Sex-Specific MicroRNAs in Neurovascular Units in Ischemic Stroke
Abstract
1. Introduction
1.1. Sex Differences in Stroke
1.2. Neurovascular Units and Ischemic Brain Injury
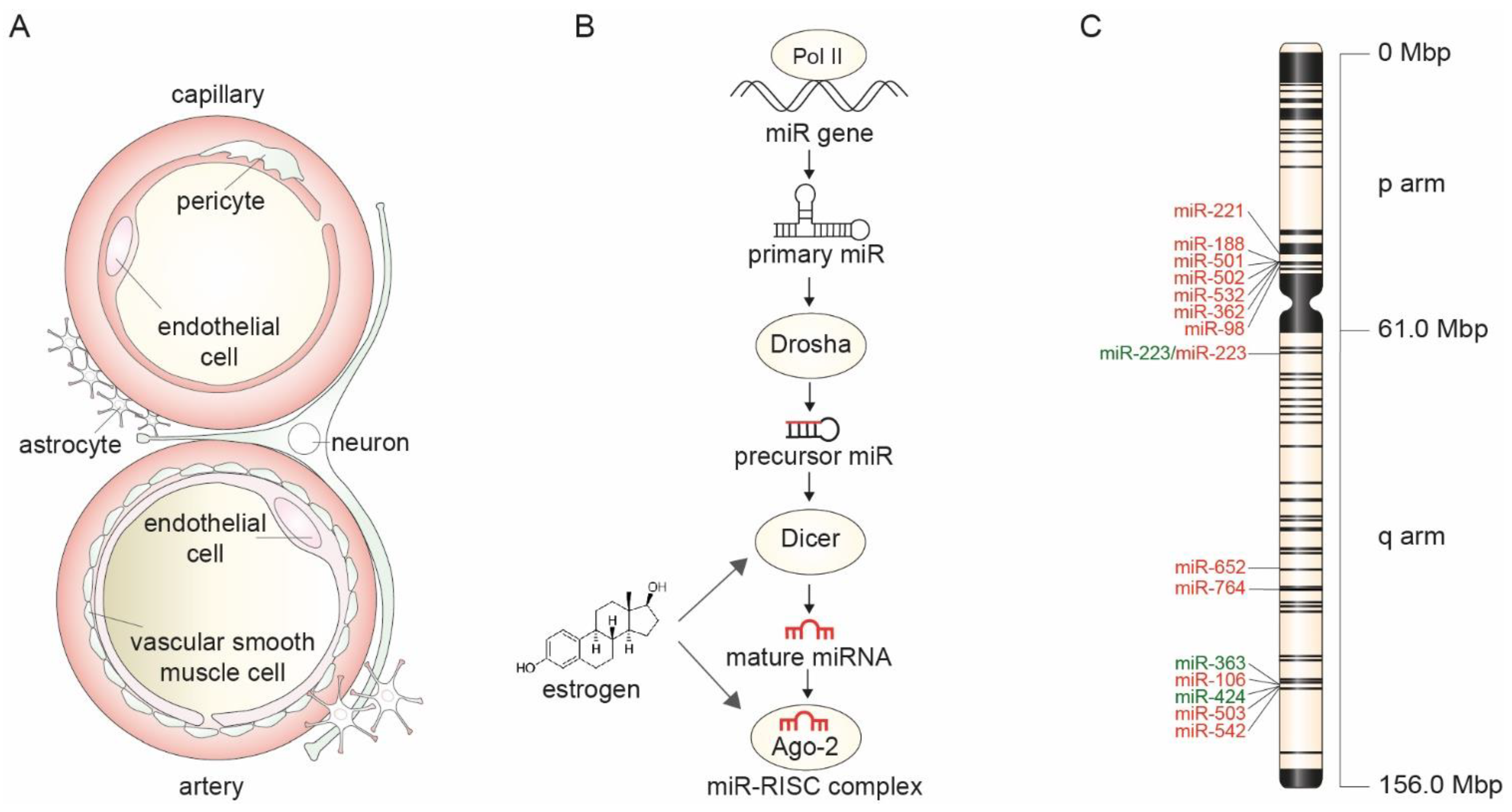
1.3. Sex-Specific MicroRNA Expression in Neurovascular Units
1.4. Purpose of the Study
2. Mechanisms of Sex-Specific MicroRNA Expression
2.1. X-Chromosome Mosaicism and Its Effect on MicroRNA Expression
2.2. Estrogen Control of MicroRNA Biogenesis
3. Stroke Promoting Mechanisms
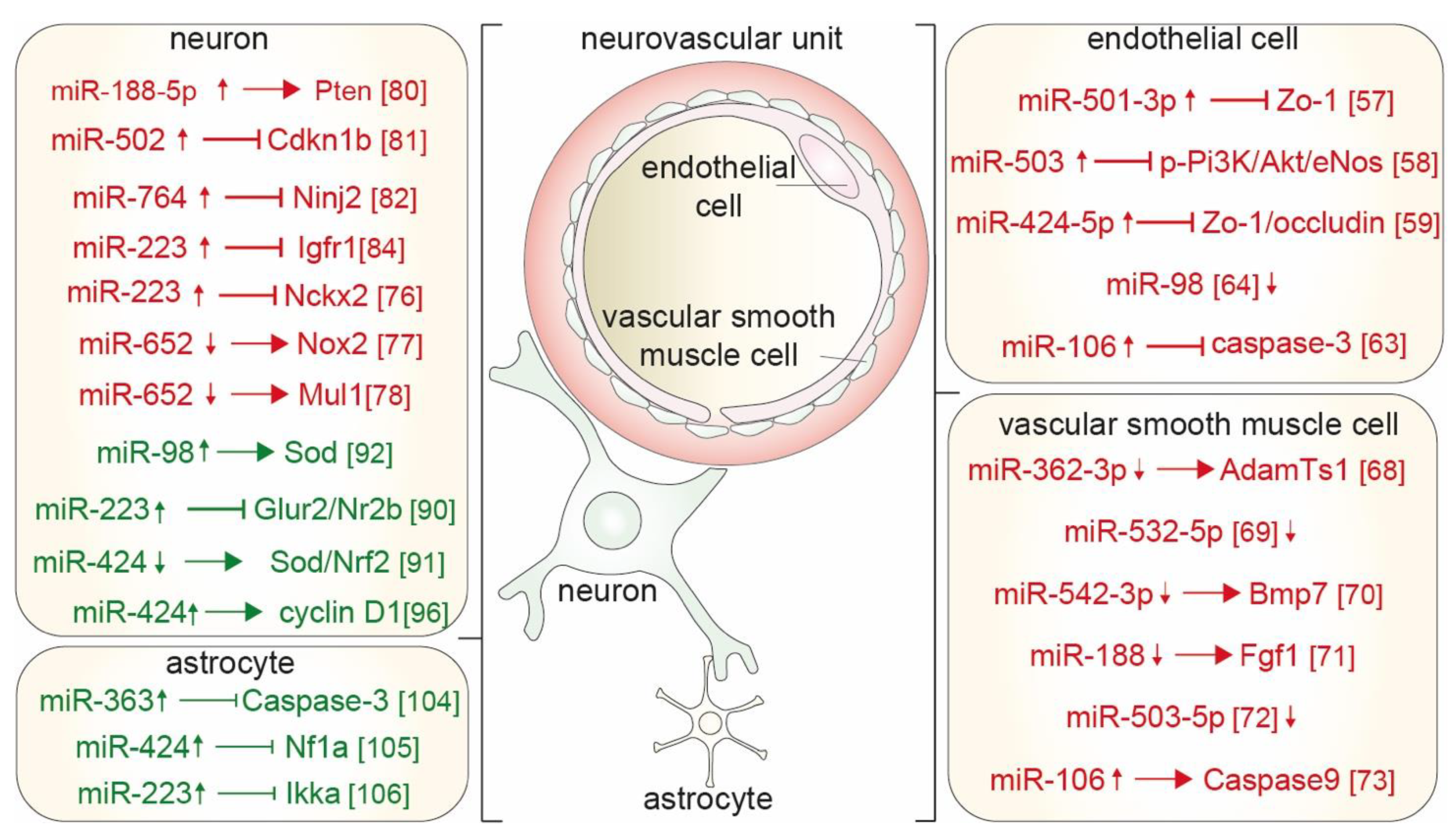
3.1. Sex-Specific MicroRNAs and Blood Brain Barrier Integrity
3.2. Sex-Specific MicroRNAs and Vascular Smooth Muscle Cell Proliferation
3.3. Sex-Specific MicroRNAs and Apoptosis of Neurons
4. Stroke Preventing or Ameliorating Mechanisms
4.1. Sex-Specific MicroRNAs Prevent Apoptosis of Neurons Following Cerebral Ischemia
4.2. Sex-Specific MicroRNAs in Astrocytes Diminish Neuronal Apoptosis and Microvascular Injury
4.3. Sex-Specific Estrogen-Regulated MiRs in Neurovascular Units Following Cerebral Ischemia
5. Actual and Potential Clinical Implications
5.1. Biomarker Potential for Stroke Diagnosis
5.2. Biomarker Potential for Stroke Severity and Progression
5.3. Biomarker Potential in Predicting the Therapeutic Response to Antiplatelet Therapy
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Leppert, M.H.; Ho, P.M.; Burke, J.; Madsen, T.E.; Kleindorfer, D.; Sillau, S.; Daugherty, S.; Bradley, C.J.; Poisson, S.N. Young women had more strokes than young men in a large, United States claims sample. Stroke 2020, 51, 3352–3355. [Google Scholar] [CrossRef]
- Arboix, A.; Cartanyà, A.; Lowak, M.; García-Eroles, L.; Parra, O.; Oliveres, M.; Massons, J. Gender differences and woman-specific trends in acute stroke: Results from a hospital-based registry (1986–2009). Clin. Neurol. Neurosurg. 2014, 127, 19–24. [Google Scholar] [CrossRef]
- Gargano, J.W.; Wehner, S.; Reeves, M.J. Do presenting symptoms explain sex differences in emergency department delays among patients with acute stroke? Stroke 2009, 40, 1114–1120. [Google Scholar] [CrossRef]
- Yu, A.Y.X.; Penn, A.M.; Lesperance, M.L.; Croteau, N.; Balshaw, R.F.; Votova, K.; Bibok, M.B.; Penn, M.; Saly, V.; Hegedus, J.; et al. Sex differences in presentation and outcome after an acute transient or minor neurologic event. JAMA Neurol. 2019, 76, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Carcel, C.; Wang, X.; Sandset, E.C.; Delcourt, C.; Arima, H.; Lindley, R.; Hackett, M.L.; Lavados, P.; Robinson, T.G.; Venturelli, P.M.; et al. Sex differences in treatment and outcome after stroke: Pooled analysis including 19,000 participants. Neurology 2019, 93, e2170–e2180. [Google Scholar] [CrossRef]
- Förster, A.; Gass, A.; Kern, R.; Wolf, M.E.; Ottomeyer, C.; Zohsel, K.; Hennerici, M.; Szabo, K. Gender differences in acute ischemic stroke: Etiology, stroke patterns and response to thrombolysis. Stroke 2009, 40, 2428–2432. [Google Scholar] [CrossRef]
- Roy-O’Reilly, M.; McCullough, L.D. Age and sex are critical factors in ischemic stroke pathology. Endocrinology 2018, 159, 3120–3131. [Google Scholar] [CrossRef]
- Peters, S.A.; Carcel, C.; Millett, E.R.; Woodward, M. Sex differences in the association between major risk factors and the risk of stroke in the UK Biobank cohort study. Neurology 2020, 95, e2715–e2726. [Google Scholar] [CrossRef]
- Malla, G.; Long, D.L.; Judd, S.E.; Irvin, M.R.; Kissela, B.M.; Lackland, D.T.; Safford, M.M.; Levine, D.A.; Howard, V.J.; Howard, G.; et al. Does the association of diabetes with stroke risk differ by age, race, and sex? Results from the reasons for geographic and racial differences in stroke (REGARDS) study. Diabetes Care 2019, 42, 1966–1972. [Google Scholar] [CrossRef]
- Peters, S.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar] [CrossRef]
- McDermott, M.; Miller, E.C.; Rundek, T.; Hurn, P.D.; Bushnell, C.D. Preeclampsia: Association with posterior reversible encepha-lopathy syndrome and stroke. Stroke 2018, 49, 524–530. [Google Scholar] [CrossRef]
