PI3Kinase Inhibition in Hormone Receptor-Positive Breast Cancer
Abstract
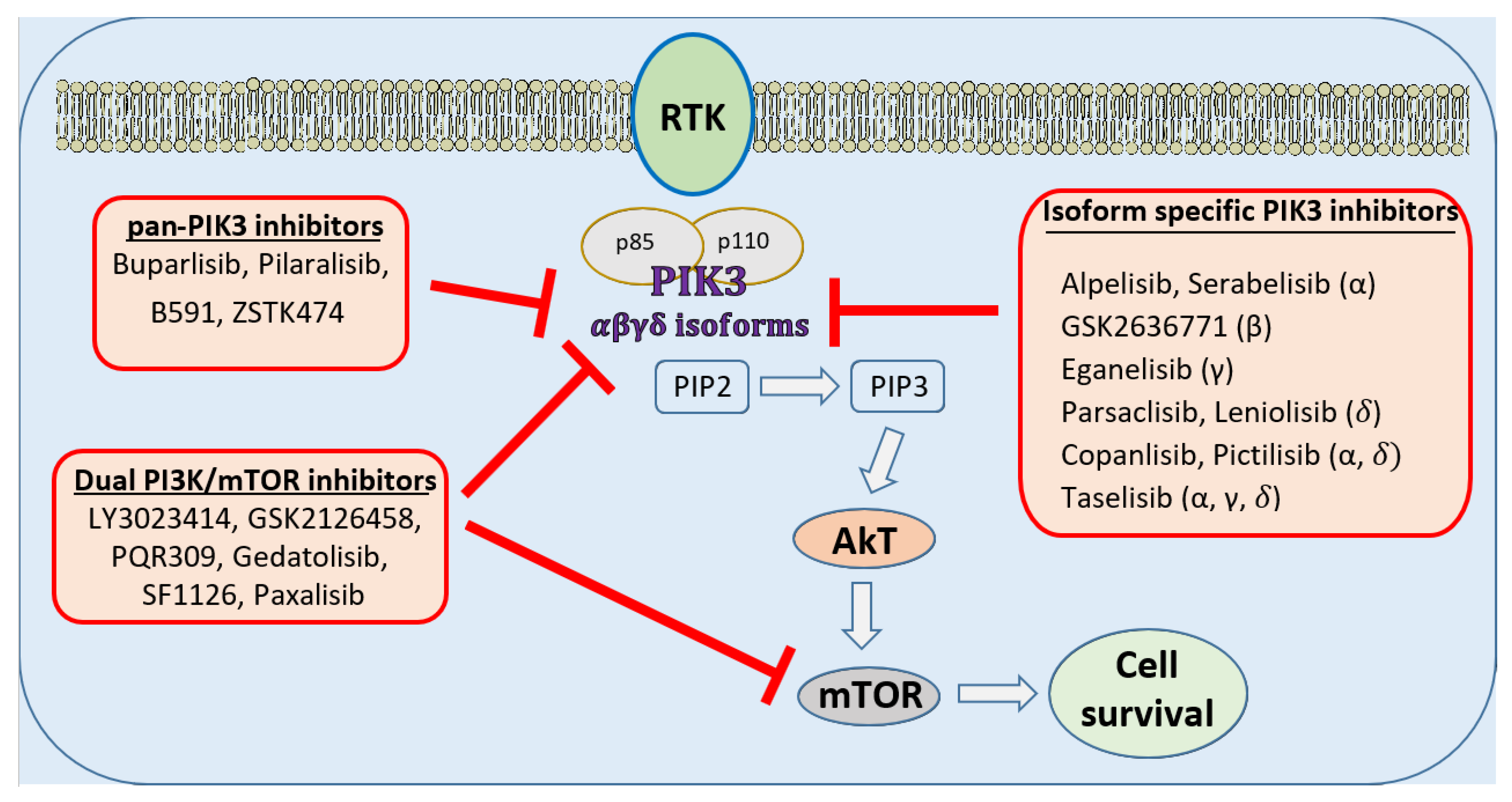
:1. Role and Discovery of PI3Kinase in Cancer
2. PI3K in Breast Cancer
3. Buparlisib (BKM120)
4. Taselisib (GDC-0032)
5. Alpelisib
6. Utility and Limitation of Current Alpelisib Data
7. Opportunity and Challenges of Adopting PI3K Inhibitors in Clinic
8. Cross Trial Comparison of Alpelisib, Taselisib and Buparlisib
9. Future Perspectives with PI3K Inhibitors in HR+ Breast Cancer
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PI3K | phosphatidylinositol-3 kinase |
| EGF | epidermal growth factor |
| HR | hormone receptor |
| ER | estrogen receptor |
| LTED | long-term estrogen deprivation |
| AI | aromatase inhibitor |
| PFS | progression free survival |
| AE | adverse event |
| G | grade |
| MBC | metastatic breast cancer |
| MTD | maximum tolerated dose |
References
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Leevers, S.J.; Vanhaesebroeck, B.; Waterfield, M.D. Signalling through phosphoinositide 3-kinases: The lipids take centre stage. Curr. Opin. Cell Biol. 1999, 11, 219–225. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Crowder, R.J.; Phommaly, C.; Tao, Y.; Hoog, J.; Luo, J.; Perou, C.M.; Parker, J.S.; Miller, M.A.; Huntsman, D.G.; Lin, L.; et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009, 69, 3955–3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.G.; Ma, C.X.; Crowder, R.J.; Guintoli, T.; Phommaly, C.; Gao, F.; Lin, L.; Ellis, M.J. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 2011, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Sansal, I.; Sellers, W.R. The biology and clinical relevance of the PTEN tumor suppressor pathway. J. Clin. Oncol. 2004, 22, 2954–2963. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Engelman, J.A.; Irie, H.Y.; Luo, J.; Brachmann, S.M.; Pearline, R.V.; Cantley, L.C.; Brugge, J.S. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005, 65, 10992–101000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.J.; Liu, Z.; Wang, L.; Shin, E.; Loda, M.F.; Roberts, T.M. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc. Natl. Acad. Sci. USA 2005, 102, 18443–18448. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, G.; Dziubinski, M.; Yang, Z.; Ethier, S.P.; Wu, G. Comprehensive analysis of oncogenic effects of PIK3CA mutations in human mammary epithelial cells. Breast Cancer Res. Treat. 2008, 112, 217–227. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loibl, S.; de la Pena, L.; Nekljudova, V.; Zardavas, D.; Michiels, S.; Denkert, C.; Rezai, M.; Bermejo, B.; Untch, M.; Lee, S.C.; et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: A randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur. J. Cancer 2017, 85, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Metzger Filho, O.; Goel, S.; Barry, W.T.; Hamilton, E.P.; Tolaney, S.M.; Yardley, D.A.; Rees, R.; Demeo, M.; Mills, C.; Hafner, M.; et al. A mouse-human phase I co-clinical trial of taselisib in combination with TDM1 in advanced HER2-positive breast cancer (MBC). J. Clin. Oncol. 2017, 35, 1030. [Google Scholar] [CrossRef]
