Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Brain Tumors and Therapeutic Management
3. Drug Delivery Strategies for Brain Tumors
3.1. Physical Drug Delivery Strategies
3.2. Chemical Drug Delivery Strategies
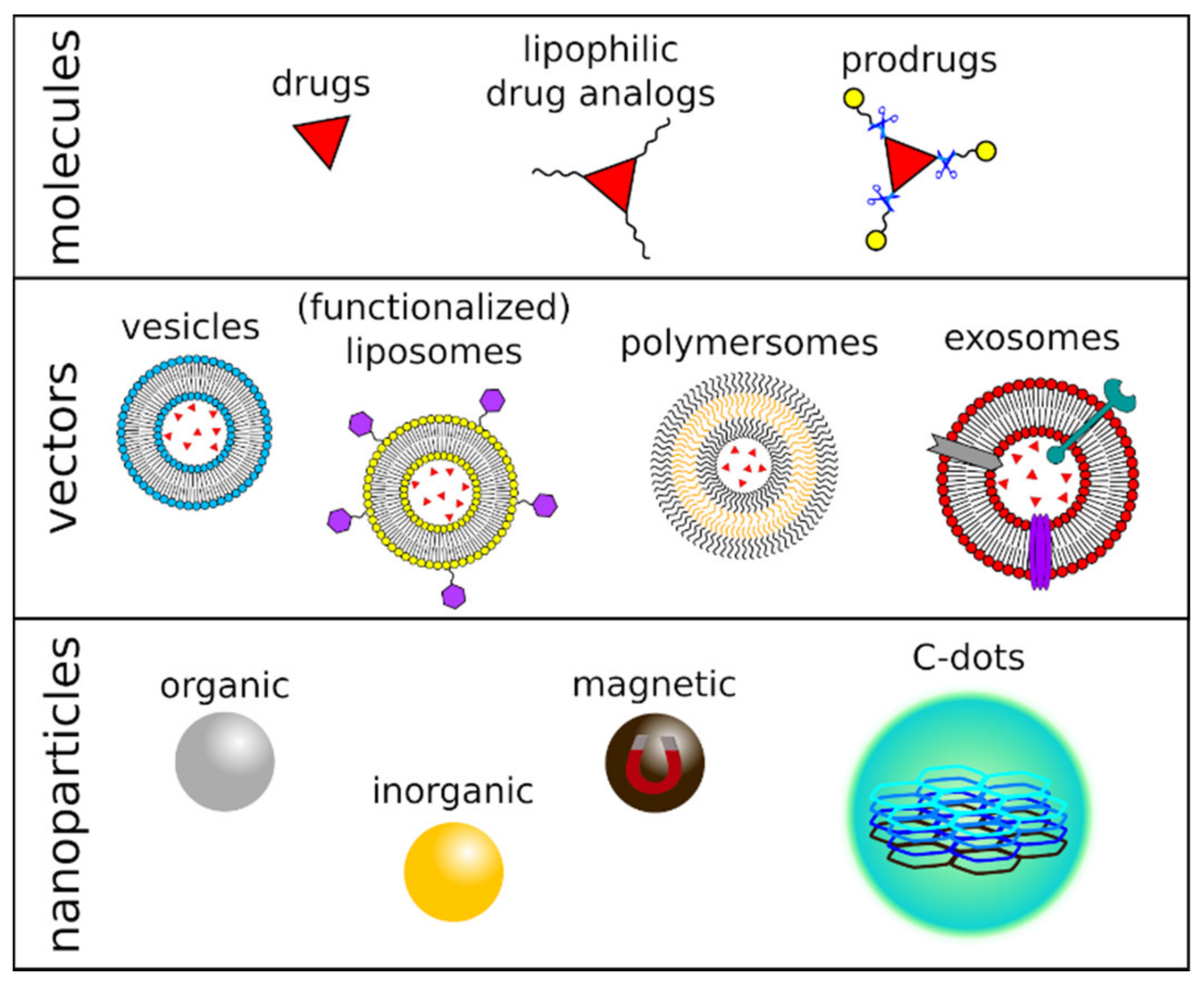
3.3. Vector-Based Drug Delivery Strategies
3.4. Nanomaterial-Based Drug Delivery Strategies
4. Carbon Dots for Drug Delivery in Brain Tumors
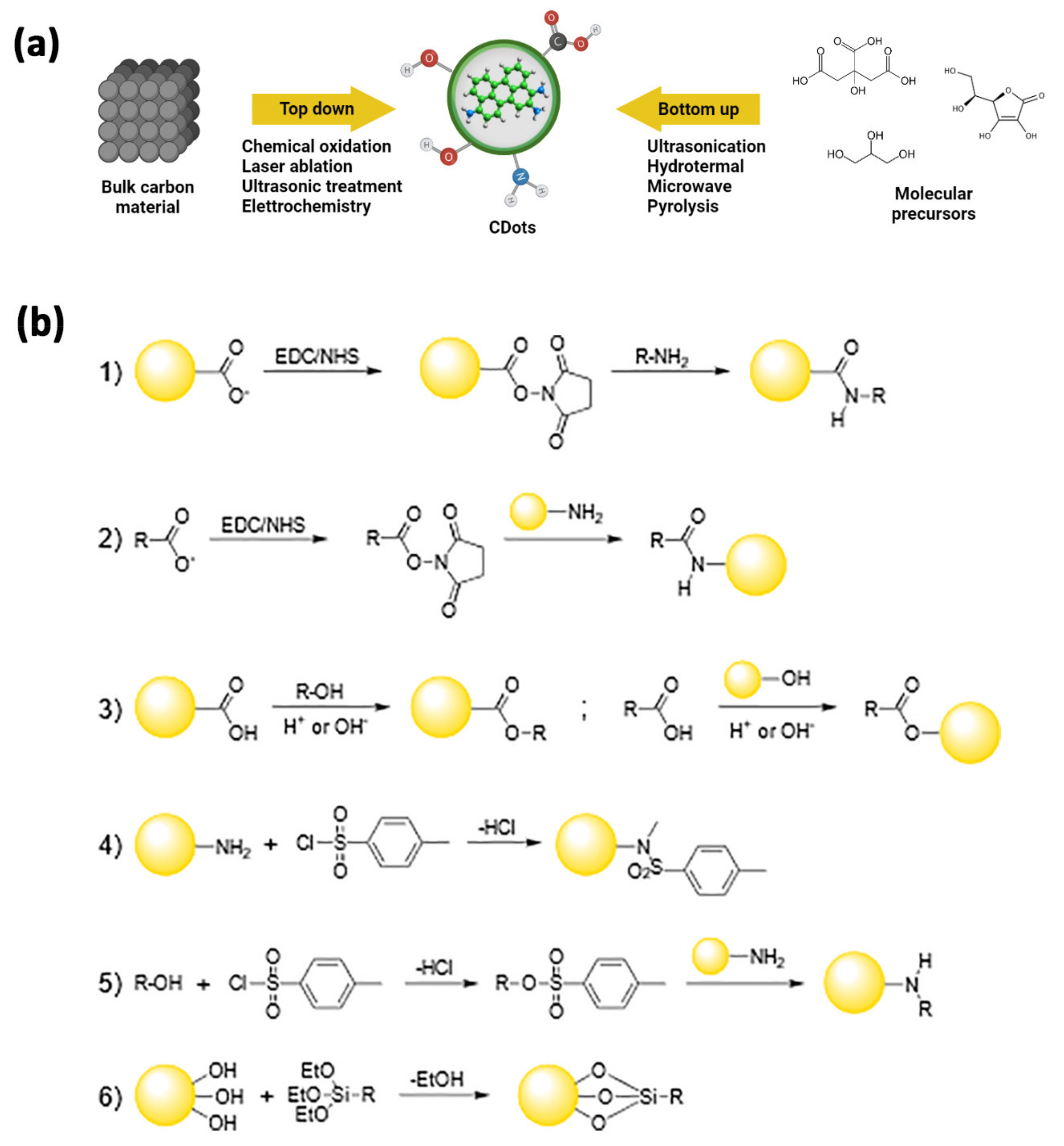
4.1. Carbon Dots
4.2. Carbon Dots Crossing the BBB
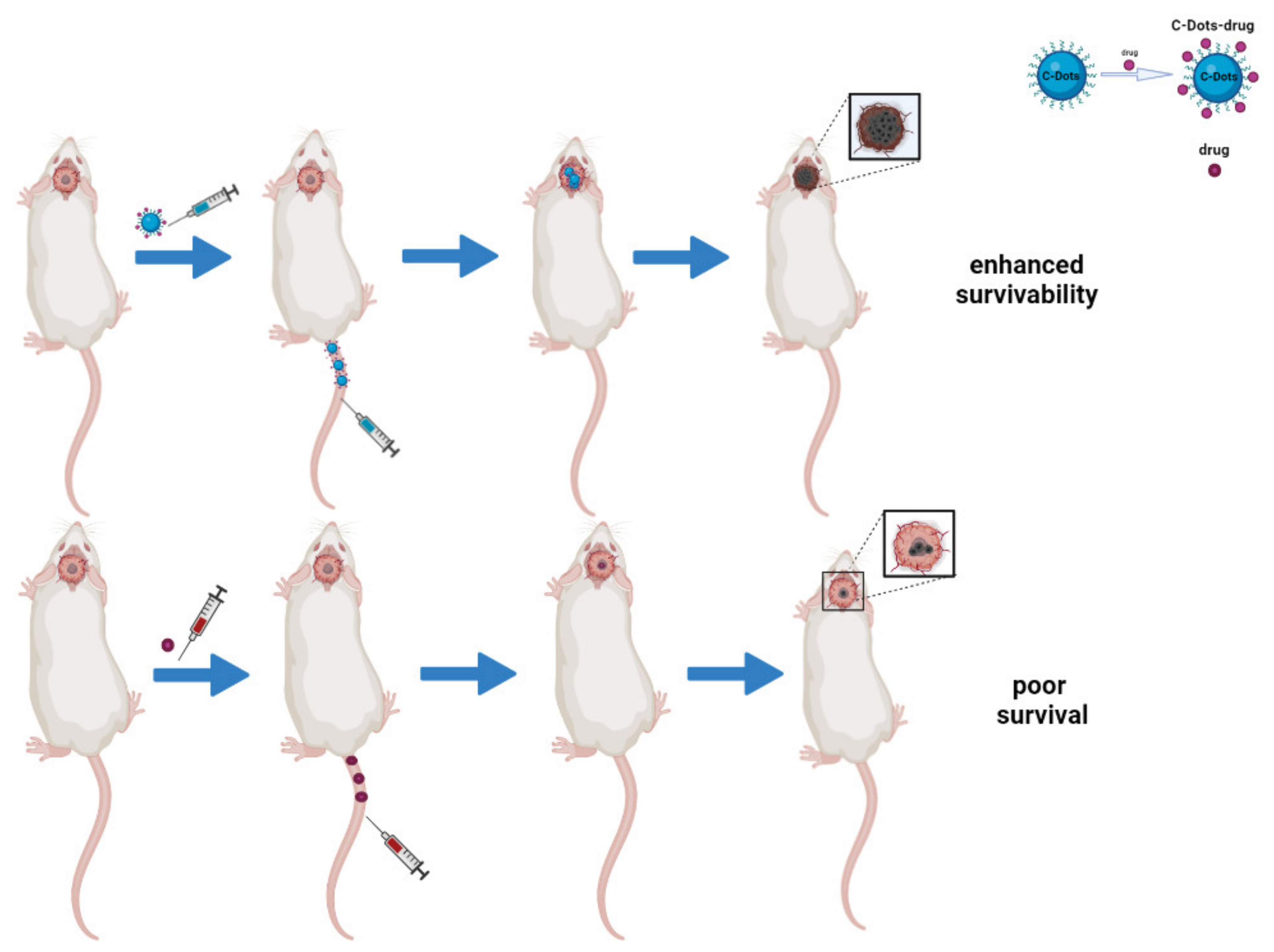
4.3. Carbon Dots as Drug Delivery Systems in Brain Tumors Treatment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Curtin, S.C.; Minino, A.M.; Anderson, R.N. Declines in Cancer Death Rates Among Children and Adolescents in the United States, 1999–2014. In NCHS Data Brief, No 257; National Center for Health Statistics: Hyattsville, MD, USA, 2016; pp. 1–8. [Google Scholar]
- McNeill, K.A. Epidemiology of Brain Tumors. Neurol. Clin. 2016, 34, 981–998. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review, 1975–2017. Available online: https://seer.cancer.gov/csr/1975_2017/index.html (accessed on 22 May 2021).