- Medlin, F.; Amiguet, M.; Eskandari, A.; Michel, P. Sex differences in acute ischaemic stroke patients: Clinical presentation, causes and outcomes. Eur. J. Neurol. 2020, 27, 1680–1688. [Google Scholar] [CrossRef]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Kaplan, L.; Chow, B.W.; Gu, C. Neuronal regulation of the blood–brain barrier and neurovascular coupling. Nat. Rev. Neurosci. 2020, 21, 416–432. [Google Scholar] [CrossRef]
- Guo, S.; Kim, W.J.; Lok, J.; Lee, S.-R.; Besancon, E.; Luo, B.-H.; Stins, M.F.; Wang, X.; Dedhar, S.; Lo, E.H. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 7582–7587. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tian, G.-F.; Peng, W.; Lou, N.; Libionka, W.; Han, X.; Nedergaard, M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2005, 9, 260–267. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, S.L.; Young, J.M.; Nelson, J.W.; Davis, C.; Koerner, I.P.; Alkayed, N.J. Mechanism of the sex difference in neuronal ischemic cell death. Neuroscience 2012, 219, 183–191. [Google Scholar] [CrossRef]
- Chisholm, N.C.; Sohrabji, F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol. Dis. 2016, 85, 245–253. [Google Scholar] [CrossRef]
- Morrison, H.W.; Filosa, J.A. Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience 2016, 339, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, C.; Zapata, L.E.C.; Mir, F.R.; Scerbo, M.J.; Arevalo, M.A.; Garcia-Segura, L.M.; Cambiasso, M.J. Estradiol-dependent axogenesis and Ngn3 expression are determined by XY sex chromosome complement in hypothalamic neurons. Sci. Rep. 2020, 10, 8223. [Google Scholar] [CrossRef]
- Morrison, H.W.; Filosa, J.A. Stroke and the neurovascular unit: Glial cells, sex differences, and hypertension. Am. J. Physiol. Physiol. 2019, 316, C325–C339. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lo, E.H. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke 2009, 40, S4–S7. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Su, B.; Zhou, Z.; Sha, J. Rapid evolution of mammalian X-linked testis microRNAs. BMC Genom. 2009, 10, 97. [Google Scholar] [CrossRef]
- Song, R.; Ro, S.; Michaels, J.D.; Park, C.; McCarrey, J.R.; Yan, W. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat. Genet. 2009, 41, 488–493. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nat. Cell Biol. 2005, 434, 400–404. [Google Scholar] [CrossRef]
- Carroll, J.S.; Meyer, C.A.; Song, J.; Li, W.; Geistlinger, T.R.; Eeckhoute, J.; Brodsky, A.S.; Keeton, E.K.; Fertuck, K.C.; Hall, G.F.; et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006, 38, 1289–1297. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogen action: Receptors, transcripts, cell signaling, and non-coding RNAs in normal physiology and disease. Mol. Cell. Endocrinol. 2015, 418, 191–192. [Google Scholar] [CrossRef]
- Florijn, B.W.; Bijkerk, R.; van der Veer, E.P.; van Zonneveld, A.J. Gender and cardiovascular disease: Are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc. Res. 2018, 114, 210–225. [Google Scholar] [CrossRef]
- Florijn, B.W.; Valstar, G.B.; Duijs, J.M.G.J.; Menken, R.; Cramer, M.J.; Teske, A.J.; Ghossein-Doha, C.; Rutten, F.H.; Spaanderman, M.E.A.; Ruijter, H.M.D.; et al. Sex-specific microRNAs in women with diabetes and left ventricular diastolic dysfunction or HFpEF associate with microvascular injury. Sci. Rep. 2020, 10, 13945. [Google Scholar] [CrossRef]
- Bijkerk, R.; Kallenberg, M.H.; Zijlstra, L.E.; Berg, B.M.V.D.; de Bresser, J.; Hammer, S.; Bron, E.E.; Achterberg, H.; van Buchem, M.A.; Berkhout-Byrne, N.C.; et al. Circulating angiopoietin-2 and angiogenic microRNAs associate with cerebral small vessel disease and cognitive decline in older patients reaching end-stage renal disease. Nephrol. Dial. Transplant. 2020, gfaa370. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Disteche, C.M. Dosage compensation of the active X chromosome in mammals. Nat. Genet. 2005, 38, 47–53. [Google Scholar] [CrossRef]
- Augui, S.; Nora, E.; Heard, E. Regulation of X-chromosome inactivation by the X-inactivation centre. Nat. Rev. Genet. 2011, 12, 429–442. [Google Scholar] [CrossRef]
