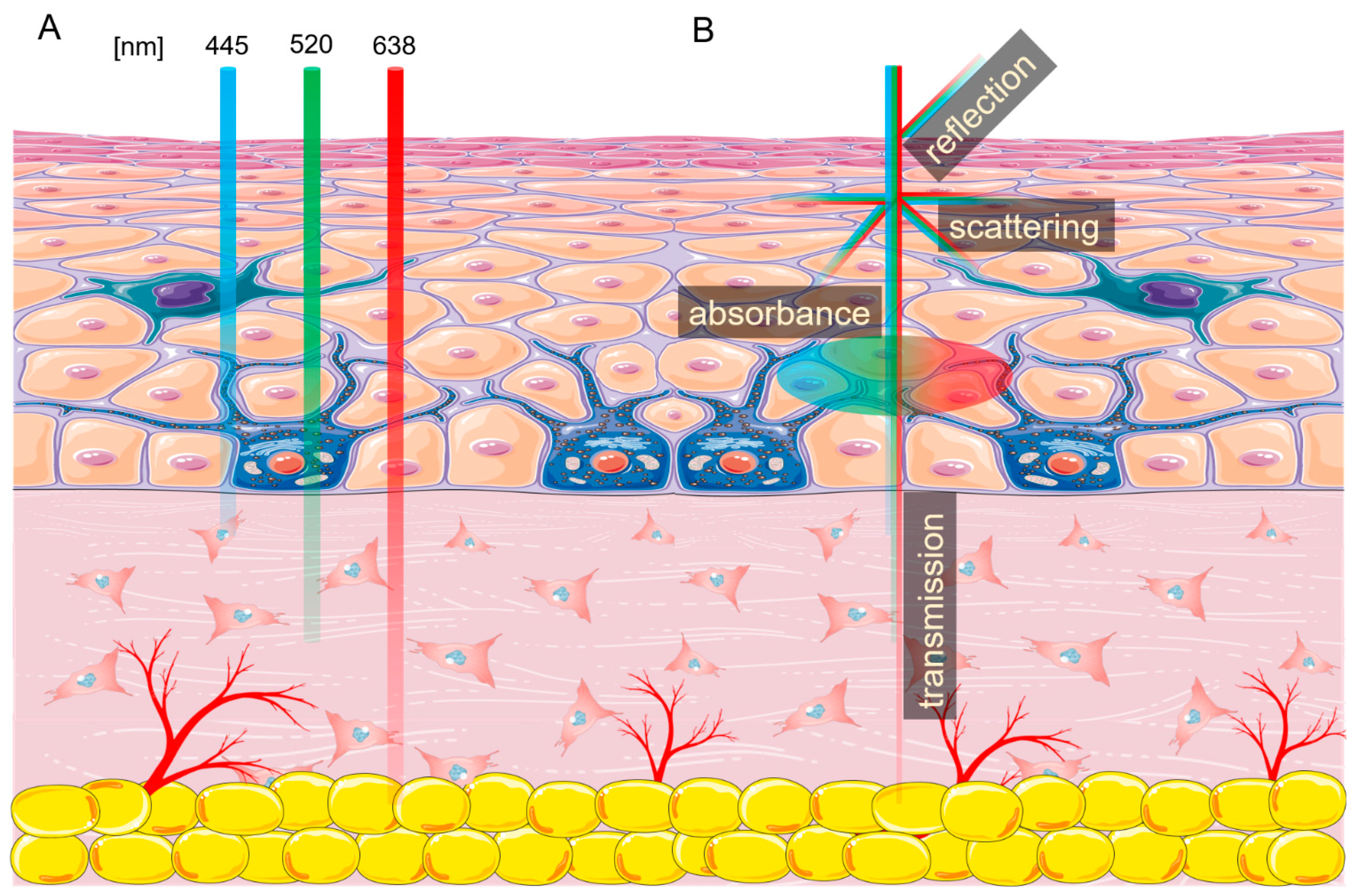
Effects of 445 nm, 520 nm, and 638 nm Laser Irradiation on the Dermal Cells
Abstract
:1. Introduction
2. Results
2.1. Biological Effects of 455 nm Laser Radiation
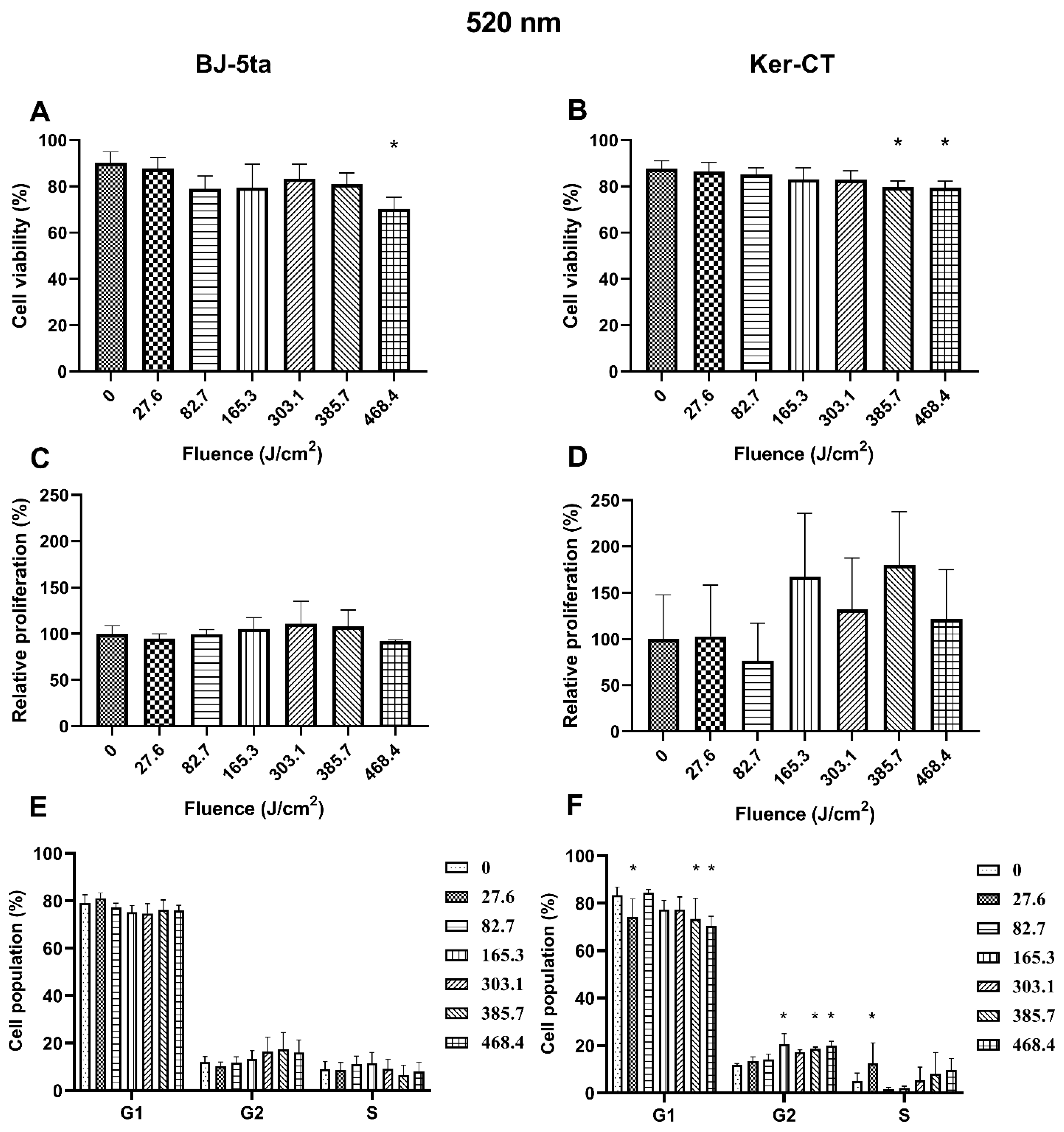
2.2. Biological Effects of 520 nm Laser Radiation
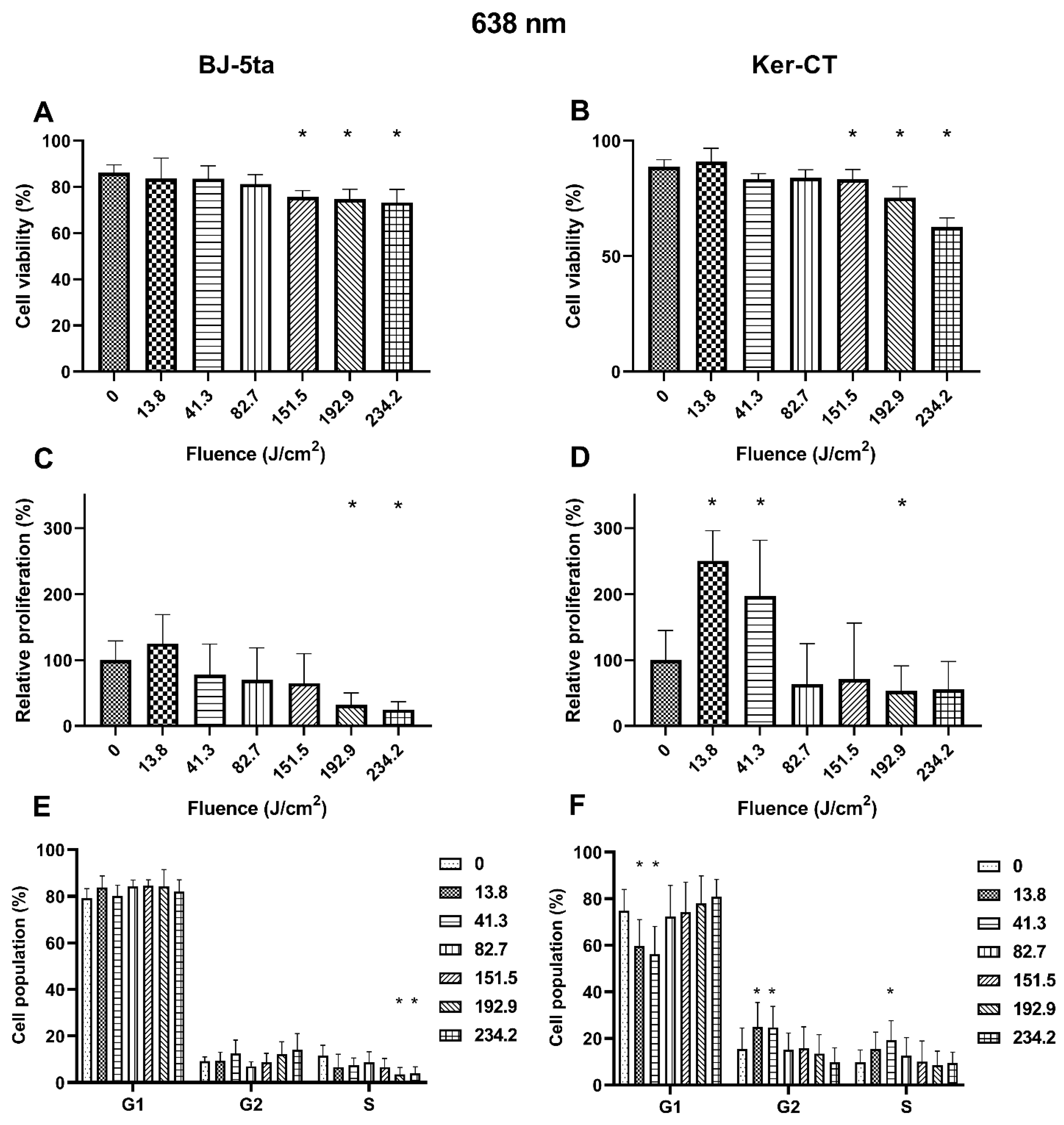
