C-Reactive Protein Controls IL-23 Production by Human Monocytes
Abstract
1. Introduction
2. Results
2.1. CRP Induces IL-23 Production by Human Monocytes
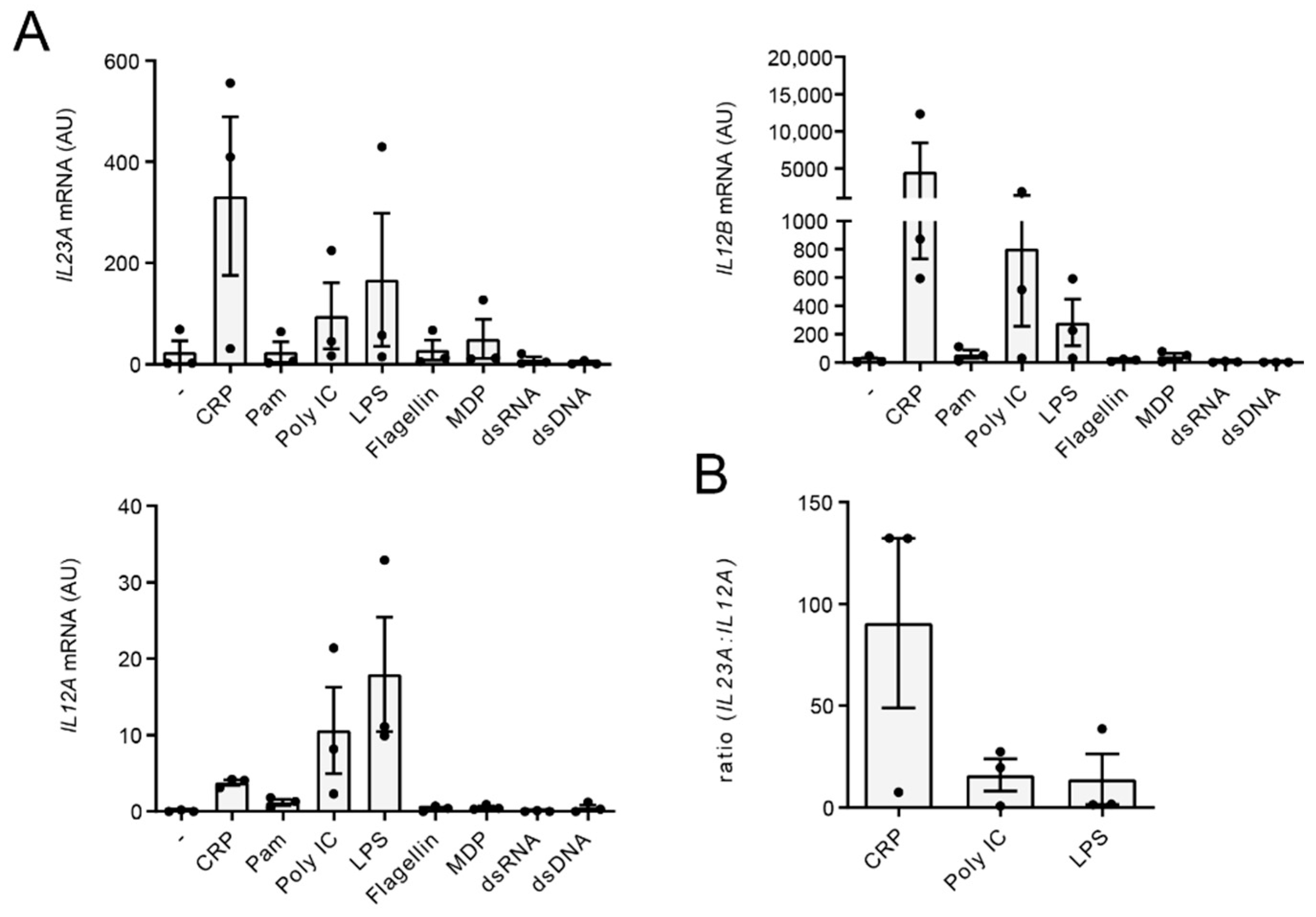
2.2. CRP Favors Gene Transcription of IL23A over IL12A
2.3. CRP-Induced IL-23 Production Is Inhibited upon TLR Co-Stimulation
2.4. IL-23 Induction by CRP Is Only Partially Dependent on Syk Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Stimulation
4.2. ELISA
4.3. Quantitative RT-PCR
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhodes, B.; Fürnrohr, B.G.; Vyse, T.J. C-reactive protein in rheumatology: Biology and genetics. Nat. Rev. Rheumatol. 2011, 7, 282–289. [Google Scholar] [CrossRef]
- Kapur, R.; Heitink-Pollé, K.M.; Porcelijn, L.; Bentlage, A.E.; Bruin, M.C.; Visser, R.; Roos, D.; Schasfoort, R.B.M.; de Haas, M.; van der Schoot, C.E.; et al. C-reactive protein enhances IgG-mediated phagocyte responses and thrombocytopenia. Blood 2015, 125, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.; Volanakis, J.E.; Briles, D.E. Blood clearance of Streptococcus pneumoniae by C-reactive protein. J. Immunol. 1987, 138, 2598–2603. [Google Scholar]
- Mold, C.; Nakayama, S.; Holzer, T.J.; Gewurz, H.; Du Clos, T.W. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J. Exp. Med. 1981, 154, 1703–1708. [Google Scholar] [CrossRef]
- Szalai, A.J.; Briles, D.E.; Volanakis, J.E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J. Immunol. 1995, 155, 2557–2563. [Google Scholar] [PubMed]
- Yother, J.; Volanakis, J.E.; Briles, D.E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in mice. J. Immunol. 1982, 128, 2374–2376. [Google Scholar]
- Szalai, A.J.; VanCott, J.L.; McGhee, J.R.; Volanakis, J.E.; Benjamin, W.H. Human C-reactive protein is protective against fatal Salmonella enterica serovar typhimurium infection in transgenic mice. Infect. Immun. 2000, 68, 5652–5656. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Pan, N.; McGowan, K.L.; Musher, D.; Martin, A.; Richards, J. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 1998, 187, 631–640. [Google Scholar] [CrossRef]
- Casey, R.; Newcombe, J.; McFadden, J.; Bodman-Smith, K.B. The acute-phase reactant C-reactive protein binds to phosphorylcholine-expressing Neisseria meningitidis and increases uptake by human phagocytes. Infect. Immun. 2008, 76, 1298–1304. [Google Scholar] [CrossRef]
- Du Clos, T.W. Pentraxins: Structure, function, and role in inflammation. ISRN Inflamm. 2013, 2013, 379040. [Google Scholar] [CrossRef] [PubMed]
- Gershov, D.; Kim, S.; Brot, N.; Elkon, K.B. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: Implications for systemic autoimmunity. J. Exp. Med. 2000, 192, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Marnell, L.; Mold, C.; Clos, T.W.D. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef]
