Abstract
Parkinson’s disease (PD) is characterized by four pathognomonic hallmarks: (1) motor and non-motor deficits; (2) neuroinflammation and oxidative stress; (3) pathological aggregates of the α-synuclein (α-syn) protein; (4) neurodegeneration of the nigrostriatal system. Recent evidence sustains that the aggregation of pathological α-syn occurs in the early stages of the disease, becoming the first trigger of neuroinflammation and subsequent neurodegeneration. Thus, a therapeutic line aims at striking back α-synucleinopathy and neuroinflammation to impede neurodegeneration. Another therapeutic line is restoring the compromised dopaminergic system using neurotrophic factors, particularly the glial cell-derived neurotrophic factor (GDNF). Preclinical studies with GDNF have provided encouraging results but often lack evaluation of anti-α-syn and anti-inflammatory effects. In contrast, clinical trials have yielded imprecise results and have reported the emergence of severe side effects. Here, we analyze the discrepancy between preclinical and clinical outcomes, review the mechanisms of the aggregation of pathological α-syn, including neuroinflammation, and evaluate the neurorestorative properties of GDNF, emphasizing its anti-α-syn and anti-inflammatory effects in preclinical and clinical trials.
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder that affects around 10 million people worldwide. Furthermore, prospective studies predict that the PD prevalence will continue increasing for the next 30 years caused by the expanding life expectancy of the general population [1,2,3]. PD is well known through the disabling motor deficits, including bradykinesia, tremor, stiffness, and postural instability, which arise from the progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) [1,4]. Besides, non-motor alterations, such as olfactory loss and mood changes, also occur in PD and can appear years or decades before the motor deficit manifestation [5].
Another pathological hallmark of PD is the presence of Lewy bodies and Lewy neurites, mainly composed of misfolded α-synuclein (α-syn) [6,7]. This protein plays different physiological roles in neuronal homeostasis, predominantly in clustering and releasing synaptic vesicles during neuronal plasticity [8]. Unfortunately, many events can modify the structure of α-syn converting it into a pathological protein that gives rise and extends the PD process [9]. Experimental evidence suggests that the neurotoxicity of α-syn comes from its transformation into insoluble aggregates, either oligomers or fibrils [10]. Aggregates of α-syn are known to activate pathological intracellular signaling in neurons and glial cells, leading to membrane disruption, mitochondrial dysfunction, homeostatic alterations, and finally, neuronal death [11]. However, the exact mechanism in the DA neurodegeneration remains unknown [9]. Recently, an increasing number of publications associate pathological α-syn with neuroinflammation, the latter unveiled through microglia activation, astrocyte reactivity, inflammatory mediator release, blood-brain barrier (BBB) breakdown, and immune cell infiltration [12,13]. Interestingly, extracellular α-syn aggregates [14] can directly stimulate microglia and astrocytes to produce pro-inflammatory cytokines and initiate neuroinflammation [12,15,16,17]. Moreover, pathological α-syn can also significantly decrease glial cell-derived neurotrophic factor (GDNF) levels and its receptors tyrosine kinase (RET) expression [18,19], thus, eradicating a vital survival pathway of DA neurons in adulthood.
It is still controversial whether the chronic inflammatory environment is the cause or the consequence of neurodegeneration. However, the current consensus is that neuroinflammation can lead to neural degeneration, for which it has become the third pathological hallmark of PD [20,21]. Moreover, the importance of neuroinflammation relies on its ability to launch a vicious circle by recruiting professional immune cells, which release harmful humoral factors that worsen neuroinflammation [22].
Given the lack of regenerative actions of the current pharmacological treatments for PD [23,24], modern therapeutics aim to reduce the neuroinflammation and the pathological α-syn aggregation, both considered causal factors behind the DA neurodegeneration.
Another modern therapeutic strategy uses neurotrophic factors that participate in the differentiation and growth of DA neurons during brain development and promote maintenance and survival in adulthood [1]. GDNF is an attractive candidate that has been tested in PD models in vitro and in vivo because of its potent neuroregenerative action and its apparent anti-α-syn and anti-inflammatory effects [25,26,27,28,29,30,31,32,33,34]. However, the outcomes of clinical trials with GDNF through various administration routes have been disappointing, and some patients have developed severe adverse side effects [35,36]. The uncertainty of GDNF as a therapeutic agent arises from the lack of knowledge about GDNF’s effects on α-syn aggregation and neuroinflammation in preclinical studies and clinical trials. Therefore, the question arises as to whether GDNF is an appropriate therapeutic agent for treating PD or just another dead end of what once seemed to be a promising idea.
In this review, we discuss the newest knowledge on the role of pathological α-syn aggregates in the neuroinflammatory process, two of the pathognomonic hallmarks of PD. In this line, we analyze possible pathophysiological mechanisms through which α-syn could cause neuroinflammation and contribute to the death of DA neurons. Besides, we discuss preclinical and clinical studies on the therapeutic use of GDNF, emphasizing its neuroregenerative action and its anti-α-syn and anti-inflammatory effects.
2. The Role of α-Synuclein in Parkinson’s Disease
2.1. Structure and Physiology of α-Synuclein
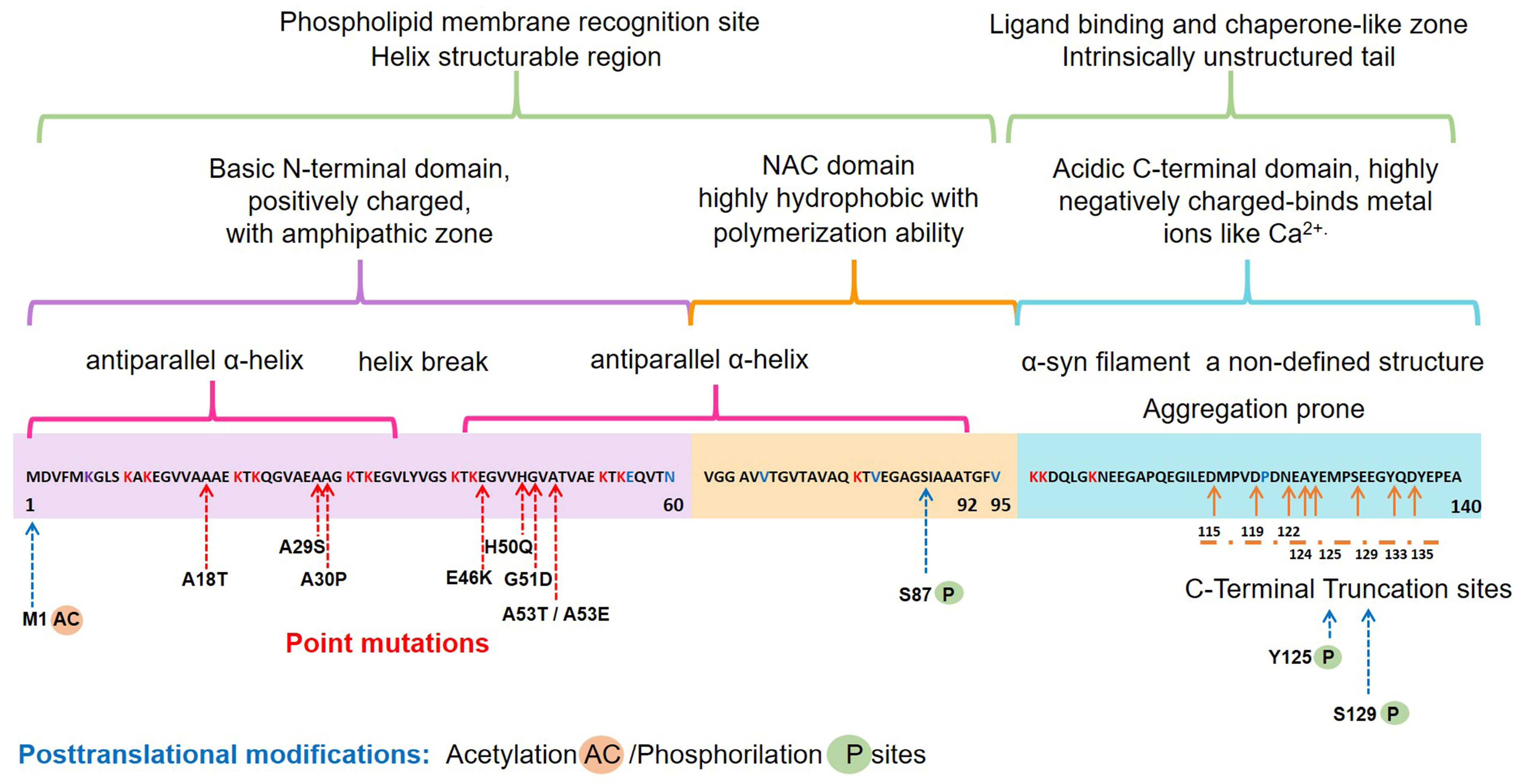
The α-syn, encoded by the SNCA1/PARK1 gene, is a ubiquitous protein that is abundantly expressed in kidneys and blood cells [37], but highly enriched in the brain, particularly in the presynaptic terminals of the neocortex, hippocampus, substantia nigra (SN), thalamus, and cerebellum. Interestingly, it has been found expressed in the cytoplasm of astrocytes and oligodendrocytes in healthy individuals [38,39]. Different scientific approaches have converged in the description of α-syn as an intrinsically disordered protein (IDP), with unusual structural properties [40]. Three distinct domains (Figure 1) confer dynamic structural flexibility and remarkable conformational plasticity [40,41,42,43]; (1) The N-terminal region (amino acids 1–65) confers an α-helical structure involved in lipid membranes binding and possibly promotes α-syn oligomerization; (2) The central region (amino acids 61–95) includes the NACore phosphate-binding loop, which has been implicated in the formation of amyloid fibrils and the stabilization of the pathogenic conformation of α-syn; (3) The C-terminal region (amino-acids 96–140) is associated with the major sites of metal binding and posttranslational modification, involved in modulating the protein structure, physiological functions, and toxicity.
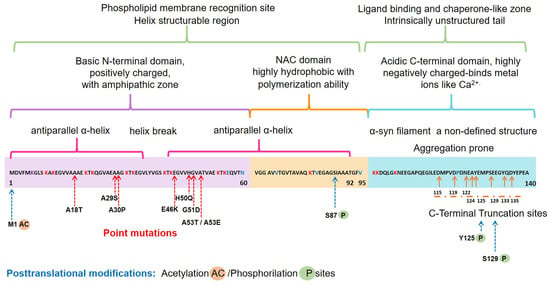
Figure 1.
Schematic representation of the native α-syn monomeric structure, highlighting features linked to its biochemical function and dysfunction. Abbreviation: NAC, non-amyloid β-component.
Concerning the physiological roles of this protein, several studies have considered the subcellular localization of α-syn, which ranges from the nucleus to mitochondria and nerve terminals, to propose the following functions [44,45,46,47,48]: (i) neuronal health maintenance; (ii) synaptic plasticity; (iii) membrane biogenesis; iv) mitigation of oxidative stress; (v) regulation of synaptic vesicle trafficking; (vi) neurotransmitter release.
2.2. Pathological α-Syn Aggregates and Prion-like Properties
Several lines of evidence have proposed that native α-syn exists predominantly as an IDP monomer, which is typically found in an unfolded state and soluble in the cytosol, minimally phosphorylated in the healthy human brain. However, this dynamic protein can convert to various conformations such as helically folded tetramers resisting aggregation, pathologic oligomers, small aggregates, protofibrils, or irreversible insoluble amyloid fibrils with a stabilizing β-sheet structure [42,49,50]. Most α-syn forms exist in a dynamic equilibrium with each other, but perturbation of neuronal homeostasis is a starting point for pathological α-syn insolubility, self-assembly, β-sheet stacking, and misfolding. Cellular environmental cues combined with genetic factors contribute to the posttranslational modifications of the unfolded monomeric α-syn that lead to dysfunctional, neurotoxic, and pathological aggregates with a high degree of β-sheet structure [51].
