Cell Surface and Functional Features of Cortical Bone Stem Cells
Abstract
1. Introduction
2. Results
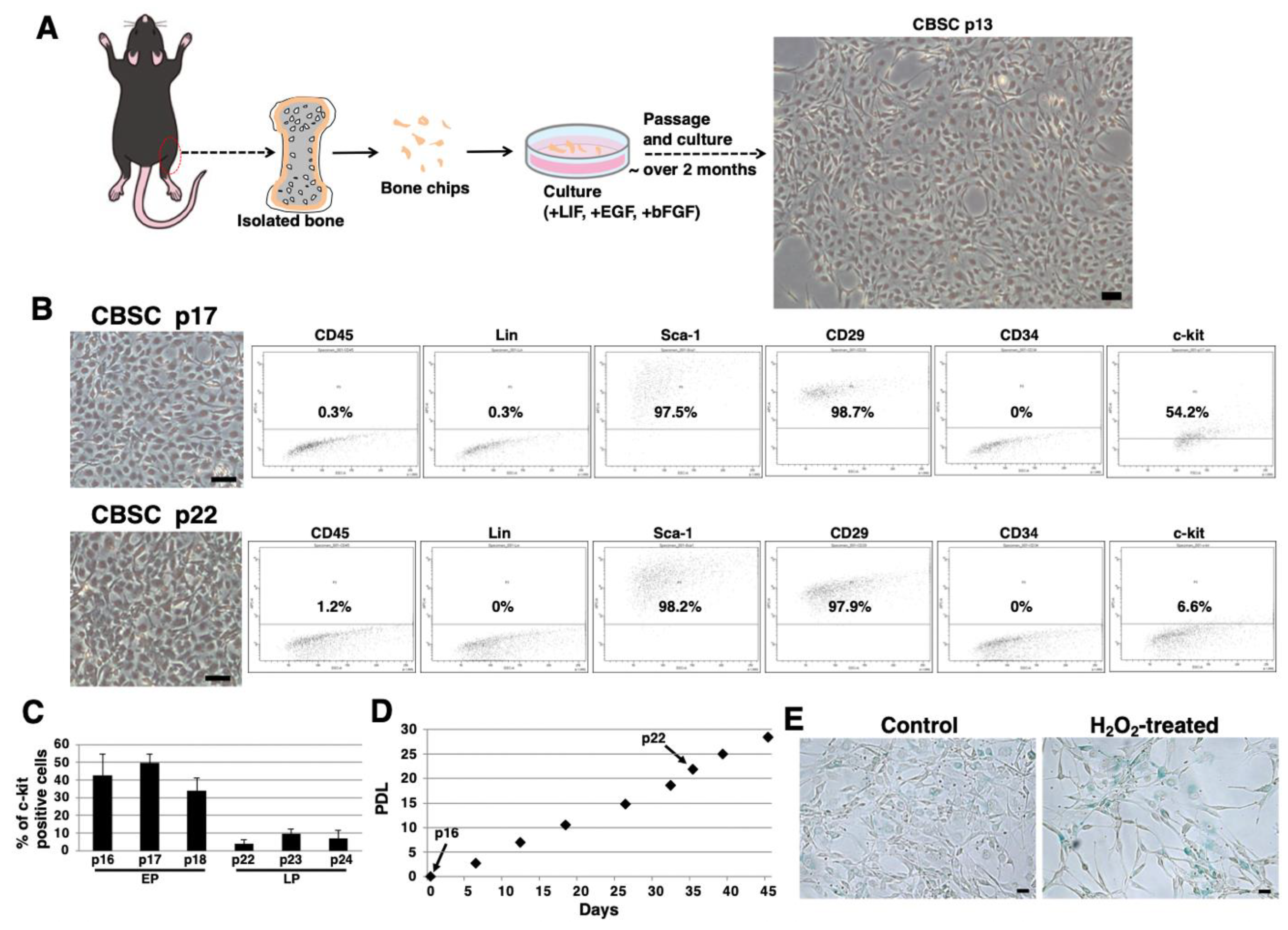
2.1. Isolation and Culture of mCBSCs
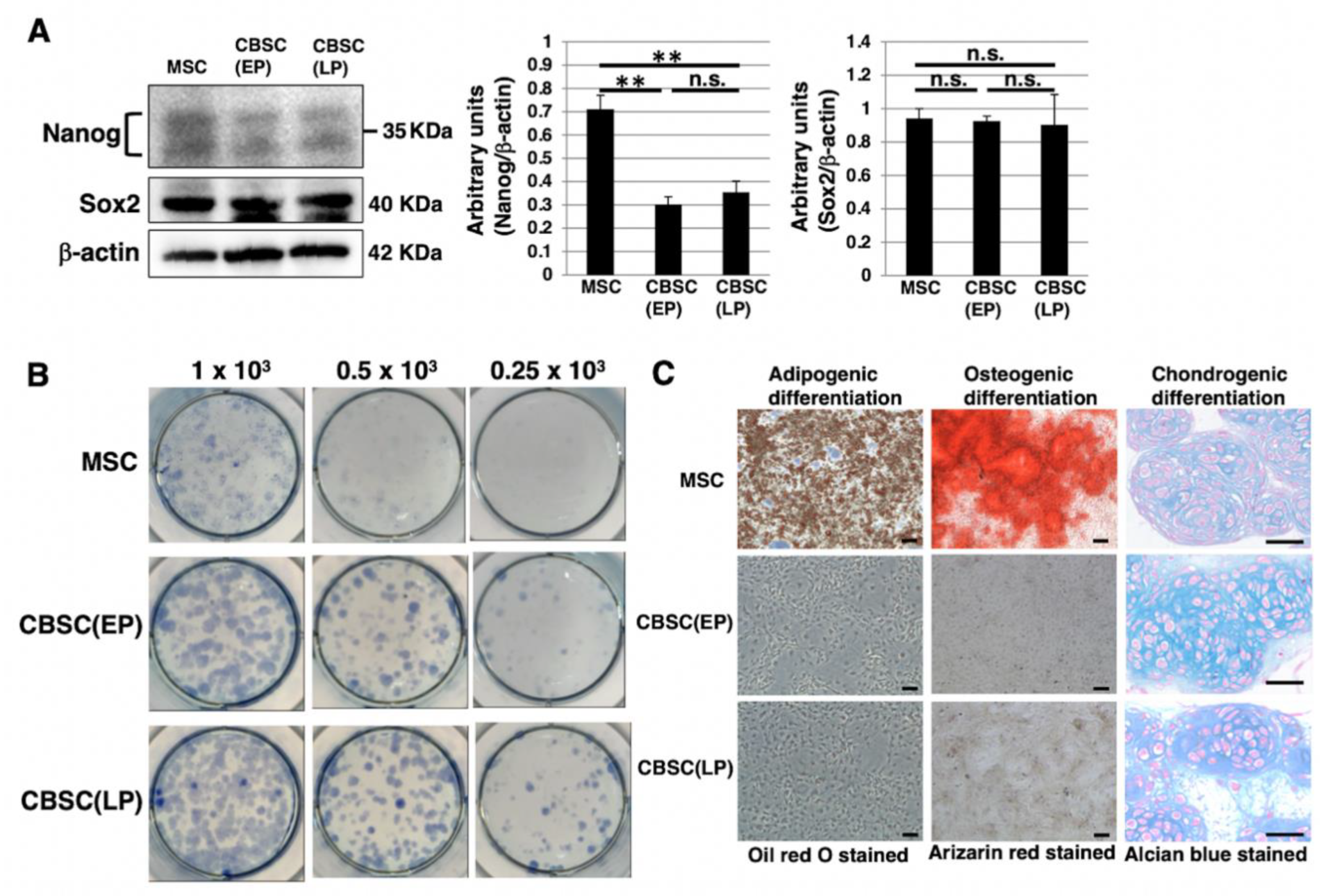
2.2. Stem-Cell-Like Features of mCBSCs
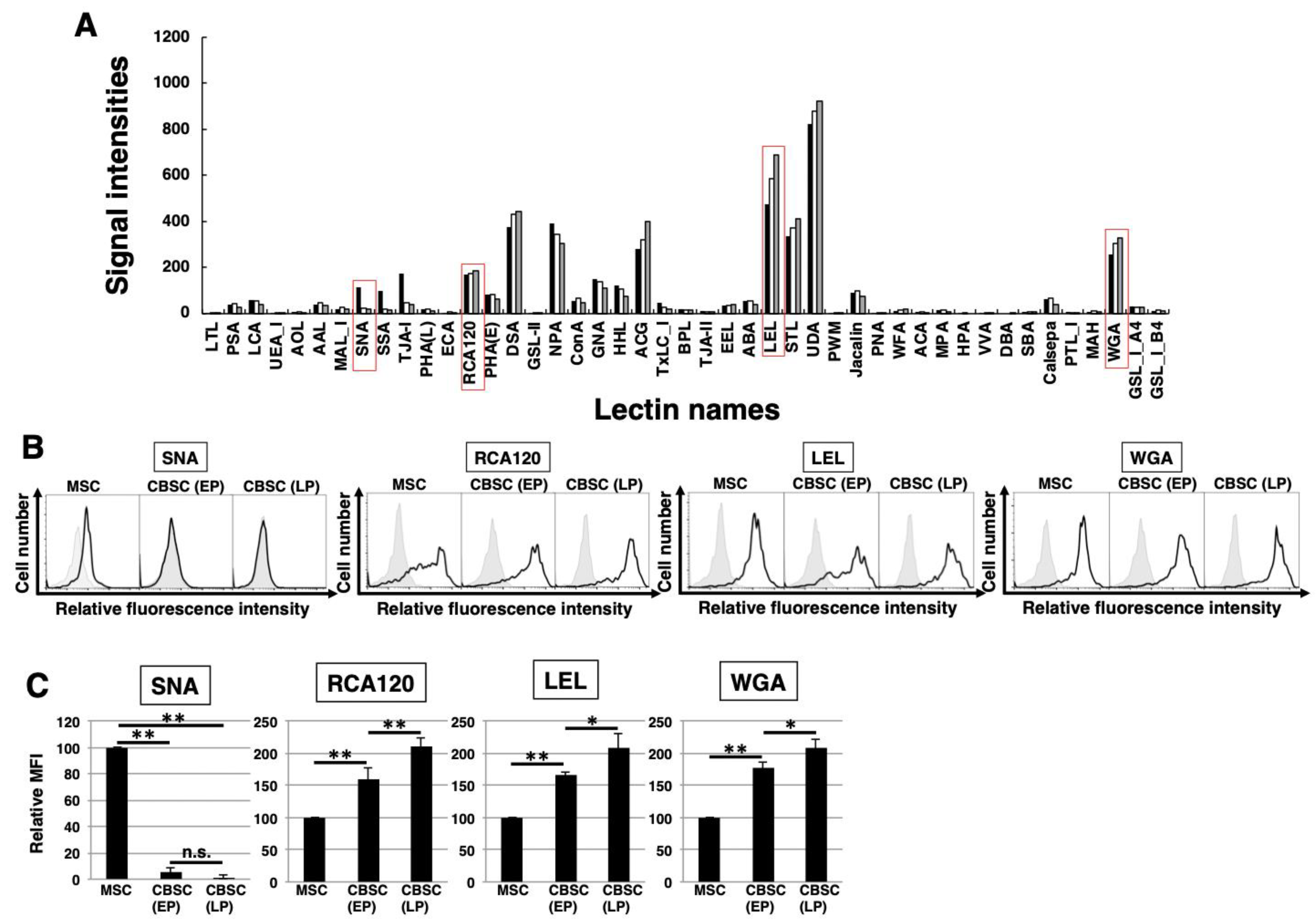
2.3. The Feature of Glycan Profiles in mCBSCs (EP and LP) and mMSCs
2.4. Comparison of Glycan Profiles between mCBSCs and mMSCs Using Statistical Analysis
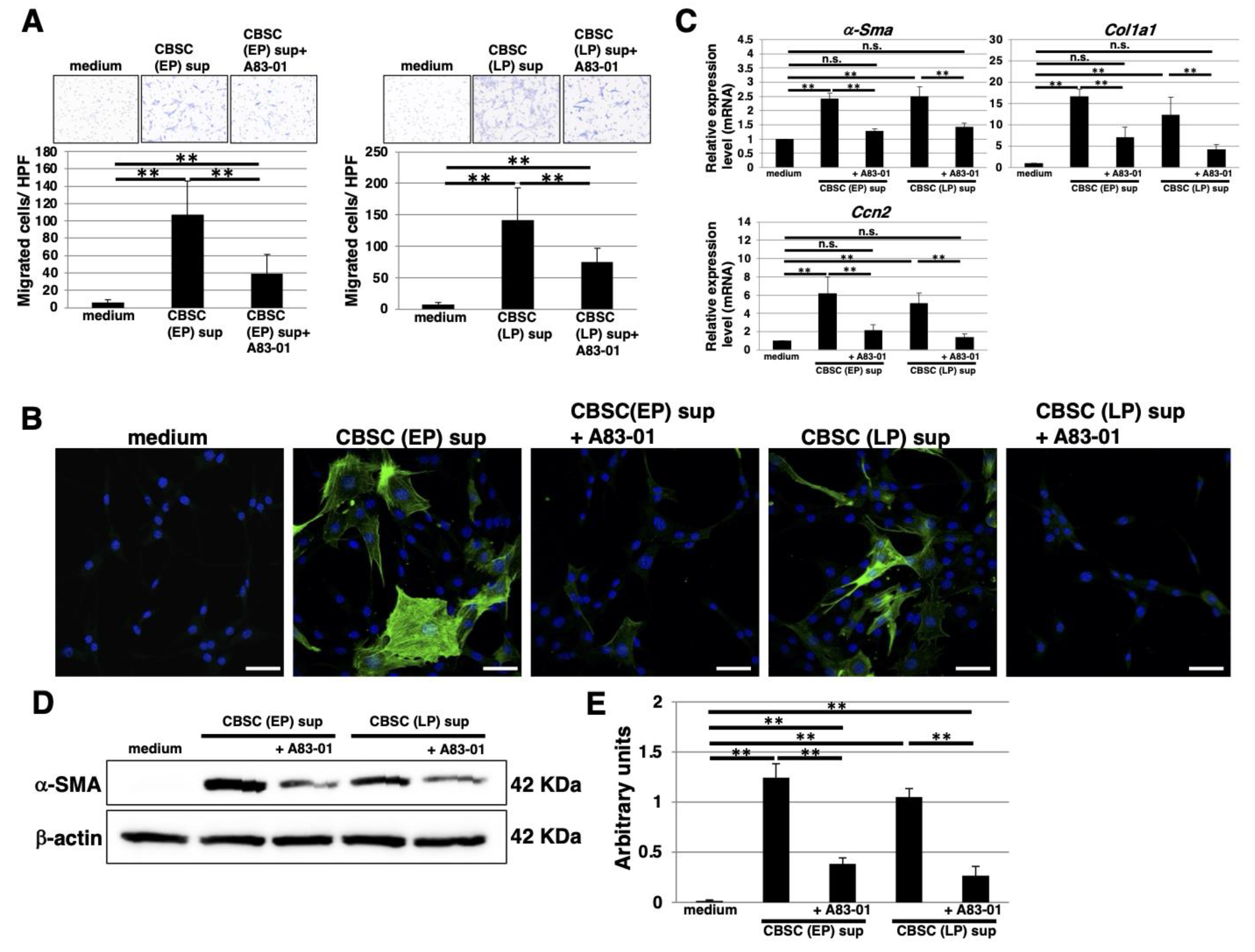
2.5. The Cell Cycle Feature and Transforming Growth Factor-β1 (TGF-β1) Expression in mCBSCs
2.6. The Functional Properties of TGF-β1 Derived from mCBSCs
3. Discussion
4. Materials and Methods
4.1. CBSC Isolation and Cell Culture
4.2. Multilineage Differentiation
4.3. Self-renewal Assay
4.4. FACS Analysis
4.5. Immunoblotting
4.6. Immunocytostaining
4.7. Lectin Array Analysis
4.8. ELISA Analysis
4.9. Migration Assay
4.10. Real-Time PCR
4.11. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Sliwa, K.; Ntusi, N. Battling Cardiovascular Diseases in a Perfect Storm. Circulation 2019, 139, 1658–1660. [Google Scholar] [CrossRef]
- Ji, S.T.; Kim, H.; Yun, J.; Chung, J.S.; Kwon, S.M. Promising Therapeutic Strategies for Mesenchymal Stem Cell-Based Cardiovascular Regeneration: From Cell Priming to Tissue Engineering. Stem Cells Int. 2017, 2017, 3945403. [Google Scholar] [CrossRef]
- Karantalis, V.; Balkan, W.; Schulman, I.H.; Hatzistergos, K.E.; Hare, J.M. Cell-based therapy for prevention and reversal of myocardial remodeling. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H256–H270. [Google Scholar] [CrossRef]
