BHLHE41/DEC2 Expression Induces Autophagic Cell Death in Lung Cancer Cells and Is Associated with Favorable Prognosis for Patients with Lung Adenocarcinoma
Abstract
:1. Introduction
2. Results
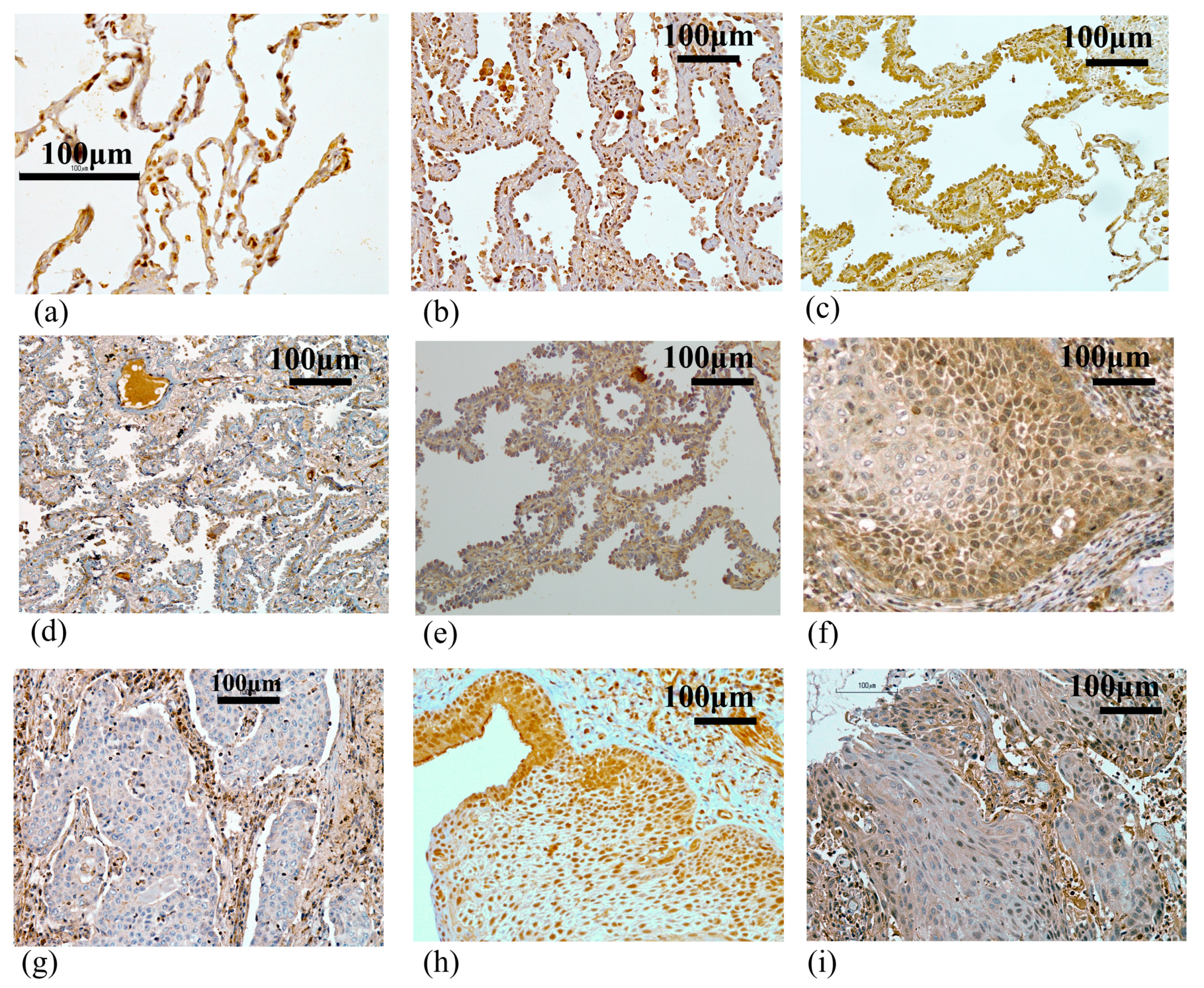
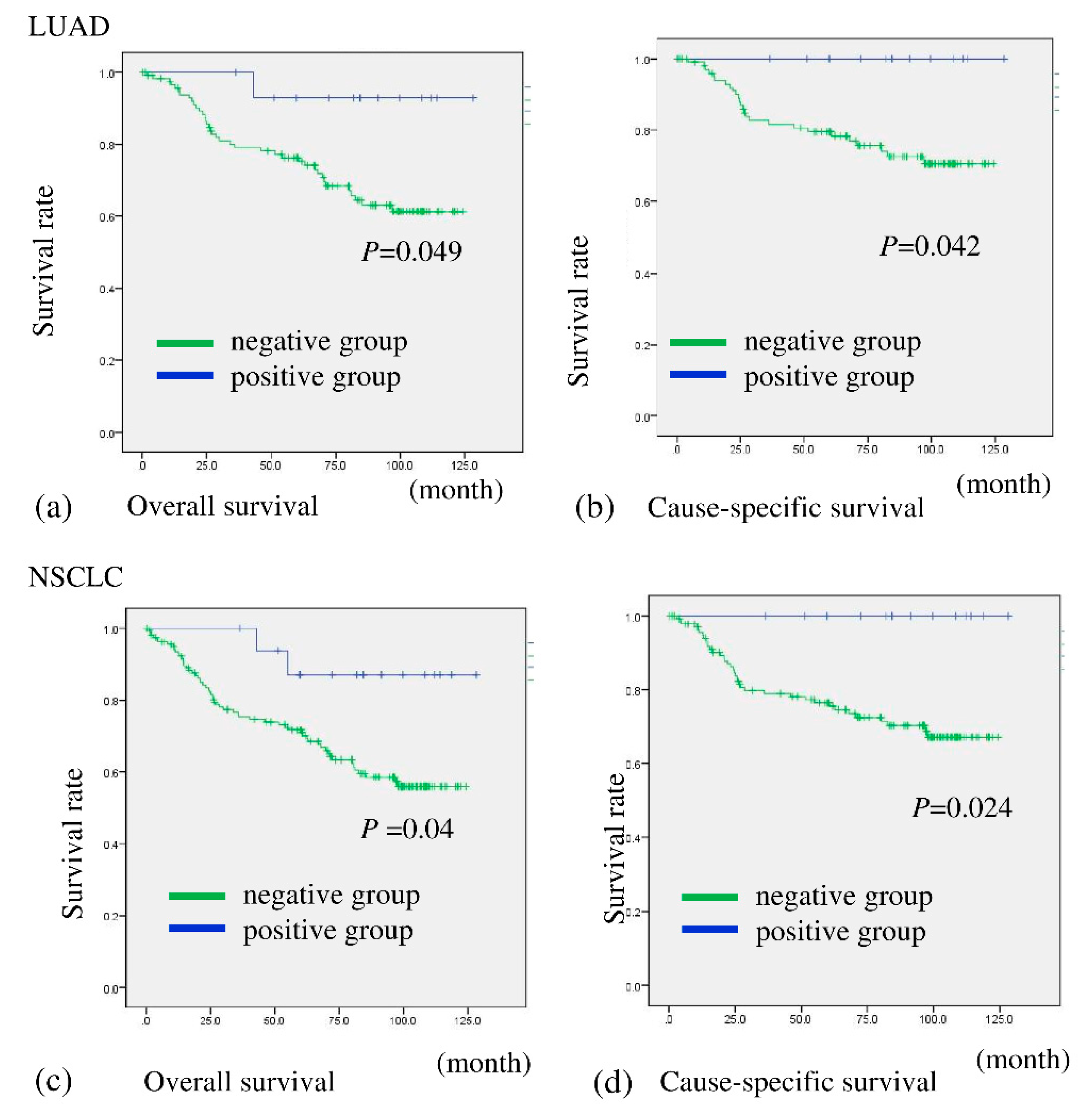
2.1. BHLHE41 Expression Is Associated with Favorable Prognosis for Patients with Lung Adenocarcinoma (LUAD) as Shown by In Silico and Immunohistochemistry (IHC) Analyses
2.2. BHLHE41 Expression Is Associated with Early-Stage Cancer
2.3. BHLHE41 Expression Suppresses Cell Proliferation and Induces Autophagy
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis of BHLHE41 Expression in Cancer
4.2. Reagents and Antibodies
4.3. Patients and Tumor Samples
4.4. IHC and Assessments
4.5. Correlation between the DI and BHLHE41 Staining of Early LUSC Cases
4.6. Cell Lines and Culture
4.7. Vectors
4.8. Establishing BHLHE41-Expressing Cells
4.9. Cell Proliferation Assay
4.10. Protein Extraction and Immunoblotting
4.11. Observation of Fluorescence Associated with ftLC3 Expression
4.12. Xenograft Model Experiments and Animal Husbandry
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Brierley, J.D.; Gospodarowicz, M.K.; Christian, W. (Eds.) Lung. In UICC TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 106–112. [Google Scholar]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Shiraishi, K.; Kunitoh, H.; Takenoshita, S.; Yokota, J.; Kohno, T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016, 107, 713–720. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef]
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018, 52 Pt 1, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Shen, M.; Noshiro, M.; Matsubara, K.; Shingu, S.; Honda, K.; Yoshida, E.; Suardita, K.; Matsuda, Y.; Kato, Y. Molecular cloning and characterization of DEC2, a new member of basic helix-loop-helix proteins. Biochem. Biophys. Res. Commun. 2001, 280, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Kawamoto, T.; Takagi, Y.; Fujimoto, K.; Sato, F.; Noshiro, M.; Kato, Y.; Honma, K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 2002, 419, 841–844. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jones, C.R.; Fujiki, N.; Xu, Y.; Guo, B.; Holder, J.L., Jr.; Rossner, M.J.; Nishino, S.; Fu, Y.H. The transcriptional repressor DEC2 regulates sleep length in mammals. Science 2009, 325, 866–870. [Google Scholar] [CrossRef] [Green Version]
