Relationship between Blood Vessels and Migration of Neuroblasts in the Olfactory Neurogenic Region of the Rodent Brain
Abstract
1. Introduction
2. Development and Vascularization of the Telencephalon and the Rostral Migratory Stream during the Embryogenesis of Rodents
2.1. Development of the Telencephalon and the RMS
2.2. Vascularization of the Telencephalon and the RMS during the Embryonic Period
3. Angiogenesis in the Brain during the Postnatal Period and Mutual Coordination between Nervous and Vascular Systems
4. Blood Vessels and Neuroblast Migration in Neurogenic Areas in the Postnatal Period
4.1. Migration of Neuroblasts in the RMS during the Postnatal Period
4.2. Region-Dependent Distinctions Relating to Blood Vessels in the RMS
4.3. Specific Arrangement of Blood Vessels in the Rodent RMS and Interspecies Differences
4.4. Blood Vessels, Neuroblasts, and Astrocytes in the RMS in the Context of Migration
4.5. Migration-Promoting Function of the Blood Vessels in the RMS
4.6. Development of a Vascular Scaffold in the RMS during Perinatal and Early Postnatal Periods
4.7. Relevance of Blood-Vessel Reorganization during the Early Postnatal Period for Migration of Neuroblasts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| BV | blood vessels |
| CNS | central nervous system |
| E | embryonic day |
| GABA | gamma-aminobutyric acid |
| GAD65, GAD67 | glutamic acid decarboxylase 65,67-kilodalton isoforms |
| GFAP | glial fibrillary acidic protein |
| IZ | intermediate zone |
| LGE | lateral ganglionic eminence |
| MAP2 | microtubule-associated protein 2 |
| MGE | medial ganglionic eminence |
| MZ | marginal zone |
| NCAM | neural cell adhesion molecule |
| OB | olfactory bulb |
| P | postnatal day |
| PNVP | perineural vascular plexus |
| PSA-NCAM | polysialylated form of neural cell adhesion molecule |
| PVVP | periventricular vascular plexus |
| RMS | rostral migratory stream |
| SGZ | subgranular zone of the gyrus dentatus of the hippocampus |
| SVZ | subventricular zone of the lateral ventricles |
| TUJ1 | neuron-specific class III beta-tubulin |
| VEGF | vascular endothelial growth factor |
| VZ | ventricular zone |
References
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Neurogenesis in the adult subventricular zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef]
- Cameron, R.S.; Rakic, P. Glial cell lineage in the cerebral cortex: Review and synthesis. Glia 1991, 4, 124–137. [Google Scholar] [CrossRef]
- Menezes, J.R.; Marins, M.; Alves, J.A.; Froes, M.M.; Hedin-Pereira, C. Cell migration in the postnatal subventricular zone. Braz. J. Med. Biol. Res. 2002, 35, 1411–1421. [Google Scholar] [CrossRef][Green Version]
- Nam, S.C.; Kim, Y.; Dryanovski, D.; Walker, A.; Goings, G.; Woolfrey, K.; Kang, S.S.; Chu, C.; Chen, A.; Erdelyi, F.; et al. Dynamic features of postnatal subventricular zone cell motility: A two-photon time-lapse study. J. Comp. Neurol. 2007, 505, 190–208. [Google Scholar] [CrossRef] [PubMed]
- Luskin, M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 1993, 11, 173–189. [Google Scholar] [CrossRef]
- Menezes, J.R.L.; Smith, C.M.; Nelson, K.C.; Luskin, M.B. The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol. Cell Neurosci. 1995, 6, 496–508. [Google Scholar] [CrossRef]
- Luskin, M.B.; Zigova, T.; Soteres, T.J.; Stewart, R.R. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol. Cell Neurosci. 1997, 8, 351–366. [Google Scholar] [CrossRef]
- Purves, D.; Lichtman, J.W. Principles of Neural Development, 1st ed.; Sinauer: Sunderland, MA, USA, 1985. [Google Scholar]
- Tavazoie, M.; Van der Veken, L.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3, 279–288. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ Stem Cell Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interaction. Cell Stem Cell 2008, 3, 289–300. [Google Scholar] [CrossRef]