Interestingly, all the known mutations associated with familial forms of PD are clustered within the N-terminal region, causing misfolding and/or aggregation of the mutant α-syn [42]. Furthermore, N-terminal acetylation could be critical for both aggregation and function [52]. In the C-terminal region, posttranslational modifications have been described that promote a tendency to protein aggregation. Examples are the C-terminal truncation, which results in increased filament assembly, and the phosphorylation at S129 (pS129), which regulates membrane-binding and enhances interactions with metal ions and other proteins (Figure 1). Highlighting that pS129 α-syn modulates key events in the pathogenesis of synucleinopathy such as: (i) variations in the fibrillar structure; (ii) different propagation properties; (iii) increase in cytotoxicity [53].
Indeed, a diversity of pathogenic properties of the misfolded conformations and accumulating aggregates of α-syn have been associated with: (i) mitochondrial dysfunction; (ii) endoplasmic reticulum stress; (iii) proteostasis dysregulation; (iv) synaptic impairment; (v) cell apoptosis; (vi) neuroinflammation; and (vii) neurodegeneration [11,54,55,56,57,58].
Notably, the pathological α-syn aggregates may spread from one neuron to another, causing Lewy pathology in the whole brain [59]. However, α-synuclein-positive inclusions have also been found in the cytoplasm of oligodendrocytes, an event that occurs in the α-synucleinopathy called multiple system atrophy. Specifically, Braak et al. (2002) suggested that pathological forms of the α-syn act in a prion-like manner, trafficking between cells in a non-random way/form [59]. They hypothesized that PD pathology initiates in the peripheral nervous system, gaining access to the central nervous system (CNS) through retrograde transport via the olfactory tract and the vagal nerve [59,60]. It has been argued that the release and propagation mechanisms of α-syn between neuroanatomically connected regions can be through exosomes, classical exocytosis, trans-synaptic junctions, tunneling nanotubes, and direct penetration [61]. Last but not least, recent studies have suggested that α-syn misfolding and aggregation trigger microglial activation, leading to neuroinflammation and cellular metabolic stress, enhancing the aggregation and spreading of α-syn and affecting its prion-like transmissibility and pathogenicity (Braak’s hypothesis) [62,63,64,65].
3. The Misfolding of α-Synuclein and Its Association with Neuroinflammation in Parkinson’s Disease
A hypothesis claims that chronic neuroinflammation can lead to neuronal damage, neuronal circuitry disturbances, and ultimately, neurodegeneration in PD [20,66]. Hence, chronic neuroinflammation is relevant when considering the pathophysiological mechanisms involved in PD progression and proposing appropriate therapeutic approaches. The brain is an organ susceptible to external stimuli. However, internal stimuli can also alter the delicate homeostasis of the neuronal microenvironment maintained by microglia and astrocytes, considered the brain’s absorptive, excretory, and defense systems [67]. These cells display a Janus-like face because they help eliminate neurotoxins and pathogens, and conversely, they can also cause neuroinflammation, neurotoxicity, and neurodegeneration. Thus, neuroinflammation is a complex pathological condition where different cells and humoral factors converge to resolve the damage as a first intention and latter they aggravate the disease in the long term. The cellular actors are activated microglia, reactive astrocytes, and infiltrated lymphocytes, whereas the humoral factors are a great variety of pro-inflammatory molecules. A resulting critical event from the flare-up between cells and humoral factors activities is the loss of BBB permeability that allows molecules to cross from one side to another of the brain [66].
In PD, microglia activation can arise from several factors or causes. The preference of activated microglia for brain areas enriched with pathological α-syn aggregates supports its close association with the neurodegeneration process in PD [68,69]. Multiple studies have shown that extracellular α-syn stimulates microglial cells to produce pro-inflammatory molecules such as interleukin (IL)-1β, IL-6, tumoral necrosis factor-alpha (TNF-α), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and reactive oxygen species (ROS) [70,71,72,73,74]. The combined neuroinflammation and oxidative stress can promote the neurodegenerative process and further aggravate it [75,76].
Furthermore, neuron-derived α-syn can stimulate astrocytes to produce and release pro-inflammatory cytokines and chemokines, which in turn can recruit activated microglial cells [12] and differentiate them to an M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotype [77]. Therefore, pathological α-syn also behaves as a chemokine to concentrate activated microglia in the affected anatomical areas in PD [78,79].
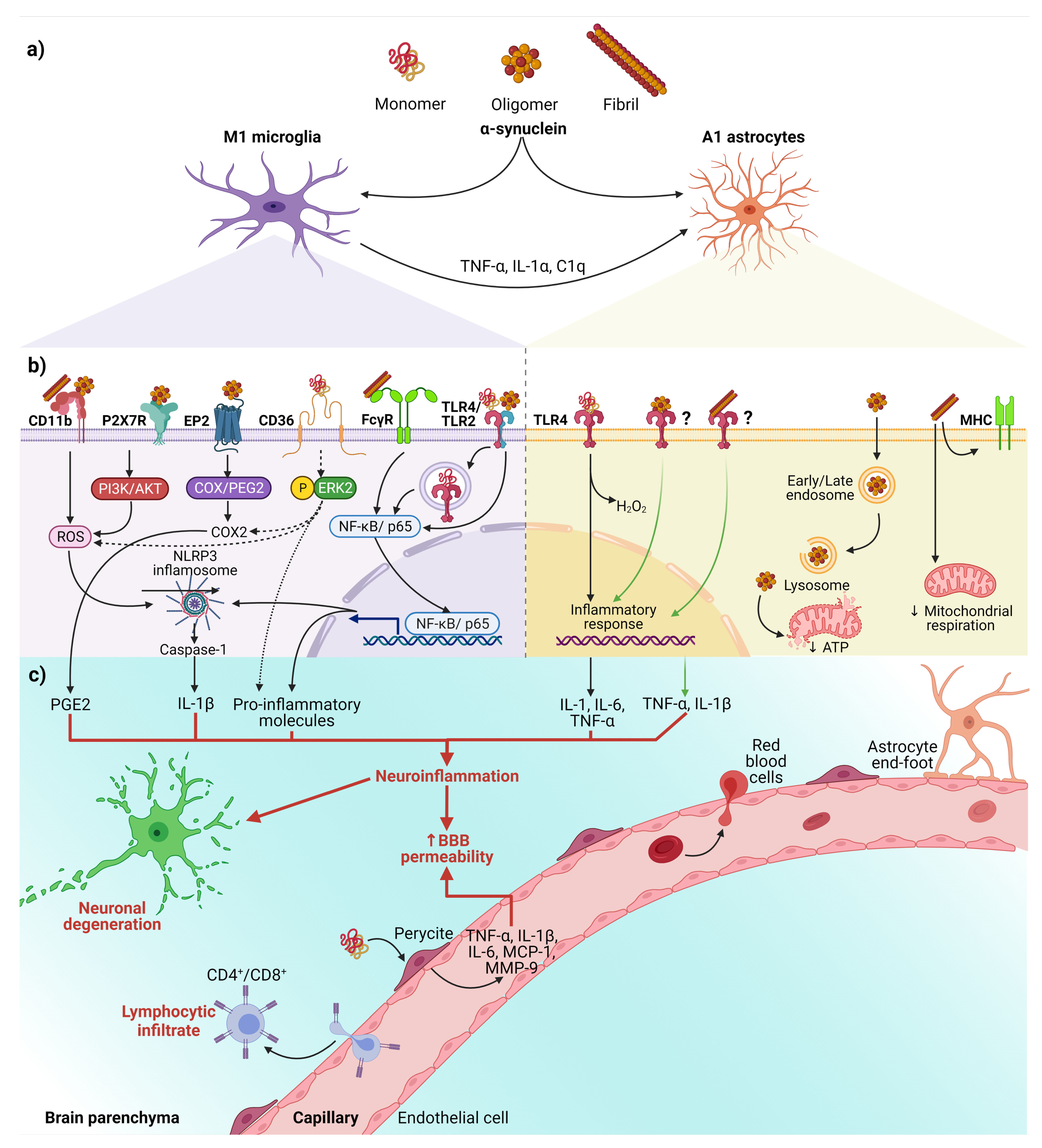
The relevance of activated microglia in neuroinflammation and neurodegeneration relies on its ability to convert physiological astrocytes into neurotoxic reactive astrocytes classified as A1 phenotype. Recently, it has been established that IL-1α, TNF, and complement component 1q (C1q) released by activated microglia are sufficient and necessary to detonate the A1 phenotype (Figure 2a) [80]. Furthermore, evidence in vitro and in vivo shows that neurotoxic reactive astrocytes A1 can kill neurons through the secretion of neurotoxins not yet identified [80,81]. Thus, the neurotoxic role of reactive astrocytes A1 has severe implications for PD and other neurodegenerative diseases. It means that neurotoxic astrocytes lost their ability to promote neuronal survival, growth, synaptogenesis, and phagocytosis. Therefore, an effective therapy must also prevent the conversion of neurotoxic reactive astrocytes A1 and block their neurotoxic activity [80,81].
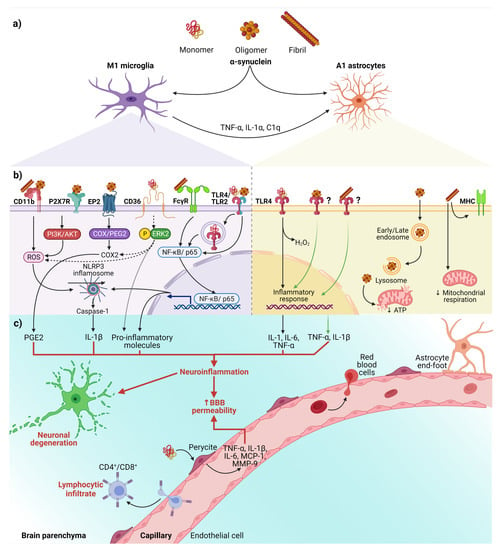
Figure 2.
The interaction between α-syn and neuroinflammation in PD. (a) Activation of glial cells by pathological α-syn aggregates; (b) Signaling pathways in microglia and astrocytes triggered by interaction with the different aggregation patterns of α-syn; (c) Neuronal and BBB dysfunction triggered by the neuroinflammatory environment. Dash lines indicate the signaling pathway activated by CD36; Question marks and faded green lines indicate the proposed mechanism for oligomeric and fibrillar α-syn interaction with astrocytes, and thick red lines indicate the proinflammatory molecules that lead to dysfunction of the BBB and neural degeneration. Abbreviations: AKT, Protein kinase B; BBB, Blood-brain barrier; C1q, complement component 1q; CD, cluster of differentiation; COX, cyclooxygenase; EP2, E prostanoid receptor 2; ERK2, extracellular signal-regulated kinase 2; FcγR, the gamma chain subunit of Fc receptor; H2O2, hydrogen peroxide; IL, interleukin; MCP-1, Monocyte Chemoattractant Protein-1; MHC, Major Histocompatibility Complex; MMP-9, Matrix metallopeptidase 9; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing 3; P2X7R, P2X7 receptor; PGE2, Prostaglandin E2; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; TLR, Toll-like receptors; TNF-α, tumor necrosis factor-alpha. Created with BioRender.com.
Besides, the interaction between α-syn with glial cells depends on α-syn aggregation state and the receptors responsible for its uptake (Figure 2b). These receptors expressed in glial cells, through which α-syn interact to trigger a neuroinflammatory environment (Table 1 and Figure 2b,c), play a critical role in early PD, considering that the α-syn aggregation process from soluble oligomers to insoluble inclusions occurs in the early phase of the disease [15,82].

Table 1.
Neuroinflammatory and neurotoxic effects triggered by pathological α-syn interaction with glial cells.
On the other hand, an in vitro study demonstrated that monomeric α-syn activates pericytes (critical cells in BBB regulation) to release pro-inflammatory molecules and matrix metalloproteinase-9 (MMP-9) [101]. These findings suggest that pericytes could exacerbate the neuroinflammatory environment and cause the BBB rupture in PD patients (Figure 2c).
