienko, Y.F.; Kirilyuk, I.A.; Prikhod’ko, S.A.; Adonin, N.Y.; Fedin, M.V. Peek Inside the Water Mixtures of Ionic Liquids at Molecular Level: Microscopic Properties Probed by EPR Spectroscopy. Int. J. Mol. Sci. 2021, 22, 11900. https://doi.org/10.3390/ijms222111900
Ivanov MY, Polienko YF, Kirilyuk IA, Prikhod’ko SA, Adonin NY, Fedin MV. Peek Inside the Water Mixtures of Ionic Liquids at Molecular Level: Microscopic Properties Probed by EPR Spectroscopy. International Journal of Molecular Sciences. 2021; 22(21):11900. https://doi.org/10.3390/ijms222111900
Chicago/Turabian StyleIvanov, Mikhail Yu., Yuliya F. Polienko, Igor A. Kirilyuk, Sergey A. Prikhod’ko, Nicolay Yu. Adonin, and Matvey V. Fedin. 2021. "Peek Inside the Water Mixtures of Ionic Liquids at Molecular Level: Microscopic Properties Probed by EPR Spectroscopy" International Journal of Molecular Sciences 22, no. 21: 11900. https://doi.org/10.3390/ijms222111900
APA StyleIvanov, M. Y., Polienko, Y. F., Kirilyuk, I. A., Prikhod’ko, S. A., Adonin, N. Y., & Fedin, M. V. (2021). Peek Inside the Water Mixtures of Ionic Liquids at Molecular Level: Microscopic Properties Probed by EPR Spectroscopy. International Journal of Molecular Sciences, 22(21), 11900. https://doi.org/10.3390/ijms222111900