- Jain, S.; Shah, A.N.; Santa-Maria, C.A.; Siziopikou, K.; Rademaker, A.; Helenowski, I.; Cristofanilli, M.; Gradishar, W.J. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2018, 171, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Janku, F.; Rodón, J.; Burris, H.A.; Mayer, I.A.; Schuler, M.; Seggewiss-Bernhardt, R.; Gil-Martin, M.; Middleton, M.R.; Baselga, J.; et al. Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2019, 5, e184475. [Google Scholar] [CrossRef] [Green Version]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- Saura, C.; Hlauschek, D.; Oliveira, M.; Zardavas, D.; Jallitsch-Halper, A.; de la Peña, L.; Nuciforo, P.; Ballestrero, A.; Dubsky, P.; Lombard, J.M.; et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019, 20, 1226–1238. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L.; et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020, 22, 120. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Abramson, V.G.; Sanders, M.E.; Mayer, E.L.; Haddad, T.C.; Nanda, R.; Van Poznak, C.; Storniolo, A.M.; Nangia, J.R.; Gonzalez-Ericsson, P.I.; et al. TBCRC 032 IB/II Multicenter Study: Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR(+) Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2020, 26, 2111–2123. [Google Scholar] [CrossRef]
- Martín, M.; Chan, A.; Dirix, L.; O’Shaughnessy, J.; Hegg, R.; Manikhas, A.; Shtivelband, M.; Krivorotko, P.; Lopez, N.B.; Campone, M.; et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann. Oncol. 2017, 28, 313–320. [Google Scholar] [CrossRef]
- Burger, M.T.; Pecchi, S.; Wagman, A.; Ni, Z.J.; Knapp, M.; Hendrickson, T.; Atallah, G.; Pfister, K.; Zhang, Y.; Bartulis, S.; et al. Identification of NVP-BKM120 as a Potent, Selective, Orally Bioavailable Class I PI3 Kinase Inhibitor for Treating Cancer. ACS Med. Chem. Lett. 2011, 2, 774–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Inada-Inoue, M.; Mitsuma, A.; Yoshino, T.; Ohtsu, A.; Suenaga, N.; Sato, M.; Kakizume, T.; Robson, M.; Quadt, C.; et al. Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci. 2014, 105, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Bendell, J.C.; Rodon, J.; Burris, H.A.; De Jonge, M.; Verweij, J.; Birle, D.; Demanse, D.; De Buck, S.S.; Ru, Q.C.; Peters, M.; et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012, 30, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Braña, I.; Siu, L.L.; De Jonge, M.J.; Homji, N.; Mills, D.; Di Tomaso, E.; Sarr, C.; Trandafir, L.; Massacesi, C.; et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Investig. N. Drugs 2014, 32, 670–681. [Google Scholar] [CrossRef]
- Mayer, I.A.; Abramson, V.G.; Isakoff, S.J.; Forero, A.; Balko, J.M.; Kuba, M.G.; Sanders, M.E.; Yap, J.T.; Van den Abbeele, A.D.; Li, Y.; et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2014, 32, 1202–1209. [Google Scholar] [CrossRef]
- Baselga, J.; Im, S.A.; Iwata, H.; Cortés, J.; De Laurentiis, M.; Jiang, Z.; Arteaga, C.L.; Jonat, W.; Clemons, M.; Ito, Y.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.A.; Kalev, D.; Egle, D.; et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar] [CrossRef]
