Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia
Abstract
1. Introduction
2. Results
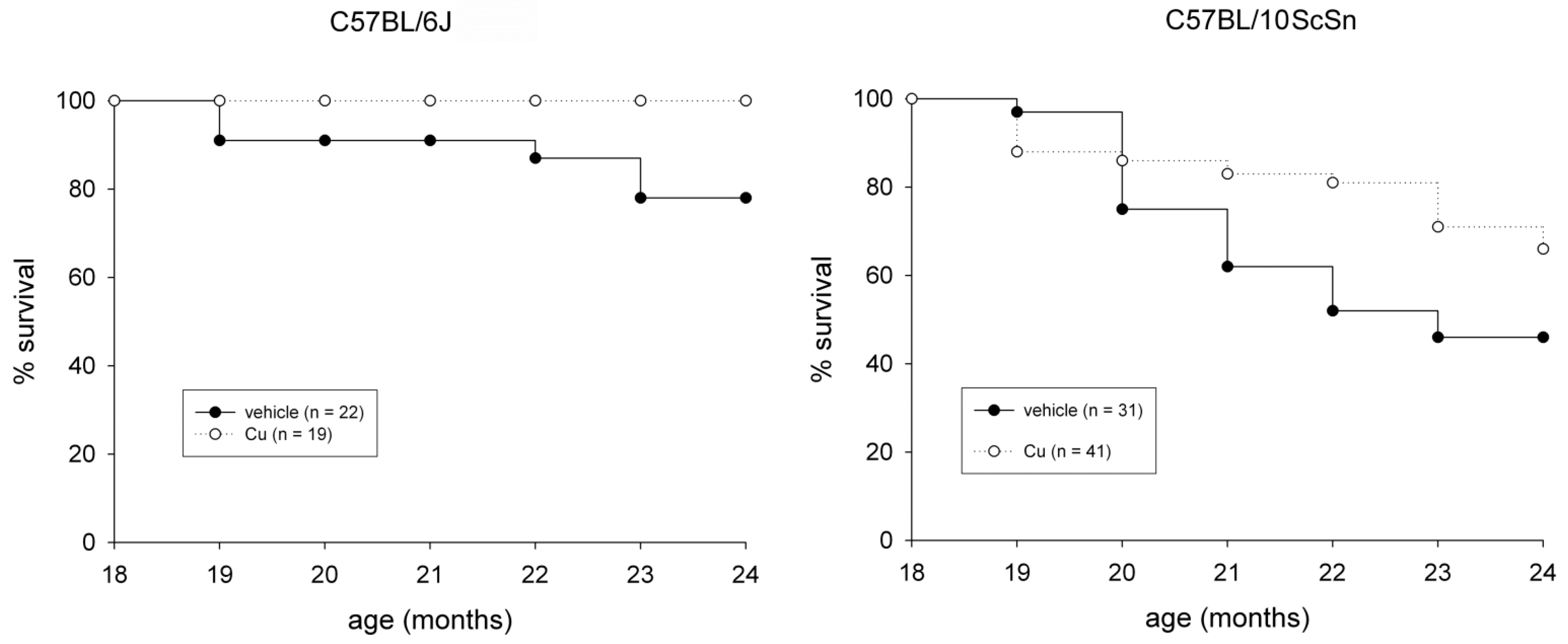
2.1. General and Muscle-Specific Effects of Chronic Curcumin Administration in Old C57BL Mice
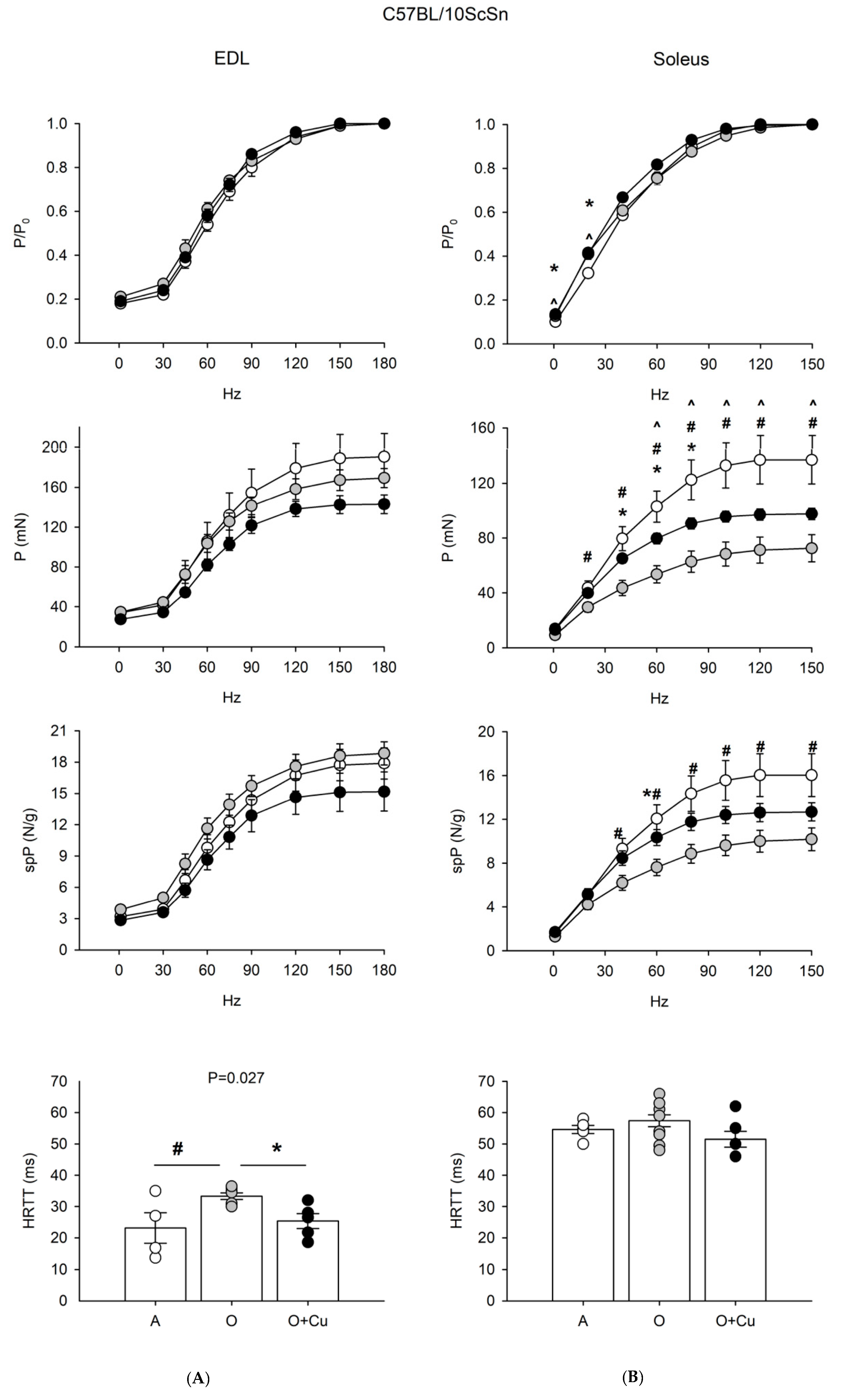
2.2. Effects of Aging and Curcumin Treatment on Muscle Contractile Properties
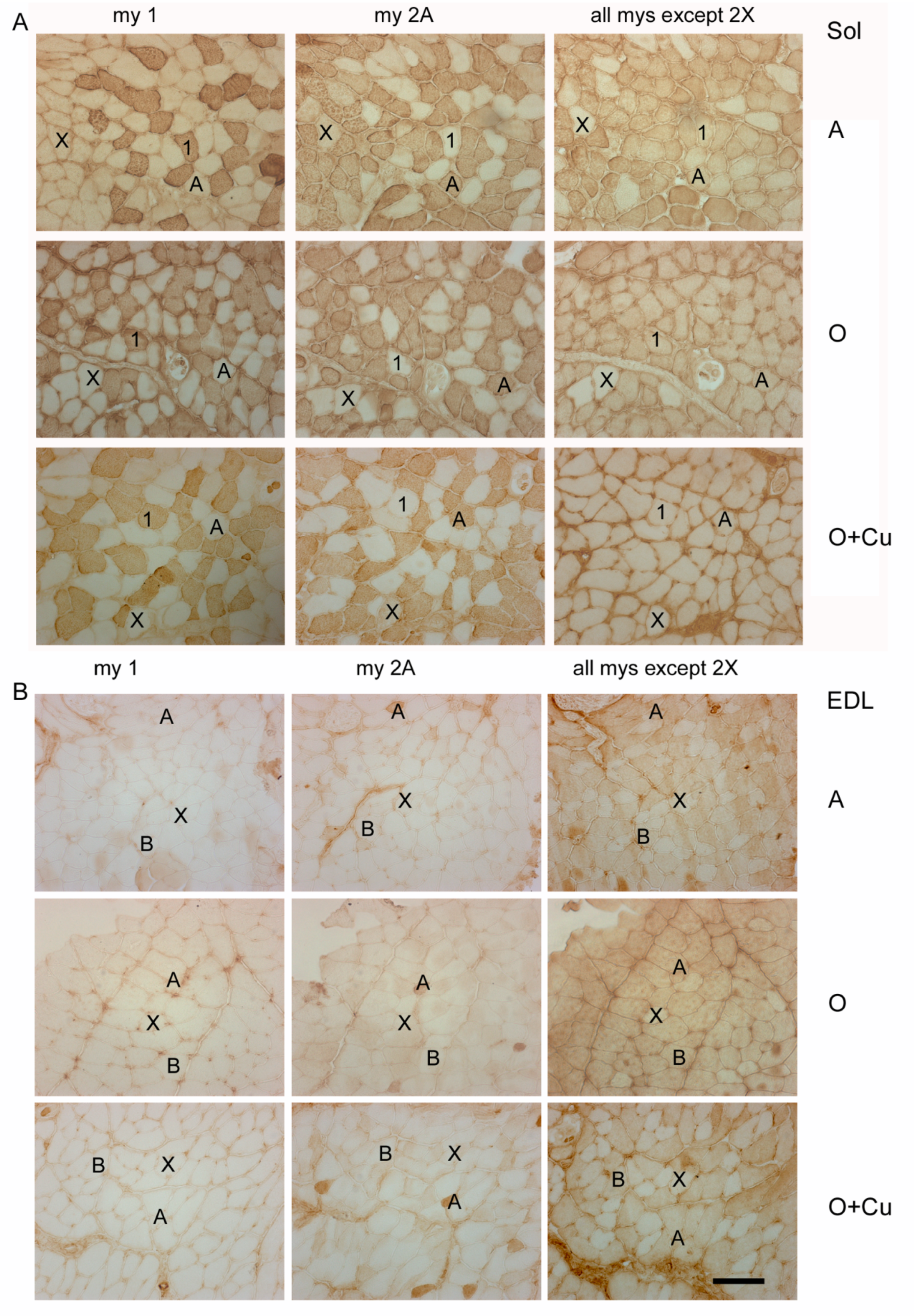
2.3. Effects of Aging and Curcumin Treatment on Myofiber Number, Type and Size
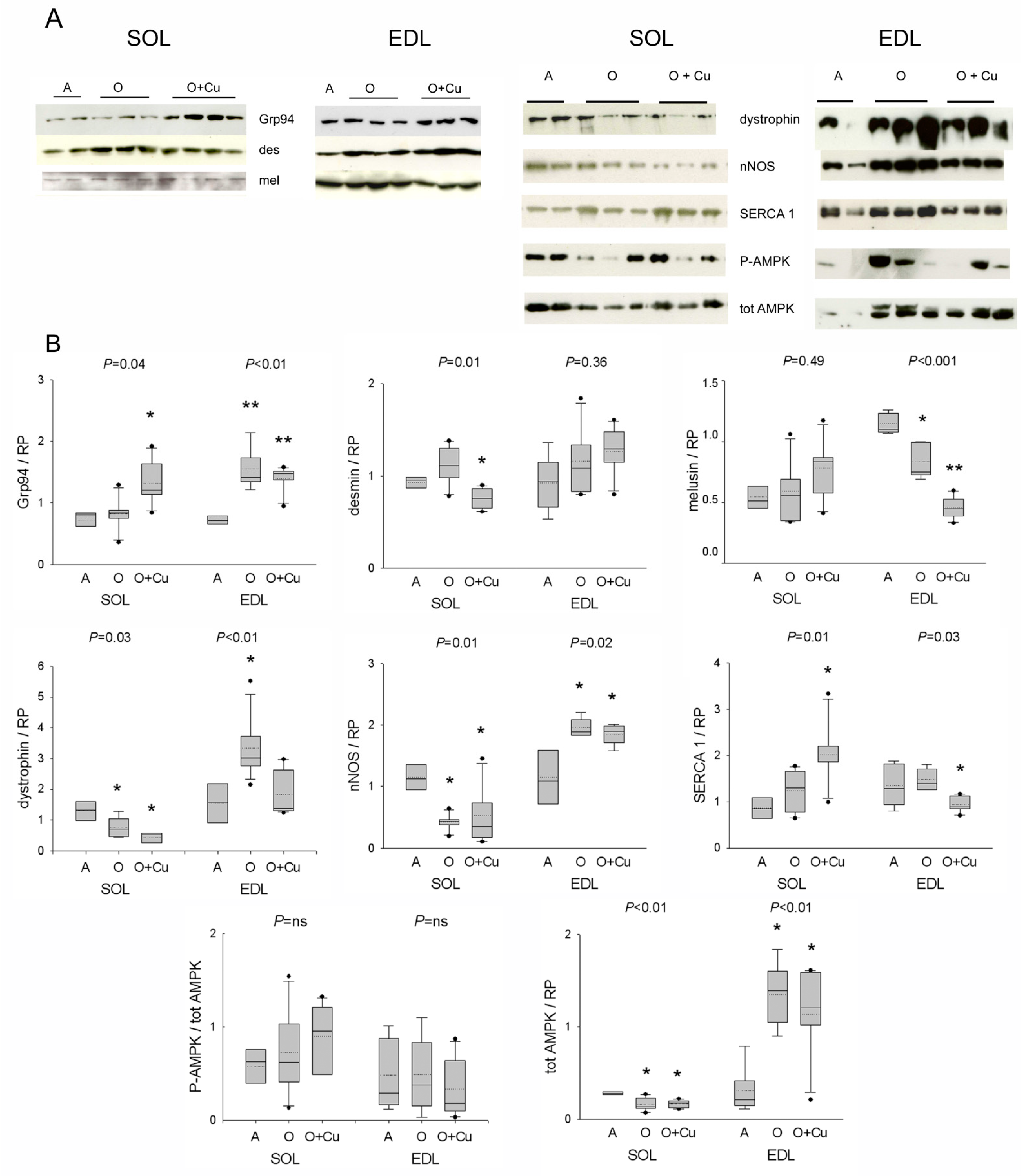
2.4. Effects of Aging and Curcumin Treatment on Costamere Muscle Proteins
2.5. Effect of Aging and Curcumin Treatment on Myofibrillar Protein Oxidation
2.6. Effects of Curcumin Treatment on Muscle Satellite Cells
3. Discussion
3.1. Strain- and Muscle-Specific Responses to Aging
3.2. Curcumin Blunts Presarcopenic and Sarcopenic Signatures
4. Materials and Methods
4.1. Curcumin Formulation
4.2. Mice
4.3. Mechanical Recordings
4.4. Antibodies and Reagents
4.5. Routine Histology, Immunofluorescence and Immunoperoxidase
- (1)
- labeling of Ki67 required fixation with 4% buffered paraformaldehyde (PFA) for 20 min at RT and epitope unmasking by two-times 5 min-boiling in citrate buffer pH 6.0;
- (2)
- labeling of activated-caspase 3 required fixation with 3% PFA for 15 min at RT;
- (3)
- labeling for dystrophin required fixation with 4% PFA for 10 min at RT;
- (4)
- labeling for phospho-nNOS-S847 and -S1417 required fixation with 4% PFA for 5 and 15 min, respectively;
- (5)
- double labeling for SERCA1 and SERCA2 required fixation with 4% PFA for 10 min. Incubation with antibodies was performed in 5% donkey serum.
4.6. SDH and NADPH-Diaphorase (NADPH-d) Histochemistry
4.7. Western Blotting
4.8. Tropomyosin Oxidation and Oxyblot
4.9. Satellite Cell Isolation and Culture
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2016, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a multifaceted hormetic agent, mediates an intricate crosstalk between mitochondrial turnover, autophagy, and apoptosis. Oxid. Med. Cell. Longev. 2020, 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.; Scapin, C.; Vitadello, M.; Florean, C.; Gorza, L. Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J. Cell. Mol. Med. 2010, 14, 970–981. [Google Scholar] [CrossRef]