- Sun, G.J.; Zhou, Y.; Stadel, R.P.; Moss, J.; Yong, J.H.A.; Ito, S.; Kawasaki, N.K.; Phan, A.T.; Oh, J.H.; Modak, N.; et al. Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 2015, 112, 9484–9489. [Google Scholar] [CrossRef] [PubMed]
- Snapyan, M.; Lemasson, M.; Brill, M.S.; Blais, M.; Massouh, M.; Ninkovic, J.; Gravel, C.; Berthod, F.; Götz, M.; Barker, P.A.; et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 2009, 29, 4172–4188. [Google Scholar] [CrossRef] [PubMed]
- Whitman, M.C.; Fan, W.; Rela, L.; Rodriguez-Gil, D.J.; Greer, C.A. Blood vessels form a migratory scaffold in the rostral migratory stream. J. Comp. Neurol. 2009, 516, 94–104. [Google Scholar] [CrossRef]
- Martončíková, M.; Fabianová, K.; Schreiberová, A.; Blaško, J.; Almašiová, V.; Račeková, E. Astrocytic and vascular scaffolding for neuroblast migration in the rostral migratory stream. Curr. Neurovasc. Res. 2014, 11, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.C.; Vadakkan, T.J.; Dickinson, M.J. A Specialized Microvascular Domain in the Mouse Stem Cell Niche. PLoS ONE 2013, 8, e53546. [Google Scholar] [CrossRef] [PubMed]
- Bozoyan, L.; Khlghatyan, J.; Saghatelyan, A. Astrocytes Control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. J. Neurosci. 2012, 32, 1687–1704. [Google Scholar] [CrossRef]
- Angelidis, A.; Račeková, E.; Arnoul, P.; Závodská, M.; Raček, A.; Martončíková, M. Disrupted migration and proliferation of neuroblasts after postnatal administration of angiogenesis inhibitor. Brain Res. 2018, 1698, 121–129. [Google Scholar] [CrossRef]
- Chen, V.S.; Morrison, J.P.; Southwell, M.F.; Foley, J.F.; Bolon, B.; Elmore, S.A. Histology Atlas of the Developing Prenatal and Postnatal Mouse Central Nervous System, with Emphasis on Prenatal Days E7.5 to E18.5. Toxicol. Pathol. 2017, 45, 705–744. [Google Scholar] [CrossRef]
- Freeman, B.G. Surface modification of neural epithelial cells during formation of the neural tube in the embryo. J. Embryol. Exp. Morph. 1972, 28, 437–448. [Google Scholar]
- Copp, A.J. Neurulation in the Cranial Region—Normal and Abnormal. J. Anat. 2005, 207, 623–635. [Google Scholar] [CrossRef] [PubMed]
- McLone, D.G.; Naidich, T.P. Developmental morphology of the subarachnoid space, brain vasculature, and contiguous structures, and the cause of the Chiari II malformation. AJNR Am. J. Neuroradiol. 1992, 13, 463–482. [Google Scholar] [PubMed]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 5115–5133. [Google Scholar] [CrossRef]
- Bayer, S.A.; Zhang, X.; Russo, R.J.; Altman, J. Three-dimensional reconstructions of the developing forebrain in rat embryos. Neuroimage 1994, 1, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Alicelebic, S.; Grbesa, D. Development of the rat telencephalon-volumetric analysis. Bosn. J. Bas. Med. Sci. 2004, 4, 11–14. [Google Scholar] [CrossRef][Green Version]
- Tucker, E.S.; Polleux, F.; LaMantia, A.S. Position and time specify the migration of a pioneering population of olfactory bulb interneurons. Dev. Biol. 2006, 297, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Shipley, M.T. Evidence that pioneer olfactory axons regulate telencephalon cell cycle kinetics to induce the formation of the olfactory bulb. Neuron 1995, 14, 91–101. [Google Scholar] [CrossRef]
- De Carlos, J.A.; Lopez-Mascaraque, L.; Valverde, F. Early olfactory fiber projections and cell migration into the rat telencephalon. Int. J. Dev. Neurosci. 1996, 14, 853–866. [Google Scholar] [CrossRef]
- Bayer, S.A.; Altman, J. CHAPTER 2—Development of the Telencephalon: Neural Stem Cells, Neurogenesis, and Neuronal Migration. In The Rat Nervous System, 3rd ed.; Paxinos, G., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2004; pp. 27–73. [Google Scholar]