2.3. Biological Effects of 638 nm Laser Radiation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
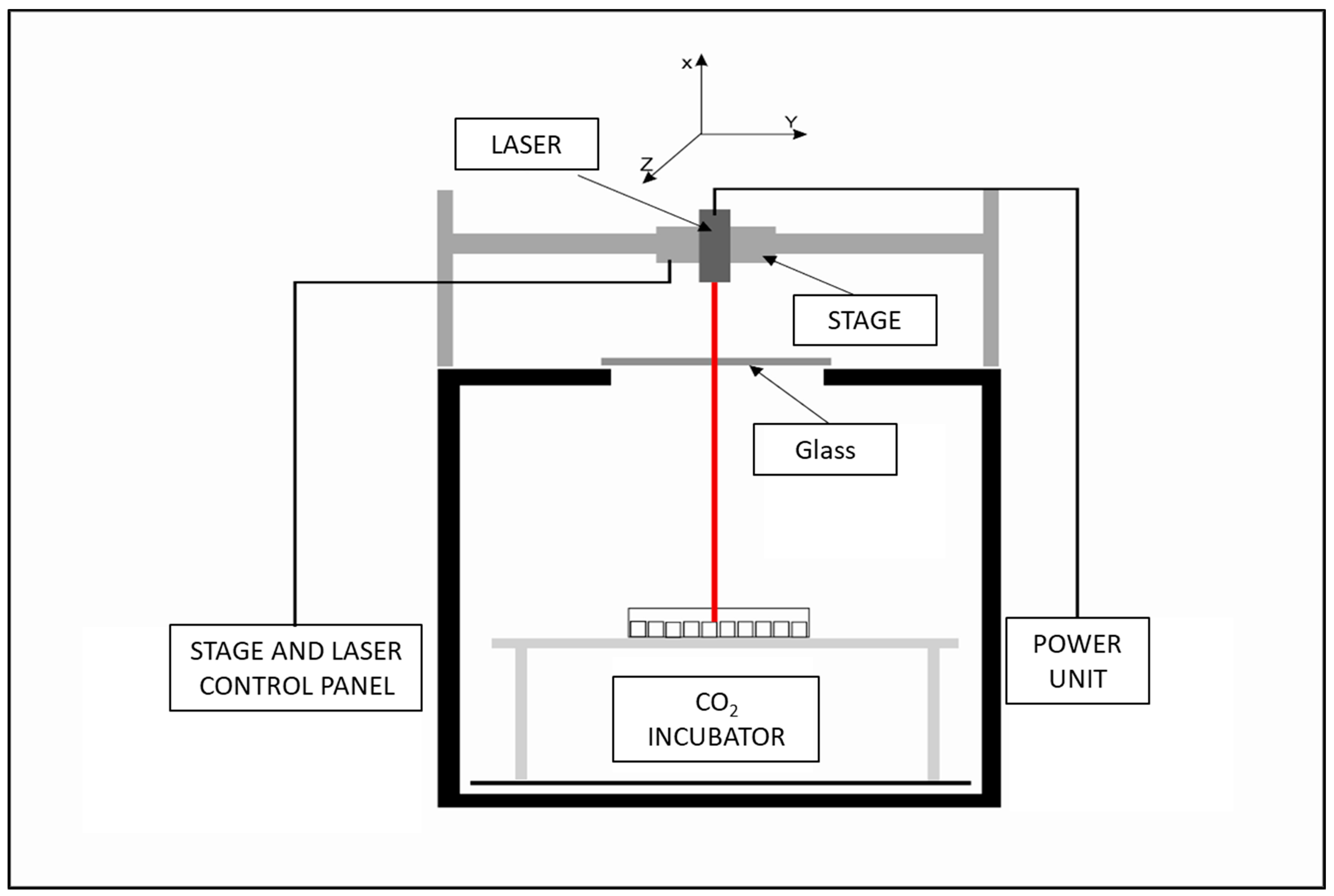
4.2. Laser Exposure System
4.3. Laser Exposure
4.4. Cell Viability
4.5. Proliferation (Ki67 Assay) and Cell Cycle
4.6. Statistical Analysis
4.7. Calculations
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cios, A.; Ciepielak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef]
- Kaneko, S. Safety Guidelines for Diagnostic and Therapeutic Laser Applications in the Neurosurgical Field. Laser Ther. 2012, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Cotler, H.B.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The Use of Low Level Laser Therapy (LLLT) For Musculoskeletal Pain. MOJ Orthop. Rheumatol. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.M.A.; Barkokebas, A.; Pires, A.P.; Barros, L.F.; Carvalho, A.A.T.; Leão, J.C. Current Use and Future Perspectives of Diagnostic and Therapeutic Lasers in Oral Medicine. Minerva Stomatol. 2008, 57, 511–517. [Google Scholar] [PubMed]
- Km, B. Lasers in Urology. Available online: https://pubmed.ncbi.nlm.nih.gov/7651053/ (accessed on 16 September 2020).
