An RNA-Seq-Based Framework for Characterizing Canine Prostate Cancer and Prioritizing Clinically Relevant Biomarker Candidate Genes
Abstract
1. Introduction
2. Results
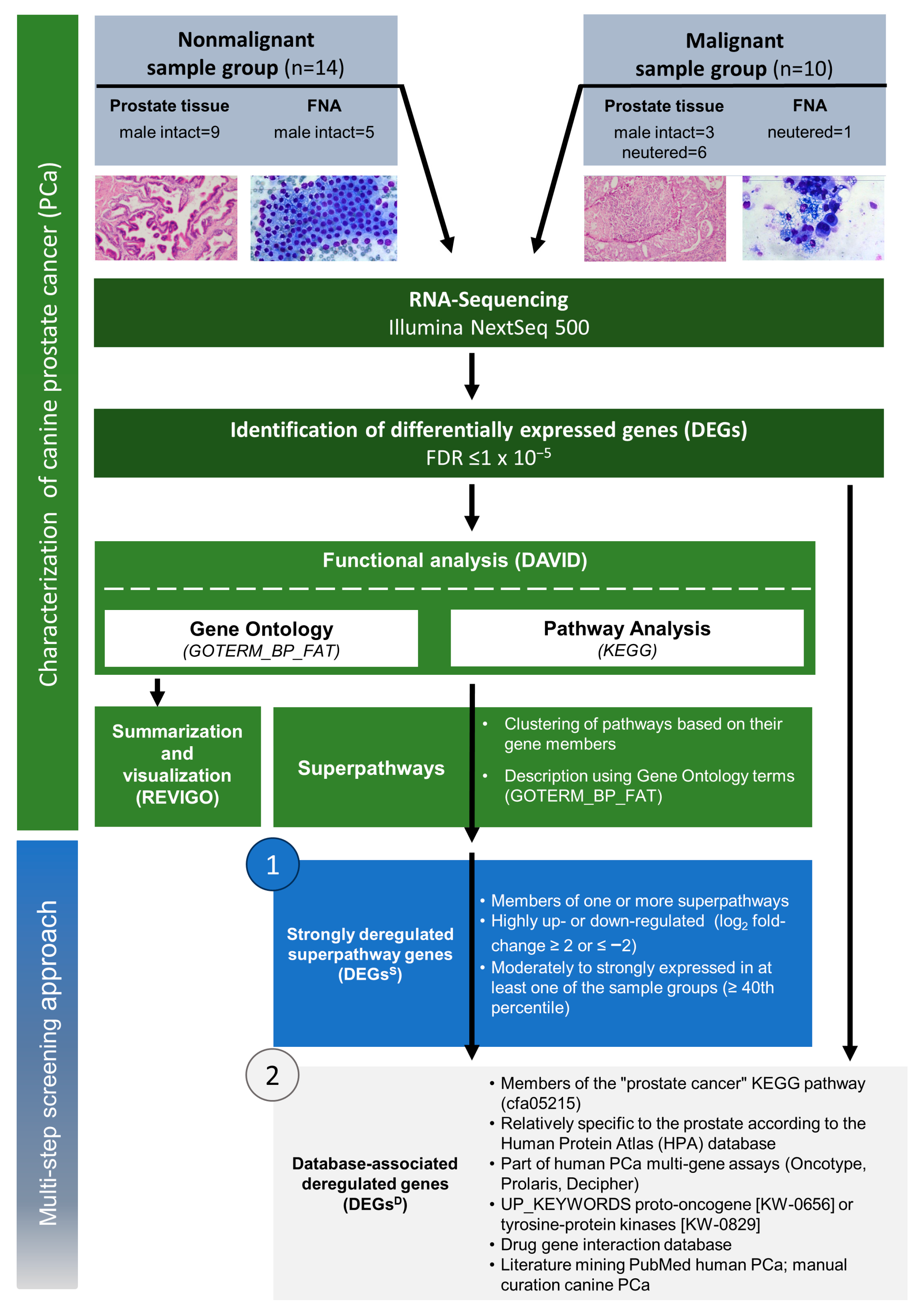
2.1. Discriminating Malignant and Nonmalignant Canine Prostate Samples
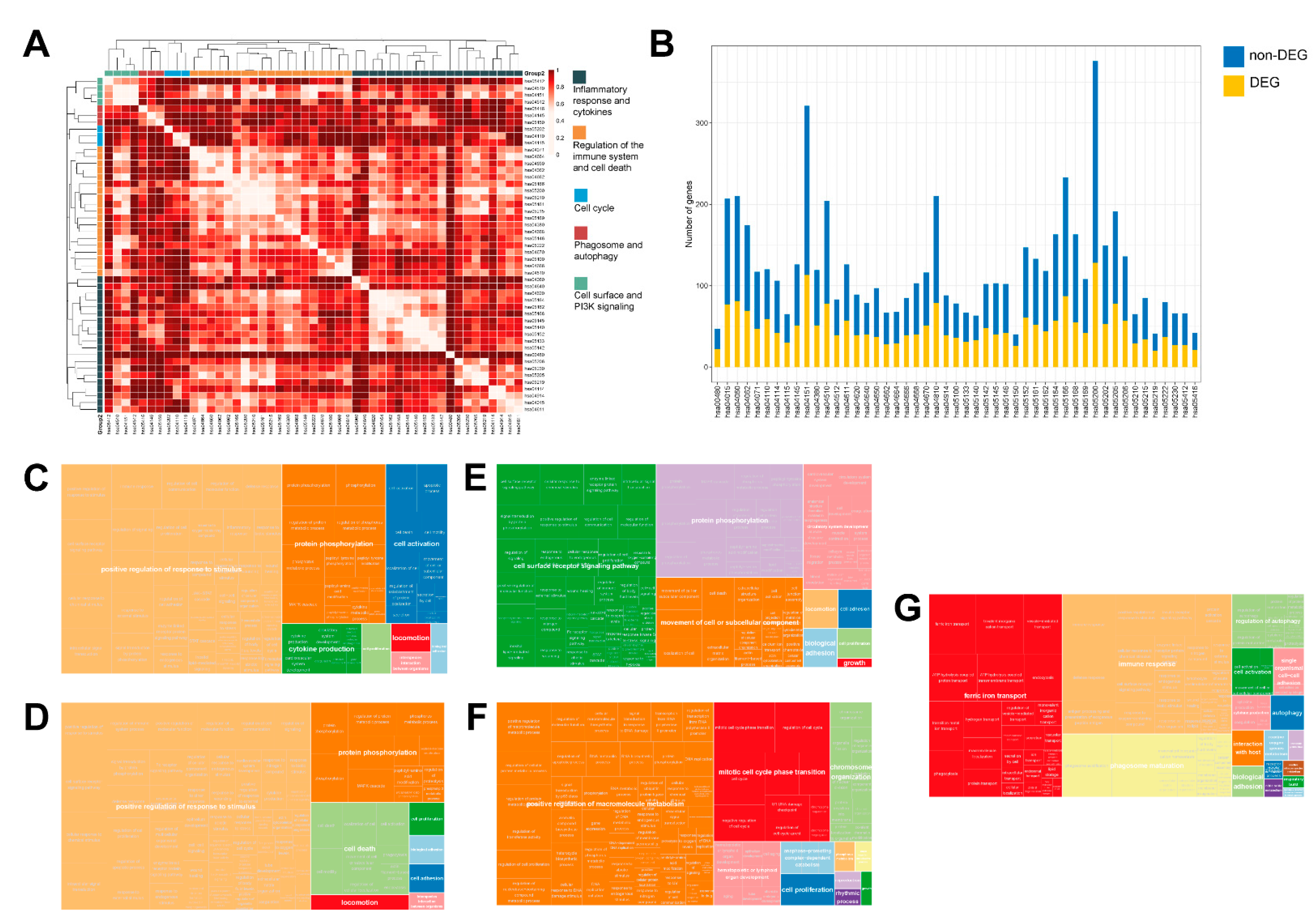
2.2. The Transcriptional Landscape of Canine PCa Is Characterized by the Deregulation of Pathways Involved in Immune Response, Cell Adhesion, PI3K Signaling, Cell Cycle, as Well as Phagosome and Autophagy
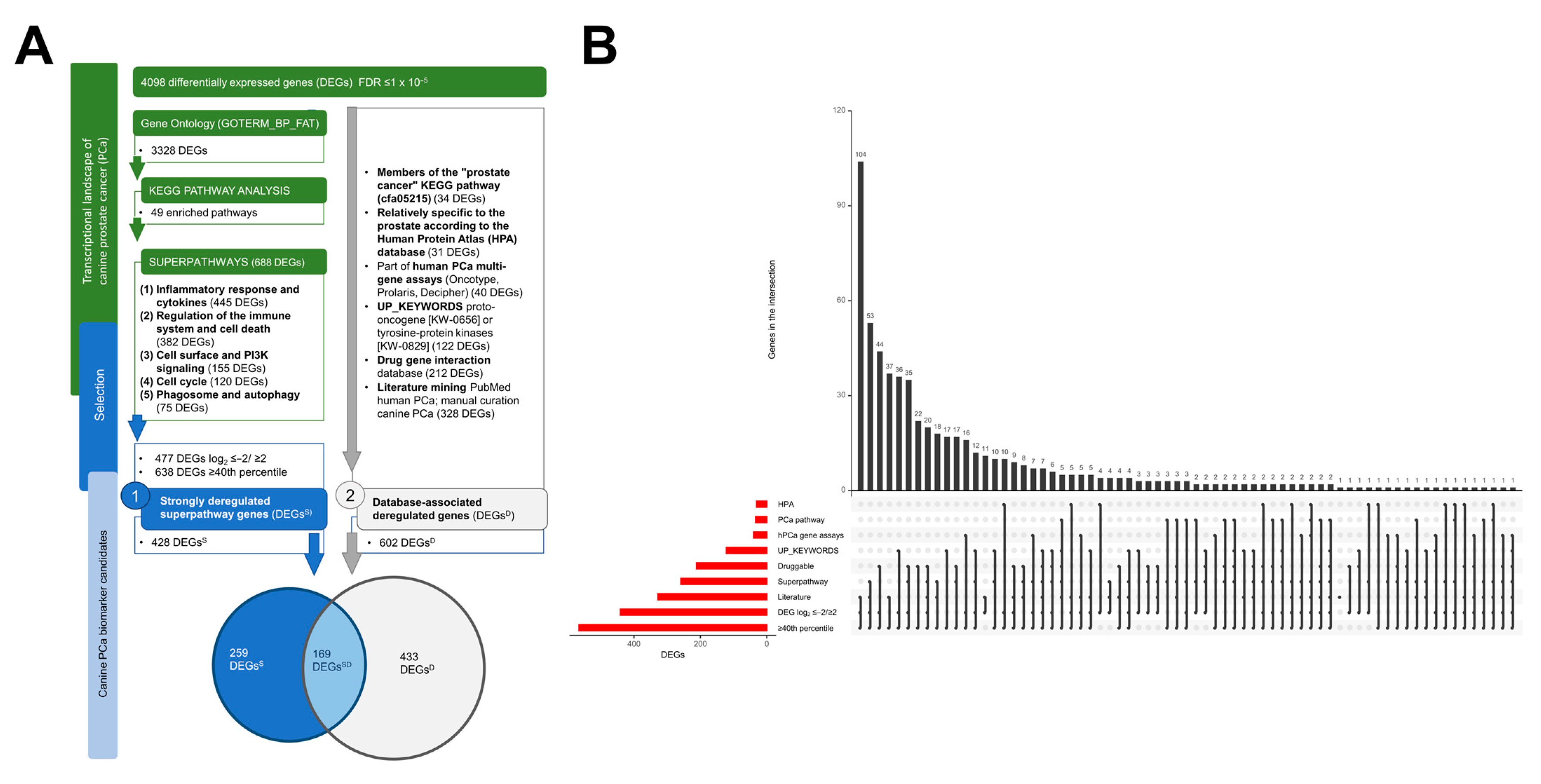
2.3. A Framework for Selecting Canine PCa Biomarker Candidates with Clinical Value
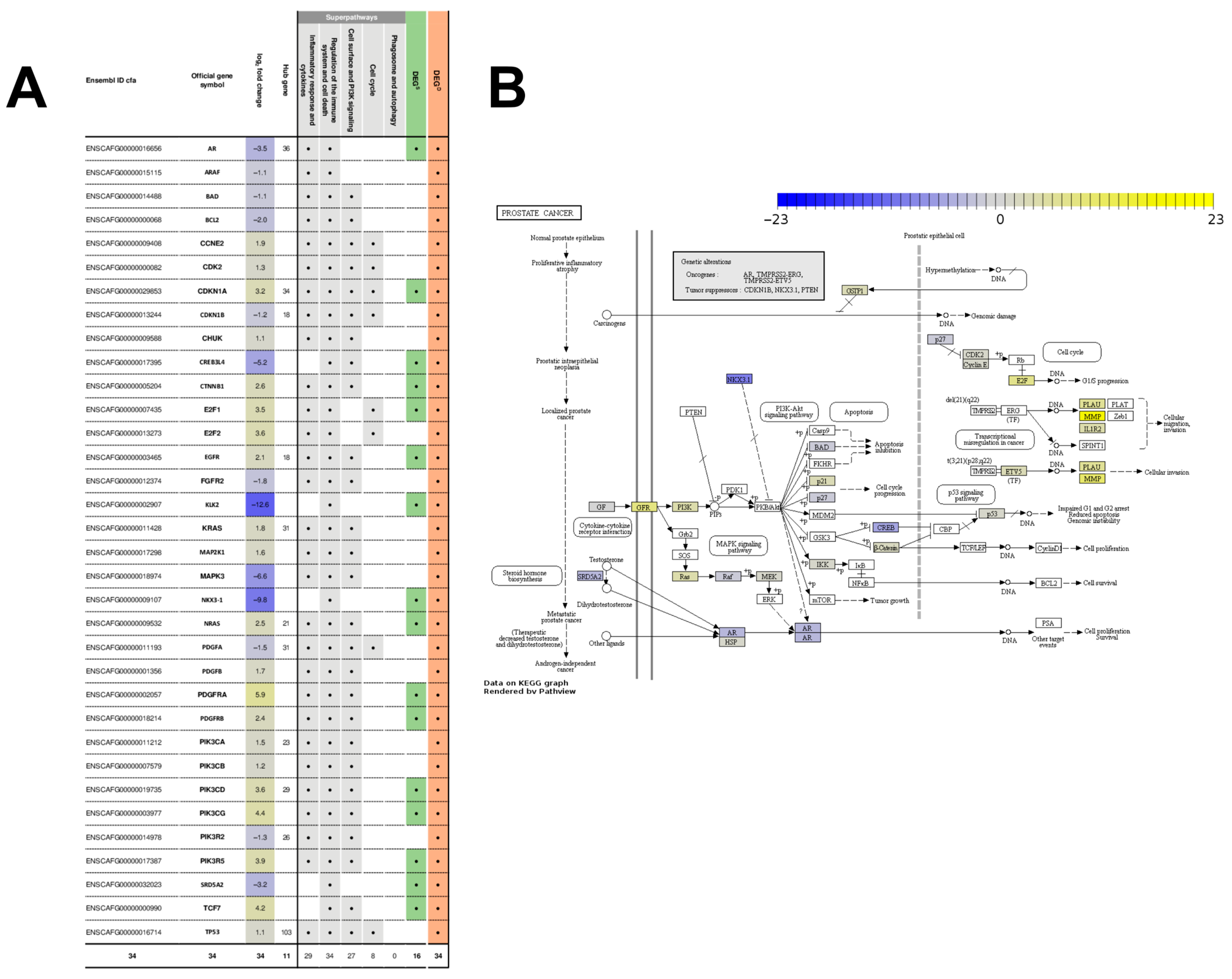
2.4. The Gene Network of 602 DEGsD Form a Tightly Interconnected Gene Network Intertwined with the “Prostate Cancer” Pathway
3. Discussion
3.1. Five Superpathways Provide a Comprehensive Insight into the Hallmarks of Canine PCa
3.2. Database-Associated Strongly Deregulated Superpathway Genes Are Reliable Biomarker Candidates for the Diagnostic and Therapeutic Work-Up