- Lu, Y.S.; Lee, K.S.; Chao, T.Y.; Tseng, L.M.; Chitapanarux, I.; Chen, S.C.; Liu, C.T.; Sohn, J.; Kim, J.H.; Chang, Y.C.; et al. A Phase Ib Study of Alpelisib or Buparlisib Combined with Tamoxifen Plus Goserelin in Premenopausal Women with HR-Positive HER2-Negative Advanced Breast Cancer. Clin. Cancer Res. 2021, 27, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Im, Y.H.; Calvo, E.; Lu, Y.S.; Hamilton, E.; Forero-Torres, A.; Bachelot, T.; Maur, M.; Fasolo, A.; Tiedt, R.; et al. Phase Ib Study of Ribociclib plus Fulvestrant and Ribociclib plus Fulvestrant plus PI3K Inhibitor (Alpelisib or Buparlisib) for HR(+) Advanced Breast Cancer. Clin. Cancer Res. 2021, 27, 418–428. [Google Scholar] [CrossRef]
- Ndubaku, C.O.; Heffron, T.P.; Staben, S.T.; Baumgardner, M.; Blaquiere, N.; Bradley, E.; Bull, R.; Do, S.; Dotson, J.; Dudley, D.; et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): A β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J. Med. Chem. 2013, 56, 4597–4610. [Google Scholar] [PubMed]
- Heffron, T.P.; Heald, R.A.; Ndubaku, C.; Wei, B.; Augistin, M.; Do, S.; Edgar, K.; Eigenbrot, C.; Friedman, L.; Gancia, E.; et al. The Rational Design of Selective Benzoxazepin Inhibitors of the α-Isoform of Phosphoinositide 3-Kinase Culminating in the Identification of (S)-2-((2-(1-Isopropyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)oxy)propanamide (GDC-0326). J. Med. Chem. 2016, 59, 985–1002. [Google Scholar] [PubMed]
- Hoeflich, K.P.; Guan, J.; Edgar, K.A.; O’Brien, C.; Savage, H.; Wilson, T.R.; Neve, R.M.; Friedman, L.S.; Wallin, J.J. The PI3K inhibitor taselisib overcomes letrozole resistance in a breast cancer model expressing aromatase. Genes. Cancer 2016, 7, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Dickler, M.N.; Saura, C.; Richards, D.A.; Krop, I.E.; Cervantes, A.; Bedard, P.L.; Patel, M.R.; Pusztai, L.; Oliveira, M.; Cardenas, A.K.; et al. Phase II Study of Taselisib (GDC-0032) in Combination with Fulvestrant in Patients with HER2-Negative, Hormone Receptor-Positive Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 4380–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baselga, J.; Dent, S.F.; Cortés, J.; Im, Y.H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Verma, S.; Wilson, T.R.; Jin, H.; et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. J. Clin. Oncol. 2018, 36 (Suppl. 18), LBA1006. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- Pascual, J.; Lim, J.S.; Macpherson, I.R.; Armstrong, A.C.; Ring, A.; Okines, A.F.; Cutts, R.J.; Herrera-Abreu, M.T.; Garcia-Murillas, I.; Pearson, A.; et al. Triplet Therapy with Palbociclib, Taselisib, and Fulvestrant in PIK3CA-Mutant Breast Cancer and Doublet Palbociclib and Taselisib in Pathway-Mutant Solid Cancers. Cancer Discov. 2021, 11, 92–107. [Google Scholar] [CrossRef]
- Jhaveri, K.; Chang, M.T.; Juric, D.; Saura, C.; Gambardella, V.; Melnyk, A.; Patel, M.R.; Ribrag, V.; Ma, C.X.; Aljumaily, R.; et al. Phase I Basket Study of Taselisib, an Isoform-Selective PI3K Inhibitor, in Patients with PIK3CA-Mutant Cancers. Clin. Cancer Res. 2021, 27, 447–459. [Google Scholar] [CrossRef]
- Fritsch, C.; Huang, A.; Chatenay-Rivauday, C.; Schnell, C.; Reddy, A.; Liu, M.; Kauffmann, A.; Guthy, D.; Erdmann, D.; De Pover, A.; et al. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol. Cancer Ther. 2014, 13, 1117–1129. [Google Scholar] [CrossRef] [Green Version]
- Bosch, A.; Li, Z.; Bergamaschi, A.; Ellis, H.; Toska, E.; Prat, A.; Tao, J.J.; Spratt, D.E.; Viola-Villegas, N.T.; Castel, P.; et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci. Transl. Med. 2015, 7, 283ra51. [Google Scholar] [CrossRef] [Green Version]