- Gulbagci, N.T.; Li, L.; Ling, B.; Gopinadhan, S.; Walsh, M.; Rossner, M.; Nave, K.A.; Taneja, R. SHARP1/DEC2 inhibits adipogenic differentiation by regulating the activity of C/EBP. EMBO Rep. 2009, 10, 79–86. [Google Scholar] [CrossRef]
- Yang, X.O.; Angkasekwinai, P.; Zhu, J.; Peng, J.; Liu, Z.; Nurieva, R.; Liu, X.; Chung, Y.; Chang, S.H.; Sun, B.; et al. Requirement for the basic helix-loop-helix transcription factor Dec2 in initial TH2 lineage commitment. Nat. Immunol. 2009, 10, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Sato, F.; Bhawal, U.K.; Yoshimura, T.; Muragaki, Y. DEC1 and DEC2 Crosstalk between Circadian Rhythm and Tumor Progression. J. Cancer 2016, 7, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sato, F.; Kawamoto, T.; Fujimoto, K.; Morohashi, S.; Akasaka, H.; Kondo, J.; Wu, Y.; Noshiro, M.; Kato, Y.; et al. Anti-apoptotic effect of the basic helix-loop-helix (bHLH) transcription factor DEC2 in human breast cancer cells. Genes Cells 2010, 15, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Asanoma, K.; Liu, G.; Yamane, T.; Miyanari, Y.; Takao, T.; Yagi, H.; Ohgami, T.; Ichinoe, A.; Sonoda, K.; Wake, N.; et al. Regulation of the Mechanism of TWIST1 Transcription by BHLHE40 and BHLHE41 in Cancer Cells. Mol. Cell Biol. 2015, 35, 4096–4109. [Google Scholar] [CrossRef] [Green Version]
- Montagner, M.; Enzo, E.; Forcato, M.; Zanconato, F.; Parenti, A.; Rampazzo, E.; Basso, G.; Leo, G.; Rosato, A.; Bicciato, S.; et al. SHARP1 suppresses breast cancer metastasis by promoting degradation of hypoxia-inducible factors. Nature 2012, 487, 380–384. [Google Scholar] [CrossRef]
- Bigot, P.; Colli, L.M.; Machiela, M.J.; Jessop, L.; Myers, T.A.; Carrouget, J.; Wagner, S.; Roberson, D.; Eymerit, C.; Henrion, D.; et al. Functional characterization of the 12p12.1 renal cancer-susceptibility locus implicates BHLHE41. Nat. Commun. 2016, 7, 12098. [Google Scholar] [CrossRef] [Green Version]
- Numata, A.; Kwok, H.S.; Kawasaki, A.; Li, J.; Zhou, Q.L.; Kerry, J.; Benoukraf, T.; Bararia, D.; Li, F.; Ballabio, E.; et al. The basic helix-loop-helix transcription factor SHARP1 is an oncogenic driver in MLL-AF6 acute myelogenous leukemia. Nat. Commun. 2018, 9, 1622. [Google Scholar] [CrossRef] [Green Version]