- Urbanek, K.; Torella, D.; Sheikh, F.; Angelis, A.D.; Nurzynska, D.; Silvestri, F.; Beltrami, C.A.; Bussani, R.; Beltrami, A.P.; Quaini, F.; et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc. Natl. Acad. Sci. USA 2005, 102, 8692–8697. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Khan, M.; Toko, H.; Bailey, B.; Cottage, C.T.; Wallach, K.; Nag, D.; Lee, A.; Siddiqi, S.; Lan, F.; et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J. Am. Coll. Cardiol. 2012, 60, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Oskouei, B.N.; Lamirault, G.; Joseph, C.; Treuer, A.V.; Landa, S.; Silva, J.D.; Hatzistergos, K.; Dauer, M.; Balkan, W.; McNiece, I.; et al. Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Transl. Med. 2012, 1, 116–124. [Google Scholar] [CrossRef]
- Duran, J.M.; Makarewich, C.A.; Sharp, T.E.; Starosta, T.; Zhu, F.; Hoffman, N.E.; Chiba, Y.; Madesh, M.; Berretta, R.M.; Kubo, H.; et al. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ. Res. 2013, 113, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res. 2014, 114, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Pätilä, T.; Lehtinen, M.; Vento, A.; Schildt, J.; Sinisalo, J.; Laine, M.; Hämmäinen, P.; Nihtinen, A.; Alitalo, R.; Nikkinen, P.; et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: A prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J. Heart Lung Transplant. 2014, 33, 567–574. [Google Scholar] [CrossRef]
- Delewi, R.; van der Laan, A.M.; Robbers, L.F.; Hirsch, A.; Nijveldt, R.; van der Vleuten, P.A.; Tijssen, J.G.; Tio, R.A.; Waltenberger, J.; Ten Berg, J.M.; et al. Long term outcome after mononuclear bone marrow or peripheral blood cells infusion after myocardial infarction. Heart 2015, 101, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Mohsin, S.; Troupes, C.D.; Starosta, T.; Sharp, T.E.; Agra, E.J.; Smith, S.; Duran, J.M.; Zalavadia, N.; Zhou, Y.; Kubo, H.; et al. Unique Features of Cortical Bone Stem Cells Associated With Repair of the Injured Heart. Circ. Res. 2015, 117, 1024–1033. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017. [Google Scholar]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- An, H.J.; Gip, P.; Kim, J.; Wu, S.; Park, K.W.; McVaugh, C.T.; Schaffer, D.V.; Bertozzi, C.R.; Lebrilla, C.B. Extensive determination of glycan heterogeneity reveals an unusual abundance of high mannose glycans in enriched plasma membranes of human embryonic stem cells. Mol. Cell. Proteom. 2012, 11, M111.010660. [Google Scholar] [CrossRef] [PubMed]
- Hasehira, K.; Tateno, H.; Onuma, Y.; Ito, Y.; Asashima, M.; Hirabayashi, J. Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol. Cell Proteomics 2012, 11, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, H.; Ullah, M.; Berger, M.; Tauber, R.; Ringe, J.; Blanchard, V. N-glycosylation profile of undifferentiated and adipogenically differentiated human bone marrow mesenchymal stem cells: Towards a next generation of stem cell markers. Stem Cells Dev. 2013, 22, 3100–3113. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Thomas-Oates, J.E.; Genever, P.G.; Ungar, D. Glycan profiling shows unvaried n-glycomes in MSC clones with distinct differentiation potentials. Front. Cell Dev. Biol. 2016, 4, 52. [Google Scholar] [CrossRef]
- Kumar, A.; Torii, T.; Ishino, Y.; Muraoka, D.; Yoshimura, T.; Togayachi, A.; Narimatsu, H.; Ikenaka, K.; Hitoshi, S. The Lewis X-related alpha1,3-fucosyltransferase, Fut10, is required for the maintenance of stem cell populations. J. Biol. Chem. 2013, 288, 28859–28868. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Shinomi, M.; Hirano, K.; Ui-Tei, K.; Nishihara, S. LacdiNAc (GalNAc beta 1-4GlcNAc) contributes to self-renewal ofmouse embryonic stemcells by regulating leukemia inhibitory factor/STAT3 signaling. Stem Cells. 