- Blanchart, A.; De Carlos, J.A.; López-Mascaraque, L. Time frame of mitral cell development in the mice olfactory bulb. J. Comp. Neurol. 2006, 496, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Ayoub, A.E.; Rakic, P.; Greer, C.A. Timing of neurogenesis is a determinant of olfactory circuitry. Nat. Neurosci. 2011, 14, 331–337. [Google Scholar] [CrossRef]
- Nomura, T.; Osumi, N. Misrouting of mitral cell progenitors in the Pax6/small eye rat telencephalon. Development 2004, 131, 787–796. [Google Scholar] [CrossRef]
- Smart, I.H. A pilot study of cell production by the ganglionic eminences of the developing mouse brain. J. Anat. 1976, 121, 71–84. [Google Scholar] [PubMed]
- Pencea, V.; Luskin, M.B. Prenatal development of the rodent rostral migratory stream. J. Comp. Neurol. 2003, 463, 402–418. [Google Scholar] [CrossRef]
- Garcia-Moreno, F.; Lopez-Mascaraque, L.; de Carlos, J.A. Early telencephalic migration topographically converging in the olfactory cortex. Cereb. Cortex 2008, 18, 1239–1252. [Google Scholar] [CrossRef]
- Hinds, J.W. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J. Comp. Neurol. 1968, 134, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Bulfone, A.; Wang, F.; Hevner, R.; Anderson, S.; Cutforth, T.; Chen, S.; Meneses, J.; Pedersen, R.; Axel, R.; Rubenstein, J.L. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron 1998, 21, 1273–1282. [Google Scholar] [CrossRef]
- Bayer, S.A. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp. Brain Res. 1983, 50, 329–340. [Google Scholar] [CrossRef]
- Inaki, K.; Nishimura, S.; Nakashiba, T.; Itohara, S.; Yoshihara, Y. Laminar organization of the developing lateral olfactory tract revealed by differential expression of cell recognition molecules. J. Comp. Neurol. 2004, 479, 243–256. [Google Scholar] [CrossRef]
- Puche, A.C.; Shipley, M.T. Radial glia development in the mouse olfactory bulb. J. Comp. Neurol. 2001, 434, 1–12. [Google Scholar] [CrossRef]
- Peretto, P.; Giachino, C.; Aimar, P.; Fasolo, A.; Bonfanti, L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. J. Comp. Neurol. 2005, 487, 407–427. [Google Scholar] [CrossRef]
- Copp, A.J.; Greene, N.D.E.; Murdoch, J.N. The Genetic Basis of Mammalian Neurulation. Nat. Rev. Genet. 2003, 4, 784–793. [Google Scholar] [CrossRef]
- Sadler, T.W. Embryology of Neural Tube Development. Am. J. Med. Genet. C Semin. Med. Genet. 2005, 135C, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.R.; Shimamura, K.; Martinez, S.; Puelles, L. Regionalisation of the prosencephalic neural plate. Annu. Rev. Neurosci. 1998, 21, 445–477. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nakamura, S.; Osumi, N. Fate mapping of the mouse prosencephalic neural plate. Dev. Biol. 2000, 219, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.W.; Rubenstein, J.L.R. Induction and dorsoventral patterning of the telencephalon. Neuron 2000, 28, 641–651. [Google Scholar] [CrossRef]
- Hébert, J.M.; Fishell, G. The genetics of early telencephalon patterning: Some assembly required. Nat. Rev. Neurosci. 2008, 9, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, D.; Garcia, C.; de Castro, F.; Chedotal, A.; Sotelo, C.; de Carlos, J.A.; Valverde, F.; López-Mascaraque, L. Evidence for intrinsic development of olfactory structures in Pax-6 mutant mice. J. Comp. Neurol. 2000, 428, 511–526. [Google Scholar] [CrossRef]
- Puelles, L.; Kuwana, E.; Puelles, E.; Bulfone, A.; Shimamura, K.; Keleher, J.; Smiga, S.; Rubenstein, J.L. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 2000, 424, 409–438. [Google Scholar] [CrossRef]