- Buckner, J.C.; Brown, P.D.; O’Neill, B.P.; Meyer, F.B.; Wetmore, C.J.; Uhm, J.H. Central Nervous System Tumors. Mayo Clin. Proc. 2007, 82, 1271–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. (Berl.) 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Ohka, F.; Natsume, A.; Wakabayashi, T. Current Trends in Targeted Therapies for Glioblastoma Multiforme. Neurol. Res. Int. 2012, 2012, 878425. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovenberg, M.S.S.; Degeling, M.H.; Tannous, B.A. Cell-Based Immunotherapy Against Gliomas: From Bench to Bedside. Mol. Ther. 2013, 21, 1297–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Chegaev, K.; Fraix, A.; Gazzano, E.; Abd-Ellatef, G.E.F.; Blangetti, M.; Rolando, B.; Conoci, S.; Riganti, C.; Fruttero, R.; Gasco, A.; et al. Light-Regulated NO Release as a Novel Strategy To Overcome Doxorubicin Multidrug Resistance. ACS Med. Chem. Lett. 2017, 8, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Marino, N.; Petralia, S.; Perez-Lloret, M.; Mosinger, J.; Conoci, S.; Sortino, S. Graphene Oxide Nanohybrid That Photoreleases Nitric Oxide. J. Mater. Chem. B 2016, 4, 5825–5830. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Nocito, G.; Calabrese, G.; Forte, S.; Petralia, S.; Esposito, E.; Conoci, S.; Puglisi, C.; Campolo, M. Carbon Dots as Promising Tools for Cancer Diagnosis and Therapy. Cancers 2021, 13, 1991. [Google Scholar] [CrossRef]
- Gong, H.; Peng, R.; Liu, Z. Carbon Nanotubes for Biomedical Imaging: The Recent Advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Leece, R.; Xu, J.; Ostrom, Q.T.; Chen, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. Global Incidence of Malignant Brain and Other Central Nervous System Tumors by Histology, 2003–2007. Neuro-Oncology 2017, 19, 1553–1564. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A Multivariate Analysis of 416 Patients with Glioblastoma Multiforme: Prognosis, Extent of Resection, and Survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma Survival in the United States before and during the Temozolomide Era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary Brain Tumours in Adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Rautio, J.; Chikhale, P.J. Drug Delivery Systems for Brain Tumor Therapy. Curr. Pharm. Des. 2004, 10, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Groothuis, D.R. The Blood-Brain and Blood-Tumor Barriers: A Review of Strategies for Increasing Drug Delivery. Neuro-Oncology 2000, 2, 45–59. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, W. Recent Advances in Brain Tumor-Targeted Nano-Drug Delivery Systems. Expert Opin. Drug Deliv. 2012, 9, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Squire, J.M.; Chew, M.; Nneji, G.; Neal, C.; Barry, J.; Michel, C. Quasi-Periodic Substructure in the Microvessel Endothelial Glycocalyx: A Possible Explanation for Molecular Filtering? J. Struct. Biol. 2001, 136, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, C.-L.; Liu, C.-M. Drug Delivery Strategies to Enhance the Permeability of the Blood-Brain Barrier for Treatment of Glioma. Drug Des. Devel. Ther. 2015, 9, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzeng, S.Y.; Green, J.J. Therapeutic Nanomedicine for Brain Cancer. Ther. Deliv. 2013, 4, 687–704. [Google Scholar] [CrossRef] [Green Version]
- Tamargo, R.J.; Langer, R.; Brem, H. 8-Interstitial Drug Delivery to the Central Nervous System Using Controlled Release Polymers: Chemotherapy for Brain Tumors. In Methods in Neurosciences; Providing Pharmacological Access to the Brain; Flanagan, T.R., Emerich, D.F., Winn, S.R., Eds.; Academic Press: Cambridge, MA, USA, 1994; Volume 21, pp. 135–149. [Google Scholar] [CrossRef]
- Brem, H.; Gabikian, P. Biodegradable Polymer Implants to Treat Brain Tumors. J. Control. Release 2001, 74, 63–67. [Google Scholar] [CrossRef]
- Doolittle, N.D.; Miner, M.E.; Hall, W.A.; Siegal, T.; Hanson, E.J.; Osztie, E.; McAllister, L.D.; Bubalo, J.S.; Kraemer, D.F.; Fortin, D.; et al. Safety and Efficacy of a Multicenter Study Using Intraarterial Chemotherapy in Conjunction with Osmotic Opening of the Blood-Brain Barrier for the Treatment of Patients with Malignant Brain Tumors. Cancer 2000, 88, 637–647. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular Mechanisms of the Blood-Brain Barrier Opening Induced by Ultrasound in Presence of Microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A.; Gottschlich, R.; Devant, R.M. A Method to Determine the Ability of Drugs to Diffuse through the Blood-Brain Barrier. Proc. Natl. Acad. Sci. USA 1994, 91, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Saeed, M.; Song, R.; Sun, T.; Jiang, C.; Yu, H. Dynamic Covalent Chemistry-Regulated Stimuli-Activatable Drug Delivery Systems for Improved Cancer Therapy. Chin. Chem. Lett. 2020, 31, 1051–1059. [Google Scholar] [CrossRef]
- Geng, W.-C.; Sessler, J.L.; Guo, D.-S. Supramolecular Prodrugs Based on Host–Guest Interactions. Chem. Soc. Rev. 2020, 49, 2303–2315. [Google Scholar] [CrossRef]