2011, 29, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Jagger, A.M.; Walker, M.; Seinkmane, E.; Fox, J.M.; Kröger, R.; Genever, P.; Ungar, D. Glycans modify mesenchymal stem cell differentiation to impact on the function of resulting osteoblasts. J. Cell Sci. 2018, 131, jcs209452. [Google Scholar] [CrossRef]
- Tateno, H.; Saito, S.; Hiemori, K.; Kiyoi, K.; Hasehira, K.; Toyoda, M.; Onuma, Y.; Ito, Y.; Akutsu, H.; Hirabayashi, J. α2-6 sialylation is a marker of the differentiation potential of human mesenchymal stem cells. Glycobiology 2016, 26, 1328–1337. [Google Scholar] [CrossRef]
- Banfi, A.; Muraglia, A.; Dozin, B.; Mastrogiacomo, M.; Cancedda, R.; Quarto, R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 2000, 28, 707–715. [Google Scholar] [CrossRef]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef]
- Schellenberg, A.; Stiehl, T.; Horn, P.; Joussen, S.; Pallua, N.; Ho, A.D.; Wagner, W. Population dynamics of mesenchymal stromal cells during culture expansion. Cytotherapy 2012, 14, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, D.E.; Shen, M.M. From blastocyst to gastrula: Gene regulatory networks of embryonic stem cells and early mouse embryogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130542. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Sasaki, N.; Itakura, Y.; Gomi, F.; Hirano, K.; Toyoda, M.; Ishiwata, T. Comparison of functional glycans between cancer stem cells and normal stem cells. Histol. Histopathol. 2019, 34, 995–1007. [Google Scholar] [CrossRef]
- Mohammadi, M.; Olsen, S.K.; Ibrahimi, O.A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005, 16, 107–137. [Google Scholar] [CrossRef]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef]