- Lee, J.T.; Bartolomei, M.S. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 2013, 152, 1308–1323. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; McClusky, R.; Itoh, Y.; Reue, K.; Arnold, A.P. X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology 2013, 154, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zheng, W.; Wu, X.; Liu, J.; Ecelbarger, C.M.; Watkins, R.; Arnold, A.P.; Sandberg, K. Sex chromosome effects unmasked in angiotensin II–induced hypertension. Hypertension 2010, 55, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.M.; Quick, S.; Ruigrok, S.R.; Graham, D.; Harris, S.E.; Verhaaren, B.F.; Fornage, M.; Seshadri, S.; Atanur, S.S.; Dominiczak, A.F.; et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci. Transl. Med. 2018, 10, eaam9507. [Google Scholar] [CrossRef] [PubMed]
- Castellano, L.; Giamas, G.; Jacob, J.; Coombes, R.C.; Lucchesi, W.; Thiruchelvam, P.; Barton, G.; Jiao, L.R.; Wait, R.; Waxman, J.; et al. The estrogen receptor-alpha-induced mi-croRNA signature regulates itself and its transcriptional response. Proc. Natl. Acad. Sci. USA 2009, 106, 15732–15737. [Google Scholar] [CrossRef]
- Bhat-Nakshatri, P.; Wang, G.; Collins, N.R.; Thomson, M.J.; Geistlinger, T.R.; Carroll, J.S.; Brown, M.; Hammond, S.; Srour, E.F.; Liu, Y.; et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009, 37, 4850–4861. [Google Scholar] [CrossRef]
- Adams, B.D.; Claffey, K.P.; White, B.A. Argonaute-2 expression is regulated by epidermal growth factor receptor and mito-gen-activated protein kinase signaling and correlates with a transformed phenotype in breast cancer cells. Endocrinology 2009, 150, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Mellios, N.; Galdzicka, M.; Ginns, E.; Baker, S.P.; Rogaev, E.; Xu, J.; Akbarian, S. Gender-specific reduction of estrogen-sensitive small RNA, miR-30b, in subjects with schizophrenia. Schizophr. Bull. 2010, 38, 433–443. [Google Scholar] [CrossRef]
- Li, P.; Wei, J.; Li, X.; Cheng, Y.; Chen, W.; Cui, Y.; Simoncini, T.; Gu, Z.; Yang, J.; Fu, X. 17β-estradiol enhances vascular endothelial Ets-1/miR-126-3p expression: The possible mechanism for attenuation of atherosclerosis. J. Clin. Endocrinol. Metab. 2016, 102, 594–603. [Google Scholar] [CrossRef]
- Stary, C.M.; Xu, L.; Li, L.; Sun, X.; Ouyang, Y.-B.; Xiong, X.; Zhao, J.; Giffard, R.G. Inhibition of miR-181a protects female mice from transient focal cerebral ischemia by targeting astrocyte estrogen receptor-α. Mol. Cell. Neurosci. 2017, 82, 118–125. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Cahill, H.; Yu, M.; Badea, T.C.; Smallwood, P.M.; Peachey, N.S.; Nathans, J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 2009, 139, 285–298. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cy-clophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef]
- Török, O.; Schreiner, B.; Schaffenrath, J.; Tsai, H.-C.; Maheshwari, U.; Stifter, S.A.; Welsh, C.; Amorim, A.; Sridhar, S.; Utz, S.G.; et al. Pericytes regulate vascular immune homeostasis in the CNS. Proc. Natl. Acad. Sci. USA 2021, 118, e2016587118. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-F.; Fischer, S.; Koshkin, A.; Laczko, E.; Fischer, D.; Ogunshola, O.O. Cell-specific metabolomic responses to injury: Novel insights into blood-brain barrier modulation. Sci. Rep. 2020, 10, 7760. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Goyal, M.S.; Blazey, T.M.; Su, Y.; Couture, L.E.; Durbin, T.J.; Bateman, R.J.; Benzinger, T.L.-S.; Morris, J.C.; Raichle, M.E.; Vlassenko, A.G. Persistent metabolic youth in the aging female brain. Proc. Natl. Acad. Sci. USA 2019, 116, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, E.S.; Azarin, S.M.; Kay, J.E.; Nessler, R.A.; Wilson, H.K.; Alahmad, A.; Palecek, S.P.; Shusta, E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012, 30, 783–791. [Google Scholar] [CrossRef]
