Protein Palmitoylation in Bovine Ovarian Follicle
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of Potentially Palmitoylated Proteins in Follicular Cells
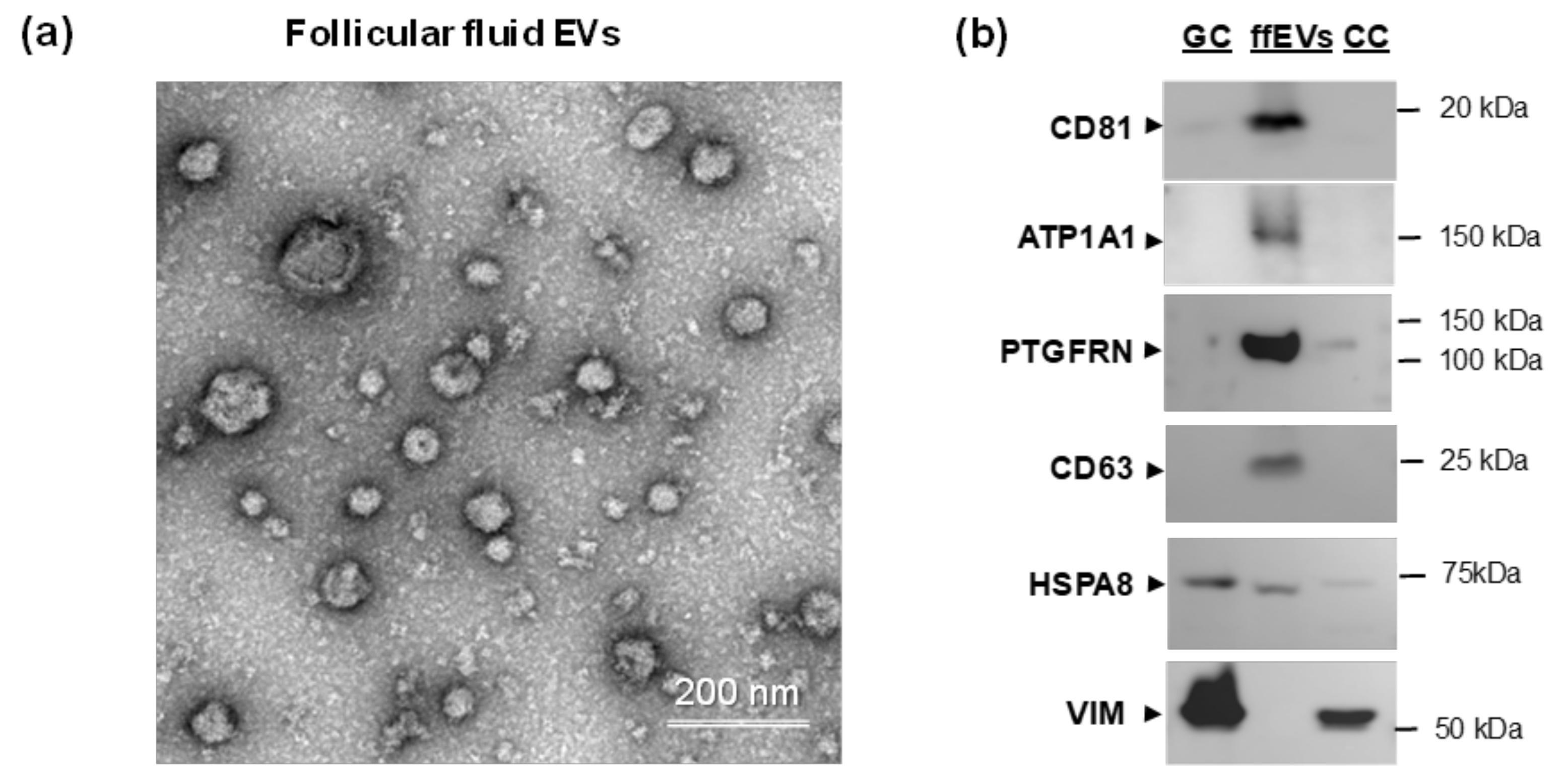
2.2. Western Blot Analysis of Palmitoyl-Proteins in Follicular Fluid Extracellular Vesicles
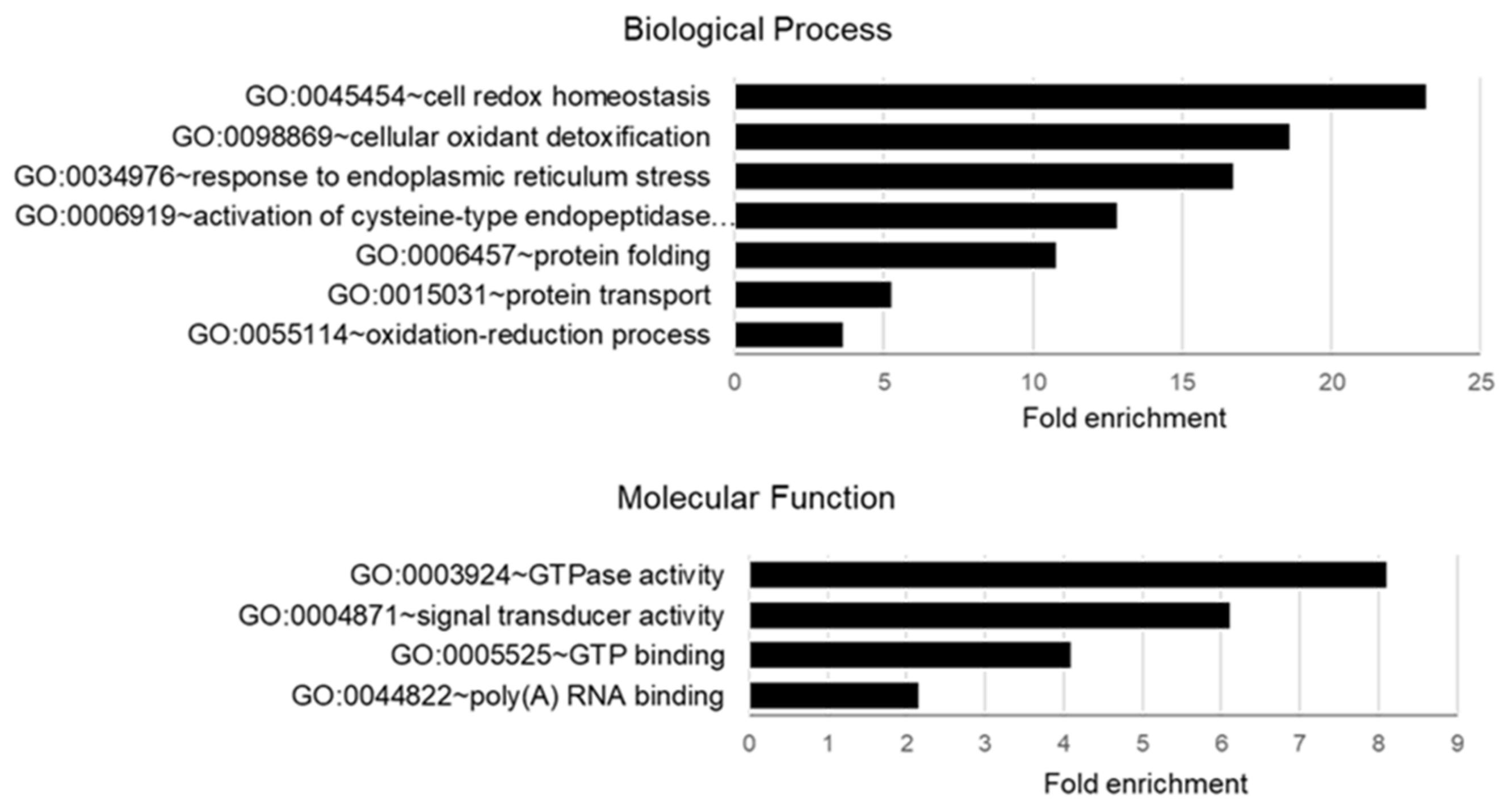
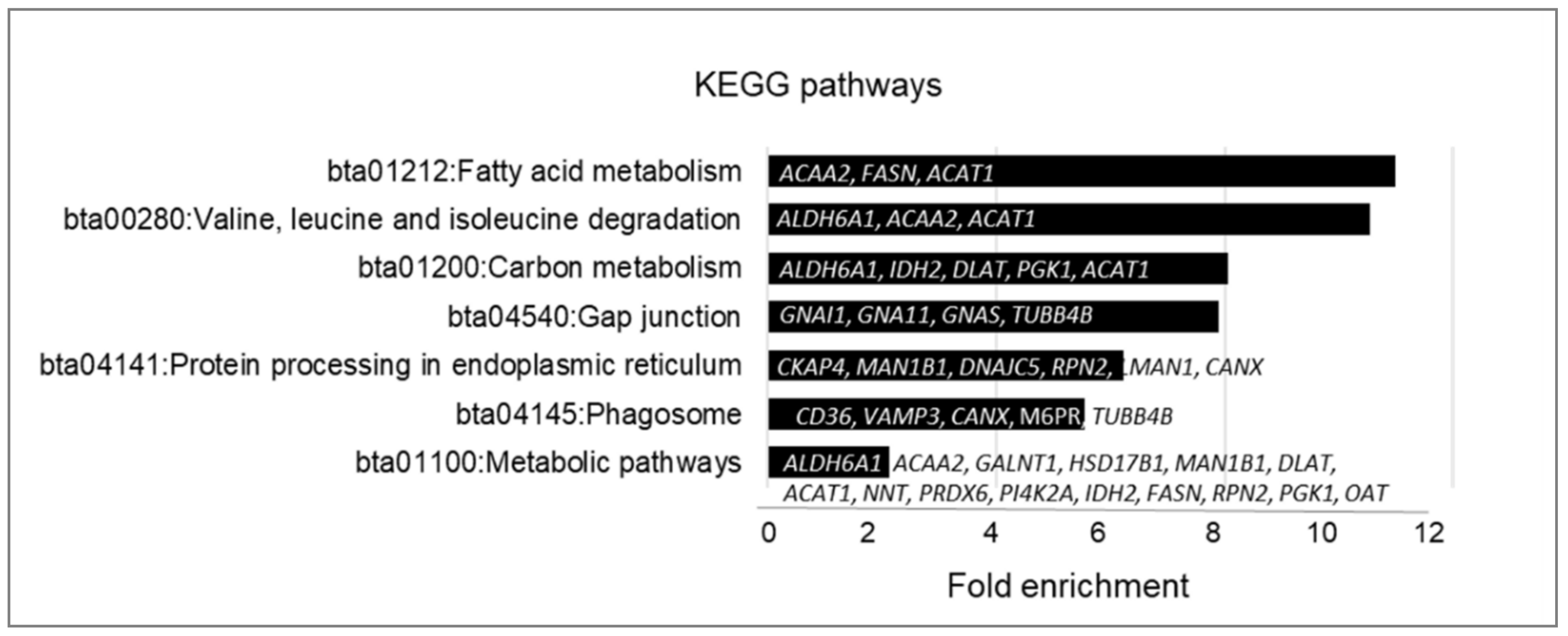
2.3. Gene Ontology Analyses of Palmitoylated Proteins
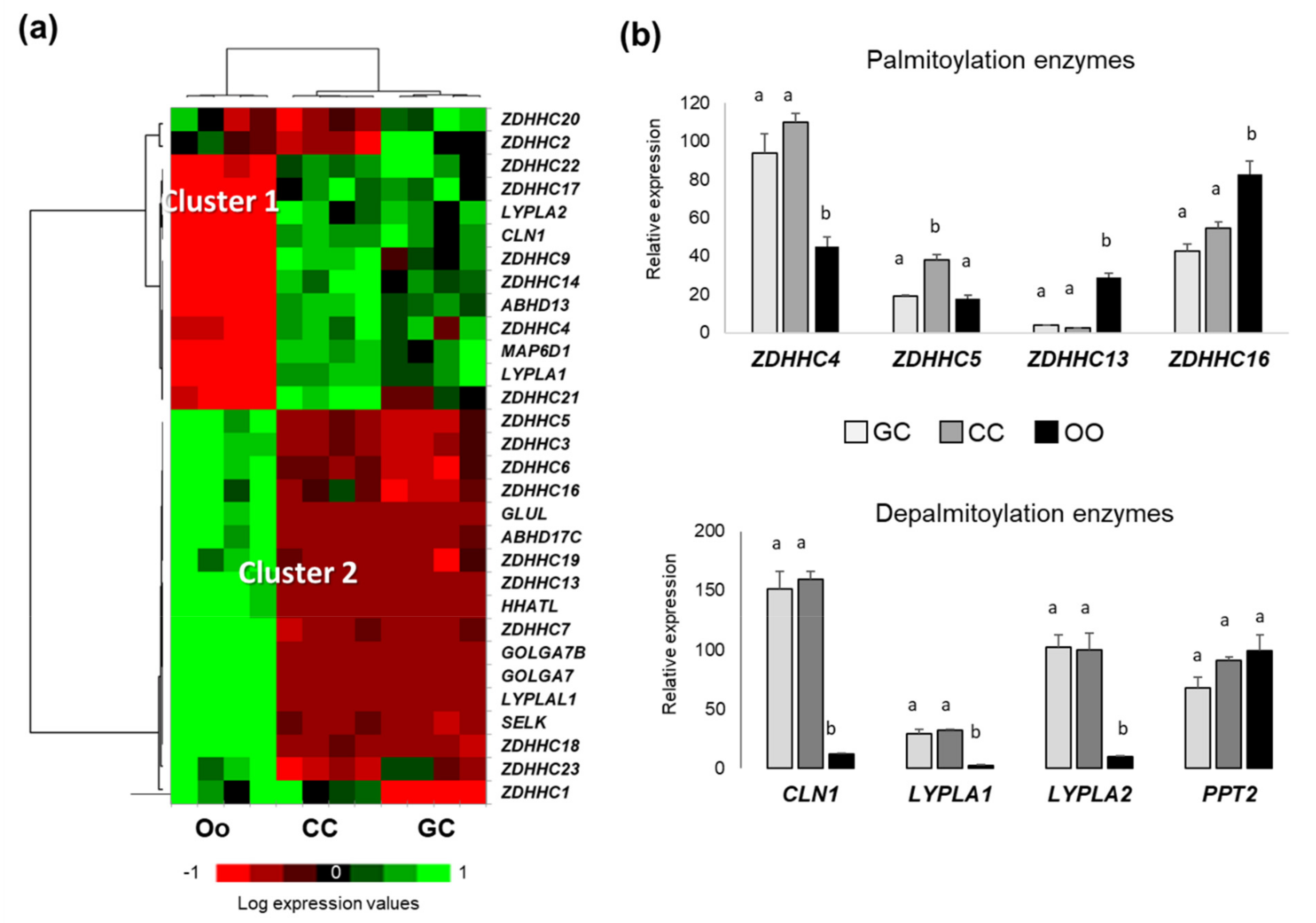
2.4. Expression of Palmitoylation and Depalmitoylation Enzymes in Bovine Ovarian Follicular Cells
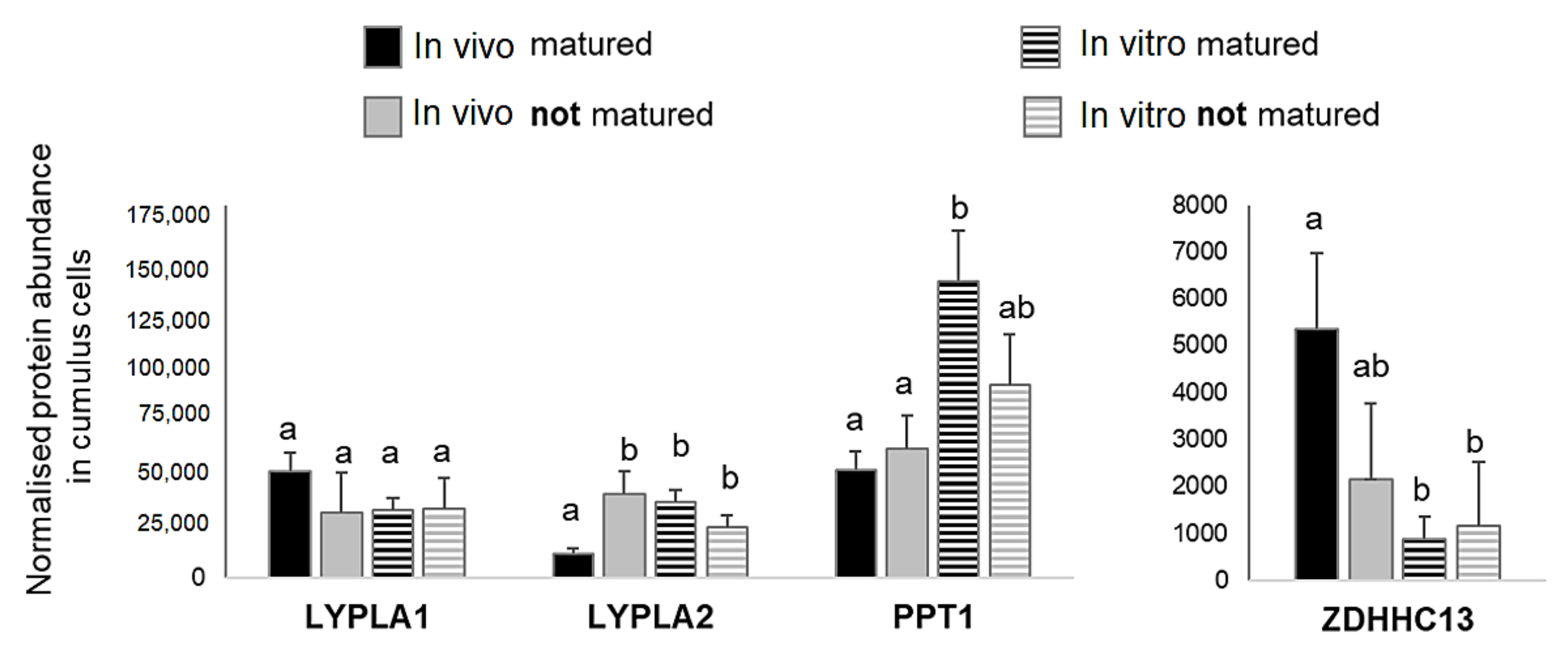
2.5. Detection of Palmitoylation and Depalmitoylation Enzymes in Bovine Follicular Cells Proteomes
2.6. Immunofluorescence Analysis of Palmitoylation and Depalmitoylation Enzymes in Bovine Follicular Cells
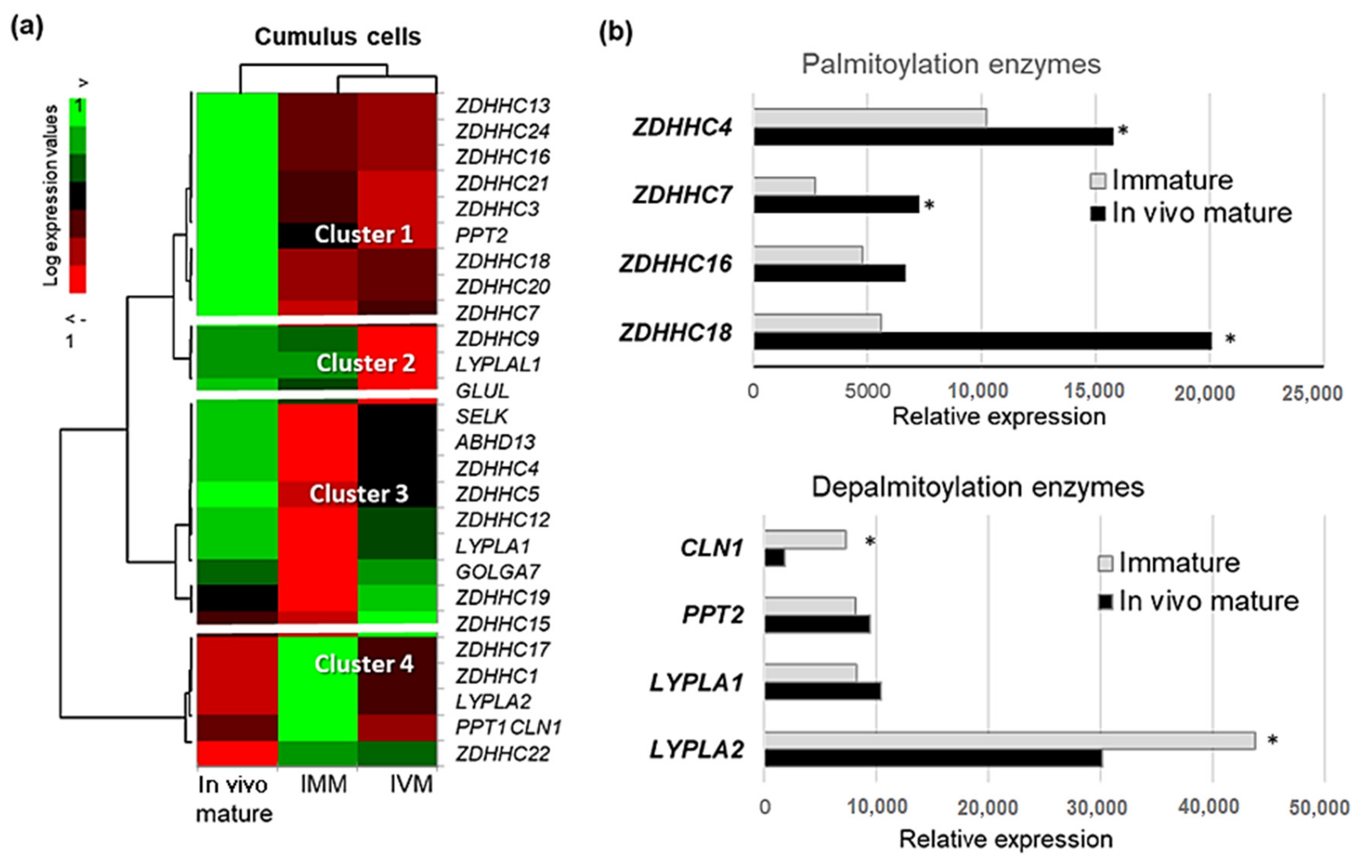
2.7. Expression of Palmitoylation and Depalmitoylation Enzymes in Cumulus Cells and Oocytes during Oocyte Maturation
3. Discussion
3.1. Palmitoylated Proteins and Their Role in Ovarian Follicular Cells
3.2. Palmitoylation Protein Sorting and Involvement in Communications between Follicular Cells
3.3. Regulation of Protein Palmitoylation within Ovarian Follicle
4. Materials and Methods
4.1. Ethics
4.2. Chemicals
4.3. Recovery of Follicular Cells and Oocytes
4.4. Analysis of Protein Palmitoylation
4.4.1. Isolation and Identification of Palmitoyl-Proteins
4.4.2. Gene Expression Analysis
4.4.3. Functional Annotation Bioinformatics Resources
4.5. Isolation of Extracellular Vesicles from Follicular Fluid
4.6. Transmission Electron Microscopy (TEM)
4.7. Total Protein Extraction and Western Blot
4.8. In Vitro Culture of Granulosa Cells
4.9. Immunofluorescence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Gene | Protein | Relative Expression | ||
|---|---|---|---|---|
| Immature | IVM | In Vivo Mature | ||
| ABHD13 | Lysophosphatidylserine lipase ABHD13 | 78.71 (a) | 271.11(b) | 338.96 (b) |
| GLUL | Glutamine synthetase | 1428.92 (ab) | 1061.75 (a) | 1568.31(b) |
| GOLGA7 | Golgin subfamily A member 7 | 6662.79 (a) | 7448.94 (a) | 7398.62 (a) |
| LYPLA1 | Acyl-protein thioesterase 1 | 8230.51 (a) | 9801.03 (a) | 10,408.22 (a) |
| LYPLA2 | Acyl-protein thioesterase 2 | 43,732.30 (a) | 35,366.24(ab) | 30,134.65(b) |
| LYPLAL1 | Lysophospholipase-like protein 1 | 230.95 (a) | 65.20 (b) | 229.32 (a) |
| CLN1 | Palmitoyl-protein thioesterase 1 | 7213.40 (a) | 1066.52 (b) | 1802.02 (c) |
| PPT2 | Lysosomal thioesterase PPT2 | 8166.68 (ab) | 6956.55 (a) | 9431.63 (b) |
| SELK | Selenoprotein K | 12,928.36 (a) | 17,856.50(b) | 21,467.59(b) |
| ZDHHC1 | Probable palmitoyltransferase ZDHHC1 | 31.00 (a) | 18.98 (b) | 13.08 (b) |
| ZDHHC3 | Palmitoyltransferase ZDHHC3 | 4209.96 (a) | 3597.84 (a) | 5936.89 (b) |
| ZDHHC4 | Palmitoyltransferase ZDHHC4 | 10,213.52 (a) | 13,393.12(b) | 15,747.39 (b) |
| ZDHHC5 | Palmitoyltransferase ZDHHC5 | 1493.68 (a) | 1844.64 (ab) | 2200.70 (b) |
| ZDHHC7 | Palmitoyltransferase ZDHHC7 | 2705.71 (a) | 4347.91(b) | 7287.39 (b) |
| ZDHHC9 | Pralmitoyltransferase ZDHHC9 | 64.93 (a) | 58.03 (a) | 65.70 (a) |
| ZDHHC12 | Probable palmitoyltransferase ZDHHC12 | 1646.18 (a) | 2138.80 (b) | 2400.89 (b) |
| ZDHHC13 | Probable palmitoyltransferase ZDHHC13 | 1758.71 (a) | 1542.11 (a) | 2768.72 (b) |
| ZDHHC15 | Palmitoyltransferase ZDHHC15 | 18.07 (a) | 20.66 (a) | 19.01 (a) |
| ZDHHC16 | Palmitoyltransferase ZDHHC16 | 4788.19 (a) | 4569.41 (a) | 6682.16 (b) |
| ZDHHC17 | Palmitoyltransferase ZDHHC17 | 1660.87 (a) | 1197.13 (b) | 1015.40 (b) |
| ZDHHC18 | Palmitoyltransferase ZDHHC18 | 5589.22 (a) | 7843.17 (b) | 20,093.53 (c) |
| ZDHHC19 | Palmitoyltransferase ZDHHC19 | 14.96 (a) | 25.59 (b) | 20.31 (b) |
| ZDHHC20 | Palmitoyltransferase ZDHHC20 | 4469.49 (a) | 5428.55 (a) | 10,346.94 (b) |
| ZDHHC21 | Palmitoyltransferase ZDHHC21 | 83.51 (a) | 72.75 (a) | 114.70 (a) |
| ZDHHC22 | Palmitoyltransferase ZDHHC22 | 180.33 (a) | 177.99 (a) | 140.04 (a) |
| ZDHHC24 | Probable palmitoyltransferase ZDHHC24 | 39,433.14 (a) | 36,372.21 (a) | 56,638.58 (b) |
References