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; Balko, J.M.; Estrada, M.V.; Sanders, M.E.; Juric, D.; Solit, D.; Berger, M.F.; Won, H.H.; et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2- Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Prowell, T.M.; Gao, J.J.; Fernandes, L.L.; Li, E.; Jiang, X.; Qiu, J.; Fan, J.; Song, P.; Yu, J.; et al. FDA Approval Summary: Alpelisib Plus Fulvestrant for Patients with HR-positive, HER2-negative, PIK3CA-mutated, Advanced or Metastatic Breast Cancer. Clin. Cancer Res. 2021, 27, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Batalini, F.; Moulder, S.L.; Winer, E.P.; Rugo, H.S.; Lin, N.U.; Wulf, G.M. Response of Brain Metastases From PIK3CA-Mutant Breast Cancer to Alpelisib. JCO Precis. Oncol. 2020, 4, 572–578. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef]
- Mosele, F.; Stefanovska, B.; Lusque, A.; Dien, A.T.; Garberis, I.; Droin, N.; Le Tourneau, C.; Sablin, M.P.; Lacroix, L.; Enrico, D.; et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann. Oncol. 2020, 31, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Signorovitch, J.; Andre, F.; Wang, R.; Lorenzo, I.; Ridolfi, A.; Park, J.; Fillbrunn, M.; Dua, A.; Rugo, H.S. PIK3CA mutation status and progression-free survival in advanced hormone receptor positive (HR+)/human endocrine receptor negative (HER2–) metastatic breast cancer (mBC): A meta-analysis of published clinical trials. J. Clin. Oncol. 2020, 38 (Suppl. 15), 1069. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Juric, D.; Turner, N.; Chia, S.; Drullinsky, P.; Prat, A.; Vázquez, R.V.; Akdere, M.; Arce, C.; et al. Abstract PD2-07: Alpelisib + letrozole in patients with PIK3CA-mutated, hormone-receptor positive (HR+), human epidermal growth factor receptor-2-negative (HER2-) advanced breast cancer (ABC) previously treated with a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) + fulvestrant: BYLieve study results. Cancer Res. 2021, 81 (Suppl. 4), PD2-07. [Google Scholar]
| Trial ID and Year of Publication | Pharmacological Agents | Cohort and Settings | Clinical Outcome | Key Adverse Effects |
|---|---|---|---|---|
| HER2+ Breast Cancer | ||||
| NCT01816594 (NeoPHOEBE), 2017 [13] | Trastuzumab +Paclitaxel with buparlisib/placebo | HER2+ breast cancer, randomized phase II, neoadjuvant | Trial suspended mainly due to liver toxicity | Liver dysfunction, diarrhea, alopecia, early fatigue, skin rash |
| NCT02390427, 2017, [14] | Taselisib + TDM1 | Metastatic HER2+ breast cancer who have received prior treatments for metastatic breast cancer, Phase I | Median progression-free survival (PFS) 7.6 months | Diarrhea, vomiting, pneumonitis, thrombocytopenia |
| NCT02038010, 2018, [15] | Alpelisib + TDM1 | HER2+ metastatic breast cancer, which has progressed on trastuzumab, Phase I | Median PFS 8.1 months, Maximum tolerated dose of Alpelisisb 250 mg daily | Easy fatigue, weight loss, hyperglycemia, hypertension, pancreatitis. |
| HR+ Breast Cancer | ||||
| NCT01219699, 2019, [16] | Alpelisib and Fulvestrant | HR+ advanced breast cancer, phase Ib | Significant clinical activity and reasonable safety profile; recommended phase II dose of alpelisib 300 mg daily | Easy fatigue, vomiting, diarrhea, hyperglycemia, skin rash. |