- Thaloor, D.; Miller, K.J.; Gephart, J.; Mitchell, P.O.; Pavlath, G.K. Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am. J. Physiol. 1999, 277, C320–C329. [Google Scholar] [CrossRef] [PubMed]
- Vitadello, M.; Germinario, E.; Ravara, B.; Libera, L.D.; Danieli, D.; Gorza, L. Curcumin counteracts loss of force and atrophy of hindlimb unloaded rat soleus by hampering neuronal nitric oxide synthase untethering from sarcolemma. J. Physiol. 2014, 592, 2637–2652. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Garcia-Villatoro, E.L.; Guzzoni, V.; Hord, J.M.; Botchlett, R.; Holly, D.; Lawler, M.S.; Janini Gomes, M.; Ryan, P.; Rodriguez, D.; et al. Effect of combined fish oil & Curcumin on murine skeletal muscle morphology and stress response proteins during mechanical unloading. Nutr. Res. 2019, 65, 17–28. [Google Scholar] [CrossRef]
- Gomes Suhett, L.; de Miranda Monteiro Santos, R.; Souza Silveira, B.K.; Gualandi Leal, A.C.; Melo de Brito, A.D.; Faries de Novaes, J.; Mattos della Lucia, C. Effects of curcumin supplementation on sport and physical exercise: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-R.; Parnell, L.D.; Ordovas, J.; Lai, C.-Q. Curcumin and aging. BioFactors 2013, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A time for action. An SCWD position paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Williams, J.; Atherton, P.J.; Larvin, M.; Lund, J.N.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a Quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Gorza, L.; Sorge, M.; Seclì, L.; Brancaccio, M. Master regulators of muscle atrophy: Role of costamere components. Cells 2021, 10, 61. [Google Scholar] [CrossRef]
- Hughes, D.; Wallace, M.A.; Baar, K. Effects of aging, exercise, and disease on force transfer in skeletal muscle. Am. J. Physiol. Metab. 2015, 309, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Valls, M.R.B.; Wilkinson, D.J.; Narici, M.V.; Smith, K.; Phillips, B.E.; Caporossi, D.; Atherton, P.J. Protein carbonylation and heat shock proteins in human skeletal muscle: Relationships to age and sarcopenia. J. Gerontol. A Boil. Sci. Med. Sci. 2014, 70, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Szentesi, P.; Csernoch, L.; Dux, L.; Keller-Pintér, A. Changes in redox signaling in the skeletal muscle with aging. Oxid. Med. Cell. Longev. 