- Greig, N.H.; Daly, E.M.; Sweeney, D.J.; Rapoport, S.I. Pharmacokinetics of Chlorambucil-Tertiary Butyl Ester, a Lipophilic Chlorambucil Derivative That Achieves and Maintains High Concentrations in Brain. Cancer Chemother. Pharmacol. 1990, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Smith, Q.R. Drug Delivery to Brain and the Role of Carrier-Mediated Transport. In Frontiers in Cerebral Vascular Biology: Transport and Its Regulation; Advances in Experimental Medicine and Biology; Drewes, L.R., Betz, A.L., Eds.; Springer: Boston, MA, USA, 1993; pp. 83–93. ISBN 978-1-4615-2920-0. [Google Scholar]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics Across the Blood-Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [Green Version]
- Blumling, J.P.I.; Silva, G.A. Targeting the Brain: Advances in Drug Delivery. Curr. Pharm. Biotechnol. 2012, 13, 2417–2426. [Google Scholar] [CrossRef]
- Xiao, G.; Gan, L.-S. Receptor-Mediated Endocytosis and Brain Delivery of Therapeutic Biologics. Int. J. Cell Biol. 2013, 2013, e703545. [Google Scholar] [CrossRef] [Green Version]
- Daniels, T.R.; Delgado, T.; Helguera, G.; Penichet, M.L. The Transferrin Receptor Part II: Targeted Delivery of Therapeutic Agents into Cancer Cells. Clin. Immunol. 2006, 121, 159–176. [Google Scholar] [CrossRef]
- Smith, Q.R. Carrier-Mediated Transport to Enhance Drug Delivery to Brain. Int. Congr. Ser. 2005, 1277, 63–74. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [Green Version]
- Vieira, D.B.; Gamarra, L.F. Getting into the Brain: Liposome-Based Strategies for Effective Drug Delivery across the Blood-Brain Barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Hau, P.; Fabel, K.; Baumgart, U.; Rümmele, P.; Grauer, O.; Bock, A.; Dietmaier, C.; Dietmaier, W.; Dietrich, J.; Dudel, C.; et al. Pegylated Liposomal Doxorubicin-Efficacy in Patients with Recurrent High-Grade Glioma. Cancer 2004, 100, 1199–1207. [Google Scholar] [CrossRef]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C.; et al. RNOP-09: Pegylated Liposomal Doxorubicine and Prolonged Temozolomide in Addition to Radiotherapy in Newly Diagnosed Glioblastoma—A Phase II Study. BMC Cancer 2009, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Ananda, S.; Nowak, A.K.; Cher, L.; Dowling, A.; Brown, C.; Simes, J.; Rosenthal, M.A. Phase 2 Trial of Temozolomide and Pegylated Liposomal Doxorubicin in the Treatment of Patients with Glioblastoma Multiforme Following Concurrent Radiotherapy and Chemotherapy. J. Clin. Neurosci. 2011, 18, 1444–1448. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, D.; Ma, X.; Wang, J.; Hou, W.; Zhang, W. Exosomes as Drug Carriers for Cancer Therapy and Challenges Regarding Exosome Uptake. Biomed. Pharmacother. 2020, 128, 110237. [Google Scholar] [CrossRef]
- Masserini, M. Nanoparticles for Brain Drug Delivery. ISRN Biochem. 2013, 2013, e238428. [Google Scholar] [CrossRef] [Green Version]
- Petkar, K.C.; Chavhan, S.S.; Agatonovik-Kustrin, S.; Sawant, K. Nanostructured Materials in Drug and Gene Delivery: A Review of the State of the Art. Crit. Rev. Ther. Drug Carr. Syst. 2011, 28, 101–164. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y. In Vivo Delivery of RNAi with Lipid-Based Nanoparticles. Annu. Rev. Biomed. Eng. 2011, 13, 507–530. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, P.S.; Banerjee, R. Nanotechnology-Based Strategies as Novel Therapies in Gliomas. Ther. Deliv. 2018, 9, 571–592. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent Progress of Drug Nanoformulations Targeting to Brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Dhermain, F.G.; Hau, P.; Lanfermann, H.; Jacobs, A.H.; van den Bent, M.J. Advanced MRI and PET Imaging for Assessment of Treatment Response in Patients with Gliomas. Lancet Neurol. 2010, 9, 906–920. [Google Scholar] [CrossRef]
- Plate, K.H.; Scholz, A.; Dumont, D.J. Tumor Angiogenesis and Anti-Angiogenic Therapy in Malignant Gliomas Revisited. Acta Neuropathol. (Berl.) 2012, 124, 763–775. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, V.P.; Jain, R.K. Strategies for Advancing Cancer Nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Nekoueian, K.; Amiri, M.; Sillanpää, M.; Marken, F.; Boukherroub, R.; Szunerits, S. Carbon-Based Quantum Particles: An Electroanalytical and Biomedical Perspective. Chem. Soc. Rev. 2019, 48, 4281–4316. [Google Scholar] [CrossRef]
- Wu, Z.L.; Liu, Z.X.; Yuan, Y.H. Carbon Dots: Materials, Synthesis, Properties and Approaches to Long-Wavelength and Multicolor Emission. J. Mater. Chem. B 2017, 5, 3794–3809. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Le, Q.V.; Jang, H.W.; Shokouhimehr, M. Carbon and Graphene Quantum Dots: A Review on Syntheses, Characterization, Biological and Sensing Applications for Neurotransmitter Determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An Electrochemical Avenue to Blue Luminescent Nanocrystals from Multiwalled Carbon Nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-L.; Zhang, Z.-L.; Huang, B.-H.; Peng, J.; Zhang, M.; Pang, D.-W. Facile Preparation of Low Cytotoxicity Fluorescent Carbon Nanocrystals by Electrooxidation of Graphite. Chem. Commun. 2008, 41, 5116–5118. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.; Wang, J.; Lim, A.; Wang, S.; Loh, K.P. One-Pot Synthesis of Fluorescent Carbon Nanoribbons, Nanoparticles, and Graphene by the Exfoliation of Graphite in Ionic Liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef]