| NCT02437318 (SOLAR 1), 2021, [17] | Alpelisib and Fulvestrant | PIK3CA-mutated HR+ advanced breast cancer | Numerical improvement of 7.9 months in median overall survival with alpelisib | Hyperglycemia, skin rash, diarrhea |
| NCT02273973 (LORELEI), 2019, [18] | Taselisib and Letrozole | HR+, HER2 negative early stage breast cancer in neoadjuvant settings, randomized phase II study | Increased objective response with the addition of taselisib. | Easy fatigue, diarrhea, hyperglycemia, skin rash, arthralgia. |
| Triple Negative Breast Cancer | ||||
| NCT01790932, 2020 [19] | Buparlisib | Triple-negative metastatic breast cancer, single-arm phase II study | clinical benefit rate of only 12% | Fatigue, nausea, anorexia, hyperglycemia |
| HER2 Negative Breast Cancer | ||||
| NCT02457910, 2020, [20] | Taselisib plus anti-androgen therapy Enzalutamide | Androgen Receptor (AR)+, metastatic breast cancer, phase Ib/II | An increase in clinical benefit rate with the combination among AR+ TNBC | Hyperglycemia, skin rash, fever, easy fatigue, vomiting |
| NCT01572727 (BELLE 4), 2017, [21] | Paclitaxel with Buparlisib/placebo | HER2 negative locally advanced or metastatic breast cancer (MBC) with no prior chemotherapy for MBC; Phase II/III | Suspended after Phase II due to futility | Diarrhea, alopecia, skin rash, nausea, hyperglycemia, reduced appetite. |
| Trial ID | Study Treatment | Phase | Breast Cancer Receptor/Mutation Type | Stage |
|---|---|---|---|---|
| NCT02626507 | Gedatolisib (PI3Kinase/mTOR pathway inhibitor), Faslodex, Palbociclib, and Zoladex | Ib | HR+ HER2 negative, PIK3CA mutant or wildtype | Neoadjuvant settings for untreated breast cancers |
| NCT01625286 | AZD533 (PI3Kinase/AKT pathway inhibitor) and paclitaxel | I/II | HR+ HER2 negative, PIK3CA mutant or wildtype | metastatic breast cancer |
| NCT02389842 | Palbociclib and Taselisib or Pictelisib | Ib | PIK3CA mutant only | advanced solid tumors |
| NCT01872260 | Ribociclib, Letrozole and alpelisib | Ib | HR+ HER2 negative PIK3CA mutant or wildtype | metastatic breast cancer |
| NCT04300790 | Alpelisib, Metformin and Fulvestrant | II | HR+ HER2 negative, PIK3CA mutant | metastatic breast cancer |
| NCT03803761 | Copanlisib (PI3Kinase inhibitor- alpha and delta) and fulvestrant | II | PIK3CA mutant or PTEN loss | Advanced metastatic breast cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, A.; Acharya, L.; O’Regan, R.; Gandhi, S.; Falkson, C. PI3Kinase Inhibition in Hormone Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 11878. https://doi.org/10.3390/ijms222111878
Dhakal A, Acharya L, O’Regan R, Gandhi S, Falkson C. PI3Kinase Inhibition in Hormone Receptor-Positive Breast Cancer. International Journal of Molecular Sciences. 2021; 22(21):11878. https://doi.org/10.3390/ijms222111878
Chicago/Turabian StyleDhakal, Ajay, Luna Acharya, Ruth O’Regan, Shipra Gandhi, and Carla Falkson. 2021. "PI3Kinase Inhibition in Hormone Receptor-Positive Breast Cancer" International Journal of Molecular Sciences 22, no. 21: 11878. https://doi.org/10.3390/ijms222111878
APA StyleDhakal, A., Acharya, L., O’Regan, R., Gandhi, S., & Falkson, C. (2021). PI3Kinase Inhibition in Hormone Receptor-Positive Breast Cancer. International Journal of Molecular Sciences, 22(21), 11878. https://doi.org/10.3390/ijms222111878