2019, 2019, 4617801. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; DeRuisseau, K.C. Effects of prolonged dietary curcumin exposure on skeletal muscle biochemical and functional responses of aged male rats. Int. J. Mol. Sci. 2019, 20, 1178. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Feregalli, B.; Togni, S.; Cornelli, U.; Giacomelli, L.; Eggenhoffner, R.; Belcaro, G. A novel phospholipid delivery system of curcumin (Meriva®) preserves muscular mass in healthy aging subjects. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 762–766. [Google Scholar]
- Ballak, S.B.; Degens, H.; de Haan, A.; Jaspers, R.T. Aging related changes in determinants of muscle force generating capacity: A comparison of muscle aging in men and male rodents. Ageing Res. Rev. 2014, 14, 43–55. [Google Scholar] [CrossRef]
- Boldrin, L.; Ross, J.A.; Whitmore, C.; Doreste, B.; Beaver, C.; Eddaoudi, A.; Pearce, D.J.; Morgan, J.E. The effect of calorie restriction on mouse skeletal muscle is sex, strain and time-dependent. Sci. Rep. 2017, 7, 5160. [Google Scholar] [CrossRef]
- Gorza, L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J. Histochem. Cytochem. 1990, 38, 257–265. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef]
- Vitadello, M.; Sorge, M.; Percivalle, E.; Germinario, E.; Danieli-Betto, D.; Turco, E.; Tarone, G.; Brancaccio, M.; Gorza, L. Loss of melusin is a novel, neuronal NO synthase/FoxO3-independent master switch of unloading-induced muscle atrophy. J. Cachexia Sarcopenia Muscle 2020, 11, 802–819. [Google Scholar] [CrossRef] [PubMed]
- Agbulut, O.; Destombes, J.; Thiesson, D.; Butler-Browne, G. Age-related appearance of tubular aggregates in the skeletal muscle of almost all male inbred mice. Histochem. Cell Biol. 2000, 114, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Lechado i Terradas, A.; Vitadello, M.; Traini, L.; Namuduri, A.V.; Gastaldello, S.; Gorza, L. Sarcolemmal loss of active nNOS (Nos1) is an oxidative stress-dependent, early event driving disuse atrophy. J. Pathol. 2018, 246, 433–446. [Google Scholar] [CrossRef]
- Bondì, M.; Germinario, E.; Pirazzini, M.; Zanetti, G.; Cencetti, F.; Donati, C.; Gorza, L.; Betto, R.; Bruni, P.; Danieli-Betto, D. Ablation of S1P3 receptor protects mouse soleus from age-related drop in muscle mass, force, and regenerative capacity. Am. J. Physiol. Cell Physiol. 2017, 313, C54–C67. [Google Scholar] [CrossRef]