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef]
- Bijlmakers, J.M.; Marsh, M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003, 13, 32–42. [Google Scholar] [CrossRef]
- Blanc, M.; David, F.; Abrami, L.; Migliozzi, D.; Armand, F.; Bürgi, J.; Van Der Goot, F.G. SwissPalm: Protein Palmitoylation database. F1000Research 2015, 4, 261. [Google Scholar] [CrossRef] [PubMed]
- Aicart-Ramos, C.; Valero, R.A.; Rodriguez-Crespo, I. Protein palmitoylation and subcellular trafficking. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 2981–2994. [Google Scholar] [CrossRef] [PubMed]
- Charollais, J.; Van Der Goot, F.G. Palmitoylation of membrane proteins. Mol. Membr. Biol. 2009, 26, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Hang, H.C. Protein S-palmitoylation in cellular differentiation. Biochem. Soc. Trans. 2017, 45, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Resh, M.D. Fatty acylation of proteins: The long and the short of it. Prog. Lipid Res. 2016, 63, 120–131. [Google Scholar] [CrossRef]
- Dunphy, T.J.; Linder, M.E. Signalling functions of protein palmitoylation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1998, 1436, 245–261. [Google Scholar] [CrossRef]
- Resh, M.D. Palmitoylation of Ligands, Receptors, and Intracellular Signaling Molecules. Sci. STKE 2006, 2006, re14. [Google Scholar] [CrossRef]
- Martin, B.R.; Wang, C.; Adibekian, A.; Tully, S.E.; Cravatt, B.F. Global profiling of dynamic protein palmitoylation. Nat. Methods 2011, 9, 84–89. [Google Scholar] [CrossRef]
- Goddard, A.D.; Watts, A. Regulation of G protein-coupled receptors by palmitoylation and cholesterol. BMC Biol. 2012, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Ko, P.; Dixon, S.J. Protein palmitoylation and cancer. EMBO Rep. 2018, 19, e46666. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Ponimaskin, E. Palmitoylation as a Functional Regulator of Neurotransmitter Receptors. Neural Plast. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.S.; Martin, D.D.O.; Butland, S.; Lavallée-Adam, M.; Calzolari, D.; Kay, C.; Yates, J.R.; Hayden, M. Curation of the Mammalian Palmitoylome Indicates a Pivotal Role for Palmitoylation in Diseases and Disorders of the Nervous System and Cancers. PLoS Comput. Biol. 2015, 11, e1004405. [Google Scholar] [CrossRef] [PubMed]
- Drisdel, R.C.; Green, W.N. Labeling and quantifying sites of protein palmitoylation. BioTechniques 2004, 36, 276–285. [Google Scholar] [CrossRef]
- Wan, J.; Roth, A.F.; O Bailey, A.; Davis, N.G. Palmitoylated proteins: Purification and identification. Nat. Protoc. 2007, 2, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.R.; Cravatt, B.F. Large-scale profiling of protein palmitoylation in mammalian cells. Nat. Methods 2009, 6, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Qanbar, R.; Bouvier, M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol. Ther. 2002, 97, 1–33. [Google Scholar] [CrossRef]
- Wang, Z.; Schey, K.L. Proteomic Analysis of S-Palmitoylated Proteins in Ocular Lens Reveals Palmitoylation of AQP5 and MP20. Investig. Opthalmol. Vis. Sci. 2018, 59, 5648–5658. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Hsieh, M.; Zamah, A.M.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.G.A.; Pereira, L.A.A.C.; Viana, J.H.M.; Russo, R.C.; Campos-Junior, P.H.A. The CC-chemokine receptor 2 is involved in the control of ovarian folliculogenesis and fertility lifespan in mice. J. Reprod. Immunol. 2020, 141, 103174. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-Y.; Sun, D.; Peng, Y.-C.; Wu, Y.-L. Regulation of the regulator of G protein signaling 2 expression and cellular localization by PKA and PKC pathways in mouse granulosa cells. Biochem. Biophys. Res. Commun. 2018, 503, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Dudler, T.; Gelb, M.H. Palmitoylation of Ha-Ras Facilitates Membrane Binding, Activation of Downstream Effectors, and Meiotic Maturation in Xenopus Oocytes. J. Biol. Chem. 1996, 271, 11541. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Miao, L.; Kuang, X.; Ma, W.; Wang, C.; Zhang, J.; Xia, G. Involvement of Protein Acyltransferase ZDHHC3 in Maintaining Oocyte Meiotic Arrest in Xenopus laevis. Biol. Reprod. 