Retinal Degeneration and Microglial Dynamics in Mature Progranulin-Deficient Mice
Abstract
:1. Introduction
2. Results
2.1. Photoreceptor Degeneration in PGRN-Deficient Mouse Retina
2.2. Subretinal Deposits in PGRN-Deficient Mouse
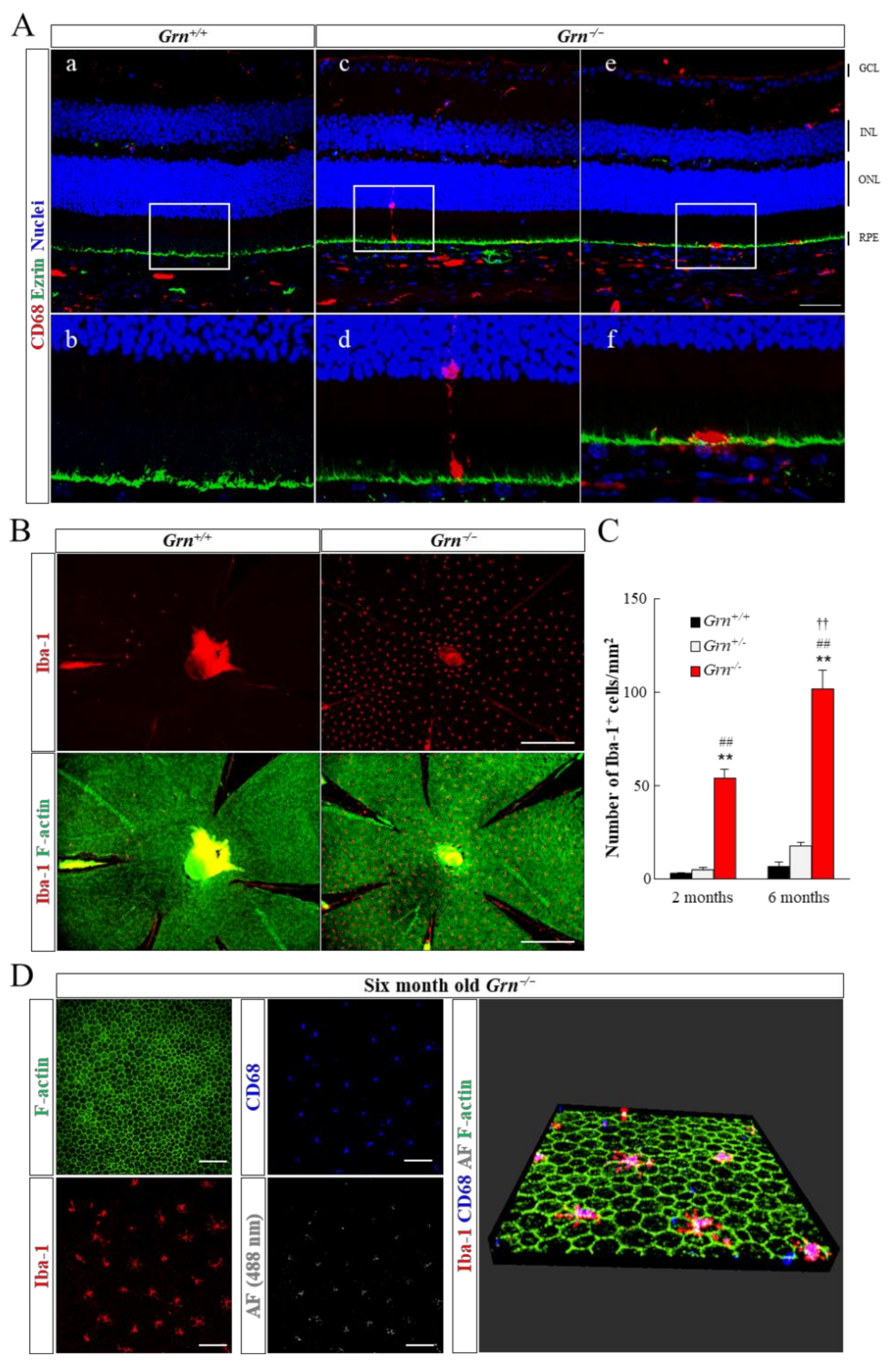
2.3. Infiltration of Retinal Microglia into Subretinal Area in PGRN-Deficient Mouse
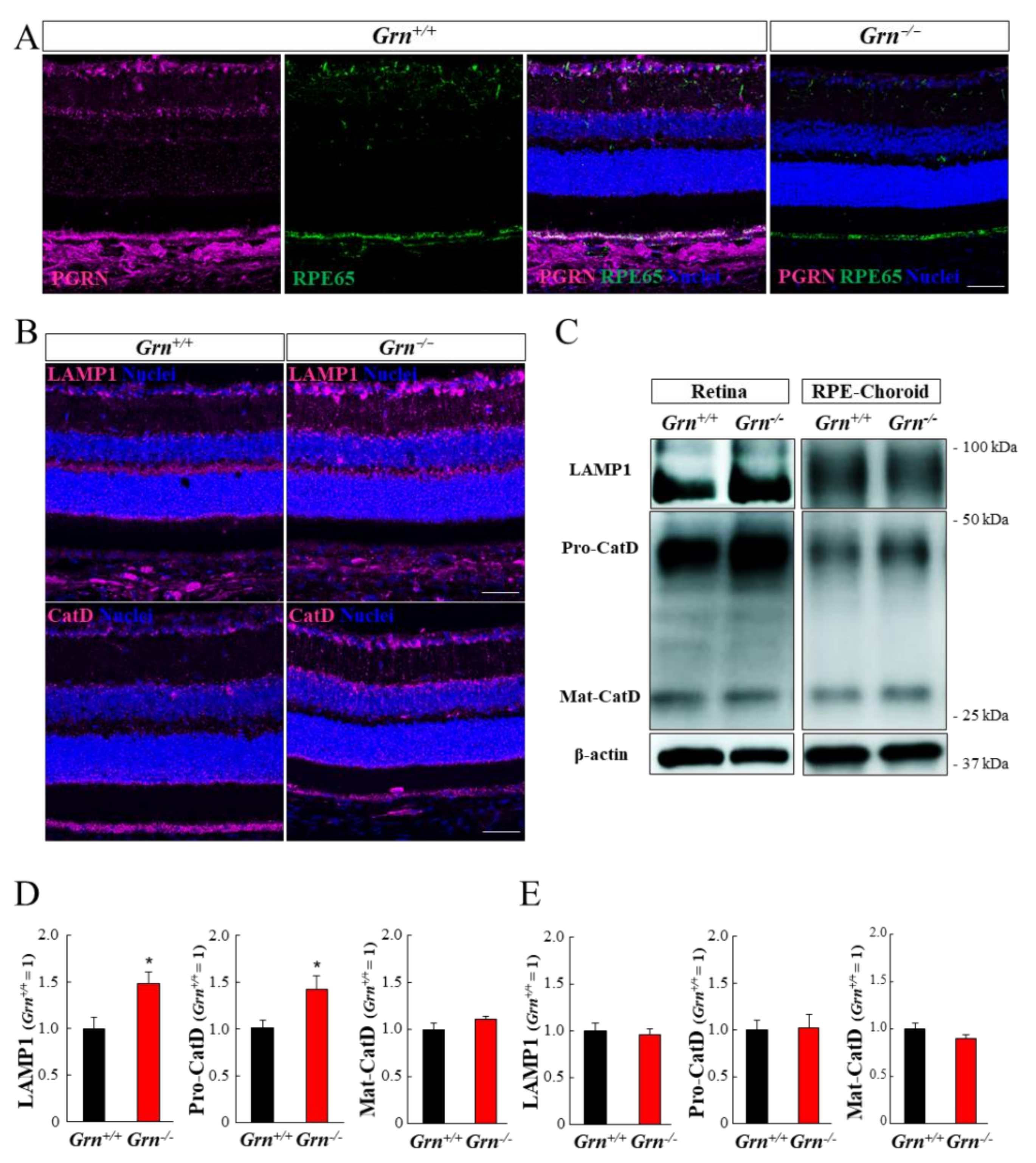
2.4. Expression Pattern of PGRN and Lysosomal Proteins in the Mouse Retina and Retinal Pigment Epithelium-Choroid Complex
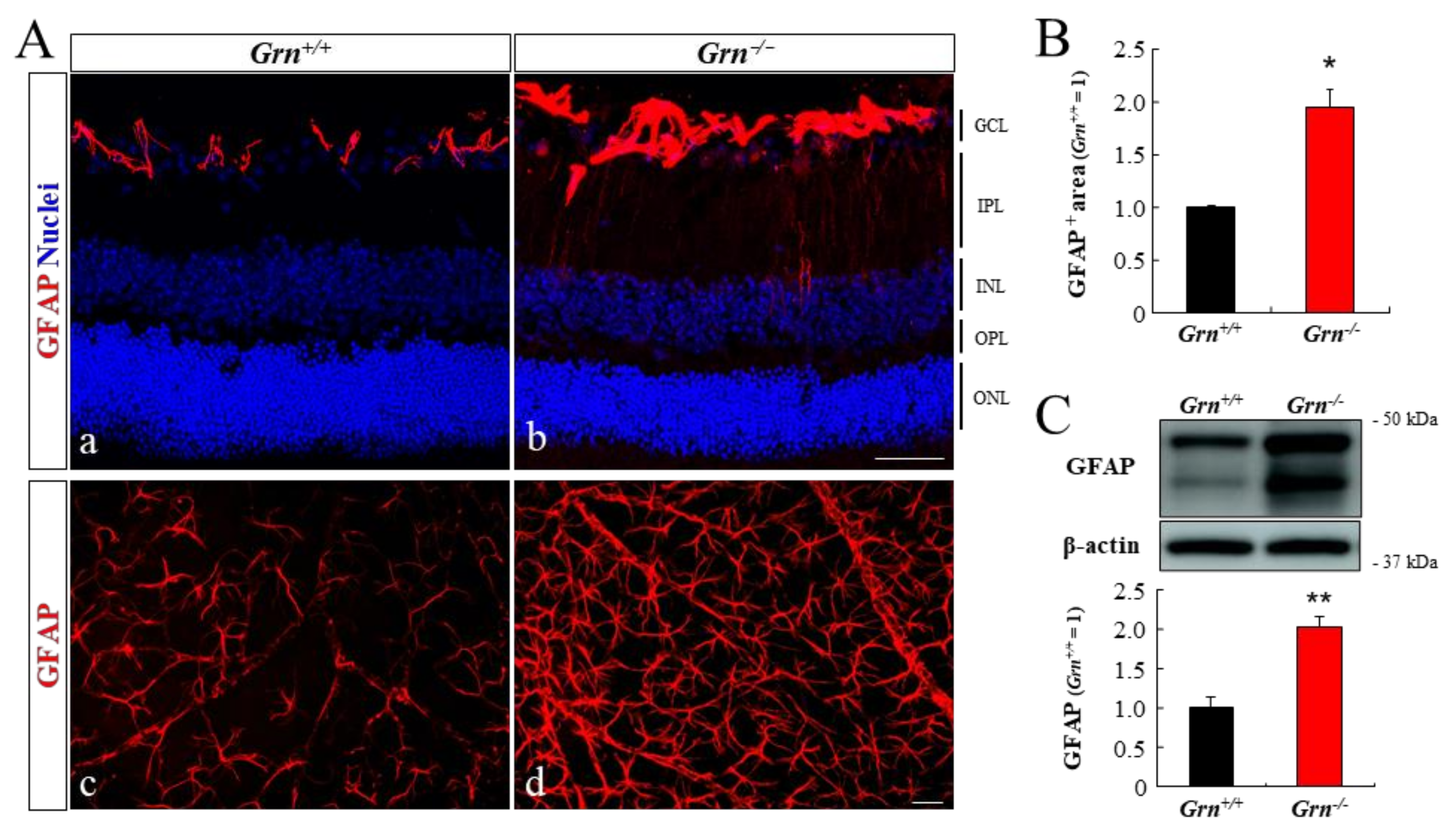
2.5. Astrogliosis in PGRN-Deficient Mouse Retina
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Fundus Photography and Optical Coherence Tomography
4.3. Scotopic-Electroretinograms
4.4. Histological Analyses
4.5. Immunohistochemistry of Ocular Sections and Flatmount
4.6. Western Blot Analysis
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kao, A.W.; McKay, A.; Singh, P.P.; Brunet, A.; Huang, E.J. Progranulin, lysosomal regulation and neurodegenerative disease. Nat. Rev. Neurosci. 2017, 18, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Paushter, D.H.; Du, H.; Feng, T.; Hu, F. The lysosomal function of progranulin, a guardian against neurodegeneration. Acta Neuropathol. 2018, 136, 1–17. [Google Scholar] [CrossRef]
- Tanaka, Y.; Suzuki, G.; Matsuwaki, T.; Hosokawa, M.; Serrano, G.; Beach, T.G.; Yamanouchi, K.; Hasegawa, M.; Nishihara, M. Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes. Hum. Mol. Genet. 2017, 26, 969–988. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Irwin, D.J.; Song, D.; Daniel, E.; Leveque, J.D.; Raquib, A.R.; Pan, W.; Ying, G.-S.; Aleman, T.S.; Dunaief, J.L.; et al. Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology 2017, 89, 1604–1611. [Google Scholar] [CrossRef]
- Atiskova, Y.; Kohlschütter, A.; Spitzer, M.S.; Dulz, S. Ophthalmological manifestations of neuronal ceroid lipofuscinoses (NCL): NCL as diseases of brain and retina-the role of ophthalmologists. Ophthalmologe 2021, 118, 113–118. [Google Scholar] [CrossRef]