- Falvella, F.S.; Colombo, F.; Spinola, M.; Campiglio, M.; Pastorino, U.; Dragani, T.A. BHLHB3: A candidate tumor suppressor in lung cancer. Oncogene 2008, 27, 3761–3764. [Google Scholar] [CrossRef] [Green Version]
- Radkiewicz, C.; Dickman, P.W.; Johansson, A.L.V.; Wagenius, G.; Edgren, G.; Lambe, M. Sex and survival in non-small cell lung cancer: A nationwide cohort study. PLoS ONE 2019, 14, e0219206. [Google Scholar] [CrossRef]
- Wainer, Z.; Wright, G.M.; Gough, K.; Daniels, M.G.; Russell, P.A.; Choong, P.; Conron, M.; Ball, D.; Solomon, B. Sex-Dependent Staging in Non-Small-Cell Lung Cancer; Analysis of the Effect of Sex Differences in the Eighth Edition of the Tumor, Node, Metastases Staging System. Clin. Lung Cancer 2018, 19, e933–e944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, H.; Kawasaki, N.; Hagiwara, M.; Ogata, A.; Kato, H. Endoscopic evaluation of centrally located early squamous cell carcinoma of the lung. Cancer 2001, 91, 1142–1147. [Google Scholar] [CrossRef]
- Nagamoto, N.; Saito, Y.; Imai, T.; Suda, H.; Hashimoto, K.; Nakada, T.; Sato, H. Roentgenographically occult bronchogenic squamous cell carcinoma: Location in the bronchi, depth of invasion and length of axial involvement of the bronchus. Tohoku J. Exp. Med. 1986, 148, 241–256. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.; Noda, T.; Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007, 3, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, V.H.; Pipinikas, C.P.; Pennycuick, A.; Lee-Six, H.; Chandrasekharan, D.; Beane, J.; Morris, T.J.; Karpathakis, A.; Feber, A.; Breeze, C.E.; et al. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat. Med. 2019, 25, 517–525. [Google Scholar] [CrossRef]
- Shoji, F.; Morodomi, Y.; Akamine, T.; Takamori, S.; Katsura, M.; Takada, K.; Suzuki, Y.; Fujishita, T.; Okamoto, T.; Maehara, Y. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer 2016, 98, 15–21. [Google Scholar] [CrossRef]
- Shoji, F.; Miura, N.; Matsubara, T.; Akamine, T.; Kozuma, Y.; Haratake, N.; Takamori, S.; Katsura, M.; Takada, K.; Toyokawa, G.; et al. Prognostic significance of immune-nutritional parameters for surgically resected elderly lung cancer patients: A multicentre retrospective study. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 389–394. [Google Scholar] [CrossRef]