Finally, the infiltrated peripheral immune cells, especially CD4+ and CD8+ T lymphocytes, extend and worsen the PD pathology (Figure 2c) [22,102]. This complication shows the critical role BBB breakdown plays in PD, thus becoming a point to control by new therapeutics. Moreover, infiltrated T lymphocytes, recognizing pathological α-syn aggregates as an antigen presented by microglial class II major histocompatibility complex (MHC-II), arise the immune response [103]. The increased infiltration of Th17 cells and reactive Th1 cells differentiated from CD4+ lymphocytes in PD brains proves the immune response’s involvement in this neuropathology [102,104]. Therefore, these antecedents support the immune response as another mechanism of neuronal death and promote the development of effective immunotherapies.
In summary, we propose the following sequential cellular events that lead to neuroinflammation in early PD (Figure 2). First, misfolded α-syn binds to microglial receptors causing differentiation of the microglia into the M1 phenotype. Then, M1 microglia release TNF-α, IL1α, and C1q inducing the conversion of astrocytes into neurotoxic reactive astrocytes A1. Both, the M1 microglia and A1 astrocytes release pro-inflammatory cytokines to open the BBB and chemokines to attract CD4+ and CD8+ cells, thus completing an immune response against misfolded α-syn. Moreover, the action of neurotrophic factors and other protective molecules released by astrocytes A2 is overpassed by the neuroinflammatory events. Altogether, the unknown molecules released by neurotoxic reactive astrocytes A1, the pro-inflammatory cytokines, and the cellular and humoral response of professional immune cells converge to kill the DA neurons in the early stages of PD. Therefore, new antiparkinsonian therapies should prevent α-syn binding to its glial receptors, block microglial M1 and astrocytic A1 activities, strengthen BBB permeability and avoid activation of the immune response. However, present therapeutic effects are insufficient for an integral PD therapy since the structural and functional restoring of the nigrostriatal dopaminergic system is not accomplished. To this purpose, different approaches based on neurotrophic factors have been tested in vitro, in vivo, and clinical trials. GDNF is an attractive therapeutic candidate because of its potent neurotrophic effects on DA neurons during development and adulthood and its potential anti-inflammatory and anti-α-syn therapeutic effects (see below).
4. Glial Cell-Derived Neurotrophic Factor in Parkinson’s Disease
4.1. Structure, Signaling Pathways, and Function
GDNF is the first neurotrophic factor member of the GDNF family ligands isolated by Lin and coworkers in 1993 from the rat glial cell line B49 [34,105]. GDNF has been proposed as a potent therapeutic agent for PD treatment based on its positive effects on the survival and morphological differentiation of embryonic midbrain DA neurons in culture [34]. GDNF is codified by the GDNF gene and translated as a precursor of 211 amino acids, which, after proteolytic processing, is secreted as a mature protein of 134 amino acids [34].
The active form of GDNF is a disulfide-bonded homodimer composed of two β-stranded finger loops and a helical heel that binds to the GDNF family receptor α1 (GFRα1) on its finger domain, forming the high-affinity GDNF-GFRα1 complex [106,107,108]. This complex can activate different signaling pathways depending on the co-receptors to which it binds, such as the receptor tyrosine kinase (RET) or the neural cell adhesion molecule (NCAM) [106,109]. In the first case, the GDNF-GFRα1 complex binds two RET molecules inducing RET dimerization and autophosphorylation of specific tyrosine residues (Y905, Y1015, Y1062, and Y1096). These phosphorylated residues operate as docking sites for signaling adaptor proteins that initiate the signaling of the mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K)/AKT, and Src pathways, which finally turn on genetic programs for neural proliferation, differentiation, migration, maintenance, survival, and neurite outgrowth [1,106,110,111]. Instead, the binding of the GDNF-GFRα1 complex with NCAM activates the focal adhesion kinase (FAK)/Fyn pathway (independently of RET signaling) that promotes axonal growth in hippocampal and cortical neurons, as well as Schwann cells migration [109]. Interestingly, multiple GDNF homodimers can simultaneously interact with syndecan-3 (independently of GFRα1 and RET signaling) and activate the Src kinase pathway, resulting in migration of cortical neurons and neurite outgrowth [112].
Under physiological conditions, GDNF is expressed in soft tissue, testis, kidney, adrenal gland, parathyroid gland, placenta, gastrointestinal tract, spinal cord, and multiple brain nuclei [113,114,115]. In the CNS, GDNF expression increases during embryonic development and decreases in adulthood, restricting itself to specific brain areas such as the cortex, hippocampus, striatum (STR), SN, thalamus, cerebellum, and spinal cord [114,116]. GDNF plays a vital role in regulating kidney morphogenesis, enteric system development, and parasympathetic neuron proliferation and migration. Of importance for this review, GDNF is well known for its potent effect on the maturation, maintenance, and survival of DA neurons [34,117,118,119]. Unfortunately, GDNF expression and signaling are dysregulated in neurodegenerative diseases, including PD [19,120]. Thus, suppression of endogenous GDNF due to PD renders it unavailable to contribute to the neural restorative process.
4.2. GDNF Alterations in Parkinson’s Disease
PD patients consistently show GDNF protein depletion in the surviving DA neurons of SNpc compared to healthy subjects, thus, partly explaining the lack of restorative effects on the nigrostriatal system [120]. Similarly, in other regions of the nigrostriatal system, the putamen nucleus shows a significant decrease in GDNF protein levels [116]. Interestingly, reduction in GDNF protein levels has also been found in the hippocampus of PD patients, associated with cognitive decline even in the absence of neuronal loss [121]. Hence, GDNF might perform physiological actions in hippocampal neurotransmission similar to those of BDNF [122]. However, in the remaining brain areas such as the cerebellum and frontal cortex, GDNF protein levels do not change [116]. On the other hand, special interest has arisen in the signaling effectors, downstream of GDNF. In the putamen of PD patients, the mRNA expression levels for GFRα1 and RET remain unchanged [123]. Moreover, gene expression analysis in the SN of sporadic PD patients exposed no significant change in RET, which was corroborated in α-syn transgenic mice [124]. Nevertheless, mRNA and gene expression give rise to many speculative mechanisms without a physiological basis since the presence of the protein was not explored.
Indeed, the protein levels for RET receptor and its downstream signaling molecule phosphor-ribosomal protein S6 (p-rpS6) were severely decreased in DA neurons with α-syn inclusions of PD patients. These findings were recapitulated in a non-human primate model overexpressing the human mutant α-syn A53T to validate the human findings. Nevertheless, the remaining RET expression in the nigral neurons is enough to activate the downstream molecule p-rpS6, and the synucleinopathy is not an impediment for GDNF to express its trophic effect [19]. The conflicting results obtained regarding GDNF downstream activation of its signaling molecules could be due to the type of method used to evaluate the presence of these molecules. Whereas no changes are observed in gene expression, assays at the protein level have challenged the earlier conclusions.
4.3. Neurotrophic Effects of GDNF in Preclinical Assays
Rodents (Table 2, a) and non-human primates (Table 2, b) with a unilateral or bilateral lesion of the nigrostriatal pathway using specific neurotoxins to model PD have been used to show the preventive and restorative effects of GDNF on DA neuron survival, levels of dopamine, and its catabolites, and motor deficits (see reviews [125,126,127,128,129,130]). However, animal models have significant limitations of applicability to the human disease process [131]. Depending on the selected model, several works have evidenced the relevance of the timing and route of GDNF administration, its concentration or dose, the type of therapy, drug delivery vehicle, and the status of PD progression [35,132,133,134,135,136,137,138]. Specifically, the timing between GDNF administration and exposure to the neurotoxic agent (PD neurotoxin-based models) is critical in achieving optimal protection [139]. In addition, the site of GDNF administration is also critical to achieving functional benefits. For example, a study explored a GDNF in the PD neurotoxin-based model the administration in the STR, SN, or lateral ventricle. The main conclusion was that the preservation of motor functions requires protection of the striatal axon terminals, which can be achieved only by intrastriatal, but not nigral or intraventricular administration of GDNF [140]. Assessment of biochemical, histological, and behavioral changes and a selection of distinct methodologies and scores for determining motor function and behavioral improvements have been used to confirm proper GDNF targeting to the SN, avoiding leakages or ligand-receptor binding in other brain areas.

Table 2.
An overview of GDNF neuroregenerative effects on PD preclinical models.
Lack of clarity on the mechanism of GDNF action could lead to an inappropriate model selection for preclinical therapeutic approaches. Many animal models have been developed to understand PD pathophysiology and propose therapeutic targets. However, not all of them mimic the pathognomonic hallmarks of PD, a requirement for qualifying as a suitable animal PD model [141,142]. Our research group recently developed an animal model by a single intranigral administration of β-sitosterol β-D-glucoside (BSSG) [143,144,145]. This animal PD model is promising because of its following characteristics: (1) development of motor and non-motor alterations; (2) slow and progressive death of DA neurons; (3) spreading and aggregation of pathological α-syn; and (4) neuroinflammation and peripheral immune infiltration. Therefore, it is a suitable animal model reproducing the four pathognomonic hallmarks of PD to test different therapies, including GDNF administration (see below). Furthermore, the outcome of the therapy assays in the stereotaxic BSSG model can predict their efficacy in clinical trials more accurately.
On a final note, the large GDNF homodimer structure impedes the BBB crossing after its systemic administration. Consequently, translational research has focused on direct GDNF protein infusion or vector-mediated GDNF delivery to the brain by several less invasive strategies that enhance vector biodistribution. In this regard, focused ultrasound-assisted delivery, which allows systemic gene delivery, is being used in clinical trials [35,146,147]. Moreover, the perspectives depend on significant improvements to (i) PD preclinical models, (ii) clinical striatal delivery methods, (iii) alternate less invasive methods and (iv) clinical designs that include early PD diagnosis for optimal patient selection. Thus, if GDNF is to be taken forward, every aspect of the clinical design must be optimized. Altogether, the evidence available preserves a cautious optimism for the future of GDNF as a promising target in the treatment of PD progression.
4.4. Anti-α-Synuclein Effect of GDNF in Parkinson’s Disease Preclinical Trials
The neuroregenerative features of GDNF have been extensively demonstrated in parkinsonian animals, but its anti-α-syn effect has been evaluated only by five studies (Figure 3) [25,27,170,171,172] whose results are contradictory (Table 3). Besides, four animal models have been used to evaluate the GDNF effect on α-syn. Two models have been developed overexpressing wild-type or A30P mutant α-syn using lentiviral or AAV transduction in the SN or STR. Another is the parkin transgenic mice that raised α-syn levels. The last model is developed by the injection of preformed fibrils (PFFs) of human α-syn in the STR, which has the advantage of triggering pathological α-syn aggregation, and then, the aggregates spread all over the brain, causing α-synucleinopathy where seeded.