- Graber, T.G.; Kim, J.-H.; Grange, R.W.; McLoon, L.K.; Thompson, L.V. C57BL/6 life span study: Age-related declines in muscle power production and contractile velocity. AGE 2015, 37, 1–16. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Blasco, A.; Gras, S.; Mòdol-Caballero, G.; Tarabal, O.; Casanovas, A.; Piedrafita, L.; Barranco, A.; Das, T.; Pereira, S.L.; Navarro, X.; et al. Motoneuron deafferentation and gliosis occur in association with neuromuscular regressive changes during ageing in mice. J. Cachexia Sarcopenia Muscle 2020, 11, 1628–1660. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Giacomello, E.; Crea, E.; Torelli, L.; Bergamo, A.; Reggiani, C.; Sava, G.; Toniolo, L. Age dependent modification of the metabolic profile of the tibialis anterior muscle fibers in C57BL/6J mice. Int. J. Mol. Sci. 2020, 21, 3923. [Google Scholar] [CrossRef]
- Crupi, A.N.; Nunnelee, J.S.; Taylor, D.J.; Thomas, A.; Vit, J.-P.; Riera, C.E.; Gottlieb, R.A.; Goodridge, H.S. Oxidative muscles have better mitochondrial homeostasis than glycolytic muscles throughout life and maintain mitochondrial function during aging. Aging 2018, 10, 3327–3352. [Google Scholar] [CrossRef]
- Pearson, T.; McArdle, A.; Jackson, M. Nitric oxide availability is increased in contracting skeletal muscle from aged mice, but does not differentially decrease muscle superoxide. Free. Radic. Biol. Med. 2015, 78, 82–88. [Google Scholar] [CrossRef][Green Version]
- Hwee, D.T.; Baehr, L.M.; Philp, A.; Baar, K.; Bodine, S.C. Maintenance of muscle mass and load-induced growth in muscle ring finger 1 null mice with age. Aging Cell 2014, 13, 92–101. [Google Scholar] [CrossRef]
- Richmonds, C.R.; Boonyapisit, K.; Kusner, L.L.; Kaminski, H.J. Nitric oxide synthase in aging rat skeletal muscle. Mech. Ageing Dev. 1999, 109, 177–189. [Google Scholar] [CrossRef]
- McDonagh, B.; Sakellariou, G.K.; Smith, N.T.; Brownridge, P.; Jackson, M.J. Differential cysteine labeling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J. Proteome Res. 2014, 13, 5008–5021. [Google Scholar] [CrossRef]