- Wichterle, H.; Garcia-Verdugo, J.M.; Herrera, D.G.; Alvarez-Buylla, A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat. Neurosci. 1999, 2, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Law, A.K.T.; Pencea, V.; Buck, C.R.; Luskin, M.B. Neurogenesis and neuronal migration in the neonatal rat forebrain anterior subventricular zone do not require GFAP-positive astrocytes. Dev. Biol. 1999, 216, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Wolff, J.R.; Goerz, C.; Bär, T.; Guldner, F.H. Common morphogenetic aspects of various organotypic microvascular patterns. Microvasc. Res. 1975, 10, 373–395. [Google Scholar] [CrossRef]
- Bär, T.; Wolff, J.R. The formation of capillary basement membranes during internal vascularization of the rat’s cerebral cortex. Z. Zellforsch. Mikrosk. Anat. 1972, 133, 231–248. [Google Scholar] [CrossRef]
- Breier, G.; Albrecht, U.; Sterrer, S.; Risau, W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992, 114, 521–532. [Google Scholar] [CrossRef]
- Carmeliet, P.; Storkebaum, E. Vascular and neuronal effects of VEGF in the nervous system: Implications for neurological disorders. Semin. Cell Dev. Biol. 2002, 13, 39–53. [Google Scholar] [CrossRef]
- Puelles, L.; Martinez-Marin, R.; Melgarejo-Otalora, P.; Ayad, A.; Valavanis, A.; Ferran, J.L. Patterned Vascularization of Embryonic Mouse Forebrain, and Neuromeric Topology of Major Human Subarachnoidal Arterial Branches: A Prosomeric Mapping. Front. Neuroanat. 2019, 13, 59. [Google Scholar] [CrossRef]
- Hogan, K.A.; Ambler, C.A.; Chapman, D.L.; Bautch, V.L. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development 2004, 131, 1503–1513. [Google Scholar] [CrossRef]
- Karakatsani, A.; Shah, B.; Ruiz de Almodovar, C. Blood Vessels as Regulators of Neural Stem Cell Properties. Front. Mol. Neurosci. 2019, 12, 85. [Google Scholar] [CrossRef]
- Vasudevan, A.; Long, J.E.; Crandall, J.E.; Rubenstein, J.L.; Bhide, P.G. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 2008, 11, 429–439. [Google Scholar] [CrossRef]
- Conradi, N.G.; Sourander, P. The early internal vascularization of the rat brain. Morphological studies on foetuses of normal and protein-deprived mothers. Acta Neuropathol. 1980, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Minocha, S.; Valloton, D.; Brunet, I.; Eichmann, A.; Hornung, J.P.; Lebrand, C. NG2 glia are required for vessel network formation during embryonic development. eLife 2015, 4, e09102. [Google Scholar] [CrossRef] [PubMed]
- Stoykova, A.; Treichel, D.; Hallonet, M.; Gruss, P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J. Neurosci. 2000, 20, 8042–8050. [Google Scholar] [CrossRef] [PubMed]
- Colín-Castelán, D.; Phillips-Farfán, B.V.; Gutiérrez-Ospina, G.; Fuentes-Farias, A.L.; Báez-Saldaña, A.; Padilla-Cortés, P.; Meléndez-Herrera, E. EphB4 is developmentally and differentially regulated in blood vessels throughout the forebrain neurogenic niche in the mouse brain: Implications for vascular remodeling. Brain Res. 2011, 1383, 90–98. [Google Scholar] [CrossRef]
- Nie, K.; Molnar, Z.; Szele, F.G. Proliferation but not migration is associated with blood vessels during development of the rostral migratory stream. Dev. Neurosci. 2010, 32, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bär, T. The vascular system of the cerebral cortex. In Advances Anatomy Embryology and Cell Biology, 1st ed.; Brodal, A., Hild, W., van Limborgh, J., Ortmann, J., Schiebler, T.H., Töndury, G., Wolff, E., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1980; Volume 59, pp. 1–26. [Google Scholar]
- Plate, K.H. Mechanisms of angiogenesis in the brain. J. Neuropathol. Exp. Neurol. 1999, 58, 313–320. [Google Scholar] [CrossRef]
- Robertson, P.L.; Du Bois, M.; Bowman, P.D.; Goldstein, G.W. Angiogenesis in the developing rat brain: An in vivo and in vitro study. Dev. Brain Res. 1985, 23, 219–223. [Google Scholar] [CrossRef]