2016, 95, 67. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Razandi, M.; Lewis, M.; Hammes, S.; Levin, E.R. Membrane-Localized Estrogen Receptor α Is Required for Normal Organ Development and Function. Dev. Cell 2014, 29, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Han, X.; Zheng, B.; DeRan, M.; Yu, J.; Jarugumilli, G.K.; Deng, H.; Pan, D.; Luo, X.; Wu, X. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat. Chem. Biol. 2016, 12, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Tabaczar, S.; Czogalla, A.; Podkalicka, J.; Biernatowska, A.; Sikorski, A.F. Protein palmitoylation: Palmitoyltransferases and their specificity. Exp. Biol. Med. 2017, 242, 1150. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Vasudevan, A.; Linder, M.E.; Deschenes, R. Thematic review series: Lipid Posttranslational Modifications. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J. Lipid Res. 2006, 47, 1118–1127. [Google Scholar] [CrossRef]
- Ohno, Y.; Kihara, A.; Sano, T.; Igarashi, Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim. Biophys. Acta 2006, 1761, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Swarthout, J.T.; Lobo, S.; Farh, L.; Croke, M.R.; Greentree, W.K.; Deschenes, R.; Linder, M.E. DHHC9 and GCP16 Constitute a Human Protein Fatty Acyltransferase with Specificity for H- and N-Ras. J. Biol. Chem. 2005, 280, 31141–31148. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Kashio, A.; Ogata, R.; Ishitomi, A.; Yamazaki, Y.; Kihara, A. Analysis of substrate specificity of human DHHC protein acyltransferases using a yeast expression system. Mol. Biol. Cell 2012, 23, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Plain, F.; Howie, J.; Kennedy, J.; Brown, E.; Shattock, M.J.; Fraser, N.J.; Fuller, W. Control of protein palmitoylation by regulating substrate recruitment to a zDHHC-protein acyltransferase. Commun. Biol. 2020, 3, 411. [Google Scholar] [CrossRef]
- Bailey, M.H.; Tokheim, C.; Porta-Pardo, E.; Sengupta, S.; Bertrand, D.; Weerasinghe, A.; Colaprico, A.; Wendl, M.C.; Kim, J.; Reardon, B.; et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018, 174, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Fierke, C.A. Understanding protein palmitoylation: Biological significance and enzymology. Sci. China Ser. B Chem. 2011, 54, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Won, S.J.; Cheung See Kit, M.; Martin, B.R. Protein depalmitoylases. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Fukata, Y.; Murakami, T.; Yokoi, N.; Fukata, M. Local Palmitoylation Cycles and Specialized Membrane Domain Organization. Curr. Top. Membr. 2016, 77, 97–141. [Google Scholar] [CrossRef]
- Yang, W.; Di Vizio, D.; Kirchner, M.; Steen, H.; Freeman, M.R. Proteome Scale Characterization of Human S-Acylated Proteins in Lipid Raft-enriched and Non-raft Membranes. Mol. Cell. Proteom. 2010, 9, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Romancino, D.P.; Buffa, V.; Caruso, S.; Ferrara, I.; Raccosta, S.; Notaro, A.; Campos, Y.; Noto, R.; Martorana, V.; Cupane, A.; et al. Palmitoylation is a post-translational modification of Alix regulating the membrane organization of exosome-like small extracellular vesicles. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2879–2887. [Google Scholar] [CrossRef]
- Mariscal, J.; Vagner, T.; Kim, M.; Zhou, B.; Chin, A.; Zandian, M.; Freeman, M.R.; You, S.; Zijlstra, A.; Yang, W.; et al. Comprehensive palmitoyl-proteomic analysis identifies distinct protein signatures for large and small cancer-derived extracellular vesicles. J. Extracell. Vesicles 2020, 9, 1764192. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Navakanitworakul, R.; Hung, W.-T.; Gunewardena, S.; Davis, J.S.; Chotigeat, W.; Christenson, L.K. Characterization and Small RNA Content of Extracellular Vesicles in Follicular Fluid of Developing Bovine Antral Follicles. Sci. Rep. 2016, 6, 25486. [Google Scholar] [CrossRef] [PubMed]