- Gupta, N.C.; Davis, C.M.; Nelson, J.W.; Young, J.M.; Alkayed, N.J. Soluble epoxide hydrolase: Sex differences and role in endothelial cell survival. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Ahnstedt, H.; Cao, L.; Krause, D.N.; Warfvinge, K.; Säveland, H.; Nilsson, O.G.; Edvinsson, L. Male-female differences in upregulation of vasoconstrictor responses in human cerebral arteries. PLoS ONE 2013, 8, e62698. [Google Scholar] [CrossRef] [PubMed]
- Holder, S.M.; Brislane, Á.; Dawson, E.; Hopkins, N.D.; Hopman, M.T.E.; Cable, N.T.; Jones, H.; Schreuder, T.H.A.; Sprung, V.; Naylor, L.; et al. Relationship between endothelial function and the eliciting shear stress stimulus in women: Changes across the lifespan differ to men. J. Am. Hear. Assoc. 2019, 8, e010994. [Google Scholar] [CrossRef]
- Toyama, K.; Spin, J.M.; Deng, A.C.; Huang, T.T.; Wei, K.; Wagenhäuser, M.U.; Yoshino, T.; Nguyen, H.; Mulorz, J.; Kundu, S.; et al. MicroRNA-mediated therapy modulating blood-brain barrier disruption improves vascular cognitive impairment. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1392–1406. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Q.; Xie, Z.; Chen, Y.; Wang, J.; Bihl, J.; Zhong, W.; Chen, Y.; Zhao, B.; Ma, X. Implication of MicroRNA503 in brain endothelial cell function and ischemic stroke. Transl. Stroke Res. 2020, 11, 1148–1164. [Google Scholar] [CrossRef]
- Lin, M.; Zhu, L.; Wang, J.; Xue, Y.; Shang, X. miR-424–5p maybe regulate blood-brain barrier permeability in a model in vitro with Abeta incubated endothelial cells. Biochem. Biophys. Res. Commun. 2019, 517, 525–531. [Google Scholar] [CrossRef]
- Baran-Gale, J.; Purvis, J.E.; Sethupathy, P. An integrative transcriptomics approach identifies miR-503 as a candidate master regulator of the estrogen response in MCF-7 breast cancer cells. RNA 2016, 22, 1592–1603. [Google Scholar] [CrossRef]
- Cicatiello, L.; Mutarelli, M.; Grober, O.M.; Paris, O.; Ferraro, L.; Ravo, M.; Tarallo, R.; Luo, S.; Schroth, G.P.; Seifert, M.; et al. Estrogen receptor α controls a gene network in luminal-like breast cancer cells comprising multiple transcription factors and microRNAs. Am. J. Pathol. 2010, 176, 2113–2130. [Google Scholar] [CrossRef]
- Hewagama, A.; Gorelik, G.; Patel, D.; Liyanarachchi, P.; McCune, W.J.; Somers, E.; Gonzalez-Rivera, T.; Cohort, T.M.L.; Strickland, F.; Richardson, B. Overexpression of X-Linked genes in T cells from women with lupus. J. Autoimmun. 2013, 41, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hu, Z.; Feng, Y.; Yu, L.; Li, X. The transcription factor IRF6 co-represses PPARγ-mediated cytoprotection in ischemic cerebrovascular endothelial cells. Sci. Rep. 2017, 7, 2150. [Google Scholar] [CrossRef]
- Bernstein, D.L.; Zuluaga-Ramirez, V.; Gajghate, S.; Reichenbach, N.L.; Polyak, B.; Persidsky, Y.; Rom, S. miR-98 reduces endothelial dysfunction by protecting blood–brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. Br. J. Pharmacol. 2019, 40, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Van Zonneveld, A.J.; Rabelink, T.J.; Bijkerk, R. miRNA-coordinated networks as promising therapeutic targets for acute kidney injury. Am. J. Pathol. 2017, 187, 20–24. [Google Scholar] [CrossRef][Green Version]
- Shanahan, C.M. Mechanisms of vascular calcification in CKD—Evidence for premature ageing? Nat. Rev. Nephrol. 2013, 9, 661–670. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Q.; Lei, J.; Wang, X.; Chen, X.; Ding, Y. MiR-362-3p inhibits the proliferation and migration of vascular smooth muscle cells in atherosclerosis by targeting ADAMTS1. Biochem. Biophys. Res. Commun. 2017, 493, 270–276. [Google Scholar] [CrossRef]
- Sun, H.; Wu, S.; Sun, B. MicroRNA-532-5p protects against atherosclerosis through inhibiting vascular smooth muscle cell pro-liferation and migration. Cardiovasc. Diagn. Ther. 2020, 10, 481–489. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Yang, S.; Qian, D. Downregulation of miR-542-3p promotes osteogenic transition of vascular smooth muscle cells in the aging rat by targeting BMP7. Hum. Genom. 2019, 13, 67. [Google Scholar] [CrossRef]