- Ohab, J.J.; Fleming, S.; Blesch, A.; Carmichael, S.T. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 2006, 26, 13007–13016. [Google Scholar] [CrossRef]
- Louissaint, A., Jr.; Rao, S.; Leventhal, C.; Goldman, S.A. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 2002, 34, 945–960. [Google Scholar] [CrossRef]
- Palmer, T.; Willhoite, A.R.; Gage, F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000, 425, 479–494. [Google Scholar] [CrossRef]
- Ward, N.L.; LaManna, J.C. The neurovascular unit and its growth factors: Coordinated response in the vascular and nervous systems. Neurol. Res. 2004, 26, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T. Adult neurogenesis and the vascular Nietzsche. Neuron 2002, 34, 856–858. [Google Scholar] [CrossRef]
- Park, J.A.; Choi, K.S.; Kim, S.Y.; Kim, K.W. Coordinated interaction of the vascular and nervous systems: From molecule- to cell-based approaches. Biochem. Biophys. Res. Commun. 2003, 311, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef]
- Leventhal, C.; Rafii, S.; Rafii, D.; Shahhar, A.; Goldman, S.A. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol. Cell. Neurosci. 1999, 13, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Raballo, R.; Rhee, J.; Lyn-Cook, R.; Leckman, J.F.; Schwartz, M.L.; Vaccarino, F.M. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J. Neurosci. 2000, 20, 5012–5023. [Google Scholar] [CrossRef]
- Alzheimer, C.; Werner, S. Fibroblast growth factors and neuroprotection. Adv. Exp. Med. Biol. 2002, 513, 335–351. [Google Scholar] [CrossRef]
- Anderson, M.F.; Aberg, M.A.; Nilsson, M.; Eriksson, P.S. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res. Dev. Brain Res. 2002, 134, 115–122. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Kang, J.Q.; Maiese, K. Erythropoietin: Cytoprotection in vascular and neuronal cells. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2003, 3, 141–154. [Google Scholar] [CrossRef]
- Acker, T.; Beck, H.; Plate, K.H. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mechanism. Dev. 2001, 108, 45–57. [Google Scholar] [CrossRef]
- Valable, S.; Bellail, A.; Lesne, S.; Liot, G.; Mackenzie, E.T.; Vivien, D.; Bernaudin, M.; Petit, E. Angiopoietin-1-induced P13-kinase activation prevents neuronal apoptosis. FASEB J. 2003, 17, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, P.A.; Drapeau, E.; Doetsch, F. Brain micro-ecologies: Neural stem cell niches in the adult mammalian brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 123–137. [Google Scholar] [CrossRef]
- Bovetti, S.; Hsieh, Y.C.; Bovolin, P.; Perroteau, I.; Kazunori, T.; Puche, A.C. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 2007, 27, 5976–5980. [Google Scholar] [CrossRef]
- Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 1972, 145, 61–84. [Google Scholar] [CrossRef]
- Rakic, P. The radial edifice of cortical architecture: From neuronal silhouettes to genetic engineering. Brain Res. Rev. 2007, 55, 204–219. [Google Scholar] [CrossRef]
- Lois, C.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Chain migration of neuronal precursors. Science 1996, 271, 978–981. [Google Scholar] [CrossRef]
- Ng, K.L.; Li, J.D.; Cheng, M.Y.; Leslie, F.M.; Lee, A.G.; Zhou, Q.Y. Dependence of olfactory bulb neurogenesis of prokinectin 2 signaling. Science 2005, 308, 1923–1927. [Google Scholar] [CrossRef]
- Paratcha, G.; Ibañez, C.F.; Ledda, F. GDNF is chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Mol. Cell Neurosci. 2006, 31, 505–514. [Google Scholar] [CrossRef]
- Wu, W.; Wong, K.; Chen, J.; Jiang, Z.; Dupuis, S.; Wu, J.Y.; Rao, Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature 1999, 400, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ba-Charvet, K.T.; Picard-Riera, N.; Tessier-Lavigne, M.; Baron-Van Evercooren, A.; Sotelo, C.; Chedotal, A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J. Neurosci. 2004, 24, 1497–1506. [Google Scholar] [CrossRef]
