Prospects and Challenges of the Study of Anti-Glycan Antibodies and Microbiota for the Monitoring of Gastrointestinal Cancer
Abstract
1. Introduction
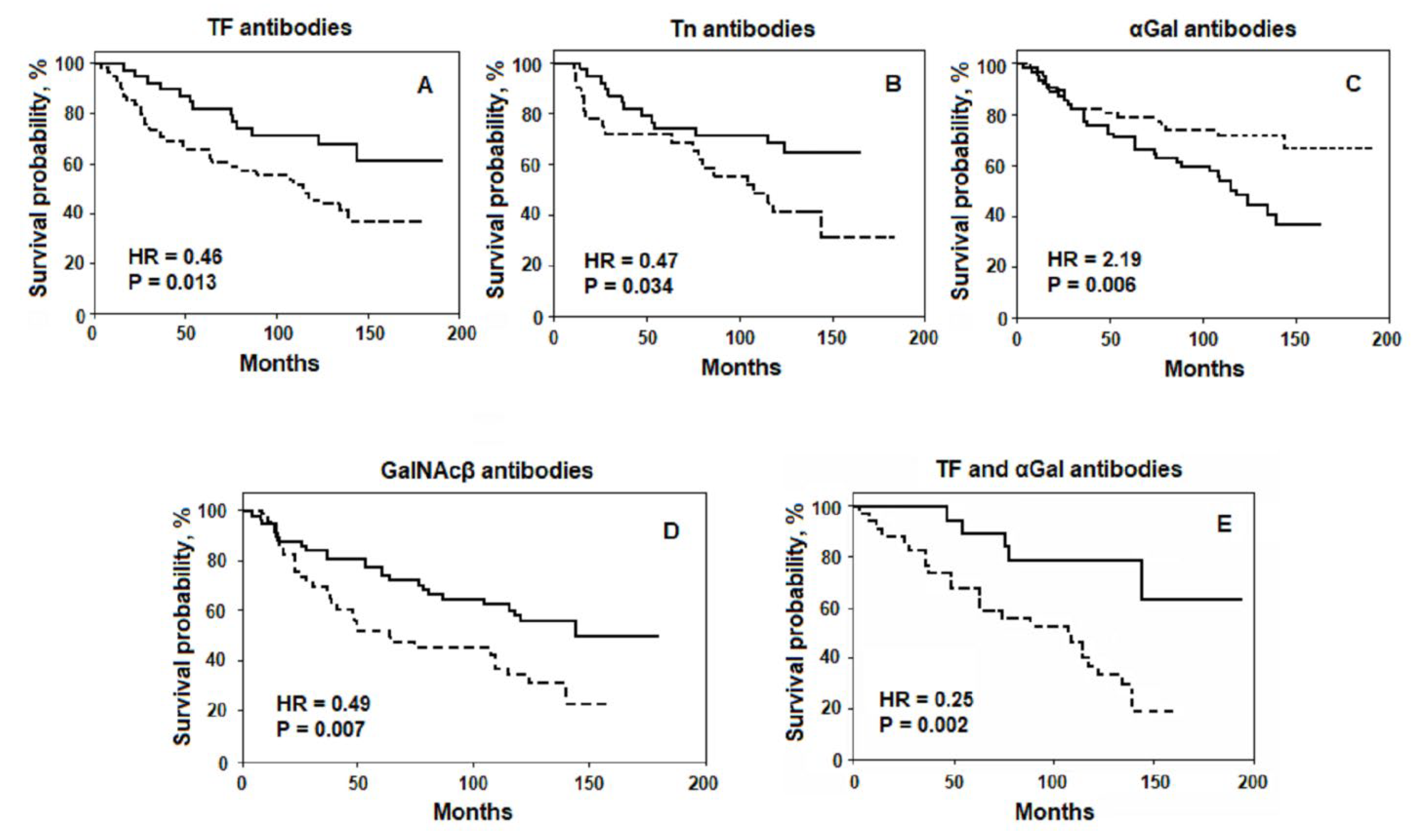
2. Association of the Level of AG Abs with Survival
3. Prognostic Potential of AG Abs
4. Study of the AG Abs Specificity
5. Paradigm of Microbial Origin of AG Abs Stimulus
6. Influence of Microbiota on Cancer Progression
6.1. Beneficial or Normalising Effects of Bacteria
6.2. Harmful Effects of Bacteria
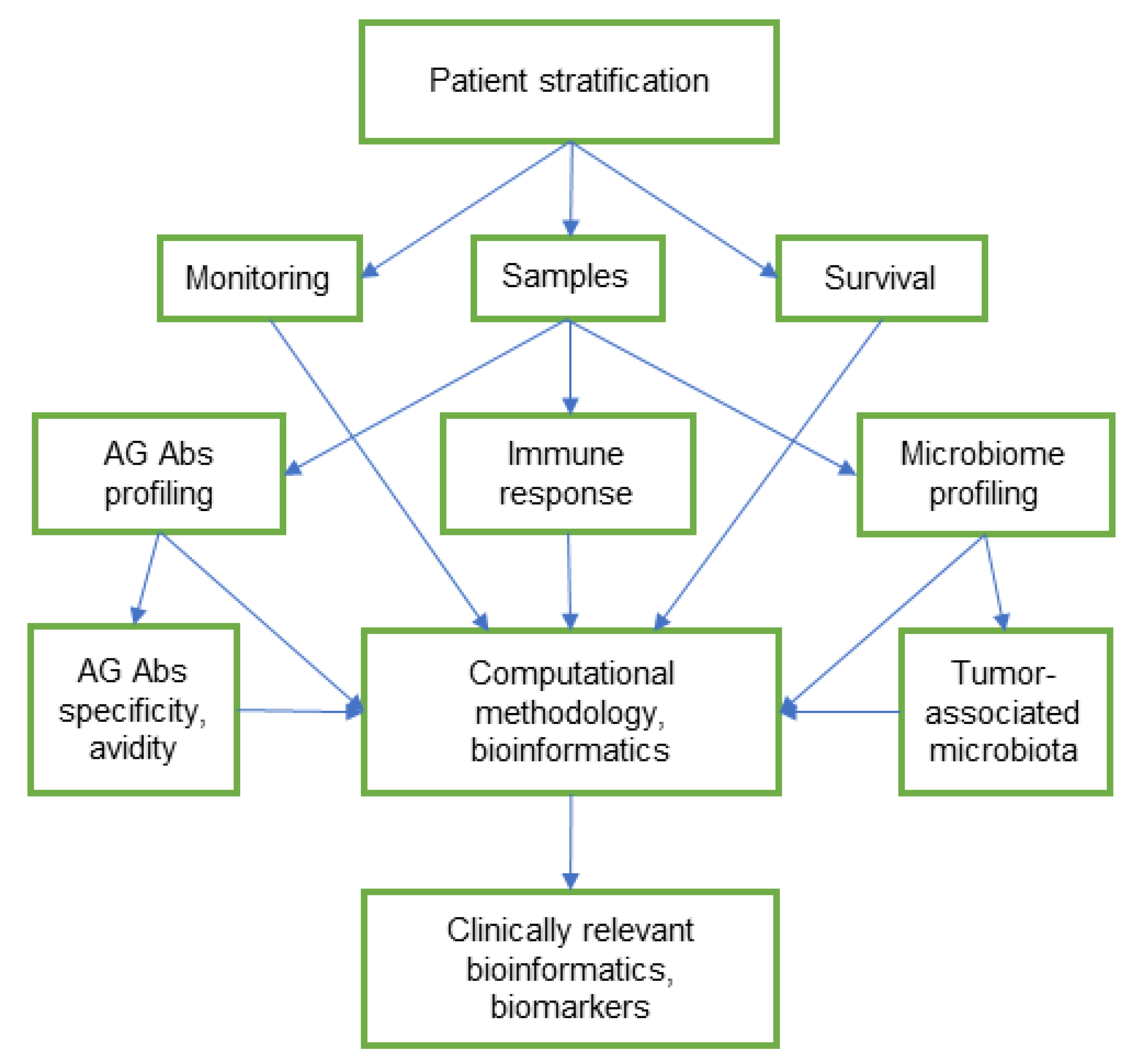
7. Prospects and Challenges of Integrative Glycome and Microbiome Research
8. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer. 2021, 1875, 188464. [Google Scholar] [CrossRef] [PubMed]
- Luetscher, R.N.D.; McKitrick, T.R.; Gao, C.; Mehta, A.Y.; McQuillan, A.M.; Kardish, R.; Boligan, K.F.; Song, X.; Lu, L.; Heimburg-Molinaro, J.; et al. Unique repertoire of anti-carbohydrate antibodies in individual human serum. Sci. Rep. 2020, 10, 15436. [Google Scholar] [CrossRef] [PubMed]
- Huflejt, M.E.; Vuskovic, M.; Vasiliu, D.; Xu, H.; Obukhova, P.; Shilova, N.; Tuzikov, A.; Galanina, O.; Arun, B.; Lu, K.; et al. Anti-carbohydrate antibodies of normal sera: Findings, surprises and challenges. Mol. Immunol. 2009, 46, 3037–3049. [Google Scholar] [CrossRef]
- Springer, G.F.; Desai, P.R.; Ghazizadeh, M.; Tegtmeyer, H. T/Tn pancarcinoma autoantigens: Fundamental, diagnostic, and prognostic aspects. Cancer Detect. Prev. 1995, 19, 173–182. [Google Scholar]
- Springer, G.F. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997, 75, 594–602. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA 2016, 88, 275–286. [Google Scholar] [CrossRef]
- Kurtenkov, O. Profiling of naturally occurring antibodies to the Thomsen-Friedenreich antigen in health and cancer: The diversity and clinical potential. Biomed. Res. Int. 2020, 23, 9747040. [Google Scholar] [CrossRef]
- Friedenreich, V. Production of a specific receptor quality in red cell corpuscules by bacterial activity. In The Thomsen Haemagglutination Phenomenon; Levin and Munksgaard: Copenhagen, Denmark, 1930; pp. 12–16. [Google Scholar]
- Springer, G.F. T and Tn, general carcinoma autoantigens. Science 1984, 224, 1198–1206. [Google Scholar] [CrossRef]
- Springer, G.F.; Desai, P.R. Tn epitopes, immunoreactive with ordinary anti-Tn antibodies, on normal, desialylated human erythrocytes and on Thomsen-Friedenreich antigen isolated therefrom. Mol. Immunol. 1985, 22, 1303–1310. [Google Scholar] [CrossRef]
- Fernandes, E.; Sores, J.; Cotton, S.; Peixoto, A.; Ferreira, D.; Freitas, R.; Reis, C.A.; Santos, L.L.; Ferreira, J.A. Esophageal, gastric and colorectal cancers: Looking beyond classical serological biomarkers towards glycoproteomics-assisted precision oncology. Theranostics 2020, 31, 4903–4928. [Google Scholar] [CrossRef]
- Kirwan, A.; Utratna, M.; O’Dwyer, M.E.; Joshi, L.; Kilcoyne, M. Glycosylation-based serum biomarkers for cancer diagnostics and prognostics. Biomed. Res. Int. 2015, 2015, 490531. [Google Scholar] [CrossRef]
- Silsirivanit, A. Glycosylation markers in cancer. Adv. Clin. Chem. 2019, 89, 189–213. [Google Scholar]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2017, 409, 395–410. [Google Scholar] [CrossRef]
- Blsakova, A.; Kveton, F.; Kasak, P.; Tkac, J. Antibodies against aberrant glycans as cancer biomarkers. Expert Rev. Mol. Diagn. 2019, 19, 1057–1068. [Google Scholar] [CrossRef]
- Tikhonov, A.; Smoldovskaya, O.; Feyzkhanova, G.; Kushlinskii, N.; Rubina, A. Glycan-specific antibodies as potential cancer biomarkers: A focus on microarray applications. Clin. Chem. Lab. Med. 2020, 58, 1611–1622. [Google Scholar] [CrossRef]
- Purohit, S.; Ferris, D.G.; Alvarez, M.; Tran, P.M.H.; Tran, L.K.H.; Mysona, D.P.; Hopkins, D.; Zhi, W.; Dun, B.; Wallbillich, J.J.; et al. Better survival is observed in cervical cancer patients positive for specific anti-glycan antibodies and receiving brachytherapy. Gynecol. Oncol. 2020, 157, 181–187. [Google Scholar] [CrossRef]