- Thomas-Rudolph, D.; Du Clos, T.W.; Snapper, C.M.; Mold, C. C-reactive protein enhances immunity to Streptococcus pneumoniae by targeting uptake to Fc gamma R on dendritic cells. J. Immunol. 2007, 178, 7283–7291. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, F.; Bruemmer, D.; Yin, F.; Takata, Y.; Wang, W.; Fishbein, M.C.; Okura, T.; Higaki, J.; Graf, K.; Fleck, E.; et al. C-reactive protein induces apoptosis in human coronary vascular smooth muscle cells. Circulation 2004, 110, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Devaraj, S.; Vasquez-Vivar, J.; Jialal, I. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J. Mol. Cell Cardiol. 2007, 43, 780–791. [Google Scholar] [CrossRef]
- Tanigaki, K.; Mineo, C.; Yuhanna, I.S.; Chambliss, K.L.; Quon, M.J.; Bonvini, E.; Shaul, P.W. C-reactive protein inhibits insulin activation of endothelial nitric oxide synthase via the immunoreceptor tyrosine-based inhibition motif of FcgammaRIIB and SHIP-1. Circ. Res. 2009, 104, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Kieser, K.J.; Kagan, J.C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017, 17, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hawkins, P.N.; Kahan, M.C.; Tennent, G.A.; Gallimore, J.R.; Graham, D.; Sabin, C.A.; Zychlinsky, A.; de Diego, J. Proinflammatory effects of bacterial recombinant human C-reactive protein are caused by contamination with bacterial products, not by C-reactive protein itself. Circ. Res. 2005, 97, e97–e103. [Google Scholar] [CrossRef]
- Taylor, K.E.; Giddings, J.C.; van den Berg, C.W. C-reactive protein-induced in vitro endothelial cell activation is an artefact caused by azide and lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1225–1230. [Google Scholar] [CrossRef]
- Newling, M.; Sritharan, L.; van der Ham, A.J.; Hoepel, W.; Fiechter, R.H.; de Boer, L.; Zaat, S.A.J.; Bisoendial, R.J.; Baeten, D.L.P.; Everts, B.; et al. C-Reactive Protein Promotes Inflammation through FcγR-Induced Glycolytic Reprogramming of Human Macrophages. J. Immunol. 2019, 203, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ballou, S.P.; Lozanski, G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine 1992, 4, 361–368. [Google Scholar] [CrossRef]
- Galve-de Rochemonteix, B.; Wiktorowicz, K.; Kushner, I.; Dayer, J.M. C-reactive protein increases production of IL-1 alpha, IL-1 beta, and TNF-alpha, and expression of mRNA by human alveolar macrophages. J. Leukoc. Biol. 1993, 53, 439–445. [Google Scholar] [CrossRef]
- Mold, C.; Du Clos, T.W. C-reactive protein increases cytokine responses to Streptococcus pneumoniae through interactions with Fc gamma receptors. J. Immunol. 2006, 176, 7598–7604. [Google Scholar] [CrossRef] [PubMed]
- Pue, C.A.; Mortensen, R.F.; Marsh, C.B.; Pope, H.A.; Wewers, M.D. Acute phase levels of C-reactive protein enhance IL-1 beta and IL-1ra production by human blood monocytes but inhibit IL-1 beta and IL-1ra production by alveolar macrophages. J. Immunol. 1996, 156, 1594–1600. [Google Scholar] [PubMed]
- Duvallet, E.; Semerano, L.; Assier, E.; Falgarone, G.; Boissier, M.C. Interleukin-23, a key cytokine in inflammatory diseases. Ann. Med. 2011, 43, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Croxford, A.L.; Mair, F.; Becher, B. IL-23, one cytokine in control of autoimmunity. Eur. J. Immunol. 2012, 42, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Jiang, T.; Zhang, W.; Ren, C.; Wang, Q.; Qin, Q.; Chen, J.; Deng, A.; Zhong, R. Increased IL-23 and IL-17 expression by peripheral blood cells of patients with primary biliary cirrhosis. Cytokine 2013, 64, 172–180. [Google Scholar] [CrossRef]