- Yoshiya, T.; Mimae, T.; Tsutani, Y.; Tsubokawa, N.; Sasada, S.; Miyata, Y.; Kushitani, K.; Takeshima, Y.; Murakami, S.; Ito, H.; et al. Prognostic Role of Subtype Classification in Small-Sized Pathologic N0 Invasive Lung Adenocarcinoma. Ann. Thorac. Surg. 2016, 102, 1668–1673. [Google Scholar] [CrossRef] [Green Version]
- Sasada, S.; Nakayama, H.; Miyata, Y.; Tsubokawa, N.; Mimae, T.; Yoshiya, T.; Murakami, S.; Ito, H.; Okada, M. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann. Thorac. Surg. 2015, 99, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Zombori, T.; Nyari, T.; Tiszlavicz, L.; Palfoldi, R.; Csada, E.; Geczi, T.; Ottlakan, A.; Pecsy, B.; Cserni, G.; Furak, J. The more the micropapillary pattern in stage I lung adenocarcinoma, the worse the prognosis-a retrospective study on digitalized slides. Virchows Arch. 2018, 472, 949–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadota, K.; Villena-Vargas, J.; Yoshizawa, A.; Motoi, N.; Sima, C.S.; Riely, G.J.; Rusch, V.W.; Adusumilli, P.S.; Travis, W.D. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am. J. Surg. Pathol. 2014, 38, 448–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuchert, M.J.; Schumacher, L.; Kilic, A.; Close, J.; Landreneau, J.R.; Pennathur, A.; Awais, O.; Yousem, S.A.; Wilson, D.O.; Luketich, J.D.; et al. Impact of angiolymphatic and pleural invasion on surgical outcomes for stage I non-small cell lung cancer. Ann. Thorac. Surg. 2011, 91, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Shiba-Ishii, A.; Kano, J.; Morishita, Y.; Sato, Y.; Minami, Y.; Noguchi, M. High expression of stratifin is a universal abnormality during the course of malignant progression of early-stage lung adenocarcinoma. Int. J. Cancer 2011, 129, 2445–2453. [Google Scholar] [CrossRef]
- Kim, Y.; Shiba-Ishii, A.; Nakagawa, T.; Husni, R.E.; Sakashita, S.; Takeuchi, T.; Noguchi, M. Ubiquitin-specific protease 8 is a novel prognostic marker in early-stage lung adenocarcinoma. Pathol. Int. 2017, 67, 292–301. [Google Scholar] [CrossRef]
- Duruisseaux, M.; Rabbe, N.; Antoine, M.; Vieira, T.; Poulot, V.; Cadranel, J.; Wislez, M. Pro-tumoural CXCL10/CXCR3-A autocrine loop in invasive mucinous lung adenocarcinoma. ERJ Open Res. 2017, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimae, T.; Okada, M.; Hagiyama, M.; Miyata, Y.; Tsutani, Y.; Inoue, T.; Murakami, Y.; Ito, A. Upregulation of notch2 and six1 is associated with progression of early-stage lung adenocarcinoma and a more aggressive phenotype at advanced