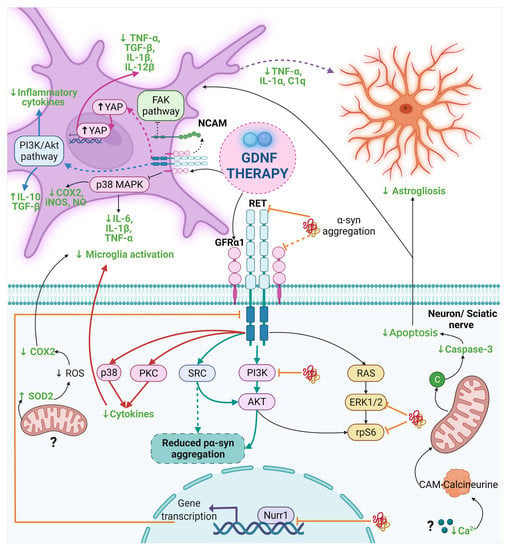
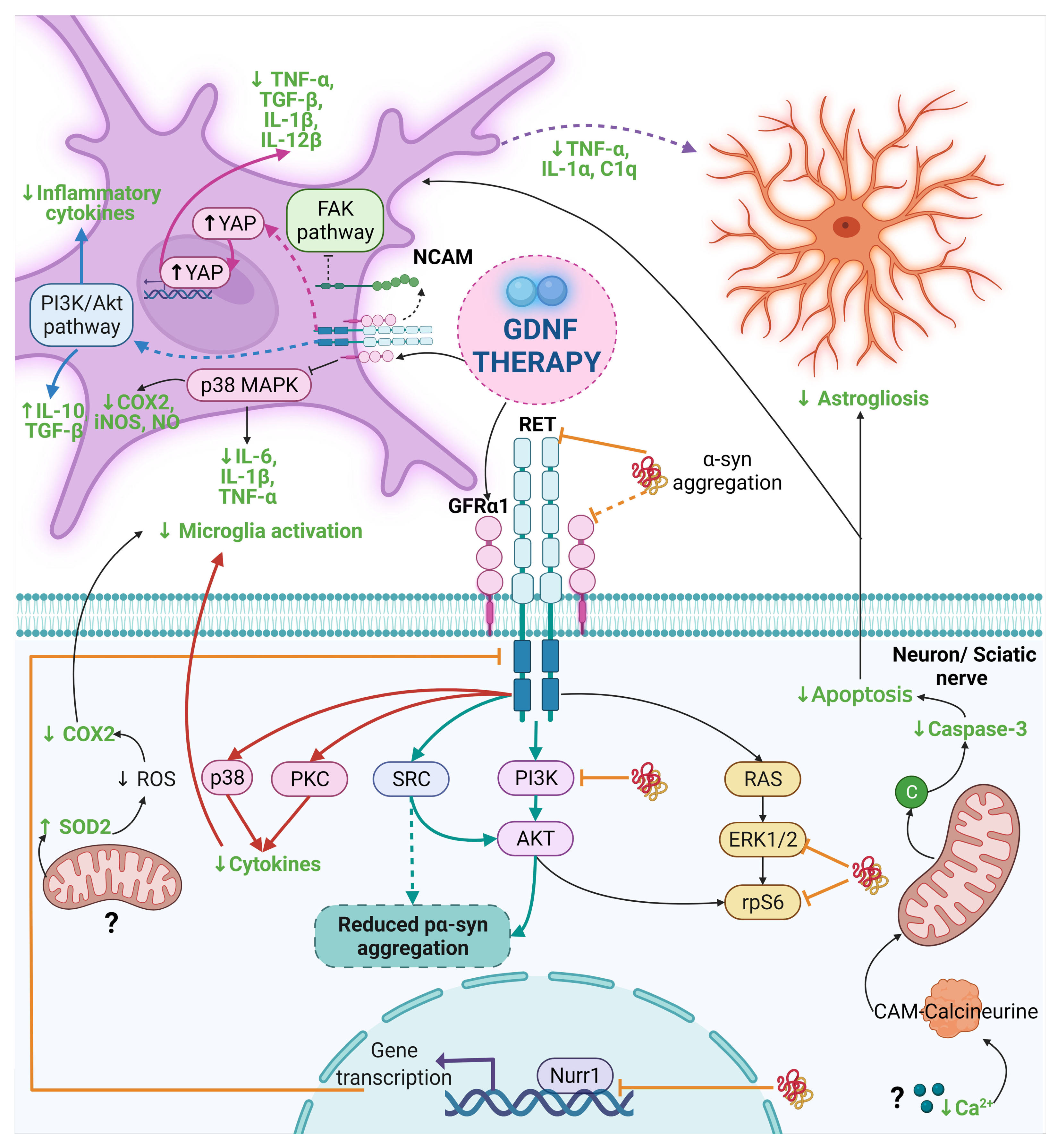
Figure 3.
The anti-α-syn and anti-inflammatory effect of GDNF in PD and other diseases. Dash lines indicate the proposed mechanism by other authors and dash thick purple lines are the proposed mechanism in this review. The mechanism of the anti-α-syn and anti-inflammatory effect of GDNF points out with thick light blue lines and black lines in Parkinson’s disease, respectively. Thick orange lines indicate the interruption in the GDNF signaling caused by α-syn. The mechanism for the anti-inflammatory effect of GDNF is highlighted with thick pink lines, thick blue lines, and thick red lines for Alzheimer’s disease, neurological diseases, and neuropathic pain, respectively. The final anti-inflammatory effect of GDNF is in a bold green letter. Abbreviations: α-syn, α-synuclein; C1q, complement component 1q; CAM, Calmodulin; COX, cyclooxygenase; FAK, Focal adhesion kinase; GDNF, Glial cell-derived neurotrophic factor; GFRα1, GDNF family receptor α1; IL, interleukin; iNOS, Inducible nitric oxide synthase; NCAM, neural cell adhesion molecule; NO, Nitric oxide; PI3K, phosphoinositide 3-kinase; PKC, Protein kinase C; RET, Receptor tyrosine kinase; ROS, reactive oxygen species; SOD2, Superoxide dismutase-2; SRC; Proto-oncogene tyrosine-protein kinase Src; TGF-β, Transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; YAP, Yes-associated protein. Created with BioRender.com.

Table 3.
GDNF therapy against α-syn pathology in preclinical trials of PD.
A line of evidence shows that GDNF therapy administrated before or after α-syn induction fails to prevent or reduce α-syn levels [170,171,172]. The GDNF failure has been speculated to depend on the stage of the neurodegeneration, being effective at the early stage, when there are sufficient surviving neurons to respond to the therapeutic effect [173]. Another explanation for the lack of GDNF neuroprotective and anti-α-syn effects in some experiments could be related to the PD model employed. Thus, there is no convincing evidence that the high levels of wild or A30P mutant α-syn in viral vector transductions can trigger all features of α-synucleinopathy, i.e., significant neurodegeneration of the nigrostriatal system, motor disabilities, and spreading of pathological α-syn aggregates [171]. This approach needs complementation with an injection of PFF to detonate α-synucleinopathy. Since the discussed studies lack this complementary approach, it is difficult to accept the outcome of GDNF failure as conclusive. Another hypothesis is that GDNF failure can be accounted for by the disruption in the GDNF/RET/Nurr1 signaling pathway caused by pathological α-syn aggregates [172]. However, findings in human brain samples of PD patients refute this hypothesis [19].
On the contrary, an anti-α-syn effect of GDNF was evidenced in Parkin Q311X(A) mice with the injection of GDNF-expressing macrophages [27]. Thus, a dual mechanism of the GDNF beneficial effects in this animal model has been taught to be an anti-inflammatory effect of M2 subtype macrophages and the GDNF/Ret pathway activation [25].
Future research on GDNF should be carried out in the stereotaxic BSSG model of PD, where GDNF effects can be evaluated on neuroinflammation, DA neurodegeneration, α-synucleinopathy, and motor and non-motor deficits.
5. Anti-Inflammatory Effects of GDNF in Preclinical Assays
5.1. GDNF Anti-Inflammatory Effects in Neurodegenerative Disease Models
In recent years, particular attention has focused on neuroinflammation and its relationship with neurodegenerative disease [174]. A few studies have investigated the anti-inflammatory properties of GDNF in neurodegenerative disease models, such as PD, Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS; Table 4 and Figure 3) [26,27,28,30,31,32,33,175,176,177]. In PD models, GDNF anti-inflammatory effect is mediated by the modulation of microglial activation [27,28,29,30,31,33]. The proposed molecular mechanisms are the activation of the GFRα1-RET complex, the possible inhibition of the FAK pathway, and the reduction in p38 phosphorylation, a component of the p38-MAPK signal transduction pathway [31,33]. In addition, GDNF administration decreases reactive astrocytes, pro-inflammatory and pro-apoptotic mediators, and oxidative stress [26,27,30,31,32]. However, a disadvantage of these PD animal models is that they present acute neuroinflammation, contrary to what was observed in PD [178].

Table 4.
Anti-inflammatory effect of GDNF in preclinical trials of Parkinson’s disease and other neurological diseases.
In the AD model, GDNF decreased the microglia-released pro-inflammatory cytokines by reducing the phosphorylation of YAP from the Hippo/YAP pathway [175]. Although the anti-inflammatory effect of GDNF on ALS is not significant, a slight decrease can be seen in the microglial inflammatory marker CD11b, which suggests that GDNF probably acts as an anti-inflammatory molecule [176]. However, different factors, such as an insufficient GDNF concentration or the neuroinflammation severity, could affect GDNF action. Finally, in the MS model, GDNF reduces the amount and size of inflammatory infiltrates in the STR and the extent of NSC astrocytic differentiation, possibly exerting these effects through the activation of myeloid dendritic cells and subsequent restriction of the expansion of T cells [177]. This GDNF effect could also block the peripheral immune cells infiltration to the brain in PD, thus reducing the neuroinflammatory process. We suggest that the decrease in reactive astrocytes could be the result of the reduction in microglial activation, due to the diminish in their release of pro-inflammatory mediators such as IL-1α, TNF-α, and C1q, which were previously mentioned as the factors that trigger the activation of A1 astrocytes (Figure 3). Given that GDNF’s anti-inflammatory properties were only recently described, many questions remain regarding its mechanism of action in different diseases. We hypothesize that the variation in the anti-inflammatory effects of GDNF could be the consequence of the different pathological properties induced by the misfolding of each set of proteins characteristic of each neurodegenerative disease. Nevertheless, the new approach using GDNF to prevent neuroinflammation at early steps in neurodegeneration could be the light at the end of the road to treat some types of neurodegenerative diseases.
5.2. GDNF Anti-Inflammatory Effect in Other Neurological Disease Models
GDNF therapy has been similarly used in other neurological diseases where negative regulation of GDNF precedes nerve damage. The rationale for the use of GDNF is based on the following properties: (i) GDNF promotes the regeneration of sensory axons; (ii) GDNF attenuates neuropathic pain; (iii) GDNF reduces the volume of the cortical infarct; (iv) GDNF promotes neurogenesis; (v) GDNF reduces the damage caused by the inflammatory process [27,184,185,186]. Table 4, b and Figure 3 summarize the anti-inflammatory effects of GDNF in certain neurological diseases and the possible associated molecular mechanisms. Importantly, GDNF therapy can be combined with other neurotrophic factors enhancing their neuroprotective properties by anti-apoptotic and antioxidant activity, and reducing excitotoxicity and the M1 microglial phenotype (with pro-inflammatory properties) at the site of damage [181].
5.3. Anti-Inflammatory Effects of GDNF Therapy on Systemic Disease Models
GDNF anti-inflammatory effects are not exclusive to the nervous system, but GDNF also acts on systemic inflammation. Peripheral GDNF comes from several cellular sources, such as enteric glial cells, plays many physiological roles, and displays beneficial actions on pathological processes in peripheral tissues (Table 4, c). It has been proposed that GDNF could have the following therapeutic effects in systemic pathological conditions [180,182,183,187,188]: (a) cell survival and restoration of the epithelial barrier reducing its permeability; (b) immunoregulation through the decrease in the expression of the transcription factor NF-κB; (c) reduction of oxidative stress; (d) attenuation of programmed cell death, including apoptosis and autophagy; and (e) decrease in the inflammatory response.
The latter anti-inflammatory property discussed widely in this review has been associated with the decrease in the level of several pro-inflammatory molecules (mainly IL-1β and TNF-α) and infiltration of immune cells [182,183]. Furthermore, GDNF can promote the phenotypic transformation of M1 macrophages to the M2 repair phenotype, modifying the expression of TNF-α and iNOS and favoring the expression of anti-inflammatory molecules such as IL-10 and IL-4, cyclooxygenase 2 (COX- 2), and TGF-β1 [182]. Therefore, it could be assumed that this transformation from M1 to M2 could also occur in microglia cells and even in astrocytes, promoting anti-inflammatory and neurogenerative effects after GDNF therapy in PD.
6. Proof of Principle of GDNF in Clinical Trials for Parkinson’s Disease
In preclinical models, GDNF has been shown to protect and restore mature DA neurons in rodent (Table 2, a) and non-human primate (Table 2, b) PD models. Generally, initial outcomes of clinical trials indicate that GDNF therapy could be of value in PD when the therapeutic agent was injected into the putamen rather than the cerebral ventricles [135,189,190].