- Yeremenko, N.; Paramarta, J.E.; Baeten, D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr. Opin. Rheumatol. 2014, 26, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019, 45, 1–8. [Google Scholar] [CrossRef]
- Duerr, R.H.; Taylor, K.D.; Brant, S.R.; Rioux, J.D.; Silverberg, M.S.; Daly, M.J.; Steinhart, A.H.; Abraham, C.; Regueiro, M.; Griffiths, A.; et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006, 314, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.; Hawkes, J.E.; Krueger, J.G. Interleukin 23 in the skin: Role in psoriasis pathogenesis and selective interleukin 23 blockade as treatment. Ther. Adv. Chronic. Dis. 2018, 9, 111–119. [Google Scholar] [CrossRef]
- Lee, E.; Trepicchio, W.L.; Oestreicher, J.L.; Pittman, D.; Wang, F.; Chamian, F.; Dhodapkar, M.; Krueger, J.G. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 2004, 199, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Tsukazaki, H.; Kaito, T. The Role of the IL-23/IL-17 Pathway in the Pathogenesis of Spondyloarthritis. Int. J. Mol. Sci. 2020, 21, 6401. [Google Scholar] [CrossRef] [PubMed]
- Cauvi, D.M.; Williams, M.R.; Bermudez, J.A.; Armijo, G.; De Maio, A. Elevated expression of IL-23/IL-17 pathway-related mediators correlates with exacerbation of pulmonary inflammation during polymicrobial sepsis. Shock 2014, 42, 246–255. [Google Scholar] [CrossRef]
- Meeks, K.D.; Sieve, A.N.; Kolls, J.K.; Ghilardi, N.; Berg, R.E. IL-23 Is Required for Protection against Systemic Infection with Listeria monocytogenes. J. Immunol. 2009, 183, 8026–8034. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Ward, A. Therapeutic potential of targeting IL-17 and IL-23 in sepsis. Clin. Transl. Med. 2012, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Butchar, J.P.; Rajaram, M.V.; Ganesan, L.P.; Parsa, K.V.; Clay, C.D.; Schlesinger, L.S.; Tridandapani, S. Francisella tularensis induces IL-23 production in human monocytes. J. Immunol. 2007, 178, 4445–4454. [Google Scholar] [CrossRef]
- Kulsantiwong, P.; Pudla, M.; Boondit, J.; Wikraiphat, C.; Dunachie, S.J.; Chantratita, N.; Utaisincharoen, P. Burkholderia pseudomallei induces IL-23 production in primary human monocytes. Med. Microbiol. Immunol. 2016, 205, 255–260. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, D.; de Paus, R.A.; van Dissel, J.T.; van de Vosse, E. Salmonella induced IL-23 and IL-1beta allow for IL-12 production by monocytes and Mphi1 through induction of IFN-gamma in CD56 NK/NK-like T cells. PLoS ONE 2009, 4, e8396. [Google Scholar] [CrossRef] [PubMed]