- Kurtenkov, O.; Klaamas, K.; Mensdorff-Pouilly, S.; Miljukhina, L.; Shljapnikova, L.; Chuzmarov, V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: Relation to survival. Acta Oncol. 2007, 46, 316–323. [Google Scholar] [CrossRef]
- Smorodin, E.; Sergeyev, B.; Klaamas, K.; Chuzmarov, V.; Kurtenkov, O. The relation of the level of serum anti-TF, -Tn and -alpha-Gal IgG to survival in gastrointestinal cancer patients. Int. J. Med. Sci. 2013, 23, 1674–1682. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L. The level of anti-(GalNAc beta) and anti-para-Forssman disaccharide IgG antibodies in patients with gastrointestinal cancer: Relation to survival. Exp. Oncol. 2013, 35, 89–92. [Google Scholar]
- Smorodin, E.P.; Sergeyev, B.L. The level of IgG antibodies reactive to TF, Tn and alpha-Gal polyacrylamide-glycoconjugates in breast cancer patients: Relation to survival. Exp. Oncol. 2016, 38, 117–121. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Chuzmarov, V.I.; Afanasyev, V.P. The relation of serum anti-(GalNAc beta) and -para-Forssman disaccharide IgG levels to the progression and histological grading of gastrointestinal cancer. Exp. Oncol. 2007, 29, 61–66. [Google Scholar] [PubMed]
- Barrow, H.; Guo, X.; Wandall, H.H.; Pedersen, J.W.; Fu, B.; Zhao, Q.; Chen, C.; Rhodes, J.M.; Yu, L.G. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin. Cancer Res. 2011, 17, 7035–7046. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, H.; Xu, S.; Qi, X. Galectins for diagnosis and prognostic assessment of human diseases: An overview of meta-analyses. Med. Sci. Monit. 2020, 26, e923901. [Google Scholar] [CrossRef]
- Hirano, K.; Matsuda, A.; Shirai, T.; Furukawa, K. Expression of LacdiNAc groups on N-glycans among human tumors is complex. Biomed. Res. Int. 2014, 2014, 981627. [Google Scholar] [CrossRef]
- Yoneyama, T.; Tobisawa, Y.; Kaneko, T.; Kaya, T.; Hatakeyama, S.; Mori, K.; Sutoh Yoneyama, M.; Okubo, T.; Mitsuzuka, K.; Duivenvoorden, W.; et al. Clinical significance of the LacdiNAc-glycosylated prostate-specific antigen assay for prostate cancer detection. Cancer Sci. 2019, 110, 2573–2589. [Google Scholar] [CrossRef]
- Bumba, L.; Laaf, D.; Spiwok, V.; Elling, L.; Křen, V.; Bojarová, P. Poly-N-acetyllactosamine neo-glycoproteins as Nanomolar ligands of human galectin-3: Binding kinetics and modeling. Int. J. Mol. Sci. 2018, 19, 372. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Kodar, K.E.; Chuzmarov, V.I.; Afanasyev, V.P. Postoperative change of anti-Thomsen-Friedenreich and Tn IgG level: The follow-up study of gastrointestinal cancer patients. World J. Gastroenterol. 2008, 14, 4352–4358. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Lilleorg, A.L.; Chuzmarov, V.I. Antibodies to tumor-associated carbohydrate epitopes in sera of cancer patients and blood donors. Exp. Oncol. 2001, 23, 109–113. [Google Scholar]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; He, J.; Li, H.; You, J.; Qin, H. Alterations in intestinal microbiota of colorectal cancer patients receiving radical surgery combined with adjuvant CapeOx therapy. Sci. China Life Sci. 2019, 62, 1178–1193. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Lipping, A.A.; Chuzmarov, V.I.; Afanasyev, V.P. The relation of serum anti-TF, Tn and alpha-Gal IgG antibody levels to cancer progression and histopathological grading. Exp. Oncol. 2002, 24, 270–273. [Google Scholar]
- Springer, G.F.; Desai, P.R. Increase in anti-T titer scores of breast-carcinoma patients following mastectomy. Naturwissenschaften 1975, 62, 587. [Google Scholar] [CrossRef]
- Smorodin, E.; Sergeyev, B.; Kurtenkov, O.; Kuznetsova, T.; Geller, J. IgG Antibodies to GlcNAcβ and asialo-GM2 (GA2) glycans as potential markers of liver damage in chronic hepatitis C and the efficacy of antiviral treatment. Dis. Markers 2018, 2018, 4639805. [Google Scholar] [CrossRef]