- Sawamoto, K.; Wichterle, H.; Gonzales-Perez, O.; Cholfin, J.A.; Yamada, M.; Spassky, N.; Murcia, N.S.; Garcia-Verdugo, J.M.; Marin, O.; Rubenstein, J.L.; et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 2006, 311, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Račeková, E.; Lievajová, K.; Danko, J.; Martončíková, M.; Flešárová, S.; Almašiová, V.; Orendáčová, J. Maternal separation induced alterations of neurogenesis in the rat rostral migratory stream. Cell Mol. Neurobiol. 2009, 29, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Colín-Castelán, D.; Ramírez-Santos, J.; Gutiérrez-Ospina, G. Differential vascular permeability along the forebrain ventricular neurogenic niche in the adult murine brain. J. Neurosci. Res. 2016, 94, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Peretto, P.; Merighi, A.; Fasolo, A.; Bonfanti, L. The subependymal layer in rodents: A site of structural plasticity and cell migration in the adult mammalian brain. Brain Res. Bull. 1999, 49, 221–243. [Google Scholar] [CrossRef]
- Kaneko, N.; Marín, O.; Koike, M.; Hirota, Y.; Uchiyama, Y.; Wu, J.Y.; Lu, Q.; Tessier-Lavigne, M.; Alvarez-Buylla, A.; Okano, H.; et al. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron 2010, 67, 213–223. [Google Scholar] [CrossRef]
- Guo, S.; Kim, W.J.; Lok, J.; Lee, S.R.; Besancon, E.; Luo, B.H.; Stins, M.F.; Wang, X.; Dedhar, S.; Lo, E.H. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Asad. Sci. USA 2008, 105, 7582–7587. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Goderie, S.K.; Jin, L.; Karanth, N.; Sun, Y.; Abramova, N.; Vincent, P.; Pumiglia, K.; Temple, S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004, 304, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Chiaramello, S.; Dalmasso, G.; Bezin, L.; Marcel, D.; Jourdan, F.; Peretto, P.; Fasolo, A.; De Marchis, S. BDNF/TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur. J. Neurosci. 2007, 26, 1780–1790. [Google Scholar] [CrossRef]
- Saghatelyan, A. Role of blood vessels in the neuronal migration. Semin. Cell Dev. Biol. 2009, 20, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Inta, D.; Alfonso, J.; von Engelhardt, J.; Kreuzberg, M.M.; Meyer, A.H.; van Hooft, J.A.; Monyer, H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc. Natl. Acad. Sci. USA 2008, 105, 20994–20999. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse, C.; Alfonso, J.; Bark, C.; Eliava, M.; Khrulev, S.; Monyer, H. Subventricular zone-derived neuroblasts use vasculature as a scaffold to migrate radially to the cortex in neonatal mice. Cereb. Cortex 2012, 22, 2285–2296. [Google Scholar] [CrossRef]
- Licht, T.; Eavri, R.; Goshen, I.; Shlomai, Y.; Mizrahi, A.; Keshet, E. VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development 2010, 137, 261–271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thored, P.; Wood, J.; Arvidsson, A.; Cammenga, J.; Kokaia, Z.; Lindvall, O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 2007, 38, 3032–3039. [Google Scholar] [CrossRef]
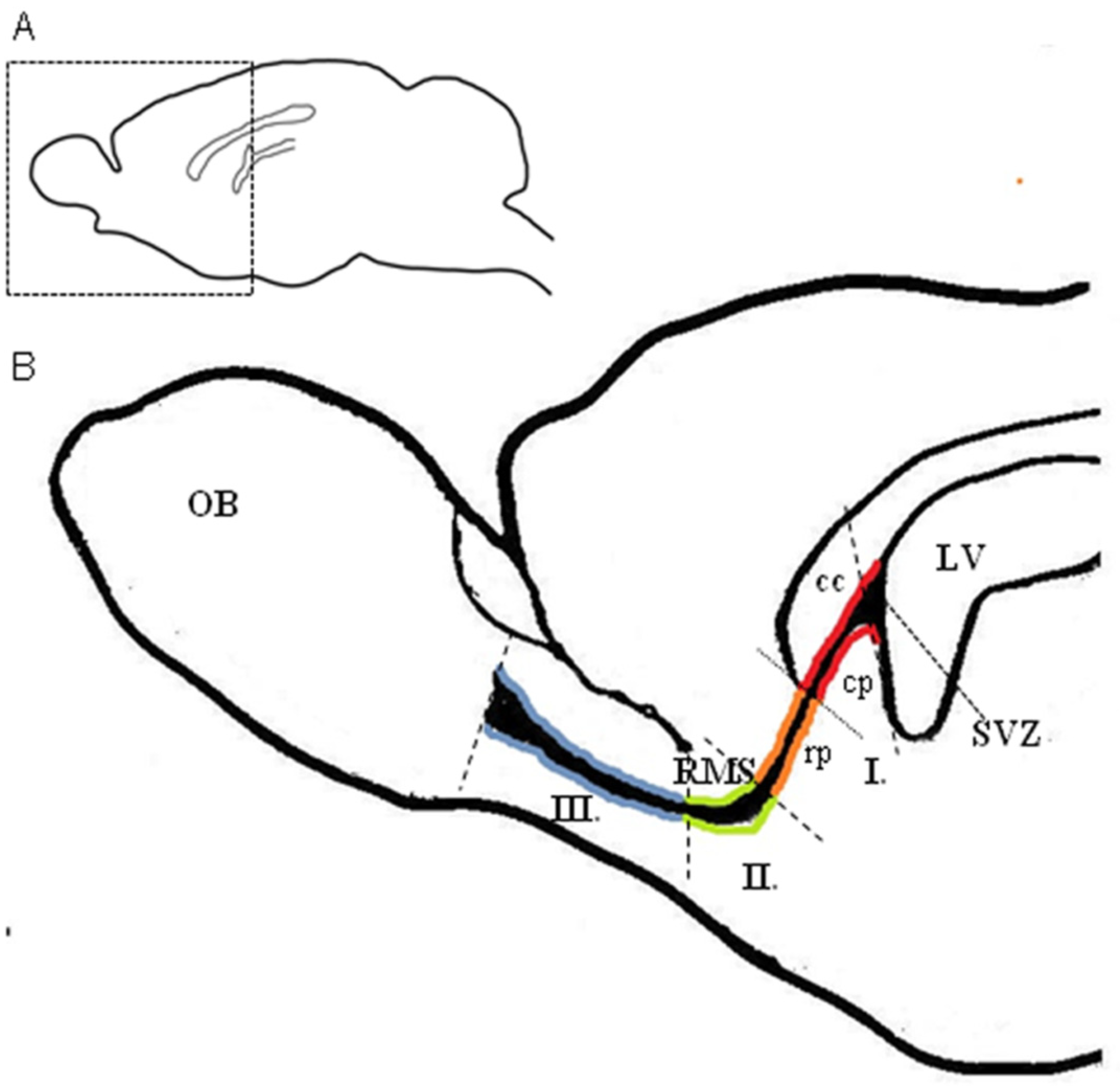
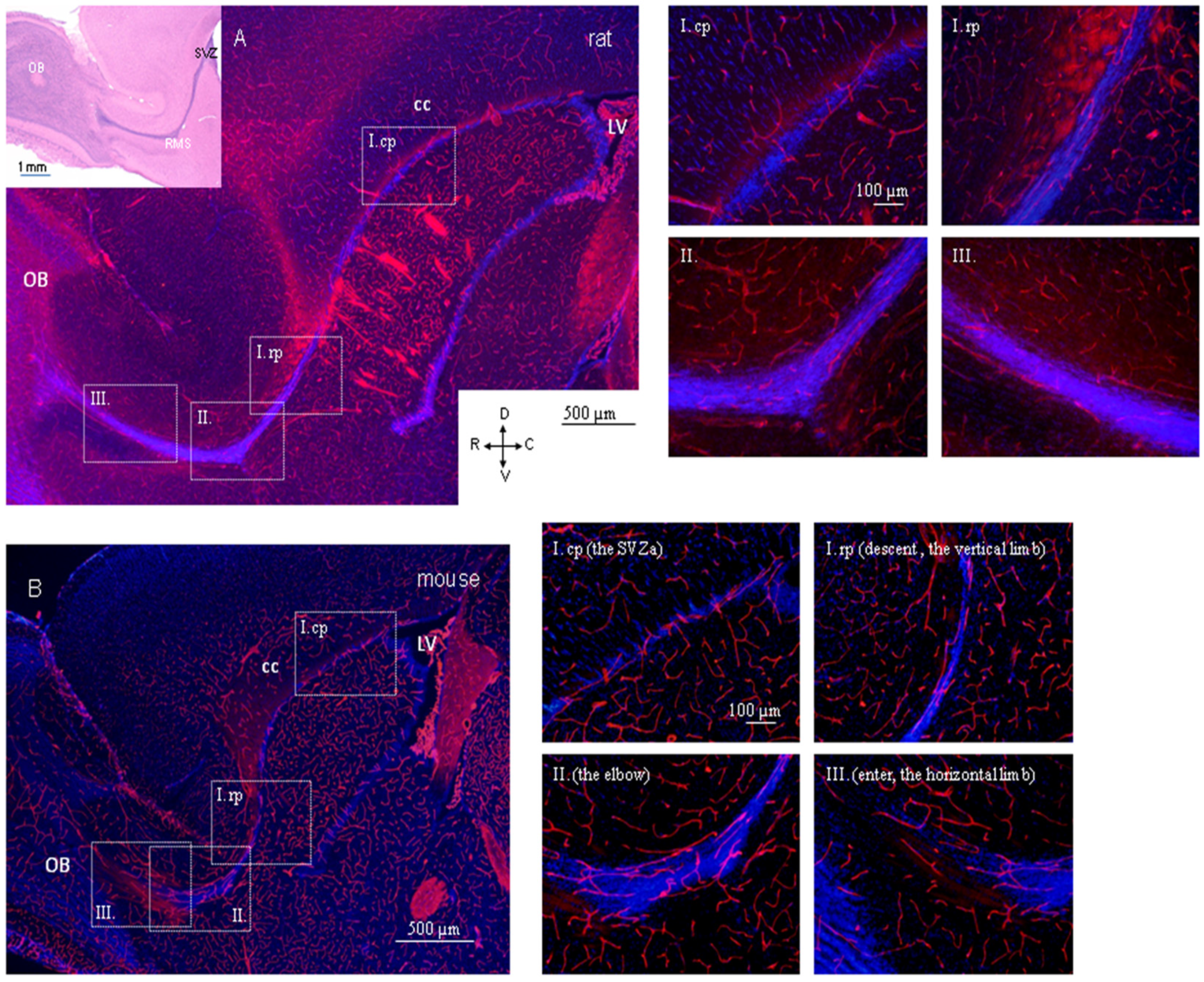
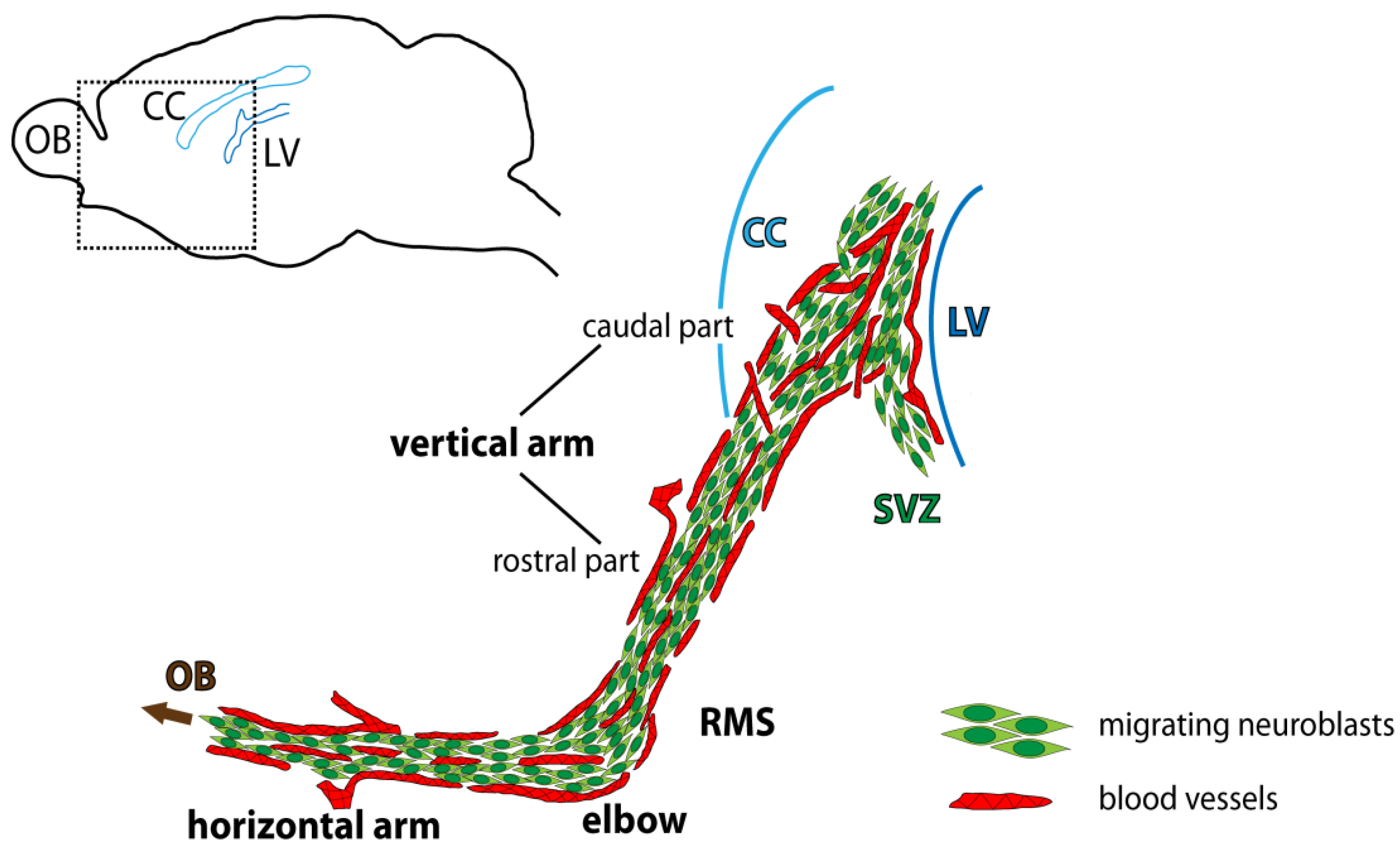
| Developmental Event | Detail | Timing | Mice Strain/ Reference | Detail | Timing | Rat Strain/ Reference |
|---|---|---|---|---|---|---|
| Establishment of CNS | Formation of neural plate | E7–E7.5 | CD1 (ICR) [22] | Neural plate stage | E9 | Sprague-Dawley [23] |
| Neurulation | Initiation at hindbrain/ cervical boundary | E8.5 | not specified [24] | Neural tube closed in upper thoracic and lower cervical region | by E10.5 | Sprague-Dawley [23] |
| Closure of the anterior neuropore | E9 | normal hy-3 [25] | Closure of the anterior neuropore | E10.5 | not specified [26] | |