- Mi, S.; Wang, P.; Lin, L. miR-188-3p inhibits vascular smooth muscle cell proliferation and migration by targeting fibroblast growth factor 1 (FGF1). Med. Sci. Monit. 2020, 26, e924394. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, H.; Liang, J.; Li, Y.; Li, X. MicroRNA-503-5p improves carotid artery stenosis by inhibiting the proliferation of vas-cular smooth muscle cells. Exp. Ther. Med. 2020, 20, 85. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.L.; Gu, J.J.; Sun, Y.Q.; Cui, H.Z.; Bu, J.Q.; Chen, Z.Y. Exosome-mediated miR-106a-3p derived from ox-LDL exposed mac-rophages accelerated cell proliferation and repressed cell apoptosis of human vascular smooth muscle cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7039–7050. [Google Scholar]
- Voigt, S.; van Os, H.; van Walderveen, M.; van der Schaaf, I.; Kappelle, L.; Broersen, A.; Velthuis, B.; de Jong, P.; Kockelkoren, R.; Kruyt, N.; et al. Sex differences in intracranial and extracranial atherosclerosis in patients with acute ischemic stroke. Int. J. Stroke 2021, 16, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal cell death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Cuomo, O.; Cepparulo, P.; Anzilotti, S.; Serani, A.; Sirabella, R.; Brancaccio, P.; Guida, N.; Valsecchi, V.; Vinciguerra, A.; Molinaro, P.; et al. Anti-miR-223-5p ameliorates ischemic damage and improves neurological function by preventing NCKX2 downregulation after ischemia in rats. Mol. Ther. Nucleic Acids 2019, 18, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.-L.; Wang, A.-P.; Song, G.-L.; Yang, Z.-B. miR-652 protects rats from cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Biomed. Pharmacother. 2020, 124, 109860. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, Q. NR4A2 exacerbates cerebral ischemic brain injury via modulating microRNA-652/Mul1 pathway. Neuropsychiatr. Dis. Treat. 2020, 16, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cui, P.; Ge, H.; Shi, Y.; Wu, X.; Ru, Z.F. miR-188-5p inhibits apoptosis of neuronal cells during oxygen-glucose deprivation (OGD)-induced stroke by suppressing PTEN. Exp. Mol. Pathol. 2020, 116, 104512. [Google Scholar] [CrossRef]
- Farajdokht, F.; Mohaddes, G.; Karimi-Sales, E.; Kafshdooz, T.; Mahmoudi, J.; Aberoumandi, S.M.; Karimi, P. Inhibition of PTEN protects PC12 cells against oxygen-glucose deprivation induced cell death through mitoprotection. Brain Res. 2018, 1692, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, X.; Huang, J.; Cui, S.; Zhang, L. CDKN1B mediates apoptosis of neuronal cells and inflammation induced by oxyhemoglobin via miR-502-5p after subarachnoid hemorrhage. J. Mol. Neurosci. 2020, 70, 1073–1080. [Google Scholar] [CrossRef]
- Araki, T.; Milbrandt, J. Ninjurin2, a novel homophilic adhesion molecule, is expressed in mature sensory and enteric neurons and promotes neurite outgrowth. J. Neurosci. 2000, 20, 187–195. [Google Scholar] [CrossRef]
- Jing, D.; Yinzhu, L.; Jinjing, P.; Lishuang, L.; Guozhuan, Z. Targeting ninjurin 2 by miR-764 regulates hydrogen peroxide (H2O2)-induced neuronal cell death. Biochem. Biophys. Res. Commun. 2018, 505, 1180–1188. [Google Scholar] [CrossRef]
- Feng, S.-J.; Zhang, X.-Q.; Li, J.-T.; Dai, X.-M.; Zhao, F. miRNA-223 regulates ischemic neuronal injury by targeting the type 1 insulin-like growth factor receptor (IGF1R). Folia Neuropathol. 2018, 56, 49–57. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; An, K.; Kwon, O.-B.; Park, S.; Cha, J.H.; Kim, M.-H.; Lee, Y.; Kim, J.-H.; Cho, K.; et al. Replenishment of microRNA-188-5p restores the synaptic and cognitive deficits in 5XFAD mouse model of Alzheimer’s disease. Sci. Rep. 2016, 6, 34433. [Google Scholar] [CrossRef]
- Alano, C.C.; Garnier, P.; Ying, W.; Higashi, Y.; Kauppinen, T.; Swanson, R. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J. Neurosci. 2010, 30, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.T.; McCullough, L.D. Pathways to ischemic neuronal cell death: Are sex differences relevant? J. Transl. Med. 2008, 6, 33. [Google Scholar] [CrossRef]