- Floss, D.M.; Schroder, J.; Franke, M.; Scheller, J. Insights into IL-23 biology: From structure to function. Cytokine Growth Factor Rev. 2015, 26, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Tindall, E.A.; Hayes, V.M. Comprehensive sequence analysis of the human IL23A gene defines new variation content and high rate of evolutionary conservation. DNA Res. 2010, 17, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, D.; Stein, M.P.; Volzer, M.; Mold, C.; Du Clos, T.W. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J. Exp. Med. 1999, 190, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Bodman-Smith, K.B.; Melendez, A.J.; Campbell, I.; Harrison, P.T.; Allen, J.M.; Raynes, J.G. C-reactive protein-mediated phagocytosis and phospholipase D signalling through the high-affinity receptor for immunoglobulin G (FcgammaRI). Immunology 2002, 107, 252–260. [Google Scholar] [CrossRef]
- Lu, J.; Marjon, K.D.; Marnell, L.L.; Wang, R.; Mold, C.; Du Clos, T.W.; Sun, P. Recognition and functional activation of the human IgA receptor (FcalphaRI) by C-reactive protein. Proc. Natl. Acad. Sci. USA 2011, 108, 4974–4979. [Google Scholar] [CrossRef] [PubMed]
- Vogelpoel, L.T.; Hansen, I.S.; Visser, M.W.; Nagelkerke, S.Q.; Kuijpers, T.W.; Kapsenberg, M.L.; de Jong, E.C.; den Dunnen, J. FcγRIIa cross-talk with TLRs, IL-1R, and IFNγR selectively modulates cytokine production in human myeloid cells. Immunobiology 2015, 220, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.S.; Hoepel, W.; Zaat, S.A.J.; Baeten, D.L.P.; den Dunnen, J. Serum IgA Immune Complexes Promote Proinflammatory Cytokine Production by Human Macrophages, Monocytes, and Kupffer Cells through FcαRI-TLR Cross-Talk. J. Immunol. 2017, 199, 4124–4131. [Google Scholar] [CrossRef]
- Curtis, M.M.; Way, S.S. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 2009, 126, 177–185. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; Wu, X.; Yang, S.; Zhang, Y.; Shu, Q.; Fang, X. Triggering Receptor Expressed on Myeloid Cells-2 Protects Aged Mice Against Sepsis by Mitigating the IL-23/IL-17A Response. Shock 2021, 56, 98–107. [Google Scholar] [CrossRef]
- Belladonna, M.L.; Vacca, C.; Volpi, C.; Giampietri, A.; Fiorett, M.C.; Puccetti, P.; Grohmann, U.; Campanile, F. IL-23 neutralization protects mice from Gram-negative endotoxic shock. Cytokine 2006, 34, 161–169. [Google Scholar] [CrossRef] [PubMed]
- McGovern, D.; Powrie, F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut 2007, 56, 1333–1336. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. IL-23 and autoimmunity: New insights into the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2009, 60, 97–110. [Google Scholar] [CrossRef]
- Oh, K.; Oh, E.H.; Baek, S.; Song, E.M.; Kim, G.U.; Seo, M.; Hwang, S.W.; Park, S.H.; Yang, D.-H.; Kim, K.-J.; et al. Elevated C-reactive protein level during clinical remission can predict poor outcomes in patients with Crohn’s disease. PLoS ONE 2017, 12, e0179266. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Stray, N.; Sauar, J.; Vatn, M.H.; Moum, B.; IBSEN Study Group. C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008, 57, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyy, D.; Rudwaleit, M.; Haibel, H.; Listing, J.; Märker-Hermann, E.; Zeidler, H.; Braun, J.; Sieper, J. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann. Rheum. Dis. 2011, 70, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Cui, L.; Yang, W.; Shi, H.; Jin, C.; Shu, R.; Li, H.; Zeng, X.; Wu, S.; Gao, X. Baseline high-sensitivity C-reactive protein predicts the risk of incident ankylosing spondylitis: Results of a community-based prospective study. PLoS ONE 2019, 14, e0211946. [Google Scholar] [CrossRef] [PubMed]