- Furue, M.K.; Na, J.; Jackson, J.P.; Okamoto, T.; Jones, M.; Baker, D.; Hata, R.I.; Moore, H.D.; Sato, J.D.; Andrews, P.W. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc. Natl. Acad. Sci. USA 2008, 105, 13409–13414. [Google Scholar] [CrossRef] [PubMed]
- Levenstein, M.E.; Berggren, W.T.; Lee, J.F.; Conard, K.R.; Llanas, R.A.; Wagner, R.J.; Smith, L.M.; Thomson, J.A. Secreted proteoglycans directly mediate human embryonic stem cell-basic fibroblast growth factor 2 interactions critical for proliferation. Stem Cells 2008, 26, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Hasehira, K.; Hirabayashi, J.; Tateno, H. Structural and quantitative evidence of α2–6-sialylated N-glycans as markers of the differentiation potential of human mesenchymal stem cells. Glycoconj. J. 2017, 34, 797–806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gharaibeh, B.; Lavasani1, M.; Cummins, J.H.; Huard, J. Terminal diff erentiation is not a major determinant for the success of stem cell therapy - cross-talk between muscle-derived stem cells and host cells. Stem Cell Res. Ther. 2011, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Michael, L.H.; Entman, M.L. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc. Res. 2000, 48, 89–100. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Hofmann, U.; Frantz, S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res. 2015, 116, 354–367. [Google Scholar] [CrossRef]
- Shevach, E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009, 30, 636–645. [Google Scholar] [CrossRef]