However, subsequent double-blind placebo-controlled trials, the most recent report being in 2019, concluded that treatment with GDNF was ineffective for PD. As a result, there has been uncertainty about whether GDNF (and related GDNF family neurotrophic factors) could serve to treat PD [35]. Many studies explored the efficacy of direct infusion of GDNF into the putamen, and while open-label studies were promising, a non-randomized study did not meet its primary endpoint [135,189]. Limitations intrinsic to direct putaminal delivery of GDNF or the vector containing the coding sequence for GDNF could be potential reasons for failure. Improved vectors for gene delivery of GDNF are considered as a potentially efficient alternative.
Table 5 summarizes current GDNF clinical trials for PD according to the clinical trials website (ClinicalTrials.gov, accessed on 29 August 2021) and publications available in the literature. Likewise, the table content emphasizes the clinical phase, the characteristics of the study, and the outcome measures or/and preliminary results. The last aspect is associated with adverse effects, safety, tolerability, analysis of laboratory studies (serum and cerebrospinal fluid; CSF), and cabinet. In most cases, only aspects related to clinical and imaging improvement were considered, and no markers were associated with neuroinflammation and/or α-syn, which could result in a critical bias when considering the efficacy of treatment.

Table 5.
GDNF current clinical trials for Parkinson disease, based on NIH ClinicalTrials.gov (accessed on 30 August 2021).
Although the strong evidence for the neuroprotective and neurodegenerative effects for GDNF was demonstrated in experimental animals and in vitro, neurorestorative effects were not observed when treating PD patients. As discussed above, we believe that the therapeutic mechanism of action of GDNF has not been sufficiently defined. Alternatively, the degenerating PD brain may be resistant to the neuroprotective potential of this neurotrophic factor. It has been hypothesized that the failure of GDNF in clinical trials may be due to the disruption in the GDNF/RET/Nurr1 signaling pathway [172]. Although this mechanism is supported by findings in experimental animals [172], others refute such a hypothesis [19]. These controversial results suggest that there are other tactical variables not inherent to the disease or resistance to GDNF that should also be taken into account in the treatment of PD patients; for instance, (i) optimal timing of drug administration during the natural course of the disease; (ii) route of administration; (iii) anatomical site of administration; (iv) administration period; (v) type of associated therapies in addition to GDNF treatment, and (vi) delivery system efficiency.
7. Conclusions and Perspectives
Despite promising results in preclinical assays, the clinical trials have so far shown disappointing results. It remains to be determined whether the lack of success in PD clinical trials reflects a lack of biological effect of the intervention, limitations of the methods of administration used (e.g., insufficient coverage of the putamen area, right dosage, effective delivery approaches), or an inherent inefficacy of the treatment at an advanced stage of the disease. In the latter case, the possibility of administering GDNF in presymptomatic or early stages of PD should be considered.
The use of GDNF in PD treatment has been mainly supported by preclinical studies in 6-OHDA and MPTP models that neither generate pathological α-syn aggregates, nor chronic neuroinflammation with the participation of neurotoxic reactive astrocytes A1. Therefore, previous GDNF therapy schemes in clinical trials were based on unsuitable preclinical models. However, some preclinical studies have shown that GDNF decreases pathological α-syn and neuroinflammation in transgenic α-syn animal models through mechanisms that remain unclear. Therefore, anti-α-syn aggregation and anti-inflammatory activity are likely effects of GDNF. As α-syn aggregates and neuroinflammation play an important role in the development of PD, we propose that GDNF could counteract these two pathological marks and help to delay or stop the progression of the disease.
Taking into account these results in animal models and Braak’s hypothesis of the prion-like spreading of α-syn, we suggest that GDNF treatment should be applied in the early stages of PD and at neuroanatomical sites where α-synucleinopathy begins. In future studies, it is important to use animal models that develop the four pathognomonic hallmarks of PD to elucidate the therapeutic mechanisms of GDNF. In this regard, the BSSG model seems suitable for evaluating new therapeutic approaches of GDNF in presymptomatic or symptomatic stages of the disease.
Finally, it is important that the clinical trials evaluate biomarkers of α-synucleinopathy and neuroinflammation in serum, CSF, and neuroimaging studies of PD patients to determine the effectiveness of GDNF therapy. The analysis and understanding of these markers will be useful to decide changes in dose and duration with GDNF-based therapy, as well as to determine both the ideal time to start and the endpoint of treatment. Applying these new considerations to GDNF therapy in future clinical trials could lead to favorable results.
Author Contributions
K.M.D.-M., D.M.-F., and L.O.S.-R. contributed to the manuscript’s conceptualization, writing, review, and editing. M.E.G.-C., C.B., V.M.B.-A. and M.-d.-C.C.-A. contributed to the writing and review of the manuscript. I.A.M.-D., J.L.-M. and M.P.-H. contributed to the review and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by UNAM-PAPIIT (IA202021 and IA210620) and Fondo Nacional de Ciencia y Tecnologia, FONDOCyT, from the Ministry of Higher Education, Science and Technology, Dominican Republic (2018-2019-2A3-208 to JL-M and MP-H).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
K.M.D.-M. was a recipient of a master’s fellowship from CONACYT.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chmielarz, P.; Saarma, M. Neurotrophic factors for disease-modifying treatments of Parkinson’s disease: Gaps between basic science and clinical studies. Pharmacol. Rep. 2020, 72, 1195–1217. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A. The burden of Parkinson’s disease: A worldwide perspective. Lancet Neurol. 2018, 17, 928–929. [Google Scholar] [CrossRef] [Green Version]
- Collaborators, G.B.D.P.s.D. Global, regional, and national burden of Parkinson’s disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Parkinson, J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 223–236, discussion 222. [Google Scholar] [CrossRef]
- Ziemssen, T.; Reichmann, H. Non-motor dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, 323–332. [Google Scholar] [CrossRef]
- Mahul-Mellier, A.L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply alpha-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [Green Version]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The Synucleinopathies: Twenty Years On. J. Parkinsons Dis. 2017, 7, S51–S69. [Google Scholar] [CrossRef] [Green Version]
- Sulzer, D.; Edwards, R.H. The physiological role of alpha-synuclein and its relationship to Parkinson’s Disease. J. Neurochem. 2019, 150, 475–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanis, L. Alpha-Synuclein in Parkinson’s disease. Cold Spring Harb0 Perspect Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [Green Version]
- Surguchov, A. Intracellular Dynamics of Synucleins: “Here, There and Everywhere”. Int. Rev. Cell. Mol. Biol. 2015, 320, 103–169. [Google Scholar] [CrossRef]
- Cascella, R.; Chen, S.W.; Bigi, A.; Camino, J.D.; Xu, C.K.; Dobson, C.M.; Chiti, F.; Cremades, N.; Cecchi, C. The release of toxic oligomers from alpha-synuclein fibrils induces dysfunction in neuronal cells. Nat. Commun. 2021, 12, 1814. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, C.; Lee, S.J. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid. Med. Cell Longev. 2010, 3, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef]
- Surguchev, A.A.; Emamzadeh, F.N.; Surguchov, A. Cell Responses to Extracellular alpha-Synuclein. Molecules 2019, 24, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.A.; Romero-Ramos, M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell. Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Ho, D.H.; Suk, J.E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.J.; et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013, 4, 1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanova, N.; Fellner, L.; Reindl, M.; Masliah, E.; Poewe, W.; Wenning, G.K. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 2011, 179, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Qin, L.; Wang, L.; Tan, J.; Zhang, H.; Tang, J.; Shen, X.; Tan, L.; Wang, C. alphasynuclein induces apoptosis of astrocytes by causing dysfunction of the endoplasmic reticulumGolgi compartment. Mol. Med. Rep. 2018, 18, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Kordower, J.H. GDNF signaling in subjects with minimal motor deficits and Parkinson’s disease. Neurobiol. Dis. 2021, 153, 105298. [Google Scholar] [CrossRef] [PubMed]
- Flores-Martinez, Y.M.; Fernandez-Parrilla, M.A.; Ayala-Davila, J.; Reyes-Corona, D.; Blanco-Alvarez, V.M.; Soto-Rojas, L.O.; Luna-Herrera, C.; Gonzalez-Barrios, J.A.; Leon-Chavez, B.A.; Gutierrez-Castillo, M.E.; et al. Acute Neuroinflammatory Response in the Substantia Nigra Pars Compacta of Rats after a Local Injection of Lipopolysaccharide. J. Immunol. Res. 2018, 2018, 1838921. [Google Scholar] [CrossRef]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef] [Green Version]
- Brochard, V.; Combadiere, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.; Brundin, P. Prion-like propagation of pathology in Parkinson disease. Handb. Clin. Neurol. 2018, 153, 321–335. [Google Scholar] [CrossRef]
- Fahn, S.; Oakes, D.; Shoulson, I.; Kieburtz, K.; Rudolph, A.; Lang, A.; Olanow, C.W.; Tanner, C.; Marek, K.; Parkinson Study, G. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004, 351, 2498–2508. [Google Scholar] [CrossRef]
- Chmielarz, P.; Er, S.; Konovalova, J.; Bandres, L.; Hlushchuk, I.; Albert, K.; Panhelainen, A.; Luk, K.; Airavaara, M.; Domanskyi, A. GDNF/RET Signaling Pathway Activation Eliminates Lewy Body Pathology in Midbrain Dopamine Neurons. Mov. Disord. 2020, 35, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lin, Y.C.; Huang, C.Y.; Wu, S.R.; Chen, C.M.; Liu, H.L. Ultrasound-responsive neurotrophic factor-loaded microbubble- liposome complex: Preclinical investigation for Parkinson’s disease treatment. J. Control. Release 2020, 321, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Haney, M.J.; Jin, Y.S.; Uvarov, O.; Vinod, N.; Lee, Y.Z.; Langworthy, B.; Fine, J.P.; Rodriguez, M.; El-Hage, N.; et al. GDNF-expressing macrophages restore motor functions at a severe late-stage, and produce long-term neuroprotective effects at an early-stage of Parkinson’s disease in transgenic Parkin Q311X(A) mice. J. Control. Release 2019, 315, 139–149. [Google Scholar] [CrossRef]
- Hernando, S.; Herran, E.; Figueiro-Silva, J.; Pedraz, J.L.; Igartua, M.; Carro, E.; Hernandez, R.M. Intranasal Administration of TAT-Conjugated Lipid Nanocarriers Loading GDNF for Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 145–155. [Google Scholar] [CrossRef]
- Zhao, Y.; Haney, M.J.; Gupta, R.; Bohnsack, J.P.; He, Z.; Kabanov, A.V.; Batrakova, E.V. GDNF-transfected macrophages produce potent neuroprotective effects in Parkinson’s disease mouse model. PLoS ONE 2014, 9, e106867. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, G.; Yang, X.; Zhang, Z.; Shi, H.; Zu, J.; Hua, F.; Shen, X. Intracerebral administration of ultrasound-induced dissolution of lipid-coated GDNF microbubbles provides neuroprotection in a rat model of Parkinson’s disease. Brain Res. Bull. 2014, 103, 60–65. [Google Scholar] [CrossRef]
- Rickert, U.; Grampp, S.; Wilms, H.; Spreu, J.; Knerlich-Lukoschus, F.; Held-Feindt, J.; Lucius, R. Glial Cell Line-Derived Neurotrophic Factor Family Members Reduce Microglial Activation via Inhibiting p38MAPKs-Mediated Inflammatory Responses. J. Neurodegener. Dis. 2014, 2014, 369468. [Google Scholar] [CrossRef] [PubMed]
- Littrell, O.M.; Granholm, A.C.; Gerhardt, G.A.; Boger, H.A. Glial cell-line derived neurotrophic factor (GDNF) replacement attenuates motor impairments and nigrostriatal dopamine deficits in 12-month-old mice with a partial deletion of GDNF. Pharmacol. Biochem. Behav. 2013, 104, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Rocha, S.M.; Cristovao, A.C.; Campos, F.L.; Fonseca, C.P.; Baltazar, G. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol. Dis. 2012, 47, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Barker, R.A.; Bjorklund, A.; Gash, D.M.; Whone, A.; Van Laar, A.; Kordower, J.H.; Bankiewicz, K.; Kieburtz, K.; Saarma, M.; Booms, S.; et al. GDNF and Parkinson’s Disease: Where Next? A Summary from a Recent Workshop. J. Parkinsons Dis. 2020, 10, 875–891. [Google Scholar] [CrossRef]
- Nutt, J.G.; Burchiel, K.J.; Comella, C.L.; Jankovic, J.; Lang, A.E.; Laws, E.R., Jr.; Lozano, A.M.; Penn, R.D.; Simpson, R.K., Jr.; Stacy, M.; et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003, 60, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Burre, J.; Sharma, M.; Sudhof, T.C. Cell Biology and Pathophysiology of alpha-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Tanji, K.; Yoshimoto, M.; Takahashi, H.; Wakabayashi, K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp. Neurol. 2002, 176, 98–104. [Google Scholar] [CrossRef]