3.3. Database-Associated Deregulated Genes Such as TP53, MYC and AR Are Crucial to Complement the DEGsSD and Tackle Clinically Relevant Questions
4. Materials and Methods
4.1. Ethical Statement and Sampling of Fresh Frozen Prostate Tissue and FNA Samples
4.2. Data Processing and Differential Gene Expression Analysis
4.3. Pathway Enrichment and Functional Analysis
4.4. Pathway Cross-Talk
4.5. Multi-Step Screening for Canine PCa Biomarker Candidates
4.6. PPI Network Construction and Identification of Hub Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waters, D.J.; Patronek, G.J.; Bostwick, D.G.; Glickman, L.T. Comparing the Age at Prostate Cancer Diagnosis in Humans and Dogs. J. Natl. Cancer Inst. 1996, 88, 1686–1687. [Google Scholar] [CrossRef]
- Schrank, M.; Romagnoli, S. Prostatic Neoplasia in the Intact and Castrated Dog: How Dangerous is Castration? Animals 2020, 10, 85. [Google Scholar] [CrossRef]
- Waters, D.J.; Sakr, W.A.; Hayden, D.W.; Lang, C.M.; McKinney, L.; Murphy, G.P.; Radinsky, R.; Ramoner, R.; Richardson, R.C.; Tindall, D.J. Workgroup 4: Spontaneous prostate carcinoma in dogs and nonhuman primates. Prostate 1998, 36, 64–67. [Google Scholar] [CrossRef]