- Rice, K.M.; Preston, D.; Neff, D.; Norton, M.; Blough, E.R. Age-related dystrophin-glycoprotein complex structure and function in the rat extensor digitorum longus and soleus muscle. J. Gerontol. A Boil. Sci. Med. Sci. 2006, 61, 1119–1129. [Google Scholar] [CrossRef]
- Capanni, C.; Squarzoni, S.; Petrini, S.; Villanova, M.; Muscari, C.; Maraldi, N.M.; Guarnieri, C.; Caldarera, C.M. Increase of neuronal nitric oxide synthase in rat skeletal muscle during ageing. Biochem. Biophys. Res. Commun. 1998, 245, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Leiter, J.R.S.; Upadhaya, R.; Anderson, J.E. Nitric oxide and voluntary exercise together promote quadriceps hypertrophy and increase vascular density in female 18-month-old mice. Am. J. Physiol. 2012, 302, C1306–C1315. [Google Scholar] [CrossRef]
- Samengo, G.; Avik, A.; Fedor, B.; Whittaker, D.; Myung, K.H.; Wehling-Henricks, M.; Tidball, J.G. Age-related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S-nitrosylation that increase myofibril degradation and sarcopenia. Aging Cell 2012, 11, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S. How postural muscle senses disuse? Early signs and signals. Int. J. Mol. Sci. 2020, 21, 5037. [Google Scholar] [CrossRef]
- Zeng, Z.; Liang, J.; Wu, L.; Zhang, H.; Lv, J.; Chen, N. Exercise-induced autophagy suppresses sarcopenia through Akt/mTOR and Akt/FoxO3a signal pathways and AMPK-mediated mitochondrial quality control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef] [PubMed]
- Hangelbroek, R.W.J.; Fazelzadeh, P.; Tieland, M.; Boekschoten, M.V.; Hooiveld, G.J.E.J.; van Duynhoven, J.P.M.; Timmons, J.A.; Verdijk, L.B.; de Groot, L.C.P.G.M.; van Loon, L.J.C.; et al. Expression of protocadherin gamma in skeletal muscle tissue is associated with age and muscle weakness. J. Cachexia Sarcopenia Muscle 2016, 7, 604–614. [Google Scholar] [CrossRef]
- Jaka, O.; Casas-Fraile, L.; Azpitarte, M.; Aiastui, A.; de Munain, A.L.; Sáenz, A. FRZB and melusin, overexpressed in LGMD2A, regulate integrin β1D isoform replacement altering myoblast fusion and the integrin-signalling pathway. Expert Rev. Mol. Med. 2017, 19, e2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, J.; Li, Y.; Xie, Y.; Shan, H.; Chen, M.; Zhang, J.; Yang, X.; Zhang, Q.; Yang, X. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1α/SIRT3 pathway involved. Chem. Biol. Interact. 2017, 277, 168–175. [Google Scholar] [CrossRef]