- Richter-Landsberg, C.; Gorath, M.; Trojanowski, J.Q.; Lee, V.M. alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J. Neurosci. Res. 2000, 62, 9–14. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of alpha-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Emamzadeh, F.N. Alpha-synuclein structure, functions, and interactions. J. Res. Med. Sci. 2016, 21, 29. [Google Scholar] [CrossRef]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease—Lessons and emerging principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, N.; Arcos-Lopez, T.; Konig, A.; Quintanar, L.; Menacho Marquez, M.; Outeiro, T.F.; Fernandez, C.O. Effects of alpha-synuclein post-translational modifications on metal binding. J. Neurochem. 2019, 150, 507–521. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhao, C.; Li, D.; Tian, Z.; Lai, Y.; Diao, J.; Liu, C. Versatile Structures of alpha-Synuclein. Front. Mol. Neurosci. 2016, 9, 48. [Google Scholar] [CrossRef]
- Twohig, D.; Nielsen, H.M. alpha-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runfola, M.; De Simone, A.; Vendruscolo, M.; Dobson, C.M.; Fusco, G. The N-terminal Acetylation of alpha-Synuclein Changes the Affinity for Lipid Membranes but not the Structural Properties of the Bound State. Sci. Rep. 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carta, A.R.; Boi, L.; Pisanu, A.; Palmas, M.F.; Carboni, E.; De Simone, A. Advances in modelling alpha-synuclein-induced Parkinson’s diseases in rodents: Virus-based models versus inoculation of exogenous preformed toxic species. J. Neurosci. Methods 2020, 338, 108685. [Google Scholar] [CrossRef]
- Li, X.; Dong, C.; Hoffmann, M.; Garen, C.R.; Cortez, L.M.; Petersen, N.O.; Woodside, M.T. Early stages of aggregation of engineered alpha-synuclein monomers and oligomers in solution. Sci. Rep. 2019, 9, 1734. [Google Scholar] [CrossRef] [PubMed]
- Killinger, B.A.; Melki, R.; Brundin, P.; Kordower, J.H. Endogenous alpha-synuclein monomers, oligomers and resulting pathology: Let’s talk about the lipids in the room. NPJ Parkinsons Dis. 2019, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Bousset, L.; Melki, R.; Otzen, D.E. alpha-synuclein oligomers and fibrils: A spectrum of species, a spectrum of toxicities. J. Neurochem. 2019, 150, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Bridi, J.C.; Hirth, F. Mechanisms of alpha-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Vinueza-Gavilanes, R.; Inigo-Marco, I.; Larrea, L.; Lasa, M.; Carte, B.; Santamaria, E.; Fernandez-Irigoyen, J.; Bugallo, R.; Aragon, T.; Aldabe, R.; et al. N-terminal acetylation mutants affect alpha-synuclein stability, protein levels and neuronal toxicity. Neurobiol. Dis. 2020, 137, 104781. [Google Scholar] [CrossRef]
- Ma, M.R.; Hu, Z.W.; Zhao, Y.F.; Chen, Y.X.; Li, Y.M. Phosphorylation induces distinct alpha-synuclein strain formation. Sci. Rep. 2016, 6, 37130. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, U. Rationally Designed Variants of alpha-Synuclein Illuminate Its in vivo Structural Properties in Health and Disease. Front. Neurosci. 2018, 12, 623. [Google Scholar] [CrossRef]
- Villar-Pique, A.; Lopes da Fonseca, T.; Outeiro, T.F. Structure, function and toxicity of alpha-synuclein: The Bermuda triangle in synucleinopathies. J. Neurochem. 2016, 139 (Suppl. 1), 240–255. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, T.A.; Baker, K.; Brooker, H.R.; Frank, S.; Mulvihill, D.P. An enhanced recombinant amino-terminal acetylation system and novel in vivo high-throughput screen for molecules affecting alpha-synuclein oligomerisation. FEBS Lett. 2017, 591, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavroeidi, P.; Xilouri, M. Neurons and Glia Interplay in alpha-Synucleinopathies. Int. J. Mol. Sci. 2021, 22, 4994. [Google Scholar] [CrossRef]
- Du, X.Y.; Xie, X.X.; Liu, R.T. The Role of alpha-Synuclein Oligomers in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8645. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Bratzke, H.; Hamm-Clement, J.; Sandmann-Keil, D.; Rub, U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J. Neurol. 2002, 249 (Suppl. 3), iii1–iii5. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, G.; Han, C.; Ma, K.; Guo, X.; Wan, F.; Kou, L.; Yin, S.; Liu, L.; Huang, J.; et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, Z.A.; Vijayaraghavan, N.; Gorion, K.M.; Riffe, C.J.; Strang, K.H.; Caldwell, J.; Giasson, B.I. Physiological C-terminal truncation of alpha-synuclein potentiates the prion-like formation of pathological inclusions. J. Biol. Chem. 2018, 293, 18914–18932. [Google Scholar] [CrossRef] [Green Version]
- Visanji, N.P.; Brooks, P.L.; Hazrati, L.N.; Lang, A.E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Heras-Garvin, A.; Stefanova, N. From Synaptic Protein to Prion: The Long and Controversial Journey of alpha-Synuclein. Front. Synaptic Neurosci. 2020, 12, 584536. [Google Scholar] [CrossRef]
- Izco, M.; Blesa, J.; Verona, G.; Cooper, J.M.; Alvarez-Erviti, L. Glial activation precedes alpha-synuclein pathology in a mouse model of Parkinson’s disease. Neurosci. Res. 2021, 170, 330–340. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [Green Version]
- Badanjak, K.; Fixemer, S.; Smajic, S.; Skupin, A.; Grunewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef] [PubMed]
- Doorn, K.J.; Moors, T.; Drukarch, B.; van de Berg, W.; Lucassen, P.J.; van Dam, A.M. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol. Commun. 2014, 2, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliday, G.M.; Stevens, C.H. Glia: Initiators and progressors of pathology in Parkinson’s disease. Mov. Disord. 2011, 26, 6–17. [Google Scholar] [CrossRef]
- Marques, O.; Outeiro, T.F. Alpha-synuclein: From secretion to dysfunction and death. Cell Death Dis. 2012, 3, e350. [Google Scholar] [CrossRef]
- Lee, S.B.; Park, S.M.; Ahn, K.J.; Chung, K.C.; Paik, S.R.; Kim, J. Identification of the amino acid sequence motif of alpha-synuclein responsible for macrophage activation. Biochem. Biophys. Res. Commun. 2009, 381, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Maguire-Zeiss, K.A.; Giuliano, R.; Prifti, L.; Venkatesh, K.; Federoff, H.J. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging 2008, 29, 1690–1701. [Google Scholar] [CrossRef] [Green Version]
- Couch, Y.; Alvarez-Erviti, L.; Sibson, N.R.; Wood, M.J.; Anthony, D.C. The acute inflammatory response to intranigral alpha-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J. Neuroinflammation 2011, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Suk, J.E.; Patrick, C.; Bae, E.J.; Cho, J.H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, A.D.; Stone, D.K.; Mosley, R.L.; Gendelman, H.E. Nitrated {alpha}-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J. Immunol. 2009, 182, 4137–4149. [Google Scholar] [CrossRef] [Green Version]
- Benner, E.J.; Banerjee, R.; Reynolds, A.D.; Sherman, S.; Pisarev, V.M.; Tsiperson, V.; Nemachek, C.; Ciborowski, P.; Przedborski, S.; Mosley, R.L.; et al. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE 2008, 3, e1376. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.R.; Federoff, H.J. Targeting Microglial Activation States as a Therapeutic Avenue in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 176. [Google Scholar] [CrossRef]
- Kim, S.; Cho, S.H.; Kim, K.Y.; Shin, K.Y.; Kim, H.S.; Park, C.H.; Chang, K.A.; Lee, S.H.; Cho, D.; Suh, Y.H. Alpha-synuclein induces migration of BV-2 microglial cells by up-regulation of CD44 and MT1-MMP. J. Neurochem. 2009, 109, 1483–1496. [Google Scholar] [CrossRef]
- Wang, S.; Chu, C.H.; Stewart, T.; Ginghina, C.; Wang, Y.; Nie, H.; Guo, M.; Wilson, B.; Hong, J.S.; Zhang, J. alpha-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E1926–E1935. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [Green Version]
- Gordon, R.; Albornoz, E.A.; Christie, D.C.; Langley, M.R.; Kumar, V.; Mantovani, S.; Robertson, A.A.B.; Butler, M.S.; Rowe, D.B.; O’Neill, L.A.; et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018, 10, aah4066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, A.F.; Varanita, T.; Herrebout, M.A.C.; Plug, B.C.; Kole, J.; Musters, R.J.P.; Teunissen, C.E.; Hoozemans, J.J.M.; Bubacco, L.; Veerhuis, R. alpha-Synuclein evokes NLRP3 inflammasome-mediated IL-1beta secretion from primary human microglia. Glia 2021, 69, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.; Wu, H.; Kagan, J.C. Gasdermin D activity in inflammation and host defense. Sci. Immunol. 2019, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Wang, X.; Chi, J.; Huang, D.; Ding, L.; Zhao, X.; Jiang, L.; Yu, Y.; Gao, F. alpha-synuclein promotes progression of Parkinson’s disease by upregulating autophagy signaling pathway to activate NLRP3 inflammasome. Exp. Ther. Med. 2020, 19, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzamko, N.; Gysbers, A.; Perera, G.; Bahar, A.; Shankar, A.; Gao, J.; Fu, Y.; Halliday, G.M. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017, 133, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Rannikko, E.H.; Weber, S.S.; Kahle, P.J. Exogenous alpha-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015, 16, 57. [Google Scholar] [CrossRef] [Green Version]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 2013, 61, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Chavarria, C.; Rodriguez-Bottero, S.; Quijano, C.; Cassina, P.; Souza, J.M. Impact of monomeric, oligomeric and fibrillar alpha-synuclein on astrocyte reactivity and toxicity to neurons. Biochem. J. 2018, 475, 3153–3169. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Standaert, D.G.; Harms, A.S. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J. Neuroinflamm. 2012, 9, 259. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Theodore, S.; Standaert, D.G. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol. Neurodegener. 2010, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Bao, X.; Zang, C.; Yang, H.; Sun, F.; Che, Y.; Wu, X.; Li, S.; Zhang, D.; Wang, Q. Integrin CD11b mediates alpha-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018, 14, 600–608. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.S.; et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef]
- Jin, J.; Shie, F.S.; Liu, J.; Wang, Y.; Davis, J.; Schantz, A.M.; Montine, K.S.; Montine, T.J.; Zhang, J. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J. Neuroinflammation 2007, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.U.; Woodling, N.S.; Wang, Q.; Panchal, M.; Liang, X.; Trueba-Saiz, A.; Brown, H.D.; Mhatre, S.D.; Loui, T.; Andreasson, K.I. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J. Clin. Investig. 2015, 125, 350–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Hoekstra, J.; Heng, X.; Kang, W.; Ding, J.; Liu, J.; Chen, S.; Zhang, J. P2X7 receptor is critical in alpha-synuclein–mediated microglial NADPH oxidase activation. Neurobiol. Aging 2015, 36, 2304–2318. [Google Scholar] [CrossRef]
- Russ, K.; Teku, G.; Bousset, L.; Redeker, V.; Piel, S.; Savchenko, E.; Pomeshchik, Y.; Savistchenko, J.; Stummann, T.C.; Azevedo, C.; et al. TNF-alpha and alpha-synuclein fibrils differently regulate human astrocyte immune reactivity and impair mitochondrial respiration. Cell Rep. 2021, 34, 108895. [Google Scholar] [CrossRef]
- Lindstrom, V.; Gustafsson, G.; Sanders, L.H.; Howlett, E.H.; Sigvardson, J.; Kasrayan, A.; Ingelsson, M.; Bergstrom, J.; Erlandsson, A. Extensive uptake of alpha-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol. Cell. Neurosci. 2017, 82, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Yamauchi, A.; Kataoka, Y. Monomeric alpha-synuclein induces blood-brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc. Res. 2019, 124, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Marxreiter, F.; Krach, F.; Fadler, T.; Grosch, J.; Maroni, M.; Graef, D.; Eberhardt, E.; Riemenschneider, M.J.; Yeo, G.W.; et al. Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease. Cell Stem Cell 2018, 23, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbarayan, M.S.; Hudson, C.; Moss, L.D.; Nash, K.R.; Bickford, P.C. T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an alpha-synuclein rat model of Parkinson’s disease. J. Neuroinflamm. 2020, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Kustrimovic, N.; Comi, C.; Magistrelli, L.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Minafra, B.; Riboldazzi, G.; et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J. Neuroinflamm. 2018, 15, 205. [Google Scholar] [CrossRef]
- Kotzbauer, P.T.; Lampe, P.A.; Heuckeroth, R.O.; Golden, J.P.; Creedon, D.J.; Johnson, E.M., Jr.; Milbrandt, J. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature 1996, 384, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Cintron-Colon, A.F.; Almeida-Alves, G.; Boynton, A.M.; Spitsbergen, J.M. GDNF synthesis, signaling, and retrograde transport in motor neurons. Cell Tissue Res. 2020, 382, 47–56. [Google Scholar] [CrossRef]
- Parkash, V.; Leppanen, V.M.; Virtanen, H.; Jurvansuu, J.M.; Bespalov, M.M.; Sidorova, Y.A.; Runeberg-Roos, P.; Saarma, M.; Goldman, A. The structure of the glial cell line-derived neurotrophic factor-coreceptor complex: Insights into RET signaling and heparin binding. J. Biol. Chem. 2008, 283, 35164–35172. [Google Scholar] [CrossRef] [Green Version]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef]
- Paratcha, G.; Ledda, F.; Ibanez, C.F. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 2003, 113, 867–879. [Google Scholar] [CrossRef] [Green Version]
- Kawai, K.; Takahashi, M. Intracellular RET signaling pathways activated by GDNF. Cell Tissue Res. 2020, 382, 113–123. [Google Scholar] [CrossRef]
- Coulpier, M.; Anders, J.; Ibanez, C.F. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: Importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J. Biol. Chem. 2002, 277, 1991–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bespalov, M.M.; Sidorova, Y.A.; Tumova, S.; Ahonen-Bishopp, A.; Magalhaes, A.C.; Kulesskiy, E.; Paveliev, M.; Rivera, C.; Rauvala, H.; Saarma, M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J. Cell Biol. 2011, 192, 153–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The GTEx Portal. Available online: https://gtexportal.org/home/gene/GDNF (accessed on 29 August 2021).