- Bovin, N.; Obukhova, P.; Shilova, N.; Rapoport, E.; Popova, I.; Navakouski, M.; Unverzagt, C.; Vuskovic, M.; Huflejt, M. Repertoire of human natural anti-glycan immunoglobulins. Do we have auto-antibodies? Biochim. Biophys. Acta 2012, 1820, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Goldstein, D.R.; Bovin, N.V.; Pochechueva, T.; Spengler, M.; Caduff, R.; Fink, D.; Vuskovic, M.I.; Huflejt, M.E.; Heinzelmann-Schwarz, V. Serum antiglycan antibody detection of nonmucinous ovarian cancers by using a printed glycan array. Int. J. Cancer 2012, 130, 138–146. [Google Scholar] [CrossRef]
- Pochechueva, T.; Chinarev, A.; Schoetzau, A.; Fedier, A.; Bovin, N.V.; Hacker, N.F.; Jacob, F.; Heinzelmann-Schwarz, V. Blood plasma-derived anti-glycan antibodies to sialylated and sulfated glycans identify ovarian cancer patients. PLoS ONE 2016, 11, e0164230. [Google Scholar] [CrossRef]
- Tuzikov, A.; Chinarev, A.; Shilova, N.; Gordeeva, E.; Galanina, O.; Ovchinnikova, T.; Schaefer, M.; Bovin, N. 40 years of glyco-polyacrylamide in glycobiology. Glycoconj. J. 2021, 38, 89–100. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Klaamas, K.V.; Izotova, J.G. The characterization of cross-reactive antibodies to Thomsen-Friedenreich α/β and related glycan-conjugates with polyacrylamide carriers in patients with gastrointestinal cancer. J. Clin. Cell. Immunol. 2011, S5. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Branovets, J.S.; Izotova, J.G.; Formanovsky, A.A. Specificity of serum anti-A(di) IgG antibodies from patients with gastrointestinal cancer. J. Immunoass. Immunochem. 2011, 32, 170–190. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Kurtenkov, O.A.; Sergeyev, B.L.; Pazynina, G.V.; Bovin, N.V. Specificity of human anti-carbohydrate IgG antibodies as probed with polyacrylamide-based glycoconjugates. Glycoconj. J. 2004, 20, 83–89. [Google Scholar] [CrossRef]
- Smorodin, E.P.; Sergeyev, B.L.; Kurtenkov, O.A. The characterization of IgG antibodies to GalNAc beta-terminated glycans of gastric cancer survivors. Exp. Oncol. 2014, 36, 38–43. [Google Scholar] [PubMed]
- Smorodin, E.P.; Kurtenkov, O.A.; Shevchuk, I.N.; Tanner, R.H. The isolation and characterization of human natural alphaGal-specific IgG antibodies applicable to the detection of alphaGal-glycosphingolipids. J. Immunoass. Immunochem. 2005, 26, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Basbaum, C.B.; Shohet, S.B.; Buehler, J.; Macher, B.A. Identification of erythrocyte Gal alpha 1-3Gal glycosphingolipids with a mouse monoclonal antibody, Gal-13. J. Biol. Chem. 1987, 262, 4683–4688. [Google Scholar] [CrossRef]
- Bhatia, R.; Gautam, S.K.; Cannon, A.; Thompson, C.; Hall, B.R.; Aithal, A.; Banerjee, K.; Jain, M.; Solheim, J.C.; Kumar, S.; et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019, 38, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Lomax-Browne, H.J.; Robertson, C.; Antonopoulos, A.; Leathem, A.J.C.; Haslam, S.M.; Dell, A.; Dwek, M.V. Serum IgA1 shows increased levels of α2,6-linked sialic acid in breast cancer. Interface Focus 2019, 9, 20180079. [Google Scholar] [CrossRef]
- Welinder, C.; Baldetorp, B.; Blixt, O.; Grabau, D.; Jansson, B. Primary breast cancer tumours contain high amounts of IgA1 immunoglobulin: An immunohistochemical analysis of a possible carrier of the tumour-associated Tn antigen. PLoS ONE 2013, 8, e61749. [Google Scholar] [CrossRef]
- Zlocowski, N.; Grupe, V.; Garay, Y.C.; Nores, G.A.; Lardone, R.D.; Irazoqui, F.J. Purified human anti-Tn and anti-T antibodies specifically recognize carcinoma tissues. Sci. Rep. 2019, 9, 8097. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Kappler, K.; Hennet, T. Emergence and significance of carbohydrate-specific antibodies. Genes Immun. 2020, 21, 224–239. [Google Scholar] [CrossRef]
- Brockhausen, I. Crossroads between bacterial and mammalian glycosyltransferases. Front. Immunol. 2014, 5, 492. [Google Scholar] [CrossRef]