- Renolleau, S.; Fau, S.; Goyenvalle, C.; Joly, L.-M.; Chauvier, D.; Jacotot, E.; Mariani, J.; Charriaut-Marlangue, C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: A role for gender. J. Neurochem. 2007, 100, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.; Li, J.; Siegel, C.; Yuan, R.; McCullough, L.D. Sex differences in caspase activation after stroke. Stroke 2009, 40, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Harraz, M.M.; Eacker, S.M.; Wang, X.; Dawson, T.M.; Dawson, V.L. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 18962–18967. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Wang, R.; Wang, P.; Tao, Z.; Gao, L.; Yan, F.; Liu, X.; Yu, S.; Ji, X.; et al. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke 2015, 46, 513–519. [Google Scholar] [CrossRef]
- Yu, S.; Zhai, J.; Yu, J.; Yang, Q.; Yang, J. miR-98-5p protects against cerebral ischemia/reperfusion injury through anti-apoptosis and anti-oxidative stress in mice. J. Biochem. 2021, 169, 195–206. [Google Scholar] [CrossRef]
- Min, X.-L.; He, M.; Shi, Y.; Xie, L.; Ma, X.-J.; Cao, Y. miR-18b attenuates cerebral ischemia/reperfusion injury through regulation of ANXA3 and PI3K/Akt signaling pathway. Brain Res. Bull. 2020, 161, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Waisman, A.; Ginhoux, F.; Greter, M.; Bruttger, J. Homeostasis of microglia in the adult brain: Review of novel microglia depletion systems. Trends Immunol. 2015, 36, 625–636. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gao, L.; Wang, R.; Liu, X.; Gao, Z.; Tao, Z.; Xu, C.; Song, J.; Ji, X.; et al. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke 2013, 44, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Hu, J.; Lv, H.; Cui, X.; Di, W. miR-221 exerts neuroprotective effects in ischemic stroke by inhibiting the proinflammatory response. J. Stroke Cerebrovasc. Dis. 2021, 30, 105489. [Google Scholar] [CrossRef]
- Pegoraro, V.; Merico, A.; Angelini, C. Micro-RNAs in ALS muscle: Differences in gender, age at onset and disease duration. J. Neurol. Sci. 2017, 380, 58–63. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, M.; Li, J.; Wu, B.; Tian, W.; Shi, L.; Zhang, J.; Sun, Z. The inverted pattern of circulating miR-221-3p and miR-222-3p associated with isolated low HDL-C phenotype. Lipids Health Dis. 2018, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Erhard, F.; Haas, J.; Lieber, D.; Malterer, G.; Jaskiewicz, L.; Zavolan, M.; Dölken, L.; Zimmer, R. Widespread context dependency of microRNA-mediated regulation. Genome Res. 2014, 24, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Kutuzov, N.; Flyvbjerg, H.; Lauritzen, M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain barrier. Proc. Natl. Acad. Sci. USA 2018, 115, E9429–E9438. [Google Scholar] [CrossRef]
- Durkee, C.A.; Araque, A. Diversity and specificity of astrocyte–neuron communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Calixto, A.; Cardona-Gómez, G.P. The role of astrocytes in neuroprotection after brain stroke: Potential in cell therapy. Front. Mol. Neurosci. 2017, 10, 88. [Google Scholar] [CrossRef]
- Selvamani, A.; Sohrabji, F. Mir363-3p improves ischemic stroke outcomes in female but not male rats. Neurochem. Int. 2017, 107, 168–181. [Google Scholar] [CrossRef]
- Zhao, H.; Li, G.; Wang, R.; Tao, Z.; Zhang, S.; Li, F.; Han, Z.; Li, L.; Liu, P.; Luo, Y. MiR-424 prevents astrogliosis after cerebral ischemia/reperfusion in elderly mice by enhancing repressive H3K27me3 via NFIA/DNMT1 signaling. FEBS J. 2019, 286, 4926–4936. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, Y.M.; Kim, D.H.; Moon, G.J.; Bang, O.Y.; Ohn, T.; Kim, H.H. Ischemic brain extract increases SDF-1 expression in astrocytes through the CXCR2/miR-223/miR-27b pathway. Biochim. Biophys. Acta 2014, 1839, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Lu, Y.; Sareddy, G.R.; Wang, J.; Zhang, Q.; Tang, F.-L.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Neuron-derived estrogen is critical for astrocyte activation and neuroprotection of the ischemic brain. J. Neurosci. 2020, 40, 7355–7374. [Google Scholar] [CrossRef]