- Bertevello, P.S.; Teixeira-Gomes, A.-P.; Labas, V.; Cordeiro, L.; Blache, M.-C.; Papillier, P.; Singina, G.; Uzbekov, R.; Maillard, V.; Uzbekova, S. MALDI-TOF Mass Spectrometry Revealed Significant Lipid Variations in Follicular Fluid and Somatic Follicular Cells but Not in Enclosed Oocytes between the Large Dominant and Small Subordinate Follicles in Bovine Ovary. Int. J. Mol. Sci. 2020, 21, 6661. [Google Scholar] [CrossRef] [PubMed]
- Hailay, T.; Hoelker, M.; Poirier, M.; Gebremedhn, S.; Rings, F.; Saeed-Zidane, M.; Wondim, D.S.; Dauben, C.; Tholen, E.; Neuhoff, C.; et al. Extracellular vesicle-coupled miRNA profiles in follicular fluid of cows with divergent post-calving metabolic status. Sci. Rep. 2019, 9, 12851. [Google Scholar] [CrossRef] [PubMed]
- Uzbekova, S.; Almiñana, C.; Labas, V.; Teixeira-Gomes, A.-P.; Combes-Soia, L.; Tsikis, G.; Carvalho, A.V.; Uzbekov, R.; Singina, G. Protein Cargo of Extracellular Vesicles from Bovine Follicular Fluid and Analysis of Their Origin from Different Ovarian Cells. Front. Veter. Sci. 2020, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, C. Exosome-mediated communication in the ovarian follicle. J. Assist. Reprod. Genet. 2016, 33, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, D.; Hailay, T.; Wondim, D.S.; Hoelker, M.; Bitseha, S.; Gebremedhn, S. Extracellular vesicle mediated molecular signaling in ovarian follicle: Implication for oocyte developmental competence. Theriogenology 2020, 150, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.; Ritter, L.; Armstrong, D. Oocyte–somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 2004, 82, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; Spears, N. The role of intra-ovarian interactions in the regulation of follicle dominance. Hum. Reprod. Update 1999, 5, 153–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wigglesworth, K.; Lee, K.-B.; O’Brien, M.J.; Peng, J.; Matzuk, M.M.; Eppig, J.J. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc. Natl. Acad. Sci. USA 2013, 110, E3723–E3729. [Google Scholar] [CrossRef]
- Lomize, A.L.; Hage, J.M.; Pogozheva, I.D. Membranome 2.0: Database for proteome-wide profiling of bitopic proteins and their dimers. Bioinformatics 2017, 34, 1061–1062. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.A.; Van Raemdonck, G.; Baggerman, G.; Bols, P.E.J.; Leroy, J.L.M.R. Proteomic changes in oocytes after in vitro maturation in lipotoxic conditions are different from those in cumulus cells. Sci. Rep. 2019, 9, 3673. [Google Scholar] [CrossRef] [PubMed]
- Gegenfurtner, K.; Flenkenthaler, F.; Fröhlich, T.; Wolf, E.; Arnold, G.J. The impact of transcription inhibition during in vitro maturation on the proteome of bovine oocytes. Biol. Reprod. 2020, 103, 1000–1011. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Walter, J.; Monthoux, C.; Fortes, C.; Grossmann, J.; Roschitzki, B.; Meili, T.; Riond, B.; Hofmann-Lehmann, R.; Naegeli, H.; Bleul, U. The bovine cumulus proteome is influenced by maturation condition and maturational competence of the oocyte. Sci. Rep. 2020, 10, 9880. [Google Scholar] [CrossRef]
- Charlier, C.; Montfort, J.; Chabrol, O.; Brisard, D.; Nguyen, T.; Le Cam, A.; Richard-Parpaillon, L.; Moreews, F.; Pontarotti, P.; Uzbekova, S.; et al. Oocyte-somatic cells interactions, lessons from evolution. BMC Genom. 2012, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Szundi, I.; Funatogawa, C.; Guo, Y.; Yan, E.; Kliger, D.S. Protein Sequence and Membrane Lipid Roles in the Activation Kinetics of Bovine and Human Rhodopsins. Biophys. J. 2017, 113, 1934–1944. [Google Scholar] [CrossRef]
- Uppal, S.; Liu, T.; Poliakov, E.; Gentleman, S.; Redmond, T.M. The dual roles of RPE65 S-palmitoylation in membrane association and visual cycle function. Sci. Rep. 2019, 9, 5218. [Google Scholar] [CrossRef]
- Shu, L.; Guo, X.; Niu, L.; Chen, X.; Cai, T.; Ding, X.; Xie, Z.; Wang, J.; Zhu, N.; Kou, T.; et al. Comprehensive characterization and proteoform analysis of the hydrophobic surfactant proteins B and C in calf pulmonary surfactant. J. Pharm. Biomed. Anal. 2019, 174, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-W.; Wang, J.; Guo, H.; Zhao, Y.-Y.; Sun, H.-H.; Li, Y.-F.; Lai, X.-Y.; Zhao, N.; Wang, X.; Xie, C.; et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 2020, 11, 4765. [Google Scholar] [CrossRef] [PubMed]