- Vadakayil, A.R.; Dandekeri, S.; Kambil, S.M.; Ali, N. M.Role of C-reactive protein as a marker of disease severity and cardiovascular risk in patients with psoriasis. Ind. Derm. Online J. 2015, 6, 322–325. [Google Scholar] [CrossRef]
- Mise-Omata, S.; Kuroda, E.; Niikura, J.; Yamashita, U.; Obata, Y.; Doi, T.S. A proximal kappaB site in the IL-23 p19 promoter is responsible for RelA- and c-Rel-dependent transcription. J. Immunol. 2007, 179, 6596–6603. [Google Scholar] [CrossRef] [PubMed]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hoepel, W.; Newling, M.; Vogelpoel, L.T.C.; Sritharan, L.; Hansen, I.S.; Kapsenberg, M.L.; Baeten, D.L.P.; Everts, B.; den Dunnen, J. FcγR-TLR Cross-Talk Enhances TNF Production by Human Monocyte-Derived DCs via IRF5-Dependent Gene Transcription and Glycolytic Reprogramming. Front. Immunol. 2019, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Mes, L.; Newling, M.; den Dunnen, J.; Hoepel, W. Physiological and Pathological Inflammation Induced by Antibodies and Pentraxins. Cells 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Newling, M.; Hoepel, W.; Vogelpoel, L.T.C.; Heineke, M.H.; van Burgsteden, J.A.; Taanman-Kueter, E.W.M.; Eggink, D.; Kuijpers, T.W.; Beaumont, T.; Van Egmond, M.; et al. Fc gamma receptor IIa suppresses type I and III interferon production by human myeloid immune cells. Eur. J. Immunol. 2018, 48, 1796–1809. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Li Yim, A.Y.F.; Griffith, G.R.; de Jonge, W.J.; Mannens, M.M.A.M.; Ferrero, E.; Henneman, P.; de Winther, M.P.J. Meta-Analysis of in vitro-Differentiated Macrophages Identifies Transcriptomic Signatures That Classify Disease Macrophages in vivo. Front. Immunol. 2019, 10, 2887. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geyer, C.E.; Newling, M.; Sritharan, L.; Griffith, G.R.; Chen, H.-J.; Baeten, D.L.P.; den Dunnen, J. C-Reactive Protein Controls IL-23 Production by Human Monocytes. Int. J. Mol. Sci. 2021, 22, 11638. https://doi.org/10.3390/ijms222111638
Geyer CE, Newling M, Sritharan L, Griffith GR, Chen H-J, Baeten DLP, den Dunnen J. C-Reactive Protein Controls IL-23 Production by Human Monocytes. International Journal of Molecular Sciences. 2021; 22(21):11638. https://doi.org/10.3390/ijms222111638
Chicago/Turabian StyleGeyer, Chiara E., Melissa Newling, Lathees Sritharan, Guillermo R. Griffith, Hung-Jen Chen, Dominique L. P. Baeten, and Jeroen den Dunnen. 2021. "C-Reactive Protein Controls IL-23 Production by Human Monocytes" International Journal of Molecular Sciences 22, no. 21: 11638. https://doi.org/10.3390/ijms222111638
APA StyleGeyer, C. E., Newling, M., Sritharan, L., Griffith, G. R., Chen, H.-J., Baeten, D. L. P., & den Dunnen, J. (2021). C-Reactive Protein Controls IL-23 Production by Human Monocytes. International Journal of Molecular Sciences, 22(21), 11638. https://doi.org/10.3390/ijms222111638