- Ward, M.E.; Chen, R.; Huang, H.-Y.; Ludwig, C.; Telpoukhovskaia, M.; Taubes, A.; Boudin, H.; Minami, S.S.; Reichert, M.; Albrecht, P.; et al. Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis. Sci. Transl. Med. 2017, 9, eaah5642. [Google Scholar] [CrossRef] [Green Version]
- Huin, V.; Barbier, M.; Bottani, A.; Lobrinus, J.A.; Clot, F.; Lamari, F.; Chat, L.; Rucheton, B.; Fluchère, F.; Auvin, S.; et al. Homozygous GRN mutations: New phenotypes and new insights into pathological and molecular mechanisms. Brain 2020, 143, 303–319. [Google Scholar] [CrossRef]
- Atiskova, Y.; Bartsch, S.; Danyukova, T.; Becker, E.; Hagel, C.; Storch, S.; Bartsch, U. Mice deficient in the lysosomal enzyme palmitoyl-protein thioesterase 1 (PPT1) display a complex retinal phenotype. Sci. Rep. 2019, 9, 14185. [Google Scholar] [CrossRef] [PubMed]
- Seigel, G.M.; Lotery, A.; Kummer, A.; Bernard, D.J.; Greene, N.D.E.; Turmaine, M.; Derksen, T.; Nussbaum, R.L.; Davidson, B.; Wagner, J.; et al. Retinal pathology and function in a Cln3 knockout mouse model of juvenile Neuronal Ceroid Lipofuscinosis (batten disease). Mol. Cell. Neurosci. 2002, 19, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, H.; Keksa-Goldsteine, V.; Ragauskas, S.; Kohlmann, P.; Singh, Y.; Savchenko, E.; Puranen, J.; Malm, T.; Kalesnykas, G.; Koistinaho, J.; et al. Retinal Degeneration In A Mouse Model Of CLN5 Disease Is Associated With Compromised Autophagy. Sci. Rep. 2017, 7, 1597. [Google Scholar] [CrossRef] [Green Version]
- Jankowiak, W.; Brandenstein, L.; Dulz, S.; Hagel, C.; Storch, S.; Bartsch, U. Retinal Degeneration in Mice Deficient in the Lysosomal Membrane Protein CLN7. Invest. Ophthalmol. Vis. Sci. 2016, 57, 4989–4998. [Google Scholar] [CrossRef] [Green Version]