- Cisar, J.O.; Sandberg, A.L.; Abeygunawardana, C.; Reddy, G.P.; Bush, C.A. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology 1995, 5, 655–662. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yang, J.; Peaker, P.E.; Kato, H.; Bush, C.A.; Cisar, J.O. Molecular and antigenic characterization of a Streptococcus oralis coaggregation receptor polysaccharide by carbohydrate engineering in Streptococcus gordonii. J. Biol. Chem. 2008, 283, 12654–12664. [Google Scholar] [CrossRef]
- Springer, G.F.; Tegtmeyer, H. Origin of anti-Thomsen-Friedenreich (T) and Tn agglutinins in man and in White Leghorn chicks. Br. J. Haematol. 1981, 47, 453–460. [Google Scholar] [CrossRef]
- Dobrochaeva, K.; Khasbiullina, N.; Shilova, N.; Antipova, N.; Obukhova, P.; Ovchinnikova, T.; Galanina, O.; Blixt, O.; Kunz, H.; Filatov, A.; et al. Specificity of human natural antibodies referred to as anti-Tn. Mol. Immunol. 2020, 120, 74–82. [Google Scholar]
- Henderson, G.; Ulsemer, P.; Schöber, U.; Löffler, A.; Alpert, C.A.; Zimmermann-Kordmann, M.; Reutter, W.; Karsten, U.; Goletz, S.; Blaut, M. Occurrence of the human tumor-specific antigen structure Galβ1-3GalNAcα- (Thomsen-Friedenreich) and related structures on gut bacteria: Prevalence, immunochemical analysis and structural confirmation. Glycobiology 2011, 21, 1277–1289. [Google Scholar] [CrossRef]
- Ulsemer, P.; Henderson, G.; Toutounian, K.; Löffler, A.; Schmidt, J.; Karsten, U.; Blaut, M.; Goletz, S. Specific humoral immune response to the Thomsen-Friedenreich tumor antigen (CD176) in mice after vaccination with the commensal bacterium Bacteroides ovatus D-6. Cancer Immunol. Immunother. 2013, 62, 875–887. [Google Scholar] [CrossRef]
- Ulsemer, P.; Toutounian, K.; Kressel, G.; Goletz, C.; Schmidt, J.; Karsten, U.; Hahn, A.; Goletz, S. Impact of oral consumption of heat-treated Bacteroides xylanisolvens DSM 23964 on the level of natural TFα-specific antibodies in human adults. Benef. Microbes 2016, 7, 485–500. [Google Scholar] [CrossRef]
- Cooke, C.L.; An, H.J.; Kim, J.; Canfield, D.R.; Torres, J.; Lebrilla, C.B.; Solnick, J.V. Modification of gastric mucin oligosaccharide expression in rhesus macaques after infection with Helicobacter pylori. Gastroenterology 2009, 137, 1061–1071.e8. [Google Scholar] [CrossRef]
- Klaamas, K.; Kurtenkov, O.; Rittenhouse-Olson, K.; Brjalin, V.; Miljukhina, L.; Shljapnikova, L.; Engstrand, L. Expression of tumor-associated Thomsen-Friedenreich antigen (T Ag) in Helicobacter pylori and modulation of T Ag specific immune response in infected individuals. Immunol. Investig. 2002, 31, 191–204. [Google Scholar] [CrossRef]
- Li, G.; Yu, S.; Xu, J.; Zhang, X.; Ye, J.; Wang, Z.; He, Y. The prognostic role of Helicobacter pylori in gastric cancer patients: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 216–224. [Google Scholar] [CrossRef]
- Coats, M.T.; Murphy, T.; Paton, J.C.; Gray, B.; Briles, D.E. Exposure of Thomsen-Friedenreich antigen in Streptococcus pneumoniae infection is dependent on pneumococcal neuraminidase A. Microb. Pathog. 2011, 50, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Emgård, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Conover, M.S.; Ruer, S.; Taganna, J.; Kalas, V.; De Greve, H.; Pinkner, J.S.; Dodson, K.W.; Remaut, H.; Hultgren, S.J. Inflammation-induced adhesin-receptor interaction provides a fitness advantage to uropathogenic E. coli during chronic infection. Cell Host Microbe 2016, 20, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Smith, D.F.; Cummings, R.D.; Boligan, K.F.; Hamilton, R.G.; Bochner, B.S.; Miescher, S.; Simon, H.U.; Pashov, A.; Vassilev, T.; et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci. Transl. Med. 2015, 7, 269ra1. [Google Scholar] [CrossRef]
- Huai, G.; Qi, P.; Yang, H.; Wang, Y. Characteristics of α-Gal epitope, anti-Gal antibody, α1,3 galactosyltransferase and its clinical exploitation (Review). Int. J. Mol. Med. 2016, 37, 11–20. [Google Scholar] [CrossRef]