- Hellmich, H.L.; Kos, L.; Cho, E.S.; Mahon, K.A.; Zimmer, A. Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech. Dev. 1996, 54, 95–105. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000168621-GDNF/tissue (accessed on 29 August 2021).
- Mogi, M.; Togari, A.; Kondo, T.; Mizuno, Y.; Kogure, O.; Kuno, S.; Ichinose, H.; Nagatsu, T. Glial cell line-derived neurotrophic factor in the substantia nigra from control and parkinsonian brains. Neurosci. Lett. 2001, 300, 179–181. [Google Scholar] [CrossRef]
- Michos, O.; Cebrian, C.; Hyink, D.; Grieshammer, U.; Williams, L.; D’Agati, V.; Licht, J.D.; Martin, G.R.; Costantini, F. Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet 2010, 6, e1000809. [Google Scholar] [CrossRef] [Green Version]
- Gianino, S.; Grider, J.R.; Cresswell, J.; Enomoto, H.; Heuckeroth, R.O. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 2003, 130, 2187–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enomoto, H.; Heuckeroth, R.O.; Golden, J.P.; Johnson, E.M.; Milbrandt, J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development 2000, 127, 4877–4889. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B.; Siegel, G.J.; Lee, J.M. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J. Chem. Neuroanat. 2001, 21, 277–288. [Google Scholar] [CrossRef]
- Virachit, S.; Mathews, K.J.; Cottam, V.; Werry, E.; Galli, E.; Rappou, E.; Lindholm, P.; Saarma, M.; Halliday, G.M.; Shannon Weickert, C.; et al. Levels of glial cell line-derived neurotrophic factor are decreased, but fibroblast growth factor 2 and cerebral dopamine neurotrophic factor are increased in the hippocampus in Parkinson’s disease. Brain Pathol. 2019, 29, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Griego, E.; Herrera-Lopez, G.; Gomez-Lira, G.; Barrionuevo, G.; Gutierrez, R.; Galvan, E.J. Functional expression of TrkB receptors on interneurones and pyramidal cells of area CA3 of the rat hippocampus. Neuropharmacology 2021, 182, 108379. [Google Scholar] [CrossRef]
- Backman, C.M.; Shan, L.; Zhang, Y.J.; Hoffer, B.J.; Leonard, S.; Troncoso, J.C.; Vonsatel, P.; Tomac, A.C. Gene expression patterns for GDNF and its receptors in the human putamen affected by Parkinson’s disease: A real-time PCR study. Mol. Cell. Endocrinol. 2006, 252, 160–166. [Google Scholar] [CrossRef]
- Su, X.; Fischer, D.L.; Li, X.; Bankiewicz, K.; Sortwell, C.E.; Federoff, H.J. Alpha-Synuclein mRNA Is Not Increased in Sporadic PD and Alpha-Synuclein Accumulation Does Not Block GDNF Signaling in Parkinson’s Disease and Disease Models. Mol. Ther. 2017, 25, 2231–2235. [Google Scholar] [CrossRef] [Green Version]
- Migliore, M.M.; Ortiz, R.; Dye, S.; Campbell, R.B.; Amiji, M.M.; Waszczak, B.L. Neurotrophic and neuroprotective efficacy of intranasal GDNF in a rat model of Parkinson’s disease. Neuroscience 2014, 274, 11–23. [Google Scholar] [CrossRef]
- Kordower, J.H.; Bjorklund, A. Trophic factor gene therapy for Parkinson’s disease. Mov. Disord. 2013, 28, 96–109. [Google Scholar] [CrossRef]
- Sullivan, A.M.; Toulouse, A. Neurotrophic factors for the treatment of Parkinson’s disease. Cytokine Growth Factor Rev. 2011, 22, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, F.; Luan, L.; Ai, Y.; Walton, A.; Gerhardt, G.A.; Gash, D.M.; Grondin, R.; Zhang, Z. Development of a stable, early stage unilateral model of Parkinson’s disease in middle-aged rhesus monkeys. Exp. Neurol. 2008, 212, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Grondin, R.; Zhang, Z.; Yi, A.; Cass, W.A.; Maswood, N.; Andersen, A.H.; Elsberry, D.D.; Klein, M.C.; Gerhardt, G.A.; Gash, D.M. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain 2002, 125, 2191–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996, 380, 252–255. [Google Scholar] [CrossRef]
- Hirsch, E.C. Animal models in neurodegenerative diseases. J. Neural. Transm. Suppl. 2007, 72, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Luz, M.; Whone, A.; Bassani, N.; Wyse, R.K.; Stebbins, G.T.; Mohr, E. The Parkinson’s Disease Comprehensive Response (PDCORE): A composite approach integrating three standard outcome measures. Brain Commun. 2020, 2, fcaa046. [Google Scholar] [CrossRef]
- Sidorova, Y.A.; Saarma, M. Can Growth Factors Cure Parkinson’s Disease? Trends Pharmacol. Sci. 2020, 41, 909–922. [Google Scholar] [CrossRef]
- Huttunen, H.J.; Saarma, M. CDNF Protein Therapy in Parkinson’s Disease. Cell Transplant. 2019, 28, 349–366. [Google Scholar] [CrossRef] [Green Version]
- Whone, A.; Luz, M.; Boca, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 2019, 142, 512–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidorova, Y.A.; Volcho, K.P.; Salakhutdinov, N.F. Neuroregeneration in Parkinson’s Disease: From Proteins to Small Molecules. Curr. Neuropharmacol. 2019, 17, 268–287. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Ai, Y.; Fischer, B.; Zhang, A.M.; Grondin, R.C.; Zhang, Z.; Gerhardt, G.A.; Gash, D.M. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp. Neurol. 2006, 202, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Kearns, C.M.; Cass, W.A.; Smoot, K.; Kryscio, R.; Gash, D.M. GDNF protection against 6-OHDA: Time dependence and requirement for protein synthesis. J. Neurosci. 1997, 17, 7111–7118. [Google Scholar] [CrossRef]
- Kirik, D.; Rosenblad, C.; Bjorklund, A. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur. J. Neurosci. 2000, 12, 3871–3882. [Google Scholar] [CrossRef]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Van Kampen, J.M.; Robertson, H.A. The BSSG rat model of Parkinson’s disease: Progressing towards a valid, predictive model of disease. EPMA J. 2017, 8, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Soto-Rojas, L.O.; Garces-Ramirez, L.; Luna-Herrera, C.; Flores-Martinez, Y.M.; Soto-Rodriguez, G.; Gatica-Garcia, B.; Lopez-Salas, F.E.; Ayala-Davila, J.; Gutierrez-Castillo, M.E.; Padilla-Viveros, A.; et al. A single intranigral administration of beta-sitosterol beta-d-glucoside elicits bilateral sensorimotor and non-motor alterations in the rat. Behav. Brain Res. 2020, 378, 112279. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rojas, L.O.; Martinez-Davila, I.A.; Luna-Herrera, C.; Gutierrez-Castillo, M.E.; Lopez-Salas, F.E.; Gatica-Garcia, B.; Soto-Rodriguez, G.; Bringas Tobon, M.E.; Flores, G.; Padilla-Viveros, A.; et al. Unilateral intranigral administration of beta-sitosterol beta-D-glucoside triggers pathological alpha-synuclein spreading and bilateral nigrostriatal dopaminergic neurodegeneration in the rat. Acta Neuropathol. Commun. 2020, 8, 56. [Google Scholar] [CrossRef]
- Luna-Herrera, C.; Martinez-Davila, I.A.; Soto-Rojas, L.O.; Flores-Martinez, Y.M.; Fernandez-Parrilla, M.A.; Ayala-Davila, J.; Leon-Chavez, B.A.; Soto-Rodriguez, G.; Blanco-Alvarez, V.M.; Lopez-Salas, F.E.; et al. Intranigral Administration of beta-Sitosterol-beta-D-Glucoside Elicits Neurotoxic A1 Astrocyte Reactivity and Chronic Neuroinflammation in the Rat Substantia Nigra. J. Immunol. Res. 2020, 2020, 5907591. [Google Scholar] [CrossRef]
- Gash, D.M.; Gerhardt, G.A.; Bradley, L.H.; Wagner, R.; Slevin, J.T. GDNF clinical trials for Parkinson’s disease: A critical human dimension. Cell Tissue Res. 2020, 382, 65–70. [Google Scholar] [CrossRef]
- Yasuhara, T.; Kameda, M.; Agari, T.; Date, I. Regenerative medicine for Parkinson’s disease. Neurol. Med. Chir. 2015, 55, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Lapchak, P.A.; Jiao, S.; Collins, F.; Miller, P.J. Glial cell line-derived neurotrophic factor: Distribution and pharmacology in the rat following a bolus intraventricular injection. Brain Res. 1997, 747, 92–102. [Google Scholar] [CrossRef]
- Martin, D.; Miller, G.; Fischer, N.; Diz, D.; Cullen, T.; Russell, D. Glial cell line-derived neurotrophic factor: The lateral cerebral ventricle as a site of administration for stimulation of the substantia nigra dopamine system in rats. Eur. J. Neurosci. 1996, 8, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Bowenkamp, K.E.; Lapchak, P.A.; Hoffer, B.J.; Miller, P.J.; Bickford, P.C. Intracerebroventricular glial cell line-derived neurotrophic factor improves motor function and supports nigrostriatal dopamine neurons in bilaterally 6-hydroxydopamine lesioned rats. Exp. Neurol. 1997, 145, 104–117. [Google Scholar] [CrossRef]
- Sullivan, A.M.; Opacka-Juffry, J.; Blunt, S.B. Long-term protection of the rat nigrostriatal dopaminergic system by glial cell line-derived neurotrophic factor against 6-hydroxydopamine in vivo. Eur. J. Neurosci. 1998, 10, 57–63. [Google Scholar] [CrossRef]
- Winkler, C.; Sauer, H.; Lee, C.S.; Bjorklund, A. Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 1996, 16, 7206–7215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Barrios, J.A.; Lindahl, M.; Bannon, M.J.; Anaya-Martinez, V.; Flores, G.; Navarro-Quiroga, I.; Trudeau, L.E.; Aceves, J.; Martinez-Arguelles, D.B.; Garcia-Villegas, R.; et al. Neurotensin polyplex as an efficient carrier for delivering the human GDNF gene into nigral dopamine neurons of hemiparkinsonian rats. Mol. Ther. 2006, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Miller, P.J.; Collins, F.; Jiao, S. Glial cell line-derived neurotrophic factor attenuates behavioural deficits and regulates nigrostriatal dopaminergic and peptidergic markers in 6-hydroxydopamine-lesioned adult rats: Comparison of intraventricular and intranigral delivery. Neuroscience 1997, 78, 61–72. [Google Scholar] [CrossRef]