- Zheng, L.; Chi, Y.; Dong, Y.; Lin, J.; Wang, B. Electrochemiluminescence of Water-Soluble Carbon Nanocrystals Released Electrochemically from Graphite. J. Am. Chem. Soc. 2009, 131, 4564–4565. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical Synthesis of Carbon Nanodots Directly from Alcohols. Chem. Eur. J. 2014, 20, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, Y.; Niu, F.; Gooding, J.J.; Liu, J. Carbon Quantum Dots Directly Generated from Electrochemical Oxidation of Graphite Electrodes in Alkaline Alcohols and the Applications for Specific Ferric Ion Detection and Cell Imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, T.; Mao, C. Fluorescent Carbon Nanoparticles Derived from Candle Soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Travas-Sejdic, J. Simple Aqueous Solution Route to Luminescent Carbogenic Dots from Carbohydrates. Chem. Mater. 2009, 21, 5563–5565. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent Carbon Nanoparticles: Synthesis, Characterization, and Bioimaging Application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.-C.; Ruan, G.; et al. Coal as an Abundant Source of Graphene Quantum Dots. Nat. Commun. 2013, 4, 2943. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Liu, C.; Zhang, Z.-L.; Pang, D.-W. Photoluminescence-Tunable Carbon Nanodots: Surface-State Energy-Gap Tuning. Adv. Mater. 2015, 27, 1663–1667. [Google Scholar] [CrossRef]
- Meng, X.; Chang, Q.; Xue, C.; Yang, J.; Hu, S. Full-Colour Carbon Dots: From Energy-Efficient Synthesis to Concentration-Dependent Photoluminescence Properties. Chem. Commun. 2017, 53, 3074–3077. [Google Scholar] [CrossRef]
- Dey, S.; Govindaraj, A.; Biswas, K.; Rao, C.N.R. Luminescence Properties of Boron and Nitrogen Doped Graphene Quantum Dots Prepared from Arc-Discharge-Generated Doped Graphene Samples. Chem. Phys. Lett. 2014, 595, 203–208. [Google Scholar] [CrossRef]
- Biazar, N.; Poursalehi, R.; Delavari, H. Optical and Structural Properties of Carbon Dots/TiO2 Nanostructures Prepared via DC Arc Discharge in Liquid. AIP Conf. Proc. 2018, 1920, 020033. [Google Scholar] [CrossRef]
- Chao-Mujica, F.J.; Garcia-Hernández, L.; Camacho-López, S.; Camacho-López, M.; Camacho-López, M.A.; Reyes Contreras, D.; Pérez-Rodríguez, A.; Peña-Caravaca, J.P.; Páez-Rodríguez, A.; Darias-Gonzalez, J.G.; et al. Carbon Quantum Dots by Submerged Arc Discharge in Water: Synthesis, Characterization, and Mechanism of Formation. J. Appl. Phys. 2021, 129, 163301. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of Carbon Quantum Dots with Tunable Photoluminescence by Rapid Laser Passivation in Ordinary Organic Solvents. Chem. Commun. 2010, 47, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Yan, L.; Si, J.; Hou, X. Femtosecond Laser-Induced Size Reduction of Carbon Nanodots in Solution: Effect of Laser Fluence, Spot Size, and Irradiation Time. J. Appl. Phys. 2015, 117, 084304. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.; Zeng, X.; Lu, Y. Preparation of Carbon Dots by Non-Focusing Pulsed Laser Irradiation in Toluene. Chem. Commun. 2016, 52, 819–822. [Google Scholar] [CrossRef]
- Nguyen, V.; Zhao, N.; Yan, L.; Zhong, P.; Nguyen, V.C.; Le, P.H. Double-Pulse Femtosecond Laser Ablation for Synthesis of Ultrasmall Carbon Nanodots. Mater. Res. Express 2020, 7, 015606. [Google Scholar] [CrossRef]
- Huang, H.; Cui, Y.; Liu, M.; Chen, J.; Wan, Q.; Wen, Y.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A One-Step Ultrasonic Irradiation Assisted Strategy for the Preparation of Polymer-Functionalized Carbon Quantum Dots and Their Biological Imaging. J. Colloid Interface Sci. 2018, 532, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, L. One-Step Sonochemical Synthesis of Versatile Nitrogen-Doped Carbon Quantum Dots for Sensitive Detection of Fe2+ Ions and Temperature in Vitro. Mater. Sci. Eng. C 2019, 101, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Qiang, R.; Yang, S.; Hou, K.; Wang, J. Synthesis of Carbon Quantum Dots with Green Luminescence from Potato Starch. New J. Chem. 2019, 43, 10826–10833. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Liu, Y. A Novel One-Step Approach to Synthesize Fluorescent Carbon Nanoparticles. Eur. J. Inorg. Chem. 2010, 2010, 4411–4414. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Zheng, M.; Hu, C.; Tan, S.; Xiao, Y.; Yang, Q.; Liu, Y. One-Step Synthesis of Amino-Functionalized Fluorescent Carbon Nanoparticles by Hydrothermal Carbonization of Chitosan. Chem. Commun. 2012, 48, 380–382. [Google Scholar] [CrossRef]