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-Level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of Wavelength and Beam Width on Penetration in Light-Tissue Interaction Using Computational Methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Cardoso, L.M.; Citta, M.; Soares, D.G.; Scheffel, D.S.; Hebling, J.; de Souza Costa, C.A. Epithelial Cell-Enhanced Metabolism by Low-Level Laser Therapy and Epidermal Growth Factor. Lasers Med. Sci. 2018, 33, 445–449. [Google Scholar] [CrossRef]
- Lister, T.; Wright, P.A.; Chappell, P.H. Optical Properties of Human Skin. JBO 2012, 17, 090901. [Google Scholar] [CrossRef]
- Patil, U.A.; Dhami, L.D. Overview of Lasers. Indian J. Plast. Surg. 2008, 41, S101–S113. [Google Scholar] [CrossRef]
- Cvetkovic´ M, P.D.; Peratta, A. Fetd Computation of the Temperature Distribution Induced Into a Human Eye by a Pulsed Laser. Prog. Electromagn. Res. 2011, 120, 403–421. [Google Scholar] [CrossRef]
- Cvetkovi´c, M.; Peratta, A.; Poljak, D. Thermal Modelling Of The Human Eye Exposed To Infrared Radiation Of 1064 Nm Nd:YAG And 2090 Nm Ho:YAG Lasers. Environ. Health Risk 2009, 14, 221–231. [Google Scholar] [CrossRef]
- Mirnezami, S.A.; Rajaei Jafarabadi, M.; Abrishami, M. Temperature Distribution Simulation of the Human Eye Exposed to Laser Radiation. J. Lasers Med. Sci. 2013, 4, 175–181. [Google Scholar]
- Husain, Z.; Alster, T.S. The Role of Lasers and Intense Pulsed Light Technology in Dermatology. Clin. Cosmet. Investig. Dermatol. 2016, 9, 29–40. [Google Scholar] [CrossRef]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological Effects of Low Level Laser Therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar]
- Martignago, C.C.S.; Oliveira, R.F.; Pires-Oliveira, D.A.A.; Oliveira, P.D.; Pacheco Soares, C.; Monzani, P.S.; Poli-Frederico, R.C. Effect of Low-Level Laser Therapy on the Gene Expression of Collagen and Vascular Endothelial Growth Factor in a Culture of Fibroblast Cells in Mice. Lasers Med. Sci. 2015, 30, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.S.; Moreira, T.O.; Paixão, D.L.; Farias, F.M.; Guimarães, O.R.; de Paoli, S.; Geller, M.; de Paoli, F. Effect of Laser Therapy on DNA Damage. Lasers Surg. Med. 2010, 42, 481–488. [Google Scholar] [CrossRef]
- Ganjali, M.; Seifalian, A.M.; Mozafari, M. Effect of Laser Irradiation on Cell Cycle and Mitosis. J. Lasers Med. Sci. 2018, 9, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Khalkhal, E.; Razzaghi, M.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Heidari Beigvand, H.; Rezaei Tavirani, M. Evaluation of Laser Effects on the Human Body After Laser Therapy. J. Lasers Med. Sci. 2020, 11, 91–97. [Google Scholar] [CrossRef]
- Alam, M.; Warycha, M. Complications of Lasers and Light Treatments. Dermatol. Ther. 2011, 24, 571–580. [Google Scholar] [CrossRef]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wikramanayake, T.C.; Stojadinovic, O.; Tomic-Canic, M. Epidermal Differentiation in Barrier Maintenance and Wound Healing. Adv. Wound Care (New Rochelle) 2014, 3, 272–280. [Google Scholar] [CrossRef]