| Completion of rostral neural tube—segmentation to primary brain vesicles | E9 | CD1 (ICR) [22] | Entire neural tube is closed | by E11, by E12 | Sprague-Dawley [23], Purdue-Wistar [27] | |
| Segmentation to secondary brain vesicles—development of telencephalic vesicles | E9.5 | CD1 (ICR) [22] | Segmentation to primary brain vesicles | E11–E12 | Purdue-Wistar [27], Fisher [28] | |
| Segmentation to secondary brain vesicles—development of telencephalic vesicles | E12–E13, E13 | Fisher [28], Purdue-Wistar [27] | ||||
| Morphogenesis of telencephalon, OB and RMS | Early ventral forebrain cells acquire general migratory capacity | E9.5–E11.5 | ICR [29] | Telencephalon wall divides to VZ, IZ, MZ | E13–E15 | Sprague-Dawley [30], Fisher [28], Wistar [31] |
| Telencephalon wall divides to VZ, IZ, MZ | E10–E10.5 | CD1 (ICR) [22] | Olfactory nerve fibers reach the telencephalon | E13, E14 | Sprague-Dawley [30], not specified [32] | |
| Onset of mitral cells production | by E11, E10–E12 | B6tgN [33], CD1 [34] | Olfactory nerve fibers penetrate the VZ of telencephalon | E14 | Wistar [31] | |
| Morphogenesis of subpallial MGE | E11–E12 | ICR [35], ICR [29], not specified [36] | Development of neurogenic anterior SVZ | from E14 | Sprague-Dawley [37] | |
| Morphogenesis of subpallial LGE | E12–E12.5 | ICR [29], not specified [36] | Differentiation of neuronal cells of prospective RMS | E14 | Sprague-Dawley [37] | |
| Generation of subpopulation of OB projection neurons in the rostral LGE of cultured embryo | E11–E12 | C57 mice [38] | Mitral cells accumulate at the base of telencephalon | E15 | Purdue-Wistar [27], Sprague-Dawley [30], Sprague-Dawley [37] | |
| Generation of main population of mitral cells | E11–E13 | CD1 [34], F1 hybrids of Balb/c females and SJL/J males [39], C57 BL/6 [40] | peak of mitral cells generation | E14–E16 | Purdue-Wistar [41] | |
| LGE cells acquire migratory capacity toward the OB | E11.5–E12.5 | ICR [29] | OB evaginations emerge in anterior telencephalon | E15 | Purdue-Wistar [27], Sprague-Dawley [30], Sprague-Dawley [37] | |
| Primordial OB detectable at the anterior tip of telencephalon | E12.5 | not specified [42] | Neuronal progenitors organize into prospective RMS | by E15 | Sprague-Dawley [37] | |
| Radial glial cells are present in the developing OB | E13.5 | not specified [43] | Neuronal cells organize into dense compact patch; GFAP positive cells emerge in forming RMS | by E16 | Sprague-Dawley [37] | |
| Main production of tufted cells | E14–E17 | F1 hybrids of Balb/c females and SJL/J males [39] | Peak of internal tufted cells generation | E16–E17 | Purdue-Wistar [41] | |
| Generation of neuronal populations of presumptive RMS originating in LGE | E14.5 | ICR [29] | RMS emerges in the rostral forebrain; neuronal patch is surrounded by non-patch cells | by E17 | not specified [32], Sprague-Dawley [37] | |
| Presence of olfactory lobes at the rostral end of telencephalon | E15–E15.5 | CD1 (ICR) [22] | OB contain evaginated parts of the lateral ventricles | E18 | Purdue-Wistar [27] | |
| Organization of neuronal cells into presumptive RMS | by E16.5 | ICR [29] | Peak of generation of tufted cells:
| E18–E19 E20–E22 | Purdue-Wistar [41] | |
| Shrinkage of lateral ventricles, rostral extensions into the OB | E16.5–E18.5 | CD1 (ICR) [22] | Peak of generation of periglomerular cells and external plexiform layer cells | P0–P7 | Purdue-Wistar [41] | |
| Main production of OB granule cells | E18–P20 | F1 hybrids of Balb/c females and SJL/J males [39] | Peak of production of granule cells (continues throughout the rest of life) | P0–P15 | Purdue-Wistar [41] | |
OB ventricles:
| P0–P1 P0–P3 | not specified [43], CD1 [44] | OB ventricles:
| P3–P4 | Wistar [44] | |