- Rondanelli, M.; Miccono, A.; Peroni, G.; Guerriero, F.; Morazzoni, P.; Riva, A.; Guido, D.; Perna, S. A systematic review on the effects of botanicals on skeletal muscle health in order to prevent sarcopenia. Evid. Based Complement. Altern. Med. 2016, 2016, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vitadello, M.; Doria, A.; Tarricone, E.; Ghirardello, A.; Gorza, L. Myofiber stress-response in myositis: Parallel investigations on patients and experimental animal models of muscle regeneration and systemic inflammation. Arthritis Res. Ther. 2010, 12, R52. [Google Scholar] [CrossRef]
- Gorza, L.; Vitadello, M. Reduced amount of the glucose-regulated protein GRP94 in skeletal myoblasts results in loss of fusion competence. FASEB J. 2000, 14, 461–475. [Google Scholar] [CrossRef]
- Barton, E.R.; Park, S.; James, J.K.; Makarewich, C.A.; Philippou, A.; Eletto, D.; Lei, H.; Brisson, B.; Ostrovsky, O.; Li, Z.; et al. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012, 26, 3691–3702. [Google Scholar] [CrossRef]
- Frasson, M.; Vitadello, M.; Brunati, A.M.; La Rocca, N.; Tibaldi, E.; Pinna, L.A.; Gorza, L.; Donella-Deana, A. Grp94 is tyr-phosphorylated by fyn in the lumen of the endoplasmic reticulum and translocates to Golgi in differentiating myoblasts. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 239–252. [Google Scholar] [CrossRef]
- Li, S.; Fu, Y.; Pang, Y.; Tong, H.; Li, S.; Yan, Y. GRP94 promotes muscle differentiation by inhibiting the PI3K/AKT/mTOR signaling pathway. J. Cell. Physiol. 2019, 234, 21211–21223. [Google Scholar] [CrossRef]
- Ham, D.J.; A Rüegg, M. Causes and consequences of age-related changes at the neuromuscular junction. Curr. Opin. Physiol. 2018, 4, 32–39. [Google Scholar] [CrossRef]
- Scapagnini, G.; Colombrita, C.; Amadio, M.; D’Agata, V.; Arcelli, E.; Sapienza, M.; Quattrone, A.; Calabrese, V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid. Redox Signal. 2006, 8, 395–403. [Google Scholar] [CrossRef]
- Caillaud, M.; Chantemargue, B.; Richard, L.; Vignaud, L.; Favreau, F.; Faye, P.-A.; Vignoles, P.; Sturtz, F.; Trouillas, P.; Vallat, J.-M.; et al. Local low dose curcumin treatment improves functional recovery and remyelination in a rat model of sciatic nerve crush through inhibition of oxidative stress. Neuropharmacol 2018, 139, 98–116. [Google Scholar] [CrossRef]