- Liu, M.; Hurn, P.D.; Roselli, C.E.; Alkayed, N.J. Role of P450 aromatase in sex-specific astrocytic cell death. Br. J. Pharmacol. 2007, 27, 135–141. [Google Scholar] [CrossRef]
- Giordano, G.; Tait, L.; Furlong, C.E.; Cole, T.B.; Kavanagh, T.J.; Costa, L.G. Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radic. Biol. Med. 2013, 58, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Senju, A.; Oguro, A.; Shimono, M.; Araki, S.; Kusuhara, K.; Itoh, K.; Tsuji, M.; Ishihara, Y. Measurement of the estradiol concentration in cerebro-spinal fluid from infants and its correlation with serum estradiol and exosomal microRNA-126-5p. Biol. Pharm. Bull. 2020, 43, 1966–1968. [Google Scholar] [CrossRef]
- Herzog, R.; Zendedel, A.; Lammerding, L.; Beyer, C.; Slowik, A. Impact of 17beta-estradiol and progesterone on inflammatory and apoptotic microRNA expression after ischemia in a rat model. J. Steroid Biochem. Mol. Biol. 2017, 167, 126–134. [Google Scholar] [CrossRef]
- Kamel, H.; Navi, B.; Sriram, N.; Hovsepian, D.A.; Devereux, R.B.; Elkind, M.S. Risk of a thrombotic event after the 6-week postpartum period. N. Engl. J. Med. 2014, 370, 1307–1315. [Google Scholar] [CrossRef]
- Gillum, L.A.; Mamidipudi, S.K.; Johnston, S.C. Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA 2000, 284, 72–78. [Google Scholar] [CrossRef]
- Florijn, B.W.; Duijs, J.M.; Klaver, M.; Kuipers, E.N.; Kooijman, S.; Prins, J.; Zhang, H.; Sips, H.C.; Stam, W.; Hanegraaf, M.; et al. Estradiol-driven metabolism in transwomen associates with reduced circulating extracellular vesicle microRNA-224/452. Eur. J. Endocrinol. 2021, 185, 539–552. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, O.J.; Kim, S.Y.; Oh, S.H.; Oh, D.; Kim, O.J.; Shin, B.S.; Kim, N.K. Association of the miR-146a, miR-149, miR-196a2, and miR-499 poly-morphisms with ischemic stroke and silent brain infarction risk. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 420–430. [Google Scholar] [CrossRef]
- Blanco-Rojas, L.; Arboix, A.; Cánovas, D.; Grau-Olivares, M.; Morera, J.C.O.; Parra, O. Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: A comparative study. BMC Neurol. 2013, 13, 203. [Google Scholar] [CrossRef]
- Abe, A.; Tanaka, M.; Yasuoka, A.; Saito, Y.; Okada, S.; Mishina, M.; Abe, K.; Kimura, K.; Asakura, T. Changes in whole-blood microRNA profiles during the onset and treatment process of cerebral infarction: A human study. Int. J. Mol. Sci. 2020, 21, 3107. [Google Scholar] [CrossRef]
- Peng, H.; Yang, H.; Xiang, X.; Li, S. ΜicroRNA-221 participates in cerebral ischemic stroke by modulating endothelial cell function by regulating the PTEN/PI3K/AKT pathway. Exp. Ther. Med. 2019, 19, 443–450. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Liao, Y.-C.; Wang, Y.-S.; Lin, H.-F.; Lin, R.-T.; Juo, S.-H.H. Serum microRNA-21 and microRNA-221 as potential biomarkers for cerebrovascular disease. J. Vasc. Res. 2013, 50, 346–354. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Huang, J.; Chen, X.; Gu, X.; Wang, Y.; Zeng, L.; Yang, G.-Y. Increase of circulating miR-223 and insulin-like growth factor-1 is associated with the pathogenesis of acute ischemic stroke in patients. BMC Neurol. 2014, 14, 77. [Google Scholar] [CrossRef]
- Khalifa, O.; Pers, Y.M.; Ferreira, R.; Sénéchal, A.; Jorgensen, C.; Apparailly, F.; Duroux-Richard, I. X-linked miRNAs associated with gender differences in rheumatoid arthritis. Int. J. Mol. Sci. 2016, 17, 1852. [Google Scholar] [CrossRef]
- Li, P.; Teng, F.; Gao, F.; Zhang, M.; Wu, J.; Zhang, C. Identification of circulating microRNAs as potential biomarkers for detecting acute ischemic stroke. Cell. Mol. Neurobiol. 2014, 35, 433–447. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Manizheh, D.; Mohammad, S.; Hasan, T.M.; Saman, N.; Laleh, R.; Mahsa, M.; Sanaz, A.K.; Shaghayegh, H.J. Can miR-503 be used as a marker in diabetic patients with ischemic stroke? BMC Endocr. Disord. 2019, 19, 42. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, J.; Xu, F. Circulating miR-29b and miR-424 as prognostic markers in patients with acute cerebral infarction. Clin. Lab. 2017, 63, 1667–1674. [Google Scholar] [CrossRef]