- Bassal, M.; Liu, J.; Jankowiak, W.; Saftig, P.; Bartsch, U. Rapid and Progressive Loss of Multiple Retinal Cell Types in Cathepsin D-Deficient Mice-An Animal Model of CLN10 Disease. Cells 2021, 10, 696. [Google Scholar] [CrossRef]
- Hafler, B.P.; Klein, Z.A.; Jimmy Zhou, Z.; Strittmatter, S.M. Progressive retinal degeneration and accumulation of autofluorescent lipopigments in Progranulin deficient mice. Brain Res. 2014, 1588, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, M.E.; Taubes, A.; Chen, R.; Miller, B.L.; Sephton, C.F.; Gelfand, J.M.; Minami, S.; Boscardin, J.; Martens, L.H.; Seeley, W.W.; et al. Early retinal neurodegeneration and impaired Ran-mediated nuclear import of TDP-43 in progranulin-deficient FTLD. J. Exp. Med. 2014, 211, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Kuse, Y.; Tsuruma, K.; Sugitani, S.; Izawa, H.; Ohno, Y.; Shimazawa, M.; Hara, H. Progranulin promotes the retinal precursor cell proliferation and the photoreceptor differentiation in the mouse retina. Sci. Rep. 2016, 6, 23811. [Google Scholar] [CrossRef] [Green Version]
- Kuse, Y.; Tsuruma, K.; Mizoguchi, T.; Shimazawa, M.; Hara, H. Progranulin deficiency causes the retinal ganglion cell loss during development. Sci. Rep. 2017, 7, 1679. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.R.; Macário, M.C.; Ramos, L.; Baldeiras, I.; Ribeiro, M.H.; Santana, I. Portuguese family with the co-occurrence of frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis phenotypes due to progranulin gene mutation. Neurobiol. Aging 2016, 41, 200.e1–200.e5. [Google Scholar] [CrossRef]
- Saszik, S.M.; Robson, J.G.; Frishman, L.J. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J. Physiol. 2002, 543, 899–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruma, K.; Yamauchi, M.; Sugitani, S.; Otsuka, T.; Ohno, Y.; Nagahara, Y.; Ikegame, Y.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; et al. Progranulin, a major secreted protein of mouse adipose-derived stem cells, inhibits light-induced retinal degeneration. Stem Cells Transl. Med. 2014, 3, 42–53. [Google Scholar] [CrossRef]