- Delisle, J.S.; Giroux, M.; Boucher, G.; Landry, J.R.; Hardy, M.P.; Lemieux, S.; Jones, R.G.; Wilhelm, B.T.; Perreault, C. The TGF-β-Smad3 pathway inhibits CD28-dependent cell growth and proliferation of CD4 T cells. Genes Immun. 2013, 14, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bevan, M.J. TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat. Immunol. 2012, 13, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Ganglioside GM1 Contributes to the State of Insulin Resistance in Senescent Human Arterial Endothelial Cells. J. Biol. Chem. 2015, 290, 25475–25486. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Itakura, Y.; Toyoda, M.; Takahashi, Y.; Yamada, M.; Umezawa, A.; Hirabayashi, J. Development of a datamining system for differential profiling of cell glycoproteins based on lectin microarray. J. Proteom. Bioinform. 2008, 1, 68–72. [Google Scholar] [CrossRef]
- Tateno, H.; Kuno, A.; Itakura, Y.; Hirabayashi, J. A versatile technology for cellular glycomics using lectin microarray. Methods Enzymol. 2010, 478, 181–195. [Google Scholar] [CrossRef]


| Lectin Name | mMSC-1 | mMSC-2 | mMSC-3 | CBSC (EP)-1 | CBSC (EP)-2 | CBSC (EP)-3 | CBSC (LP)-1 | CBSC (LP)-2 | CBSC (LP)-3 |
|---|---|---|---|---|---|---|---|---|---|
| LTL*2 | 0.1 | 0.1 | 1.3 | 0.2 | 0.1 | 0.6 | 0.0 | 0.0 | 0.1 |
| PSA*2 | 29.6 | 33.1 | 50.5 | 41.3 | 45.4 | 36.8 | 23.5 | 28.5 | 23.8 |
| LCA*2 | 46.2 | 49.9 | 74.4 | 56.6 | 59.2 | 50.1 | 35.7 | 41.8 | 34.2 |
| UEA_I | 0.1 | 0.0 | 3.5 | 0.0 | 0.1 | 0.5 | 0.0 | 0.1 | 0.3 |
| AOL*2 | 2.8 | 2.5 | 15.7 | 5.8 | 6.5 | 7.2 | 2.0 | 3.5 | 6.1 |
| AAL*2 | 28.6 | 28.9 | 54.6 | 48.2 | 52.4 | 38.6 | 36.4 | 40.4 | 31.4 |
| MAL_I*2 | 13.1 | 14.6 | 21.9 | 27.2 | 30.6 | 21.1 | 18.8 | 22.1 | 16.6 |
| SNA*1, *2 | 105.2 | 104.2 | 129.8 | 25.2 | 23.2 | 22.0 | 16.7 | 20.5 | 20.7 |
| SSA*1, *2 | 89.2 | 84.6 | 112.9 | 17.7 | 18.2 | 16.3 | 11.1 | 15.2 | 12.7 |
| TJA-I*1, *2 | 153.2 | 160.4 | 197.7 | 45.0 | 45.0 | 47.5 | 34.8 | 44.8 | 40.9 |