- Miao, H.; Wang, L.; Zhuo, Y.; Zhou, Z.; Yang, X. Label-Free Fluorimetric Detection of CEA Using Carbon Dots Derived from Tomato Juice. Biosens. Bioelectron. 2016, 86, 83–89. [Google Scholar] [CrossRef]
- Barua, S.; Gogoi, S.; Khan, R. Fluorescence Biosensor Based on Gold-Carbon Dot Probe for Efficient Detection of Cholesterol. Synth. Met. 2018, 244, 92–98. [Google Scholar] [CrossRef]
- Zhao, C.; Jiao, Y.; Hua, J.; Yang, J.; Yang, Y. Hydrothermal Synthesis of Nitrogen-Doped Carbon Quantum Dots as Fluorescent Probes for the Detection of Dopamine. J. Fluoresc. 2018, 28, 269–276. [Google Scholar] [CrossRef]
- Wang, X.; Yang, P.; Feng, Q.; Meng, T.; Wei, J.; Xu, C.; Han, J. Green Preparation of Fluorescent Carbon Quantum Dots from Cyanobacteria for Biological Imaging. Polymers 2019, 11, 616. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave Synthesis of Fluorescent Carbon Nanoparticles with Electrochemiluminescence Properties. Chem. Commun. 2009, 34, 5118–5120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Microwave Assisted One-Step Green Synthesis of Cell-Permeable Multicolor Photoluminescent Carbon Dots without Surface Passivation Reagents. J. Mater. Chem. 2011, 21, 2445–2450. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, N.C.; Khan, S.; Tiwari, S.; Chaudhary, A.; Nandi, C.K. Paper Strip Based and Live Cell Ultrasensitive Lead Sensor Using Carbon Dots Synthesized from Biological Media. Sens. Actuators B Chem. 2016, 232, 107–114. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Zhang, P.; Liu, L. Zwitterionic Nanogels Crosslinked by Fluorescent Carbon Dots for Targeted Drug Delivery and Simultaneous Bioimaging. Acta Biomater. 2016, 40, 254–262. [Google Scholar] [CrossRef]
- Choi, Y.; Thongsai, N.; Chae, A.; Jo, S.; Kang, E.B.; Paoprasert, P.; Park, S.Y.; In, I. Microwave-Assisted Synthesis of Luminescent and Biocompatible Lysine-Based Carbon Quantum Dots. J. Ind. Eng. Chem. 2017, 47, 329–335. [Google Scholar] [CrossRef]
- Liu, L.; Mi, Z.; Hu, Q.; Li, C.; Li, X.; Feng, F. Green Synthesis of Fluorescent Carbon Dots as an Effective Fluorescence Probe for Morin Detection. Anal. Methods 2019, 11, 353–358. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On–Off–On Fluorescent Carbon Dot Nanosensor for Recognition of Chromium(VI) and Ascorbic Acid Based on the Inner Filter Effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef]
- Wei, X.-M.; Xu, Y.; Li, Y.-H.; Yin, X.-B.; He, X.-W. Ultrafast Synthesis of Nitrogen-Doped Carbon Dots via Neutralization Heat for Bioimaging and Sensing Applications. RSC Adv. 2014, 4, 44504–44508. [Google Scholar] [CrossRef]
- Martindale, B.C.M.; Hutton, G.A.M.; Caputo, C.A.; Reisner, E. Solar Hydrogen Production Using Carbon Quantum Dots and a Molecular Nickel Catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. [Google Scholar] [CrossRef]
- Fong, J.F.Y.; Chin, S.F.; Ng, S.M. A Unique “Turn-on” Fluorescence Signalling Strategy for Highly Specific Detection of Ascorbic Acid Using Carbon Dots as Sensing Probe. Biosens. Bioelectron. 2016, 85, 844–852. [Google Scholar] [CrossRef]
- Loo, A.H.; Sofer, Z.; Bouša, D.; Ulbrich, P.; Bonanni, A.; Pumera, M. Carboxylic Carbon Quantum Dots as a Fluorescent Sensing Platform for DNA Detection. ACS Appl. Mater. Interfaces 2016, 8, 1951–1957. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface Modification and Chemical Functionalization of Carbon Dots: A Review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-Doped Carbon Quantum Dots: Surface Chemistry Dependent Antibacterial Activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef]
- Esteves da Silva, J.C.G.; Gonçalves, H.M.R. Analytical and Bioanalytical Applications of Carbon Dots. TrAC Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Luo, P.G.; Yang, F.; Yang, S.-T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.-P. Carbon-Based Quantum Dots for Fluorescence Imaging of Cells and Tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.-T.; Kang, Z. One-Step Ultrasonic Synthesis of Water-Soluble Carbon Nanoparticles with Excellent Photoluminescent Properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Yu, H.; Kang, Z.; Lee, S.-T. Synthesis of Fluorescent Carbon Nanoparticles Directly from Active Carbon via a One-Step Ultrasonic Treatment. Mater. Res. Bull. 2011, 46, 147–151. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, S.; Dong, Y.; Wang, J.; Ge, X.; Sui, L. The Preparation of Ethylenediamine-Modified Fluorescent Carbon Dots and Their Use in Imaging of Cells. Luminescence 2015, 30, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Gualandi, L.; Noja, S.D.; Filippini, G.; Bosi, S.; Prato, M. Synthesis and Applications of Amino-Functionalized Carbon Nanomaterials. Chem. Commun. 2020, 56, 12698–12716. [Google Scholar] [CrossRef] [PubMed]
- Algarra, M.; Campos, B.B.; Radotić, K.; Mutavdžić, D.; Bandosz, T.; Jiménez-Jiménez, J.; Rodriguez-Castellón, E.; da Silva, J.C.G.E. Luminescent Carbon Nanoparticles: Effects of Chemical Functionalization, and Evaluation of Ag+ Sensing Properties. J. Mater. Chem. A 2014, 2, 8342–8351. [Google Scholar] [CrossRef]