- Galili, U.; Clark, M.R.; Shohet, S.B.; Buehler, J.; Macher, B.A. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1-3Gal epitope in primates. Proc. Natl. Acad. Sci. USA 1987, 84, 1369–1373. [Google Scholar] [CrossRef]
- Bernth Jensen, J.M.; Petersen, M.S.; Ellerman-Eriksen, S.; Møller, B.K.; Jensenius, J.C.; Sørensen, U.B.S.; Thiel, S. Abundant human anti-Galα3Gal antibodies display broad pathogen reactivity. Sci. Rep. 2020, 10, 4611. [Google Scholar] [CrossRef]
- Galili, U.; Mandrell, R.E.; Hamadeh, R.M.; Shohet, S.B.; Griffiss, J.M. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 1988, 56, 1730–1737. [Google Scholar] [CrossRef]
- Bernth Jensen, J.M.; Skeldal, S.; Petersen, M.S.; Møller, B.K.; Hoffmann, S.; Jensenius, J.C.; Skov Sørensen, U.B.; Thiel, S. The human natural anti-αGal antibody targets common pathogens by broad-spectrum polyreactivity. Immunology 2021, 162, 434–451. [Google Scholar] [CrossRef]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Cremonesi, E.; Governa, V.; Garzon, J.F.G.; Mele, V.; Amicarella, F.; Muraro, M.G.; Trella, E.; Galati-Fournier, V.; Oertli, D.; Däster, S.R.; et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut 2018, 67, 1984–1994. [Google Scholar] [CrossRef]
- Rodriguez, R.M.; Khadka, V.S.; Menor, M.; Hernandez, B.Y.; Deng, Y. Tissue-associated microbial detection in cancer using human sequencing data. BMC Bioinform. 2020, 21, 523. [Google Scholar] [CrossRef]
- Buchta Rosean, C.; Feng, T.Y.; Azar, F.N.; Rutkowski, M.R. Impact of the microbiome on cancer progression and response to anti-cancer therapies. Adv. Cancer Res. 2019, 143, 255–294. [Google Scholar]
- Choudhry, H. The microbiome and its implications in cancer immunotherapy. Molecules 2021, 26, 206. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, inflammation, and cancer. Cancer J. 2014, 20, 181–189. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, X.; Guo, Y.; Yan, J.; Abuduwaili, A.; Aximujiang, K.; Yan, J.; Wu, M. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 2021, 40, 42. [Google Scholar] [CrossRef]
- Drago, L. Probiotics and colon cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Wu, W.; Qin, H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol. Med. Rep. 2015, 12, 6119–6127. [Google Scholar] [CrossRef]
- Longhi, G.; van Sinderen, D.; Ventura, M.; Turroni, F. Microbiota and cancer: The emerging beneficial role of bifidobacteria in cancer immunotherapy. Front. Microbiol. 2020, 11, 575072. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Adiamah, A.; Kushairi, A.; Varadhan, K.K.; Krznaric, Z.; Kulkarni, A.D.; Neal, K.R.; Lobo, D.N. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. Ann. Surg. 2020, 271, 1036–1047. [Google Scholar] [CrossRef]
- Amitay, E.L.; Carr, P.R.; Gies, A.; Laetsch, D.C.; Brenner, H. Probiotic/synbiotic treatment and postoperative complications in colorectal cancer patients: Systematic review and meta-analysis of randomized controlled trials. Clin. Transl. Gastroenterol. 2020, 11, e00268. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Łuksza, M.; Zhao, J.N.; Makarov, V.; Moral, J.A.; Remark, R.; Herbst, B.; Askan, G.; Bhanot, U.; Senbabaoglu, Y.; et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017, 551, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Mulet-Margalef, N.; Ochoa-De-Olza, M.; Napoli, S.; Mas, J.; Laquente, B.; Alemany, L.; Duell, E.J.; Nuciforo, P.; Moreno, V. Tumor-associated microbiome: Where do we stand? Int. J. Mol. Sci. 2021, 22, 1446. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pan, J.; Xu, F.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Bacteria-based cancer immunotherapy. Adv. Sci. 2021, 8, 2003572. [Google Scholar] [CrossRef]
- Gupta, H.; Youn, G.S.; Shin, M.J.; Suk, K.T. Role of gut microbiota in hepatocarcinogenesis. Microorganisms 2019, 7, 121. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef]