- Mignon, C.; Uzunbajakava, N.E.; Castellano-Pellicena, I.; Botchkareva, N.V.; Tobin, D.J. Differential Response of Human Dermal Fibroblast Subpopulations to Visible and Near-Infrared Light: Potential of Photobiomodulation for Addressing Cutaneous Conditions. Lasers Surg. Med. 2018. [Google Scholar] [CrossRef]
- Mamalis, A.; Garcha, M.; Jagdeo, J. Light Emitting Diode-Generated Blue Light Modulates Fibrosis Characteristics: Fibroblast Proliferation, Migration Speed, and Reactive Oxygen Species Generation. Lasers Surg. Med. 2015, 47, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Darzynkiewicz, Z. Cell Cycle Dependent Expression and Stability of the Nuclear Protein Detected by Ki-67 Antibody in HL-60 Cells. Cell Prolif. 1992, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Zhao, H.; Zhang, S.; Lee, M.Y.W.T.; Lee, E.Y.C.; Zhang, Z. Initiation and Termination of DNA Replication during S Phase in Relation to Cyclins D1, E and A, P21WAF1, Cdt1 and the P12 Subunit of DNA Polymerase δ Revealed in Individual Cells by Cytometry. Oncotarget 2015, 6, 11735–11750. [Google Scholar] [CrossRef] [PubMed]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of Blue Light Irradiation on Human Dermal Fibroblasts. J. Photochem. Photobiol. B 2011, 103, 118–125. [Google Scholar] [CrossRef]
- Freije, A.; Ceballos, L.; Coisy, M.; Barnes, L.; Rosa, M.; De Diego, E.; Blanchard, J.M.; Gandarillas, A. Cyclin E Drives Human Keratinocyte Growth into Differentiation. Oncogene 2012, 31, 5180–5192. [Google Scholar] [CrossRef]
- Zanet, J.; Freije, A.; Ruiz, M.; Coulon, V.; Sanz, J.R.; Chiesa, J.; Gandarillas, A. A Mitosis Block Links Active Cell Cycle with Human Epidermal Differentiation and Results in Endoreplication. PLoS ONE 2010, 5, e15701. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-Light Irradiation Regulates Proliferation and Differentiation in Human Skin Cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef]
- Jurczyszyn, K.; Trzeciakowski, W.; Woźniak, Z.; Ziółkowski, P.; Trafalski, M. Assessment of Effects of Laser Light Combining Three Wavelengths (450, 520 and 640 Nm) on Temperature Increase and Depth of Tissue Lesions in an Ex Vivo Study. Materials 2020, 13, 5340. [Google Scholar] [CrossRef]
- Rieder, C.L.; Maiato, H. Stuck in Division or Passing through: What Happens When Cells Cannot Satisfy the Spindle Assembly Checkpoint. Dev. Cell 2004, 7, 637–651. [Google Scholar] [CrossRef]