- Lai, I.P.-J.; Harroun, S.G.; Chen, S.-Y.; Unnikrishnan, B.; Li, Y.-J.; Huang, C.-C. Solid-State Synthesis of Self-Functional Carbon Quantum Dots for Detection of Bacteria and Tumor Cells. Sens. Actuators B Chem. 2016, 228, 465–470. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Kuo, Y.-C.; Chang, C.-J.; Lin, Y.-S.; Chiu, T.-C.; Hu, C.-C. Highly Sensitive Sensing of Hydroquinone and Catechol Based on β-Cyclodextrin-Modified Carbon Dots. RSC Adv. 2018, 8, 19381–19388. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, D.; Guo, Y.; Yang, C.; Iqbal, A.; Liu, W.; Qin, W.; Yan, D.; Guo, H. Imidazole Derivative-Functionalized Carbon Dots: Using as a Fluorescent Probe for Detecting Water and Imaging of Live Cells. Dalton Trans. 2015, 44, 5547–5554. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Tang, R.; Wu, H.; Wang, B.; Tan, M.; Yuan, J. Preparation of Europium Complex-Conjugated Carbon Dots for Ratiometric Fluorescence Detection of Copper(II) Ions. New J. Chem. 2014, 38, 5721–5726. [Google Scholar] [CrossRef]
- Niu, W.-J.; Shan, D.; Zhu, R.-H.; Deng, S.-Y.; Cosnier, S.; Zhang, X.-J. Dumbbell-Shaped Carbon Quantum Dots/AuNCs Nanohybrid as an Efficient Ratiometric Fluorescent Probe for Sensing Cadmium (II) Ions and l-Ascorbic Acid. Carbon 2016, 96, 1034–1042. [Google Scholar] [CrossRef]
- Cooper, O.; Eftekhari, E.; Carter, J.; Mallard, B.; Kaur, J.; Kiefel, M.J.; Haselhorst, T.; Li, Q.; Tiralongo, J. Fluorescent Carbon Dots Functionalized with Self-Assembled Glycan Monolayers for Probing Interactions across the Glyco-Interactome. ACS Appl. Nano Mater. 2020, 3, 7804–7817. [Google Scholar] [CrossRef]
- Li, F.; Cai, Q.; Hao, X.; Zhao, C.; Huang, Z.; Zheng, Y.; Lin, X.; Weng, S. Insight into the DNA Adsorption on Nitrogen-Doped Positive Carbon Dots. RSC Adv. 2019, 9, 12462–12469. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Gao, K.; Lin, Z.; Wu, W. Amine-Capped Carbon Dots as a Nanosensor for Sensitive and Selective Detection of Picric Acid in Aqueous Solution via Electrostatic Interaction. Anal. Methods 2013, 5, 6228–6233. [Google Scholar] [CrossRef]
- Yang, W.; Ni, J.; Luo, F.; Weng, W.; Wei, Q.; Lin, Z.; Chen, G. Cationic Carbon Dots for Modification-Free Detection of Hyaluronidase via an Electrostatic-Controlled Ratiometric Fluorescence Assay. Anal. Chem. 2017, 89, 8384–8390. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, Y.; Zhong, J.; Yang, J.; Zheng, J.; Cheng, J.; Song, R.; Yi, C. Facile Synthesis of Gadolinium (III) Chelates Functionalized Carbon Quantum Dots for Fluorescence and Magnetic Resonance Dual-Modal Bioimaging. Carbon 2015, 93, 742–750. [Google Scholar] [CrossRef]
- Lan, M.; Zhang, J.; Chui, Y.-S.; Wang, P.; Chen, X.; Lee, C.-S.; Kwong, H.-L.; Zhang, W. Carbon Nanoparticle-Based Ratiometric Fluorescent Sensor for Detecting Mercury Ions in Aqueous Media and Living Cells. ACS Appl. Mater. Interfaces 2014, 6, 21270–21278. [Google Scholar] [CrossRef]
- Sun, T.; Zheng, M.; Xie, Z.; Jing, X. Supramolecular Hybrids of Carbon Dots with Doxorubicin: Synthesis, Stability and Cellular Trafficking. Mater. Chem. Front. 2017, 1, 354–360. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, M.; Zhang, F.; Xu, B.; Tian, W.; Xie, Z. Supramolecular Hybrids of AIEgen with Carbon Dots for Noninvasive Long-Term Bioimaging. Chem. Mater. 2016, 28, 8825–8833. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, L. Modern Methods for Delivery of Drugs across the Blood–Brain Barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Zhao, A.; Chen, Z.; Zhao, C.; Gao, N.; Ren, J.; Qu, X. Recent Advances in Bioapplications of C-Dots. Carbon 2015, 85, 309–327. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Dallman, J.; Baker, J.; Othman, A.M.; Blackwelder, P.L.; Leblanc, R.M. Crossing the Blood–Brain–Barrier with Transferrin Conjugated Carbon Dots: A Zebrafish Model Study. Colloids Surf. B Biointerfaces 2016, 145, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seven, E.S.; Seven, Y.B.; Zhou, Y.; Poudel-Sharma, S.; Diaz-Rucco, J.J.; Cilingir, E.K.; Mitchell, G.S.; Dyken, J.D.V.; Leblanc, R.M. Crossing the Blood–Brain Barrier with Carbon Dots: Uptake Mechanism and in Vivo Cargo Delivery. Nanoscale Adv. 2021, 3, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Sun, H. Novel Properties and Applications of Carbon Nanodots. Nanoscale Horiz. 2018, 3, 565–597. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Leblanc, R.M. Method To Determine Protein Concentration in the Protein–Nanoparticle Conjugates Aqueous Solution Using Circular Dichroism Spectroscopy. Anal. Chem. 2015, 87, 6455–6459. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, S.; Han, X.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Determination of the Composition, Encapsulation Efficiency and Loading Capacity in Protein Drug Delivery Systems Using Circular Dichroism Spectroscopy. Anal. Chim. Acta 2016, 937, 113–118. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Chen, Z.; Shin, D.M. Advances of Cancer Therapy by Nanotechnology. Cancer Res. Treat. 2009, 41, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple Conjugated Carbon Dots as a Nano-Drug Delivery Model for Glioblastoma Brain Tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Henna, T.K.; Raphey, V.R.; Sankar, R.; Ameena Shirin, V.K.; Gangadharappa, H.V.; Pramod, K. Carbon Nanostructures: The Drug and the Delivery System for Brain Disorders. Int. J. Pharm. 2020, 587, 119701. [Google Scholar] [CrossRef]