- Tanaka, M.; Kuse, Y.; Nakamura, S.; Hara, H.; Shimazawa, M. Potential effects of progranulin and granulins against retinal photoreceptor cell degeneration. Mol. Vis. 2019, 25, 902–911. [Google Scholar] [PubMed]
- Okunuki, Y.; Mukai, R.; Pearsall, E.A.; Klokman, G.; Husain, D.; Park, D.-H.; Korobkina, E.; Weiner, H.L.; Butovsky, O.; Ksander, B.R.; et al. Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment. Proc. Natl. Acad. Sci. USA 2018, 115, E6264–E6273. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penfold, P.L.; Madigan, M.C.; Gillies, M.C.; Provis, J.M. Immunological and aetiological aspects of macular degeneration. Prog. Retin. Eye Res. 2001, 20, 385–414. [Google Scholar] [CrossRef]
- Gupta, N.; Brown, K.E.; Milam, A.H. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003, 76, 463–471. [Google Scholar] [CrossRef]
- Von Eisenhart-Rothe, P.; Grubman, A.; Greferath, U.; Fothergill, L.J.; Jobling, A.I.; Phipps, J.A.; White, A.R.; Fletcher, E.L.; Vessey, K.A. Failure of Autophagy-Lysosomal Pathways in Rod Photoreceptors Causes the Early Retinal Degeneration Phenotype Observed in Cln6nclf Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5082–5097. [Google Scholar] [CrossRef] [Green Version]
- Raoul, W.; Feumi, C.; Keller, N.; Lavalette, S.; Houssier, M.; Behar-Cohen, F.; Combadière, C.; Sennlaub, F. Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice. Ophthalmic Res. 2008, 40, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Vessey, K.A.; Waugh, M.; Jobling, A.I.; Phipps, J.A.; Ho, T.; Trogrlic, L.; Greferath, U.; Fletcher, E.L. Assessment of Retinal Function and Morphology in Aging Ccl2 Knockout Mice. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1238–1252. [Google Scholar] [CrossRef] [Green Version]