- Elsalem, L.; Jum’ah, A.A.; Alfaqih, M.A.; Aloudat, O. The bacterial microbiota of gastrointestinal cancers: Role in cancer pathogenesis and therapeutic perspectives. Clin. Exp. Gastroenterol. 2020, 13, 151–185. [Google Scholar] [CrossRef]
- Villéger, R.; Lopès, A.; Veziant, J.; Gagnière, J.; Barnich, N.; Billard, E.; Boucher, D.; Bonnet, M. Microbial markers in colorectal cancer detection and/or prognosis. World J. Gastroenterol. 2018, 24, 2327–2347. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of oral bacteria in the development of oral squamous cell carcinoma. Cancers 2020, 12, 2797. [Google Scholar] [CrossRef]
- Nosho, K.; Sukawa, Y.; Adachi, Y.; Ito, M.; Mitsuhashi, K.; Kurihara, H.; Kanno, S.; Yamamoto, I.; Ishigami, K.; Igarashi, H.; et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J. Gastroenterol. 2016, 22, 557–566. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Heidarzadeh, S.; Jahangiri, S.; Farhood, B.; Mortezaee, K.; Khanlarkhani, N.; Negahdari, B. Fusobacterium nucleatum and colorectal cancer: A mechanistic overview. J. Cell. Physiol. 2019, 234, 2337–2344. [Google Scholar] [CrossRef]
- Lee, J.A.; Yoo, S.Y.; Oh, H.J.; Jeong, S.; Cho, N.Y.; Kang, G.H.; Kim, J.H. Differential immune microenvironmental features of microsatellite-unstable colorectal cancers according to Fusobacterium nucleatum status. Cancer Immunol. Immunother. 2021, 70, 47–59. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef]
- Riley, D.R.; Sieber, K.B.; Robinson, K.M.; White, J.R.; Ganesan, A.; Nourbakhsh, S.; Dunning Hotopp, J.C. Bacteria-human somatic cell lateral gene transfer is enriched in cancer samples. PLoS Comput. Biol. 2013, 9, e1003107. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef]
- Gur, C.; Maalouf, N.; Shhadeh, A.; Berhani, O.; Singer, B.B.; Bachrach, G.; Mandelboim, O. Fusobacterium nucleatum supresses anti-tumor immunity by activating CEACAM1. Oncoimmunology 2019, 8, e1581531. [Google Scholar] [CrossRef]
- Kaplan, C.W.; Ma, X.; Paranjpe, A.; Jewett, A.; Lux, R.; Kinder-Haake, S.; Shi, W. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect. Immun. 2010, 78, 4773–4778. [Google Scholar] [CrossRef]
- Chen, T.; Li, Q.; Wu, J.; Wu, Y.; Peng, W.; Li, H.; Wang, J.; Tang, X.; Peng, Y.; Fu, X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 2018, 67, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Fu, X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl. Oncol. 2019, 12, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef]
- Leung, P.H.M.; Subramanya, R.; Mou, Q.; Lee, K.T.; Islam, F.; Gopalan, V.; Lu, C.T.; Lam, A.K. Characterization of mucosa-associated microbiota in matched cancer and non-neoplastic mucosa from patients with colorectal cancer. Front. Microbiol. 2019, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, S.C.; Zhang, W.B.; Zhang, H.R.; Li, Y.; Zhang, Y.R.; Nie, J.L.; Chu, X.D.; Chen, C.S.; Jiang, H.P.; Pan, J.H. Clinicopathological and prognostic significance of Fusobacterium nucleatum infection in colorectal cancer: A meta-analysis. J. Cancer 2021, 12, 1583–1591. [Google Scholar] [CrossRef]
- Boehm, E.T.; Thon, C.; Kupcinskas, J.; Steponaitiene, R.; Skieceviciene, J.; Canbay, A.; Malfertheiner, P.; Link, A. Fusobacterium nucleatum is associated with worse prognosis in Lauren’s diffuse type gastric cancer patients. Sci. Rep. 2020, 10, 16240. [Google Scholar] [CrossRef]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, K.; Zhang, P.; Zheng, J.; Min, C.; Li, X. Research progress of pancreas-related microorganisms and pancreatic cancer. Front. Oncol. 2021, 10, 604531. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef]
- Yamamura, K.; Izumi, D.; Kandimalla, R.; Sonohara, F.; Baba, Y.; Yoshida, N.; Kodera, Y.; Baba, H.; Goel, A. Intratumoral Fusobacterium nucleatum levels predict therapeutic response to neoadjuvant chemotherapy in esophageal squamous cell carcinoma. Clin. Cancer Res. 2019, 25, 6170–6179. [Google Scholar] [CrossRef]