stages. Clin. Cancer Res. 2012, 18, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvayrac, O.; Pradines, A.; Raymond-Letron, I.; Rouquette, I.; Bousquet, E.; Lauwers-Cances, V.; Filleron, T.; Cadranel, J.; Beau-Faller, M.; Casanova, A.; et al. RhoB determines tumor aggressiveness in a murine EGFRL858R-induced adenocarcinoma model and is a potential prognostic biomarker for Lepidic lung cancer. Clin. Cancer Res. 2014, 20, 6541–6550. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, B.; Santoni-Rugiu, E.; Illemann, M.; Kriegbaum, M.C.; Laerum, O.D.; Ploug, M. Expression of C4.4A in precursor lesions of pulmonary adenocarcinoma and squamous cell carcinoma. Int. J. Cancer 2012, 130, 2734–2739. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.; Muley, T.; Meister, M.; Dienemann, H.; Christensen, I.J.; Santoni-Rugiu, E.; Laerum, O.D.; Ploug, M. Ly6/uPAR-related protein C4.4A as a marker of solid growth pattern and poor prognosis in lung adenocarcinoma. J. Thorac. Oncol. 2013, 8, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef] [Green Version]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grampp, S.; Schmid, V.; Salama, R.; Lauer, V.; Kranz, F.; Platt, J.L.; Smythies, J.; Choudhry, H.; Goppelt-Struebe, M.; Ratcliffe, P.J.; et al. Multiple renal cancer susceptibility polymorphisms modulate the HIF pathway. PLoS Genet. 2017, 13, e1006872. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.; Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) Lung. In TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 138–144. [Google Scholar]
- Johmura, Y.; Shimada, M.; Misaki, T.; Naiki-Ito, A.; Miyoshi, H.; Motoyama, N.; Ohtani, N.; Hara, E.; Nakamura, M.; Morita, A.; et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol. Cell 2014, 55, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, M.; Sumizawa, T.; Mutoh, M.; Chen, Z.S.; Terada, K.; Furukawa, T.; Yang, X.L.; Gao, H.; Miura, N.; Sugiyama, T.; et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000, 60, 1312–1316. [Google Scholar] [PubMed]
- Owatari, S.; Akune, S.; Komatsu, M.; Ikeda, R.; Firth, S.D.; Che, X.F.; Yamamoto, M.; Tsujikawa, K.; Kitazono, M.; Ishizawa, T.; et al. Copper-transporting P-type ATPase, ATP7A, confers multidrug resistance and its expression is related to resistance to SN-38 in clinical colon cancer. Cancer Res. 2007, 67, 4860–4868. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Number | % | ||