- Bertevello, P.S.; Teixeira-Gomes, A.-P.; Seyer, A.; Carvalho, A.V.; Labas, V.; Blache, M.-C.; Banliat, C.; Cordeiro, L.; Duranthon, V.; Papillier, P.; et al. Lipid Identification and Transcriptional Analysis of Controlling Enzymes in Bovine Ovarian Follicle. Int. J. Mol. Sci. 2018, 19, 3261. [Google Scholar] [CrossRef]
- Russell, D.L.; Gilchrist, R.B.; Brown, H.M.; Thompson, J.G. Bidirectional communication between cumulus cells and the oocyte: Old hands and new players? Theriogenology 2016, 86, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D. Driving folliculogenesis by the oocyte-somatic cell dialog: Lessons from genetic models. Theriogenology 2016, 86, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2018, 47, D516–D519. [Google Scholar] [CrossRef]
- Hannoush, N.R.; Arenas-Ramirez, N. Imaging the lipidome: Omega-alkynyl fatty acids for detection and cellular visualization of lipid-modified proteins. ACS Chem. Biol. 2009, 4, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Hyttel, P.; Fair, T.; Callesen, H.; Greve, T. Oocyte growth, capacitation and final maturartion in cattle. Theriogenology 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Sirard, M.-A.; Richard, F.; Blondin, P.; Robert, C. Contribution of the oocyte to embryo quality. Theriogenology 2006, 65, 126–136. [Google Scholar] [CrossRef]
- Tomek, W.; Torner, H.; Kanitz, W. Comparative analysis of protein synthesis, transcription and cytoplasmic polyadenylation of mRNA during maturation of bovine oocytes in vitro. Reprod. Domest. Anim. 2002, 37, 86–91. [Google Scholar] [CrossRef]
- Bhojwani, M.; Rudolph, E.; Kanitz, W.; Zuehlke, H.; Schneider, F.; Tomek, W. Molecular Analysis of Maturation Processes by Protein and Phosphoprotein Profiling during In Vitro Maturation of Bovine Oocytes: A Proteomic Approach. Cloning Stem Cells 2006, 8, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, L.A.; Egbert, J.R. Regulation of Mammalian Oocyte Meiosis by Intercellular Communication Within the Ovarian Follicle. Annu. Rev. Physiol. 2017, 79, 237–260. [Google Scholar] [CrossRef]
- Salaun, C.; Greaves, J.; Chamberlain, L.H. The intracellular dynamic of protein palmitoylation. J. Cell Biol. 2010, 191, 1229–1238. [Google Scholar] [CrossRef]
- McCormick, P.; Dumaresq-Doiron, K.; Pluviose, A.-S.; Pichette, V.; Tosato, G.; Lefrancois, S. Palmitoylation Controls Recycling in Lysosomal Sorting and Trafficking. Traffic 2008, 9, 1984–1997. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gonzalo, O.; Delgado, I.F.; Sanchez-Madrid, F. Post-translational add-ons mark the path in exosomal protein sorting. Experientia 2017, 75, 1–19. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.L.; Chura, L.R.; Liang, X.; Lane, M.; Norman, R.; Robker, R. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 2012, 97, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, F.; Brouwers, J.F.; van de Lest, C.H.; Wubbolts, R.; Aardema, H.; Priore, P.; Roelen, B.A.; Helms, J.B.; Gadella, B.M. The Cumulus Cell Layer Protects the Bovine Maturing Oocyte Against Fatty Acid-Induced Lipotoxicity1. Biol. Reprod. 2015, 92, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Van Hoeck, V.; Bols, P.E.; Binelli, M.; Leroy, J.L. Reduced oocyte and embryo quality in response to elevated non-esterified fatty acid concentrations: A possible pathway to subfertility? Anim. Reprod. Sci. 2014, 149, 19–29. [Google Scholar] [CrossRef]
- Baldwin, A.C.; Green, C.D.; Olson, L.K.; Moxley, M.A.; Corbett, J.A. A role for aberrant protein palmitoylation in FFA-induced ER stress and β-cell death. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1390–E1398. [Google Scholar] [CrossRef]
- Chen, Y.; Griffiths, A.; Wang, J.; Zhang, T.; Song, Q.; Song, Z. Inositol-requiring enzyme 1α links palmitate-induced mTOR activation and lipotoxicity in hepatocytes. Am. J. Physiol. Physiol. 2020, 319, C1130–C1140. [Google Scholar] [CrossRef] [PubMed]