- Al-Hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Yeh, Y.M.; Yu, H.Y.; Chin, C.Y.; Hsu, C.W.; Liu, H.; Huang, P.J.; Hu, S.N.; Liao, C.T.; Chang, K.P.; et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Kurt, M.; Yumuk, Z. Diagnostic accuracy of Fusobacterium nucleatum IgA and IgG ELISA test in colorectal cancer. Sci. Rep. 2021, 11, 1608. [Google Scholar] [CrossRef]
- Wang, H.F.; Li, L.F.; Guo, S.H.; Zeng, Q.Y.; Ning, F.; Liu, W.L.; Zhang, G. Evaluation of antibody level against Fusobacterium nucleatum in the serological diagnosis of colorectal cancer. Sci. Rep. 2016, 6, 33440. [Google Scholar] [CrossRef]
- Butt, J.; Jenab, M.; Pawlita, M.; Overvad, K.; Tjonneland, A.; Olsen, A.; Boutron-Ruault, M.C.; Carbonnel, F.; Mancini, F.R.; Kaaks, R.; et al. Antibody responses to Fusobacterium nucleatum proteins in prediagnostic blood samples are not associated with risk of developing colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1552–1555. [Google Scholar] [CrossRef]
- Alkharaan, H.; Lu, L.; Gabarrini, G.; Halimi, A.; Ateeb, Z.; Sobkowiak, M.J.; Davanian, H.; Fernández Moro, C.; Jansson, L.; Del Chiaro, M.; et al. Circulating and salivary antibodies to Fusobacterium nucleatum are associated with cystic pancreatic neoplasm malignancy. Front. Immunol. 2020, 11, 2003. [Google Scholar] [CrossRef]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef]
- Wouters, M.C.A.; Nelson, B.H. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef]
- Mima, K.; Sukawa, Y.; Nishihara, R.; Qian, Z.R.; Yamauchi, M.; Inamura, K.; Kim, S.A.; Masuda, A.; Nowak, J.A.; Nosho, K.; et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015, 1, 653–661. [Google Scholar] [CrossRef]
- Olsen, I.; Yilmaz, Ö. Possible role of Porphyromonas gingivalis in orodigestive cancers. J. Oral Microbiol. 2019, 11, 1563410. [Google Scholar] [CrossRef]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, J.; Binder Gallimidi, A.; Saba, E.; Pandi, K.; Eli Berchoer, L.; Hermano, E.; Angabo, S.; Makkawi, H.A.; Khashan, A.; Daoud, A.; et al. Intracellular Porphyromonas gingivalis promotes the tumorigenic behavior of pancreatic carcinoma cells. Cancers 2020, 12, 2331. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Jia, Y.; Chen, X.; Li, H.; Wang, Z.; Cheng, B. Intracellular Porphyromonas gingivalis promotes the proliferation of colorectal cancer cells via the MAPK/ERK signaling pathway. Front. Cell. Infect. Microbiol. 2020, 10, 584798. [Google Scholar] [CrossRef] [PubMed]
- Sztukowska, M.N.; Ojo, A.; Ahmed, S.; Carenbauer, A.L.; Wang, Q.; Shumway, B.; Jenkinson, H.F.; Wang, H.; Darling, D.S.; Lamont, R.J. Porphyromonas gingivalis initiates a mesenchymal-like transition through ZEB1 in gingival epithelial cells. Cell. Microbiol. 2016, 18, 844–858. [Google Scholar] [CrossRef]
- Vincents, B.; Guentsch, A.; Kostolowska, D.; von Pawel-Rammingen, U.; Eick, S.; Potempa, J.; Abrahamson, M. Cleavage of IgG1 and IgG3 by gingipain K from Porphyromonas gingivalis may compromise host defense in progressive periodontitis. FASEB J. 2011, 25, 3741–3750. [Google Scholar] [CrossRef]
- Khalaf, H.; Bengtsson, T. Altered T-cell responses by the periodontal pathogen Porphyromonas gingivalis. PLoS ONE 2012, 7, e45192. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, X.; Peng, X.; Li, M.; Ren, B.; Cheng, G.; Cheng, L. Porphyromonas gingivalis promotes immunoevasion of oral cancer by protecting cancer from macrophage attack. J. Immunol. 2020, 205, 282–289. [Google Scholar] [CrossRef]
- Groeger, S.; Denter, F.; Lochnit, G.; Schmitz, M.L.; Meyle, J. Porphyromonas gingivalis cell wall components induce programmed death ligand 1 (PD-L1) expression on human oral carcinoma cells by a receptor-interacting protein kinase 2 (RIP2)-dependent mechanism. Infect. Immun. 2020, 88, e00051-20. [Google Scholar] [CrossRef]