|---|---|---|---|
| Gender | Male | 96 | 54.2 |
| Female | 81 | 45.8 | |
| Age | Median | 70 | |
| Range | 38–86 | ||
| Operation | Pneumonectomy | 2 | 1.1 |
| Lobectomy | 154 | 87.0 | |
| Segmentectomy | 21 | 11.9 | |
| p stage | IA | 83 | 46.9 |
| IB | 38 | 21.5 | |
| IIA | 22 | 12.4 | |
| IIB | 11 | 6.2 | |
| IIIA | 23 | 13.0 | |
| Histology | Adenocarcinoma | 132 | 74.6 |
| Squamous cell carcinoma | 43 | 24.3 | |
| Others | 2 | 1.1 |
| Clinicopathologic Factors | Expression of BHLHE41 | |||||
|---|---|---|---|---|---|---|
| Positive | % | Negative | % | p Value | ||
| n = 15 | (11.4) | n = 117 | (88.6) | |||
| Age≥ | <70 years | 8 | (13.6) | 51 | (86.4) | 0.475 |
| ≥70 years | 7 | (9.6) | 66 | (90.4) | ||
| Gender | female | 10 | (13.0) | 67 | (87.0) | 0.487 |
| male | 5 | (9.1) | 50 | (90.9) | ||
| Tumor size | ≤30 mm | 13 | (14.6) | 76 | (85.4) | 0.076 |
| >30 mm | 2 | (4.7) | 41 | (95.3) | ||
| Pleural invasion | No | 15 | (14.3) | 90 | (85.7) | 0.026 * |
| Yes | 0 | (0.0) | 27 | (100.0) | ||
| Pulmonary metastasis | No | 15 | (12.0) | 110 | (88.0) | 0.421 |
| Yes | 0 | (0.0) | 7 | (100.0) | ||
| T factor | T1 | 14 | (16.5) | 71 | (83.5) | 0.013 * |
| ≥T2 | 1 | (2.1) | 46 | (97.9) | ||
| N factor | N0 | 13 | (12.0) | 95 | (88.0) | 0.46 |
| N1/2 | 2 | (8.3) | 22 | (91.7) | ||
| Stage | IA | 13 | (17.3) | 62 | (82.7) | 0.013 * |
| ≥IB | 2 | (3.5) | 55 | (96.5) | ||
| Histology | AIS | 7 | (46.7) | 8 | (53.3) | <0.001 * |
| Invasive | 8 | (6.8) | 109 | (93.2) | ||
| Clinicopathological Factors | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Dead | Alive | HR (95%CI) | p Value | HR (95%CI) | p Value | ||
| Age | ≥70 years | 73 | 25 | 48 | 1.70 | 0.11 | 1.53 | 0.21 |
| <70 years | 59 | 14 | 45 | (0.88–3.28) | (0.79–2.96) | |||
| Gender Male | male | 55 | 25 | 30 | 3.33 | <0.001 * | 2.91 | 0.002 * |
| female | 77 | 14 | 63 | (1.72–6.43) | (1.49–5.66) | |||
| Tumor size | >30 mm | 43 | 19 | 24 | 2.11 | 0.02 * | ||
| ≤30 mm | 89 | 20 | 69 | (1.13–3.96) | ||||
| Pleural invasion | Yes | 27 | 13 | 14 | 2.56 | 0.006 * | ||
| No | 105 | 26 | 79 | (1.32–4.99) | ||||
| Pulmonary metastasis | Yes | 7 | 3 | 4 | 1.69 | 0.39 | ||
| No | 125 | 36 | 89 | (0.52–5.48) | ||||
| T factor | ≥T2 | 47 | 20 | 27 | 2.06 | 0.024 * | ||
| T1 | 85 | 19 | 66 | (1.10–3.85) | ||||
| N factor | N1/2 | 24 | 15 | 9 | 3.95 | <0.001 * | ||