- Zhang, W.; Sigdel, G.; Mintz, K.J.; Seven, E.S.; Zhou, Y.; Wang, C.; Leblanc, R.M. Carbon Dots: A Future Blood-Brain Barrier Penetrating Nanomedicine and Drug Nanocarrier. Int. J. Nanomed. 2021, 16, 5003–5016. [Google Scholar] [CrossRef]
- Lu, S.; Guo, S.; Xu, P.; Li, X.; Zhao, Y.; Gu, W.; Xue, M. Hydrothermal Synthesis of Nitrogen-Doped Carbon Dots with Real-Time Live-Cell Imaging and Blood-Brain Barrier Penetration Capabilities. Int. J. Nanomed. 2016, 11, 6325–6336. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Amat, D.; Peng, Z.; Vanni, S.; Raskin, S.; De Angulo, G.; Othman, A.M.; Graham, R.M.; Leblanc, R.M. Transferrin Conjugated Nontoxic Carbon Dots for Doxorubicin Delivery to Target Pediatric Brain Tumor Cells. Nanoscale 2016, 8, 16662–16669. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Qiao, L.; Sun, T.; Zheng, X.; Zheng, M.; Xie, Z. Exploring the Optimal Ratio of D-Glucose/l-Aspartic Acid for Targeting Carbon Dots toward Brain Tumor Cells. Mater. Sci. Eng. C 2018, 85, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Qian, J.; Shen, S.; Zhu, J.; Jiang, X.; He, Q.; Gao, H. A Simple One-Step Method to Prepare Fluorescent Carbon Dots and Their Potential Application in Non-Invasive Glioma Imaging. Nanoscale 2014, 6, 10040–10047. [Google Scholar] [CrossRef] [PubMed]
- Mintz, K.J.; Mercado, G.; Zhou, Y.; Ji, Y.; Hettiarachchi, S.D.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Dallman, J.; Leblanc, R.M. Tryptophan Carbon Dots and Their Ability to Cross the Blood-Brain Barrier. Colloids Surf. B Biointerfaces 2019, 176, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Su, W.; Wu, H.; Yuan, T.; Yuan, C.; Liu, J.; Deng, G.; Gao, X.; Chen, Z.; Bao, Y.; et al. Targeted Tumour Theranostics in Mice via Carbon Quantum Dots Structurally Mimicking Large Amino Acids. Nat. Biomed. Eng. 2020, 4, 704–716. [Google Scholar] [CrossRef] [PubMed]
| Strategy | Synthetic Method | Refs |
|---|---|---|
| top-down | electrochemical synthesis | [68,69,70,71,72,73] |
| chemical oxidation | [74,75,76,77,78,79] | |
| arc discharge | [61,80,81,82] | |
| laser ablation | [62,83,84,85,86] | |
| bottom-up | ultrasound treatment | [87,88,89,114,115] |
| hydrothermal treatment | [90,91,92,93,94,95] | |
| microwave-assisted synthesis | [96,97,98,99,100,101] | |
| pyrolysis | [102,103,104,105,106] |
| C-Dots | Size (nm) | Drug Loaded | Ligand Attached | In Vitro/In Vivo | Administration Mode | Refs. |
|---|---|---|---|---|---|---|
| C-dot/PEI | 2.6 | None | None | Primary rat microvascular endothelial cells and astrocytes | Medium | [145] |
| C-dot/Trans-Dox | 2–6 | doxorubicin | transferrin | SJGBM2 and CHLA266 (pediatric brain tumor cells) | Medium | [146] |
| C-dot/Dex-Asp | 2.3–2.5 | None | None | C6 glioma cells/mouse | Medium/i.v. in tail vein | [147,148] |
| C-dot/Trans | 5 | transferrin | Zebrafish | [135] | ||
| C-dot/Trans-Temo-Epi | 2.6–3.5 | Temozolomide epirubicin | transferrin | SJGBM2, CHLA266, CHLA200 (pediatric brain tumor cells) and U87 (adult glioblastoma cells) | Medium | [141] |
| C-dot/Gly | <5 | None | None | C6 glioma cells/mouse | Medium/Medium/i.v. in tail vein | [149] |
| C-dot/Dex-Fluo | 2.4–2.5 | None | None | Zebrafish and rat | i.v. into the heart and i.v. in tail vein | [136] |
| C-dot/Try-ureaC-dot/Try-EDA | 9.0–10.8 | None | None | Zebrafish | i.v. into the heart | [150] |
| LAAMC-dots | 2.5 | None | None | Mouse | i.v. in tail vein | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. https://doi.org/10.3390/ijms222111783
Calabrese G, De Luca G, Nocito G, Rizzo MG, Lombardo SP, Chisari G, Forte S, Sciuto EL, Conoci S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. International Journal of Molecular Sciences. 2021; 22(21):11783. https://doi.org/10.3390/ijms222111783
Chicago/Turabian StyleCalabrese, Giovanna, Giovanna De Luca, Giuseppe Nocito, Maria Giovanna Rizzo, Sofia Paola Lombardo, Giulia Chisari, Stefano Forte, Emanuele Luigi Sciuto, and Sabrina Conoci. 2021. "Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors" International Journal of Molecular Sciences 22, no. 21: 11783. https://doi.org/10.3390/ijms222111783
APA StyleCalabrese, G., De Luca, G., Nocito, G., Rizzo, M. G., Lombardo, S. P., Chisari, G., Forte, S., Sciuto, E. L., & Conoci, S. (2021). Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. International Journal of Molecular Sciences, 22(21), 11783. https://doi.org/10.3390/ijms222111783