- Kathayat, R.S.; Cao, Y.; Elvira, P.D.; Sandoz, P.; Zaballa, M.E.; Springer, M.Z.; Drake, L.E.; MacLeod, K.F.; Van Der Goot, F.G.; Dickinson, B.C. Active and dynamic mitochondrial S-depalmitoylation revealed by targeted fluorescent probes. Nat. Commun. 2018, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Razandi, M.; Deschenes, R.J.; Levin, E.R. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol. Biol. Cell 2012, 23, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-F.; Chen, Y.-J.; Liu, K.-M.; Haddad, A.N.S.; Song, I.-W.; Roan, H.-Y.; Chen, L.-Y.; Yen, J.J.Y.; Wu, J.-Y.; Chen, Y.-T. Role of S-Palmitoylation by ZDHHC13 in Mitochondrial function and Metabolism in Liver. Sci. Rep. 2017, 7, 2182. [Google Scholar] [CrossRef] [PubMed]
- Soyombo, A.A.; Hofmann, S.L. Molecular Cloning and Expression of Palmitoyl-protein Thioesterase 2 (PPT2), a Homolog of Lysosomal Palmitoyl-protein Thioesterase with a Distinct Substrate Specificity. J. Biol. Chem. 1997, 272, 27456–27463. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Rizos, D.; Ward, F.; Boland, M.P. Factors influencing oocyte and embryo quality in cattle. Reprod. Nutr. Dev. 2001, 41, 427–437. [Google Scholar] [CrossRef]
- Humblot, P.; Holm, P.; Lonergan, P.; Wrenzycki, C.; Lequarré, A.-S.; Joly, C.G.; Herrmann, D.; Lopes, A.; Rizos, D.; Niemann, H.; et al. Effect of stage of follicular growth during superovulation on developmental competence of bovine oocytes. Theriogenology 2005, 63, 1149–1166. [Google Scholar] [CrossRef]
- Brown, H.M.; Dunning, K.R.; Sutton-McDowall, M.; Gilchrist, R.B.; Thompson, J.G.; Russell, D.L. Failure to launch: Aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction 2016, 153, R109–R120. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.; Kolker, E.; Aebersold, R. Empirical Statistical Model To Estimate the Accuracy of Peptide Identifications Made by MS/MS and Database Search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A Statistical Model for Identifying Proteins by Tandem Mass Spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially Modified Protein Abundance Index (emPAI) for Estimation of Absolute Protein Amount in Proteomics by the Number of Sequenced Peptides per Protein. Mol. Cell. Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Llinares, M.B.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2018, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Simpson, R.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
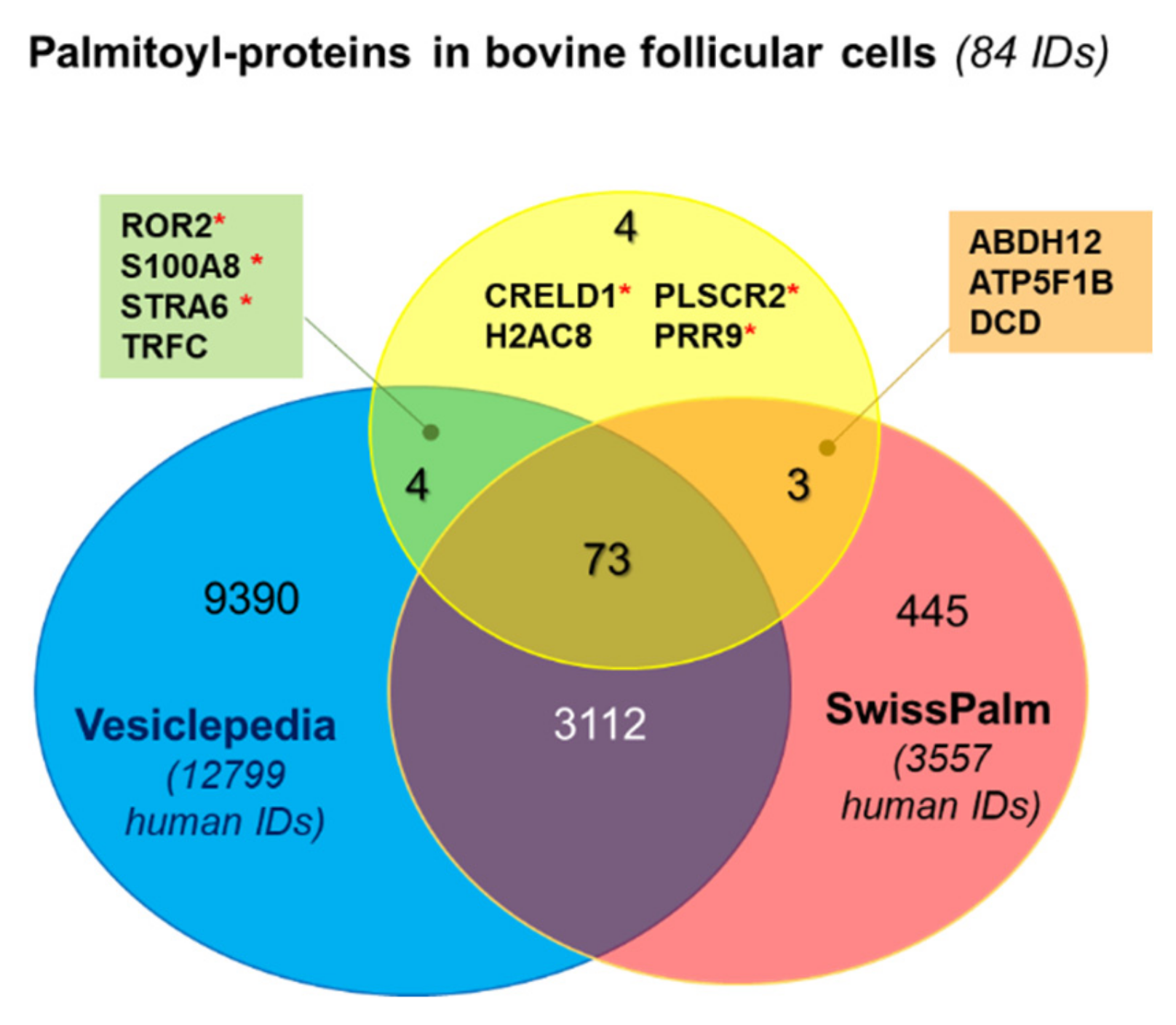


| ID | Protein Name | Palmitoylation (Human) | EVs Cargo | Membranome | +HA Enrichment Fold |
|---|---|---|---|---|---|
| ABHD12 | abhydrolase domain containing 12. lysophospholipase | + | 56 | ||
| ACAA2 | 3-ketoacyl-CoA thiolase. mitochondrial (acetyl-CoA acyltransferase 2) | + | yes | only +HA | |
| ACAT1 | acetyl-CoA acetyltransferase 1 | + | yes | 3.9 | |
| AIFM1 | apoptosis inducing factor mitochondria associated 1 | + | yes | 2.2 | |
| ALDH6A1 | aldehyde dehydrogenase 6 family member A1 | + | yes | only +HA | |
| APMAP | adipocyte plasma membrane associated protein | + | TMP | 3.2 | |
| ATP1A1 | sodium/potassium-transporting ATPase subunit alpha-1 | + | yes | 2.1 | |
| ATP5F1B | ATP synthase F1 subunit beta | + | 3.7 | ||
| B3GNTL1 | UDP-GlcNAc:betaGal beta-1.3-N-acetylglucosaminyltransferase-like protein 1 (alias B3GNT8) | + | yes | TTMP | only +HA |
| BLVRA | biliverdin reductase A | + | yes | only +HA | |
| CANX | calnexin | ++ | yes | TMP | 42 |
| CD36 | platelet glycoprotein 4 (CD36 antigen) | + | yes | only +HA | |
| CD58 | lymphocyte function-associated antigen 3 | ++ | yes | TMP | only +HA |
| CD81 | CD81 antigen (target of antiproliferative antibody 1) | ++ | yes | only +HA | |
| CKAP4 | cytoskeleton associated protein 4 | ++ | yes | TMP | 21 |
| CLGN | calmegin | + | yes | TMP | only +HA |
| CRELD1 | cysteine rich with EGF like domains 1 | predict | 4.4 | ||
| CTNND1 | catenin delta 1 | ++ | yes | only +HA | |
| DCD | dermcidin isoform 1 preproprotein | + | 6.01 | ||