| Expression of GFAP in astrocytes | P6–P13 | CD1 [44] | Expression of GFAP in astrocytes | P6–P9 | Wistar [44] | |
| glial tubes emerge in the RMS | P21–P25 | CD1 [44] | Glial tubes emerge in the RMS | P21–P25 | Wistar [44] |
| Developmental Event | Detail | Timing | Mice Strain/ Reference | Detail | Timing | Rat Strain/ Reference |
|---|---|---|---|---|---|---|
| Vascularization of telencephalon: Vasculogenesis | Initiation of PNVP in the mesoderm surrounding the neural tube | E8.5 | Swiss albino [60] | PNVP covers lateroventral surface of the rostral neural tube | E11 | Sprague-Dawley [56] |
| PNVP covers the surface of telencephalon | E9 | CD1 [63] | Degeneration of capillaries in the meningeal plexus | after E15 | Sprague-Dawley [56] | |
| Region over telencephalic roof plate remains devoid of PNVP | E8.5–E9.5 | Swiss albino [60] | end of leptomeningeal vasculature sprouting into the cerebral cortex | P8–P15 | Sprague-Dawley/Wistar [69] | |
| Whole neural tube is covered by PNVP | E11.5, E10–E12 | Swiss albino [60], normal hy-3 [25] | ||||
| Superficial vasculature condenses to tubular vessels | by E12 | normal hy-3 [25] | ||||
| Vascularization of telencephalon: Angiogenesis | Formation of PVVP in ventral telencephalon | E9–E10 | CD1 [63] | Onset of internal vascularization of telencephalon | E13 | Fisher [28] |
| Progression of PVVP from ventral to dorsal telencephalon | E11 | CD1 [63] | Formation of deep vascular plexus of the VZ (PVVP) | E13 | not specified [64] | |
| PVVP emerges in dorsal subpallium and in ventral pallium | E11.5 | Swiss albino [60] | Decrease in proliferation of endothelial cells in telencephalon | after P20 | Sprague-Dawley/Wistar [69] | |
| Simple loops of vascular plexi surround the rostral extension of lateral ventricles | E14 | C57Bl/6 [68] | ||||
| Early phase of angiogenesis in dorsal telencephalon (sprouting, branching) | E14.5–E16.5 | C57BL/6 [65] | ||||
| Late phase of angiogenesis in the dorsal telencephalon (fusion of branches) | E16.5–E18.5 | C57BL/6 [65] | ||||
| Cease of angiogenesis in mice brain | after P20 | not specified [70] | ||||
| Vascularization of neurogenic region of forebrain | presence of short, straight, unbranched BV | E14.5 | CD1 [67] | |||
| BV are tangentially oriented in the presumptive RMS | E16 | C57BL/6 [68] | ||||
| BV are longitudinaly organized in the RMS—parallel to each other | by E16–P4 | C57Bl/6 [68] | ||||
| BV are longer, branched, tangentially oriented, follow longitudinal axis of forming RMS, more frequent along the border of RMS | E17.5 | CD1 [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martončíková, M.; Alexovič Matiašová, A.; Ševc, J.; Račeková, E. Relationship between Blood Vessels and Migration of Neuroblasts in the Olfactory Neurogenic Region of the Rodent Brain. Int. J. Mol. Sci. 2021, 22, 11506. https://doi.org/10.3390/ijms222111506
Martončíková M, Alexovič Matiašová A, Ševc J, Račeková E. Relationship between Blood Vessels and Migration of Neuroblasts in the Olfactory Neurogenic Region of the Rodent Brain. International Journal of Molecular Sciences. 2021; 22(21):11506. https://doi.org/10.3390/ijms222111506
Chicago/Turabian StyleMartončíková, Marcela, Anna Alexovič Matiašová, Juraj Ševc, and Enikő Račeková. 2021. "Relationship between Blood Vessels and Migration of Neuroblasts in the Olfactory Neurogenic Region of the Rodent Brain" International Journal of Molecular Sciences 22, no. 21: 11506. https://doi.org/10.3390/ijms222111506
APA StyleMartončíková, M., Alexovič Matiašová, A., Ševc, J., & Račeková, E. (2021). Relationship between Blood Vessels and Migration of Neuroblasts in the Olfactory Neurogenic Region of the Rodent Brain. International Journal of Molecular Sciences, 22(21), 11506. https://doi.org/10.3390/ijms222111506