| PHA(L)*2 | 11.8 | 12.5 | 27.4 | 18.9 | 21.3 | 19.0 | 9.0 | 12.7 | 12.7 |
| ECA*2 | 1.1 | 1.4 | 5.7 | 5.3 | 5.6 | 5.2 | 2.9 | 5.3 | 4.4 |
| RCA120 | 148.3 | 151.8 | 194.2 | 162.8 | 167.2 | 187.2 | 164.3 | 191.9 | 192.9 |
| PHA(E)*2 | 66.1 | 70.0 | 107.9 | 74.1 | 80.4 | 94.3 | 54.3 | 60.7 | 74.6 |
| DSA*1 | 354.7 | 354.1 | 405.5 | 426.4 | 424.0 | 443.8 | 423.0 | 454.3 | 450.3 |
| GSL-II*2 | 0.5 | 1.1 | 4.0 | 1.7 | 2.4 | 3.7 | 0.1 | 0.8 | 2.0 |
| NPA*1 | 400.6 | 413.7 | 369.2 | 373.9 | 372.4 | 302.9 | 326.5 | 313.9 | 282.0 |
| ConA*1, *2 | 48.8 | 53.1 | 58.7 | 69.1 | 71.0 | 58.7 | 45.4 | 51.0 | 41.2 |
| GNA | 144.5 | 162.0 | 145.7 | 152.2 | 164.2 | 105.9 | 122.0 | 147.0 | 72.1 |
| HHL*2 | 90.0 | 89.5 | 165.5 | 111.7 | 102.1 | 103.8 | 68.3 | 67.8 | 81.0 |
| ACG*2 | 341.5 | 331.2 | 193.7 | 349.2 | 324.7 | 298.2 | 456.7 | 396.0 | 355.6 |
| TxLC_I*1, *2 | 33.2 | 36.7 | 62.0 | 24.1 | 26.8 | 26.1 | 16.1 | 18.2 | 17.8 |
| BPL | 12.6 | 11.9 | 23.9 | 14.0 | 17.0 | 18.1 | 14.9 | 17.8 | 17.0 |
| TJA-II | 5.9 | 5.5 | 17.4 | 4.2 | 4.3 | 6.1 | 1.0 | 9.5 | 5.3 |
| EEL | 33.0 | 35.1 | 39.7 | 34.3 | 38.5 | 32.3 | 35.8 | 37.0 | 37.3 |
| ABA*2 | 37.9 | 42.8 | 71.0 | 54.5 | 55.4 | 57.3 | 35.5 | 39.7 | 44.0 |
| LEL*1, *2 | 486.8 | 474.2 | 469.9 | 577.2 | 540.0 | 625.4 | 645.6 | 656.2 | 747.8 |
| STL*2 | 376.3 | 336.5 | 305.0 | 349.0 | 342.0 | 409.7 | 402.3 | 424.4 | 405.9 |
| UDA | 984.6 | 965.7 | 600.8 | 854.2 | 838.9 | 925.6 | 958.4 | 804.8 | 987.9 |
| PWM | 0.1 | 0.0 | 1.8 | 0.7 | 0.3 | 1.4 | 0.2 | 0.9 | 0.3 |
| Jacalin*2 | 85.1 | 85.3 | 99.3 | 99.7 | 103.6 | 93.8 | 78.2 | 81.8 | 63.5 |
| PNA | 0.0 | 0.0 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 |
| WFA*1 | 7.1 | 6.7 | 15.0 | 16.0 | 18.0 | 16.1 | 15.3 | 23.7 | 15.6 |
| ACA*2 | 3.4 | 4.0 | 12.7 | 6.8 | 7.5 | 6.5 | 2.6 | 4.6 | 4.9 |