- Ma, J.; Yu, H.; Liu, J.; Chen, Y.; Wang, Q.; Xiang, L. Curcumin promotes nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats. Neurosci. Lett. 2016, 610, 139–143. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; Van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Kang, C. Senolytics and senostatics: A two-pronged approach to target cellular senescence for delaying aging and age-related diseases. Mol. Cells 2019, 42, 821–827. [Google Scholar] [PubMed]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, Ł.; Sikora, E. The role of curcumin in the modulation of ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef] [PubMed]
- Germinario, E.; Bondì, M.; Cencetti, F.; Donati, C.; Nocella, M.; Colombini, B.; Betto, R.; Bruni, P.; Bagni, M.A.; Danieli-Betto, D. S1P3 receptor influences key physiological properties of fast-twitch extensor digitorum longus muscle. J. Appl. Physiol. 2016, 120, 1288–1300. [Google Scholar] [CrossRef] [PubMed]


| C57BL Strain | Age (mo) | Curcumin Treatment (6 mo) | BW (g) | Soleus Weight (mg) | Soleus MW/BW | EDL Weight (mg) | EDL MW/BW |
|---|---|---|---|---|---|---|---|
| 6J | |||||||
| (n = 8) | 6 | - | 30.9 ± 1.3 | 8.9 ± 0.5 | 0.28 ± 0.01 | 10.0 ± 0.5 | 0.34 ± 0.02 |
| (n = 10) | 24 | - | 33.1 ± 0.6 | 8.6 ± 0.4 | 0.25 ± 0.01 | 11.4 ± 0.4 | 0.34 ± 0.01 |
| (n = 8) | 24 | + | 32.9 ± 0.5 | 8.7 ± 0.3 | 0.26 ± 0.01 | 11.8 ± 0.7 | 0.34 ± 0.02 |
| 10ScSn | |||||||
| (n = 9) | 6 | - | 31.8 ± 1.5 | 8.3 ± 0.1 | 0.28 ± 0.01 | 10.3 ± 0.3 | 0.30 ± 0.01 |
| (n = 8) | 24 | - | 35.4 ± 1.6 | 6.9 ± 0.5 | 0.19 ± 0.01 ** | 9.0 ± 0.2 * | 0.25 ± 0.01 * |
| (n = 9) | 24 | + | 32.4 ± 0.5 | 8.1 ± 0.5 | 0.24 ± 0.01 | 9.7 ± 0.3 | 0.29 ± 0.01 |
| C57BL Strain (n Animal) | Age (mo) | Curcumin Treatment (6 mo) | Major Fiber Type Percentage | Minimal Feret’s Diameter (m) | Myofiber Total Number | ||
|---|---|---|---|---|---|---|---|
| Soleus | Type 1 | Type 2A | Type 1 | Type 2A | |||
| 6J | |||||||
| (n = 5) | 6 | - | 38.8 ± 3.3 | 54.2 ± 4.2 | 38.4 ± 1.5 | 35.7 ± 2.4 | 887.2 ± 65.7 |
| (n = 4) | 24 | - | 32.0 ± 1.6 | 65.6 ± 1.5 | 49.8 ± 5.6 | 44.2 ± 2.4 *ac | 796.2 ± 137.9 |