- Makabe, K.; Sugita, S.; Mandai, M.; Futatsugi, Y.; Takahashi, M. Microglia dynamics in retinitis pigmentosa model: Formation of fundus whitening and autofluorescence as an indicator of activity of retinal degeneration. Sci. Rep. 2020, 10, 14700. [Google Scholar] [CrossRef]
- Wang, S.K.; Xue, Y.; Cepko, C.L. Microglia modulation by TGF-β1 protects cones in mouse models of retinal degeneration. J. Clin. Invest. 2020, 130, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Matha, V.; Derocq, D.; Prébois, C.; Katunuma, N.; Liaudet-Coopman, E. Processing of human cathepsin D is independent of its catalytic function and auto-activation: Involvement of cathepsins L and B. J. Biochem. 2006, 139, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kleinberger, G.; Capell, A.; Haass, C.; Van Broeckhoven, C. Mechanisms of granulin deficiency: Lessons from cellular and animal models. Mol. Neurobiol. 2013, 47, 337–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M. Increased lysosomal biogenesis in activated microglia and exacerbated neuronal damage after traumatic brain injury in progranulin-deficient mice. Neuroscience 2013, 250, 8–19. [Google Scholar] [CrossRef]
- Elia, L.P.; Mason, A.R.; Alijagic, A.; Finkbeiner, S. Genetic Regulation of Neuronal Progranulin Reveals a Critical Role for the Autophagy-Lysosome Pathway. J. Neurosci. 2019, 39, 3332–3344. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Nakamura, S.; Otsu, W.; Shimazawa, M.; Hara, H. Progranulin deficiency in Iba-1+ myeloid cells exacerbates choroidal neovascularization by perturbation of lysosomal function and abnormal inflammation. J. Neuroinflamm. 2021, 18, 164. [Google Scholar] [CrossRef]
- Lui, H.; Zhang, J.; Makinson, S.R.; Cahill, M.K.; Kelley, K.W.; Huang, H.