- De Souza, C.P.; Hashmi, S.B.; Yang, X.; Osmani, S.A. Regulated Inactivation of the Spindle Assembly Checkpoint without Functional Mitotic Spindles. EMBO J. 2011, 30, 2648–2661. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Han, S.I.; Oh, S.Y.; Kang, H.S. Cellular Responses to Mild Heat Stress. Cell. Mol. Life Sci. 2005, 62, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Rennekampff, H.-O.; Alharbi, Z. Burn Injury: Mechanisms of Keratinocyte Cell Death. Med. Sci. 2021, 9, 51. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Simões, A.; Corrêa, L.; Aranha, A.C.C.; Giudice, F.S.; Hamblin, M.R.; Sousa, S.C.O.M. Low-Level Laser Irradiation Promotes the Proliferation and Maturation of Keratinocytes during Epithelial Wound Repair. J. Biophotonics 2015, 8, 795–803. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jekal, S.-J.; Kwon, P.-S. 630 Nm Light Emitting Diode Irradiation Improves Dermal Wound Healing in Rats. J. Korean Phys. Ther. 2015, 27, 140–146. [Google Scholar] [CrossRef]
- Szymanski, L.; Cios, A.; Lewicki, S.; Szymanski, P.; Stankiewicz, W. Fas/FasL Pathway and Cytokines in Keratinocytes in Atopic Dermatitis – Manipulation by the Electromagnetic Field. PLoS ONE 2018, 13, e0205103. [Google Scholar] [CrossRef]
- Złotek, U.; Jakubczyk, A.; Rybczyńska-Tkaczyk, K.; Ćwiek, P.; Baraniak, B.; Lewicki, S. Characteristics of New Peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as Inhibitors of Enzymes Involved in Metabolic Syndrome and Antimicrobial Potential. Molecules 2020, 25, 2492. [Google Scholar] [CrossRef]
- Szymański, Ł.; Jęderka, K.; Cios, A.; Ciepelak, M.; Lewicka, A.; Stankiewicz, W.; Lewicki, S. A Simple Method for the Production of Human Skin Equivalent in 3D, Multi-Cell Culture. Int. J. Mol. Sci. 2020, 21, 4644. [Google Scholar] [CrossRef]

| BJ-5ta | Ker-CT | |
|---|---|---|
| 445 nm | <59.7 J/cm2 | >204.1 J/cm2 |
| 520 nm | <468.4 J/cm2 | <385.7 J/cm2 |
| 638 nm | <151.5 J/cm2 | <151.5 J/cm2 |
| Wavelength (nm) | Power during Continuous-Wave Operation (mW) | Effective Power (mW) | Beam Diameter (mm) |
|---|---|---|---|
| 445 | 500 | 200 | 5 |
| 520 | 1000 | 600 | 5 |
| 638 | 700 | 300 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymański, Ł.; Ciepielak, M.; Cios, A.; Palusińska, M.; Stankiewicz, W.; Lewicki, S. Effects of 445 nm, 520 nm, and 638 nm Laser Irradiation on the Dermal Cells. Int. J. Mol. Sci. 2021, 22, 11605. https://doi.org/10.3390/ijms222111605
Szymański Ł, Ciepielak M, Cios A, Palusińska M, Stankiewicz W, Lewicki S. Effects of 445 nm, 520 nm, and 638 nm Laser Irradiation on the Dermal Cells. International Journal of Molecular Sciences. 2021; 22(21):11605. https://doi.org/10.3390/ijms222111605
Chicago/Turabian StyleSzymański, Łukasz, Martyna Ciepielak, Aleksandra Cios, Małgorzata Palusińska, Wanda Stankiewicz, and Sławomir Lewicki. 2021. "Effects of 445 nm, 520 nm, and 638 nm Laser Irradiation on the Dermal Cells" International Journal of Molecular Sciences 22, no. 21: 11605. https://doi.org/10.3390/ijms222111605
APA StyleSzymański, Ł., Ciepielak, M., Cios, A., Palusińska, M., Stankiewicz, W., & Lewicki, S. (2021). Effects of 445 nm, 520 nm, and 638 nm Laser Irradiation on the Dermal Cells. International Journal of Molecular Sciences, 22(21), 11605. https://doi.org/10.3390/ijms222111605