| (n = 4) | 24 | + | 41.6 ± 3.6 | 56.3 ± 3.6 | 42.0 ± 2.5 | 39.8 ± 2.1 | 765.0 ± 64.3 |
| 10ScSn | |||||||
| (n = 6) | 6 | - | 37.5 ± 2.7 | 56.8 ± 1.7 | 40.2 ± 1.6 | 36.1 ± 1.0 | 1067.6 ± 30.5 |
| (n = 6) | 24 | - | 47.9 ± 3.8 *a | 47.8 ± 3.5 *a | 35.7 ± 0.7 *ac | 35.5 ± 0.6 | 905.3 ± 47.3 *a |
| (n = 5) | 24 | + | 52.7 ± 2.8 *a | 46.3 ± 3.0 *a | 40.9 ± 0.7 | 40.8 ± 0.9 ** | 872.0 ± 48.5 *a |
| EDL | Type 2X | Type 2B/2BX | Type 2X | Type 2B/2BX | |||
| 6J | |||||||
| (n = 5) | 6 | - | 17.5 ± 2.4 | 74.1 ± 3.8 | 29.7 ± 2.2 | 37.6 ± 2.5 | 962.2 ± 49.8 |
| (n = 4) | 24 | - | 18.6 ± 3.9 | 72.5 ± 3.4 | 28.9 ± 1.6 | 36.2 ± 1.6 | 841.0 ± 38.1 |
| (n = 4) | 24 | + | 16.2 ± 4.2 | 72.6 ± 6.7 | 32.8 ± 0.9 | 40.1 ± 1.1 | 893.2 ± 19.5 |
| 10ScSn | |||||||
| (n = 5) | 6 | - | 21.8 ± 1.6 | 72.6 ± 2.3 | 26.4 ± 1.8 | 39.2 ± 2.4 | 999.2 ± 72.7. |
| (n = 6) | 24 | - | 9.3 ± 1.9 ** | 85.6 ± 1.9 ** | 28.6 ± 2.8 | 36.8 ± 1.7 | 826.8 ± 30.0 *ac |
| (n = 5) | 24 | + | 20.4 ± 2.3 | 77.1 ± 2.2 | 26.9 ± 1.0 | 34.8 ± 1.7 | 927.8 ± 32.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorza, L.; Germinario, E.; Tibaudo, L.; Vitadello, M.; Tusa, C.; Guerra, I.; Bondì, M.; Salmaso, S.; Caliceti, P.; Vitiello, L.; et al. Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia. Int. J. Mol. Sci. 2021, 22, 11789. https://doi.org/10.3390/ijms222111789
Gorza L, Germinario E, Tibaudo L, Vitadello M, Tusa C, Guerra I, Bondì M, Salmaso S, Caliceti P, Vitiello L, et al. Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia. International Journal of Molecular Sciences. 2021; 22(21):11789. https://doi.org/10.3390/ijms222111789
Chicago/Turabian StyleGorza, Luisa, Elena Germinario, Lucia Tibaudo, Maurizio Vitadello, Chiara Tusa, Irene Guerra, Michela Bondì, Stefano Salmaso, Paolo Caliceti, Libero Vitiello, and et al. 2021. "Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia" International Journal of Molecular Sciences 22, no. 21: 11789. https://doi.org/10.3390/ijms222111789
APA StyleGorza, L., Germinario, E., Tibaudo, L., Vitadello, M., Tusa, C., Guerra, I., Bondì, M., Salmaso, S., Caliceti, P., Vitiello, L., & Danieli-Betto, D. (2021). Chronic Systemic Curcumin Administration Antagonizes Murine Sarcopenia and Presarcopenia. International Journal of Molecular Sciences, 22(21), 11789. https://doi.org/10.3390/ijms222111789