| MPA*2 | 11.4 | 12.0 | 20.0 | 15.6 | 16.1 | 12.1 | 7.1 | 9.4 | 6.8 |
| HPA*2 | 0.0 | 0.0 | 3.3 | 0.3 | 0.6 | 0.8 | 0.0 | 0.0 | 0.0 |
| VVA | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| DBA | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| SBA | 2.5 | 3.8 | 8.8 | 6.1 | 6.4 | 5.4 | 4.2 | 7.1 | 4.4 |
| Calsepa*2 | 54.3 | 56.4 | 75.3 | 69.4 | 72.1 | 62.2 | 42.7 | 48.4 | 32.3 |
| PTL_I | 3.4 | 3.8 | 7.9 | 3.5 | 4.7 | 3.4 | 2.9 | 3.6 | 2.2 |
| MAH*2 | 5.1 | 5.6 | 11.9 | 13.6 | 13.9 | 11.0 | 4.6 | 7.7 | 6.3 |
| WGA*1 | 247.6 | 263.2 | 256.9 | 304.0 | 331.3 | 286.0 | 341.9 | 341.1 | 311.2 |
| GSL_I_A4 | 23.6 | 21.8 | 41.6 | 26.3 | 30.8 | 27.5 | 26.8 | 29.2 | 26.6 |
| GSL_I_B4 | 10.0 | 10.2 | 16.1 | 13.9 | 16.2 | 10.5 | 12.7 | 16.2 | 7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, N.; Itakura, Y.; Mohsin, S.; Ishigami, T.; Kubo, H.; Chiba, Y. Cell Surface and Functional Features of Cortical Bone Stem Cells. Int. J. Mol. Sci. 2021, 22, 11849. https://doi.org/10.3390/ijms222111849
Sasaki N, Itakura Y, Mohsin S, Ishigami T, Kubo H, Chiba Y. Cell Surface and Functional Features of Cortical Bone Stem Cells. International Journal of Molecular Sciences. 2021; 22(21):11849. https://doi.org/10.3390/ijms222111849
Chicago/Turabian StyleSasaki, Norihiko, Yoko Itakura, Sadia Mohsin, Tomoaki Ishigami, Hajime Kubo, and Yumi Chiba. 2021. "Cell Surface and Functional Features of Cortical Bone Stem Cells" International Journal of Molecular Sciences 22, no. 21: 11849. https://doi.org/10.3390/ijms222111849
APA StyleSasaki, N., Itakura, Y., Mohsin, S., Ishigami, T., Kubo, H., & Chiba, Y. (2021). Cell Surface and Functional Features of Cortical Bone Stem Cells. International Journal of Molecular Sciences, 22(21), 11849. https://doi.org/10.3390/ijms222111849