-Y.; Shang, Y.; Oldham, M.C.; Martens, L.H.; Gao, F.; et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell 2016, 165, 921–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, D.; Valapala, M.; Shang, P.; Hose, S.; Grebe, R.; Lutty, G.A.; Zigler, J.S.; Kaarniranta, K.; Handa, J.T. Lysosomes: Regulators of autophagy in the retinal pigmented epithelium. Exp. Eye Res. 2016, 144, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Murase, H.; Tsuruma, K.; Kuse, Y.; Shimazawa, M.; Hara, H. Progranulin increases phagocytosis by retinal pigment epithelial cells in culture. J. Neurosci. Res. 2017, 95, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Han, J.; Dalvi, S.; Manian, K.; Winschel, L.; Volland, S.; Soto, C.A.; Galloway, C.A.; Spencer, W.; Roll, M.; et al. A human model of Batten disease shows role of CLN3 in phagocytosis at the photoreceptor-RPE interface. Commun. Biol. 2021, 4, 161. [Google Scholar] [CrossRef]
- Kayasuga, Y.; Chiba, S.; Suzuki, M.; Kikusui, T.; Matsuwaki, T.; Yamanouchi, K.; Kotaki, H.; Horai, R.; Iwakura, Y.; Nishihara, M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav. Brain Res. 2007, 185, 110–118. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Nakamura, S.; Shimazawa, M.; Hara, H. Retinal Degeneration and Microglial Dynamics in Mature Progranulin-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11557. https://doi.org/10.3390/ijms222111557
Takahashi K, Nakamura S, Shimazawa M, Hara H. Retinal Degeneration and Microglial Dynamics in Mature Progranulin-Deficient Mice. International Journal of Molecular Sciences. 2021; 22(21):11557. https://doi.org/10.3390/ijms222111557
Chicago/Turabian StyleTakahashi, Kei, Shinsuke Nakamura, Masamitsu Shimazawa, and Hideaki Hara. 2021. "Retinal Degeneration and Microglial Dynamics in Mature Progranulin-Deficient Mice" International Journal of Molecular Sciences 22, no. 21: 11557. https://doi.org/10.3390/ijms222111557
APA StyleTakahashi, K., Nakamura, S., Shimazawa, M., & Hara, H. (2021). Retinal Degeneration and Microglial Dynamics in Mature Progranulin-Deficient Mice. International Journal of Molecular Sciences, 22(21), 11557. https://doi.org/10.3390/ijms222111557