- Kirik, D.; Georgievska, B.; Rosenblad, C.; Bjorklund, A. Delayed infusion of GDNF promotes recovery of motor function in the partial lesion model of Parkinson’s disease. Eur. J. Neurosci. 2001, 13, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, E.; Montero-Menei, C.N.; Ansorena, E.; Lanciego, J.L.; Aymerich, M.S.; Blanco-Prieto, M.J. Effective GDNF brain delivery using microspheres—A promising strategy for Parkinson’s disease. J. Control. Release 2009, 135, 119–126. [Google Scholar] [CrossRef]
- Fletcher, A.M.; Kowalczyk, T.H.; Padegimas, L.; Cooper, M.J.; Yurek, D.M. Transgene expression in the striatum following intracerebral injections of DNA nanoparticles encoding for human glial cell line-derived neurotrophic factor. Neuroscience 2011, 194, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Yurek, D.M.; Flectcher, A.M.; Kowalczyk, T.H.; Padegimas, L.; Cooper, M.J. Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplant. 2009, 18, 1183–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandoso, L.; Ponce, S.; Manuel, I.; Arrue, A.; Ruiz-Ortega, J.A.; Ulibarri, I.; Orive, G.; Hernandez, R.M.; Rodriguez, A.; Rodriguez-Puertas, R.; et al. Long-term survival of encapsulated GDNF secreting cells implanted within the striatum of parkinsonized rats. Int. J. Pharm. 2007, 343, 69–78. [Google Scholar] [CrossRef]
- Garbayo, E.; Ansorena, E.; Lanciego, J.L.; Blanco-Prieto, M.J.; Aymerich, M.S. Long-term neuroprotection and neurorestoration by glial cell-derived neurotrophic factor microspheres for the treatment of Parkinson’s disease. Mov. Disord. 2011, 26, 1943–1947. [Google Scholar] [CrossRef] [Green Version]
- Yue, P.; Miao, W.; Gao, L.; Zhao, X.; Teng, J. Ultrasound-Triggered Effects of the Microbubbles Coupled to GDNF Plasmid-Loaded PEGylated Liposomes in a Rat Model of Parkinson’s Disease. Front. Neurosci. 2018, 12, 222. [Google Scholar] [CrossRef] [Green Version]
- Iravani, M.M.; Costa, S.; Jackson, M.J.; Tel, B.C.; Cannizzaro, C.; Pearce, R.K.; Jenner, P. GDNF reverses priming for dyskinesia in MPTP-treated, L-DOPA-primed common marmosets. Eur. J. Neurosci. 2001, 13, 597–608. [Google Scholar] [CrossRef]
- Costa, S.; Iravani, M.M.; Pearce, R.K.; Jenner, P. Glial cell line-derived neurotrophic factor concentration dependently improves disability and motor activity in MPTP-treated common marmosets. Eur. J. Pharmacol. 2001, 412, 45–50. [Google Scholar] [CrossRef]
- Gerhardt, G.A.; Cass, W.A.; Huettl, P.; Brock, S.; Zhang, Z.; Gash, D.M. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain. Res. 1999, 817, 163–171. [Google Scholar] [CrossRef]
- Zhang, Z.; Miyoshi, Y.; Lapchak, P.A.; Collins, F.; Hilt, D.; Lebel, C.; Kryscio, R.; Gash, D.M. Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J. Pharmacol. Exp. Ther. 1997, 282, 1396–1401. [Google Scholar] [PubMed]
- Ai, Y.; Markesbery, W.; Zhang, Z.; Grondin, R.; Elseberry, D.; Gerhardt, G.A.; Gash, D.M. Intraputamenal infusion of GDNF in aged rhesus monkeys: Distribution and dopaminergic effects. J. Comp. Neurol. 2003, 461, 250–261. [Google Scholar] [CrossRef]
- Grondin, R.; Cass, W.A.; Zhang, Z.; Stanford, J.A.; Gash, D.M.; Gerhardt, G.A. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J. Neurosci. 2003, 23, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Maswood, N.; Grondin, R.; Zhang, Z.; Stanford, J.A.; Surgener, S.P.; Gash, D.M.; Gerhardt, G.A. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged Rhesus monkeys. Neurobiol. Aging 2002, 23, 881–889. [Google Scholar] [CrossRef]
- Kells, A.P.; Eberling, J.; Su, X.; Pivirotto, P.; Bringas, J.; Hadaczek, P.; Narrow, W.C.; Bowers, W.J.; Federoff, H.J.; Forsayeth, J.; et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J. Neurosci. 2010, 30, 9567–9577. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, C.; Deglon, N.; Pralong, W.; Aebischer, P. Lentiviral nigral delivery of GDNF does not prevent neurodegeneration in a genetic rat model of Parkinson’s disease. Neurobiol. Dis. 2004, 17, 283–289. [Google Scholar] [CrossRef]
- Decressac, M.; Ulusoy, A.; Mattsson, B.; Georgievska, B.; Romero-Ramos, M.; Kirik, D.; Bjorklund, A. GDNF fails to exert neuroprotection in a rat alpha-synuclein model of Parkinson’s disease. Brain 2011, 134, 2302–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decressac, M.; Kadkhodaei, B.; Mattsson, B.; Laguna, A.; Perlmann, T.; Bjorklund, A. alpha-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med. 2012, 4, 163ra156. [Google Scholar] [CrossRef]
- Quintino, L.; Avallone, M.; Brannstrom, E.; Kavanagh, P.; Lockowandt, M.; Garcia Jareno, P.; Breger, L.S.; Lundberg, C. GDNF-mediated rescue of the nigrostriatal system depends on the degree of degeneration. Gene Ther. 2019, 26, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Qing, J.; Liu, X.; Wu, Q.; Zhou, M.; Zhang, Y.; Mazhar, M.; Huang, X.; Wang, L.; He, F. Hippo/YAP Pathway Plays a Critical Role in Effect of GDNF Against Abeta-Induced Inflammation in Microglial Cells. DNA Cell Biol. 2020, 39, 1064–1071. [Google Scholar] [CrossRef]
- Van Dyke, J.M.; Smit-Oistad, I.M.; Macrander, C.; Krakora, D.; Meyer, M.G.; Suzuki, M. Macrophage-mediated inflammation and glial response in the skeletal muscle of a rat model of familial amyotrophic lateral sclerosis (ALS). Exp. Neurol. 2016, 277, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Deng, L.; Wang, Y.; Yin, L.; Yang, C.; Du, J.; Yuan, Q. GDNF Enhances Therapeutic Efficiency of Neural Stem Cells-Based Therapy in Chronic Experimental Allergic Encephalomyelitis in Rat. Stem Cells Int. 2016, 2016, 1431349. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Z.; Chen, A.; Fa, Z.; Ding, Z.; Xie, J.; Sun, Y.; Zhang, R.; Wang, Q. Adipose-Derived Stem Cells Modulate BV2 Microglial M1/M2 Polarization by Producing GDNF. Stem Cells Dev. 2020, 29, 714–727. [Google Scholar] [CrossRef]
- Chou, A.K.; Yang, M.C.; Tsai, H.P.; Chai, C.Y.; Tai, M.H.; Kwan, A.L.; Hong, Y.R. Adenoviral-mediated glial cell line-derived neurotrophic factor gene transfer has a protective effect on sciatic nerve following constriction-induced spinal cord injury. PLoS ONE 2014, 9, e92264. [Google Scholar] [CrossRef]
- Lin, C.H.; Wang, C.H.; Hsu, S.L.; Liao, L.Y.; Lin, T.A.; Hsueh, C.M. Molecular Mechanisms Responsible for Neuron-Derived Conditioned Medium (NCM)-Mediated Protection of Ischemic Brain. PLoS ONE 2016, 11, e0146692. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, S.; Wang, Y.; Zhang, X.; Chen, L.; Sun, D. GDNF enhances the anti-inflammatory effect of human adipose-derived mesenchymal stem cell-based therapy in renal interstitial fibrosis. Stem Cell Res. 2019, 41, 101605. [Google Scholar] [CrossRef]
- Zhang, D.K.; He, F.Q.; Li, T.K.; Pang, X.H.; Cui, D.J.; Xie, Q.; Huang, X.L.; Gan, H.T. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J. Pathol. 2010, 222, 213–222. [Google Scholar] [CrossRef]
- Shinoda, M.; Fukuoka, T.; Takeda, M.; Iwata, K.; Noguchi, K. Spinal glial cell line-derived neurotrophic factor infusion reverses reduction of Kv4.1-mediated A-type potassium currents of injured myelinated primary afferent neurons in a neuropathic pain model. Mol. Pain 2019, 15, 1744806919841196. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.Z.; Chiou, A.L.; Williams, L.R.; Hoffer, B.J. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J. Neurosci. 1997, 17, 4341–4348. [Google Scholar] [CrossRef]
- Hayward, N.J.; Harding, M.; Lloyd, S.A.; McKnight, A.T.; Hughes, J.; Woodruff, G.N. The effect of CCKB/gastrin antagonists on stimulated gastric acid secretion in the anaesthetized rat. Br. J. Pharmacol. 1991, 104, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Steinkamp, M.; Geerling, I.; Seufferlein, T.; von Boyen, G.; Egger, B.; Grossmann, J.; Ludwig, L.; Adler, G.; Reinshagen, M. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology 2003, 124, 1748–1757. [Google Scholar] [CrossRef]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Bunnage, M.; Plaha, P.; Svendsen, C.N.; Heywood, P.; Gill, S.S. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: A two-year outcome study. Ann. Neurol. 2005, 57, 298–302. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/history/NCT04167540?V_3=View#StudyPageTop (accessed on 29 August 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03652363 (accessed on 30 August 2021).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01621581 (accessed on 28 August 2021).
- Whone, A.L.; Boca, M.; Luz, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00006488 (accessed on 31 August 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).