- Li, G.; Ma, Q.; Wang, R.; Fan, Z.; Tao, Z.; Liu, P.; Zhao, H.; Luo, Y. Diagnostic and immunosuppressive potential of elevated mir-424 levels in circulating immune cells of ischemic stroke patients. Aging Dis. 2018, 9, 172–781. [Google Scholar] [CrossRef]
- Elkind, M.S.V.; Luna, J.M.; McClure, L.A.; Zhang, Y.; Coffey, C.S.; Roldan, A.; Del Brutto, O.H.; Pretell, E.J.; Pettigrew, L.C.; Meyer, B.C.; et al. C-reactive protein as a prognostic marker after lacunar stroke: Levels of inflammatory markers in the treatment of stroke study. Stroke 2014, 45, 707–716. [Google Scholar] [CrossRef]
- Kaplan, R.C.; McGinn, A.P.; Baird, A.E.; Hendrix, S.L.; Kooperberg, C.; Lynch, J.; Rosenbaum, D.M.; Johnson, K.C.; Strickler, H.D.; Wassertheil-Smoller, S. Inflammation and hemostasis biomarkers for predicting stroke in postmenopausal women: The women′s health initiative observational study. J Stroke Cerebrovasc. Dis. 2008, 17, 344–355. [Google Scholar] [CrossRef]
- Cui, C.; Yang, W.; Shi, J.; Zhou, Y.; Yang, J.; Cui, Q.; Zhou, Y. Identification and analysis of human sex-biased microRNAs. Genom. Proteom. Bioinform. 2018, 16, 200–211. [Google Scholar] [CrossRef]
- Leng, X.; Leung, T.W.; Wong, K.L. Antiplatelet therapy after stroke: Should it differ in the acute and chronic phase after stroke. Curr. Opin. Neurol. 2018, 31, 14–22. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Tantry, U.S. Antiplatelet drug resistance and variability in response: The role of antiplatelet therapy monitoring. Antiplatelet Anticoagul. Ther. 2012, 19, 45–112. [Google Scholar] [CrossRef]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef]
- Kaudewitz, D.; Skroblin, P.; Bender, L.H.; Barwari, T.; Willeit, P.; Pechlaner, R.; Sunderland, N.P.; Willeit, K.; Morton, A.C.; Armstrong, P.; et al. Association of microRNAs and YRNAs with platelet function. Circ. Res. 2016, 118, 420–432. [Google Scholar] [CrossRef] [PubMed]
- De Boer, H.C.; van Solingen, C.; Prins, J.; Duijs, J.M.; Huisman, M.V.; Rabelink, T.J.; van Zonneveld, A.J. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur. Heart J. 2013, 34, 3451–3457. [Google Scholar] [CrossRef]
- Duan, X.; Zhan, Q.; Song, B.; Zeng, S.; Zhou, J.; Long, Y.; Lu, J.; Li, Z.; Yuan, M.; Chen, X.; et al. Detection of platelet microRNA expression in patients with diabetes mellitus with or without ischemic stroke. J. Diabetes Complicat. 2014, 28, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Phillips, C.M.; Martinez-Revollar, G.; Keep, R.F.; Andjelkovic, A.V. Involvement of epigenetic mechanisms and non-coding RNAs in blood-brain barrier and neurovascular unit injury and recovery after stroke. Front. Neurosci. 2019, 13, 864. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, K.; Hassan, S.H.; Zhang, X.; Tang, X.; Pu, H.; Stetler, R.A.; Chen, J.; Yin, K.-J. Endothelium-targeted deletion of microRNA-15a/16-1 promotes poststroke angiogenesis and improves long-term neurological recovery. Circ. Res. 2020, 126, 1040–1057. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florijn, B.W.; Bijkerk, R.; Kruyt, N.D.; van Zonneveld, A.J.; Wermer, M.J.H. Sex-Specific MicroRNAs in Neurovascular Units in Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 11888. https://doi.org/10.3390/ijms222111888
Florijn BW, Bijkerk R, Kruyt ND, van Zonneveld AJ, Wermer MJH. Sex-Specific MicroRNAs in Neurovascular Units in Ischemic Stroke. International Journal of Molecular Sciences. 2021; 22(21):11888. https://doi.org/10.3390/ijms222111888
Chicago/Turabian StyleFlorijn, Barend W., Roel Bijkerk, Nyika D. Kruyt, Anton Jan van Zonneveld, and Marieke J. H. Wermer. 2021. "Sex-Specific MicroRNAs in Neurovascular Units in Ischemic Stroke" International Journal of Molecular Sciences 22, no. 21: 11888. https://doi.org/10.3390/ijms222111888
APA StyleFlorijn, B. W., Bijkerk, R., Kruyt, N. D., van Zonneveld, A. J., & Wermer, M. J. H. (2021). Sex-Specific MicroRNAs in Neurovascular Units in Ischemic Stroke. International Journal of Molecular Sciences, 22(21), 11888. https://doi.org/10.3390/ijms222111888