| DLAT | dihydrolipoamide S-acetyltransferase | + | yes | only +HA | |
| DNAJC5 | DnaJ heat shock protein family (Hsp40) member C5 | ++ | yes | only +HA | |
| ECE1 | endothelin converting enzyme 1 | ++ | yes | TMP | only +HA |
| EPHX1 | epoxide hydrolase 1 | + | yes | TMP | 7.2 |
| ERGIC3 | endoplasmic reticulum-Golgi intermediate compartment protein 3 | ++ | yes | TMP | only +HA |
| ERP44 | endoplasmic reticulum protein 44 | + | yes | 2.8 | |
| FASN | fatty acid synthase | + | yes | 5.5 | |
| GALNT1 | polypeptide N-acetylgalactosaminyltransferase 1 | + | yes | TMP | only +HA |
| GLG1 | golgi glycoprotein 1 | + | yes | TMP | only +HA |
| GNA11 | guanine nucleotide-binding protein subunit alpha-11 | + | yes | only +HA | |
| GNA13 | G protein subunit alpha 13 | ++ | yes | only +HA | |
| GNAI1 | guanine nucleotide-binding protein G(i) subunit alpha-1 | ++ | yes | 2.1 | |
| GNAS | guanine nucleotide-binding protein G(s) subunit alpha XLas-like isoform X1 | ++ | yes | only +HA | |
| H2AC8 | H2A clustered histone 8 | nd | 11 | ||
| HSD17B1 | estradiol 17-beta-dehydrogenase 1 | + | yes | 4.1 | |
| IDH2 | isocitrate dehydrogenase [NADP]. mitochondrial precursor | + | yes | 2.5 | |
| IGSF8 | immunoglobulin superfamily member 8 | ++ | yes | TMP | only +HA |
| IMMT | inner membrane mitochondrial protein | + | yes | TMP | 3.3 |
| ITGA6 | integrin subunit alpha 6 | ++ | yes | TMP | 510 |
| LDHC | lactate dehydrogenase C | + | yes | only +HA | |
| LMAN1 | Lectin, mannose binding 1 (ERGIC-53) | + | yes | TMP | 2.8 |
| LSR | lipolysis stimulated lipoprotein receptor | + | yes | TMP | 36 |
| M6PR | mannose-6-phosphate receptor, cation dependent | ++ | yes | TMP | only +HA |
| MAN1B1 | endoplasmic reticulum mannosyl-oligosaccharide 1.2-alpha-mannosidase | + | yes | TMP | only +HA |
| MBLAC2 | metallo-beta-lactamase domain containing 2 | + | yes | only +HA | |
| MLEC | malectin | ++ | yes | TMP | 5.9 |
| MMP14 | matrix metallopeptidase 14 | ++ | yes | TMP | only +HA |
| MYOF | myoferlin | + | yes | TMP | 39 |
| NNT | nicotinamide nucleotide transhydrogenase | + | yes | 7.5 | |
| OAT | ornithine aminotransferase | + | yes | 9.7 | |
| PGK1 | phosphoglycerate kinase 1 | + | yes | 2.6 | |
| PHB | prohibitin | + | yes | 4.3 | |
| PI4K2A | phosphatidylinositol 4-kinase type 2-alpha | ++ | yes | only +HA | |
| PLOD1 | procollagen-lysine.2-oxoglutarate 5-dioxygenase 1 | + | yes | only +HA | |
| PLSCR2 | phospholipid scramblase 1 | predict | TMP | 14 | |
| PLSCR3 | phospholipid scramblase 3 | + | yes | TMP | only +HA |
| PLXNB2 | plexin-B2 precursor | + | yes | TMP | only +HA |
| PRAF2 | PRA1 domain family member 2 | + | yes | only +HA | |
| PRDX6 | peroxiredoxin 6 | + | yes | only +HA | |
| PRR9 | proline-rich protein 9 | predict | only +HA | ||
| PTBP1 | polypyrimidine tract binding protein 1 | ++ | yes | 3.3 | |
| PTGFRN | prostaglandin F2 receptor inhibitor | + | yes | TMP | 42 |
| RAP2A | ras-related protein Rap-2a | ++ | yes | only +HA | |
| ROR2 | tyrosine-protein kinase transmembrane receptor ROR2 | predict | yes | TMP | only +HA |
| RPN2 | ribophorin II | + | yes | 3.3 | |
| S100A8 | S100 calcium binding protein A8 | predict | yes | only +HA | |
| S100A9 | protein S100-A9 | + | yes | only +HA | |
| SCAMP2 | secretory carrier membrane protein 2 | + | yes | only +HA | |
| SCAMP3 | secretory carrier membrane protein 3 | + | yes | only +HA | |
| SCARB2 | scavenger receptor class B member 2 | + | yes | 13 | |
| SCCPDH | saccharopine dehydrogenase-like oxidoreductase | + | yes | 14 | |
| SELENBP1 | selenium-binding protein 1 | + | yes | 2.0 | |
| SERPINH1 | serpin family H member 1 | + | yes | 3.7 | |
| SIGMAR1 | sigma non-opioid intracellular receptor 1 | + | yes | TMP | only +HA |
| SLC44A1 | choline transporter-like protein 1 isoform X1 | ++ | yes | only +HA | |
| SORT1 | sortilin | + | yes | TMP | only +HA |
| STRA6 | receptor for retinol uptake STRA6 | predict | yes | only +HA | |
| TMX1 | thioredoxin related transmembrane protein 1 | ++ | yes | TMP | 6.4 |
| TMX3 | thioredoxin related transmembrane protein 3 | + | yes | TMP | 37 |