- Song, J.M.; Woo, B.H.; Lee, J.H.; Yoon, S.; Cho, Y.; Kim, Y.D.; Park, H.R. Oral administration of Porphyromonas gingivalis, a major pathogen of chronic periodontitis, promotes resistance to paclitaxel in mouse xenografts of oral squamous cell carcinoma. Int. J. Mol. Sci. 2019, 20, 2494. [Google Scholar] [CrossRef]
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J.; et al. Porphyromonas gingivalis promotes oral squamous cell carcinoma progression in an immune microenvironment. J. Dent. Res. 2020, 99, 666–675. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Ma, Z.; Liang, S.; Shan, T.; Zhang, M.; Zhu, X.; Zhang, P.; Liu, G.; Zhou, F.; et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agent. Cancer 2016, 11, 3. [Google Scholar] [CrossRef]
- Gao, S.G.; Yang, J.Q.; Ma, Z.K.; Yuan, X.; Zhao, C.; Wang, G.C.; Wei, H.; Feng, X.S.; Qi, Y.J. Preoperative serum immunoglobulin G and A antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer 2018, 18, 17. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Hong, X.; Zhang, Y.; Zou, X. Databases and bioinformatic tools for glycobiology and glycoproteomics. Int. J. Mol. Sci. 2020, 21, 6727. [Google Scholar] [CrossRef]
- Geissner, A.; Reinhardt, A.; Rademacher, C.; Johannssen, T.; Monteiro, J.; Lepenies, B.; Thépaut, M.; Fieschi, F.; Mrázková, J.; Wimmerova, M.; et al. Microbe-focused glycan array screening platform. Proc. Natl. Acad. Sci. USA 2019, 116, 1958–1967. [Google Scholar] [CrossRef]
- Von Gunten, S.; Smith, D.F.; Cummings, R.D.; Riedel, S.; Miescher, S.; Schaub, A.; Hamilton, R.G.; Bochner, B.S. Intravenous immunoglobulin contains a broad repertoire of anticarbohydrate antibodies that is not restricted to the IgG2 subclass. J. Allergy Clin. Immunol. 2009, 123, 1268–1276.e15. [Google Scholar] [CrossRef] [PubMed]
- Miho, E.; Yermanos, A.; Weber, C.R.; Berger, C.T.; Reddy, S.T.; Greiff, V. Computational strategies for dissecting the high-dimensional complexity of adaptive immune repertoires. Front. Immunol. 2018, 9, 224. [Google Scholar] [CrossRef]
- Sanschagrin, S.; Yergeau, E. Next-generation sequencing of 16S ribosomal RNA gene amplicons. J. Vis. Exp. 2014, 90, 51709. [Google Scholar] [CrossRef]
- Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; De Maio, G.; Ulivi, P. Heterogeneity in colorectal cancer: A challenge for personalized medicine? Int. J. Mol. Sci. 2018, 19, 3733. [Google Scholar] [CrossRef]
- Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Santos, L.L.; Ferreira, J.A. Protein glycosylation and tumor microenvironment alterations driving cancer hallmarks. Front. Oncol. 2019, 9, 380. [Google Scholar] [CrossRef]
| AG IgGs | Total n cases | Increase ≥ 2, n | Decrease ≥ 2, n | Change in % |
|---|---|---|---|---|
| TF (Galβ1-3GalNAcα) | 109 | 8 | 3 | 10 |
| Tn (GalNAcα) | 109 | 8 | 7 | 14 |
| αGal (Galα1-3Galβ) | 109 | 12 | 18 | 28 |
| GalNAcβ | 85 | 1 | 1 | 2 |
| PFdi (GalNAcβ1-3GalNAcβ) | 85 | 7 | 8 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smorodin, E.P. Prospects and Challenges of the Study of Anti-Glycan Antibodies and Microbiota for the Monitoring of Gastrointestinal Cancer. Int. J. Mol. Sci. 2021, 22, 11608. https://doi.org/10.3390/ijms222111608
Smorodin EP. Prospects and Challenges of the Study of Anti-Glycan Antibodies and Microbiota for the Monitoring of Gastrointestinal Cancer. International Journal of Molecular Sciences. 2021; 22(21):11608. https://doi.org/10.3390/ijms222111608
Chicago/Turabian StyleSmorodin, Eugeniy P. 2021. "Prospects and Challenges of the Study of Anti-Glycan Antibodies and Microbiota for the Monitoring of Gastrointestinal Cancer" International Journal of Molecular Sciences 22, no. 21: 11608. https://doi.org/10.3390/ijms222111608
APA StyleSmorodin, E. P. (2021). Prospects and Challenges of the Study of Anti-Glycan Antibodies and Microbiota for the Monitoring of Gastrointestinal Cancer. International Journal of Molecular Sciences, 22(21), 11608. https://doi.org/10.3390/ijms222111608