| N0 | 108 | 24 | 84 | (2.07–7.56) | ||||
| Pathological stage | ≥IB | 57 | 27 | 30 | 3.60 | <0.001 * | 2.69 | 0.005 * |
| IA | 75 | 12 | 63 | (1.82–7.11) | (1.34–5.38) | |||
| Histology | invasive | 117 | 39 | 78 | 24.46 | 0.14 | ||
| AIS | 15 | 0 | 15 | (0.35–1709) | ||||
| BHLHE41 | negative | 117 | 38 | 79 | 5.82 | 0.082 | 3.67 | 0.20 |
| positive | 15 | 1 | 14 | (0.80–42.39) | (0.49–27.35) | |||
| DI: Depth of Invasion | Expression of BHLHE41 | ||
|---|---|---|---|
| n | Positive | Negative | |
| DI 1 | 0 | 0 | 0 |
| DI 2 | 2 | 2 | 0 |
| DI 3 | 6 | 4 | 2 |
| DI 4 | 7 | 1 | 6 |
| DI 5 | 3 | 0 | 3 |
| Total | 18 | 7 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagata, T.; Minami, K.; Yamamoto, M.; Hiraki, T.; Idogawa, M.; Fujimoto, K.; Kageyama, S.; Tabata, K.; Kawahara, K.; Ueda, K.; et al. BHLHE41/DEC2 Expression Induces Autophagic Cell Death in Lung Cancer Cells and Is Associated with Favorable Prognosis for Patients with Lung Adenocarcinoma. Int. J. Mol. Sci. 2021, 22, 11509. https://doi.org/10.3390/ijms222111509
Nagata T, Minami K, Yamamoto M, Hiraki T, Idogawa M, Fujimoto K, Kageyama S, Tabata K, Kawahara K, Ueda K, et al. BHLHE41/DEC2 Expression Induces Autophagic Cell Death in Lung Cancer Cells and Is Associated with Favorable Prognosis for Patients with Lung Adenocarcinoma. International Journal of Molecular Sciences. 2021; 22(21):11509. https://doi.org/10.3390/ijms222111509
Chicago/Turabian StyleNagata, Toshiyuki, Kentaro Minami, Masatatsu Yamamoto, Tsubasa Hiraki, Masashi Idogawa, Katsumi Fujimoto, Shun Kageyama, Kazuhiro Tabata, Kohichi Kawahara, Kazuhiro Ueda, and et al. 2021. "BHLHE41/DEC2 Expression Induces Autophagic Cell Death in Lung Cancer Cells and Is Associated with Favorable Prognosis for Patients with Lung Adenocarcinoma" International Journal of Molecular Sciences 22, no. 21: 11509. https://doi.org/10.3390/ijms222111509
APA StyleNagata, T., Minami, K., Yamamoto, M., Hiraki, T., Idogawa, M., Fujimoto, K., Kageyama, S., Tabata, K., Kawahara, K., Ueda, K., Ikeda, R., Kato, Y., Komatsu, M., Tanimoto, A., Furukawa, T., & Sato, M. (2021). BHLHE41/DEC2 Expression Induces Autophagic Cell Death in Lung Cancer Cells and Is Associated with Favorable Prognosis for Patients with Lung Adenocarcinoma. International Journal of Molecular Sciences, 22(21), 11509. https://doi.org/10.3390/ijms222111509