| TMX4 | thioredoxin related transmembrane protein 4 | + | yes | TMP | 14 |
| TRFC | transferrin receptor protein 1 (p90, CD71) | nd | yes | only +HA | |
| TUBB4B | tubulin beta-4B chain | ++ | yes | 3.56 | |
| TUFM | Tu translation elongation factor, mitochondrial | + | yes | 3.1 | |
| VAMP3 | vesicle associated membrane protein 3 | + | yes | TMP | only +HA |
| VIM | vimentin | + | yes | 3.73 |
| Cellular Component | Number of Proteins (IDs) | Background (Number of Proteins) | Fold Enrichment | p-Value, BH Method |
|---|---|---|---|---|
| Lysosome | 51 | 1620 | 6.0 | 1.14 × 10−27 |
| ACAT1; AIFM1; APMAP; ATP1A1; CANX; CD36; CD81; CKAP4; CTNND1; DCD; DLAT; DNAJC5; ECE1; EPHX1; ERGIC3; ERP44; GLG1; GNA11; GNA13; GNAI1; GNAS; HSD17B1; IDH2; IGSF8; ITGA6; LMAN1; M6PR; MBLAC2; MLEC; MYOF; PGK1; PHB; PI4K2A; PLSCR3; PRDX6; PTBP1; PTGFRN; RAP2A; S100A8; S100A9; SCAMP2; SCARB2; SCCPDH; SIGMAR1; SLC44A1; SORT1; STRA6; TMX1; TMX4; TUBB4B; TUFM | ||||
| Exosomes | 46 | 2043 | 4.3 | 5.46 × 10−18 |
| ACAA2; ACAT1; ATP1A1; BLVRA; CANX; CD36; CD58; CD81; CKAP4; CTNND1; DCD; ECE1; ERP44; FASN; GLG1; GNA11; GNA13; GNAI1; GNAS; IGSF8; ITGA6; LMAN1; LSR; MBLAC2; MYOF; PGK1; PHB; PLOD1; PLSCR3; PLXNB2; PRDX6; PTBP1; PTGFRN; RAP2A; S100A8; S100A9; SCAMP2; SCAMP3; SCARB2; SELENBP1; SLC44A1; SORT1; TUBB4B; TUFM; VAMP3; VIM | ||||
| Membrane | 15 | 350 | 8.1 | 9.22 × 10−8 |
| APMAP; ATP1A1; CANX; CD81; CKAP4; GLG1; LMAN1; MLEC; MMP14; MYOF; RPN2; SCAMP2; SCAMP3; SCARB2; SLC44A1 | ||||
| Plasma membrane | 41 | 3479 | 2.2 | 4.09 × 10−6 |
| ATP1A1; CANX; CD36; CD58; CD81; CKAP4; CRELD1; CTNND1; DNAJC5; ECE1; GLG1; GNA11; GNA13; GNAI1; GNAS; IGSF8; ITGA6; LDHC; LSR; M6PR; MMP14; MYOF; PHB; PLSCR2; PLSCR3; PLXNB2; PTGFRN; RAP2A; ROR2; S100A8; S100A9; SCAMP2; SCAMP3; SCARB2; SELENBP1; SERPINH1; SIGMAR1; SORT1; TMX1; VAMP3; VIM | ||||
| Golgi apparatus | 20 | 897 | 4.2 | 4.08 × 10−6 |
| CANX; CD36; CKAP4; DCD; ECE1; ERGIC3; FASN; GALNT1; GLG1; GNAI1; LMAN1; LSR; M6PR; PI4K2A; PTGFRN; SCAMP2; SCAMP3; SELENBP1; SORT1; VIM | ||||
| Endoplasmic reticulum | 22 | 1104 | 3.8 | 4.35 × 10−6 |
| CANX; CKAP4; CLGN; CRELD1; DNAJC5; EPHX1; ERP44; GALNT1; LMAN1; MAN1B1; MLEC; PI4K2A; PLOD1; PLXNB2; PTGFRN; RPN2; SERPINH1; SIGMAR1; SORT1; STRA6; TMX1; VIM | ||||
| Mitochondrion | 22 | 1259 | 3.3 | 3.75 × 10−5 |
| ACAA2; ACAT1; AIFM1; ALDH6A1; ATP1A1; CANX; DLAT; FASN; GLG1; IDH2; IMMT; LDHC; NNT; OAT; PGK1; PHB; PLSCR3; RPN2; SCCPDH; TMX1; TUFM; VIM | ||||
| ER-Golgi intermediate compartment | 4 | 30 | 25.3 | 0.00175 |
| ERGIC3; ERP44; LMAN1; SERPINH1 | ||||
| Cytoskeleton | 10 | 427 | 4.4 | 0.00647 |
| CD36; CKAP4; DCD; IMMT; ITGA6; MLEC; PHB; TUBB4B; TUFM; VIM | ||||
| Trans-Golgi network membrane | 2 | 3 | 126.2 | 0.00647 |
| GNAS, SCAMP2 | ||||
| Recycling endosome membrane | 2 | 7 | 54.2 | 0.04065 |
| RAP2A; SCAMP2 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzbekova, S.; Teixeira-Gomes, A.-P.; Marestaing, A.; Jarrier-Gaillard, P.; Papillier, P.; Shedova, E.N.; Singina, G.N.; Uzbekov, R.; Labas, V. Protein Palmitoylation in Bovine Ovarian Follicle. Int. J. Mol. Sci. 2021, 22, 11757. https://doi.org/10.3390/ijms222111757
Uzbekova S, Teixeira-Gomes A-P, Marestaing A, Jarrier-Gaillard P, Papillier P, Shedova EN, Singina GN, Uzbekov R, Labas V. Protein Palmitoylation in Bovine Ovarian Follicle. International Journal of Molecular Sciences. 2021; 22(21):11757. https://doi.org/10.3390/ijms222111757
Chicago/Turabian StyleUzbekova, Svetlana, Ana-Paula Teixeira-Gomes, Aurélie Marestaing, Peggy Jarrier-Gaillard, Pascal Papillier, Ekaterina N. Shedova, Galina N. Singina, Rustem Uzbekov, and Valerie Labas. 2021. "Protein Palmitoylation in Bovine Ovarian Follicle" International Journal of Molecular Sciences 22, no. 21: 11757. https://doi.org/10.3390/ijms222111757
APA StyleUzbekova, S., Teixeira-Gomes, A.-P., Marestaing, A., Jarrier-Gaillard, P., Papillier, P., Shedova, E. N., Singina, G. N., Uzbekov, R., & Labas, V. (2021). Protein Palmitoylation in Bovine Ovarian Follicle. International Journal of Molecular Sciences, 22(21), 11757. https://doi.org/10.3390/ijms222111757