- Gamlem, H.; Nordstoga, K.; Glattre, E. Canine neoplasia–introductory paper. Apmis 2008, 116, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.E.; Northrup, N. Prostate cancer in dogs: Comparative and clinical aspects. Vet. J. 2009, 180, 149–162. [Google Scholar] [CrossRef]
- Palmieri, C.; Lean, F.; Akter, S.; Romussi, S.; Grieco, V. A retrospective analysis of 111 canine prostatic samples: Histopathological findings and classification. Res. Veter. Sci. 2014, 97, 568–573. [Google Scholar] [CrossRef]
- Sun, F.; Báez-Díaz, C.; Sánchez-Margallo, F.M. Canine prostate models in preclinical studies of minimally invasive interventions: Part I, canine prostate anatomy and prostate cancer models. Transl. Androl. Urol. 2017, 6, 538. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alves, C.E.; Vicente, I.; Calazans, S.G.; Amorim, R.L. Canine Prostate Cancer: Would the Dog be an Important Model for the Study of New Drugs? Am. J. Drug Discov. Dev. 2013, 3, 220–224. [Google Scholar] [CrossRef]
- Palmieri, C.; Foster, R.; Grieco, V.; Fonseca-Alves, C.; Wood, G.; Culp, W.; Escobar, H.M.; De Marzo, A.; Laufer-Amorim, R. Histopathological Terminology Standards for the Reporting of Prostatic Epithelial Lesions in Dogs. J. Comp. Pathol. 2019, 171, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, P.E.; Fonseca-Alves, C.E.; Rivera-Calderón, L.G.; Carvalho, M.; Kuasne, H.; Rogatto, S.; Laufer-Amorim, R. Deregulation of E-cadherin, β-catenin, APC and Caveolin-1 expression occurs in canine prostate cancer and metastatic processes. Res. Veter. Sci. 2018, 118, 254–261. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Palmieri, C.; Laufer-Amorim, R. Investigation of c-KIT and Ki67 expression in normal, preneoplastic and neoplastic canine prostate. BMC Veter. Res. 2017, 13, 380. [Google Scholar] [CrossRef]
- Winkler, S.; Escobar, H.M.; Meyer, B.; Simon, D.; Eberle, N.; Baumgartner, W.; Loeschke, S.; Nolte, I.; Bullerdiek, J. HMGA2 expression in a canine model of prostate cancer. Cancer Genet. Cytogenet. 2007, 177, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.E.; Nadella, M.V.P.; Toribio, R.E.; Leav, I.; Rosol, T.J. Canine Prostate Carcinomas Express Markers of Urothelial and Prostatic Differentiation. Veter. Pathol. 2004, 41, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Moulay, M.; Liu, W.; Willenbrock, S.; Sterenczak, K.A.; Carlson, R.; Ngezahayo, A.; Escobar, H.M.; Nolte, I. Evaluation of stem cell marker gene expression in canine prostate carcinoma- and prostate cyst-derived cell lines. Anticancer. Res. 2013, 33, 5421–5431. [Google Scholar]
- Lai, C.-L.; Ham, R.V.D.; van Leenders, G.; van der Lugt, J.; Mol, J.A.; Teske, E. Histopathological and immunohistochemical characterization of canine prostate cancer. Prostate 2008, 68, 477–488. [Google Scholar] [CrossRef]
- Rivera-Calderón, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Drigo, S.A.; Vasconcelos, R.D.O.; Laufer-Amorim, R. Alterations in PTEN, MDM2, TP53 and AR protein and gene expression are associated with canine prostate carcinogenesis. Res. Veter. Sci. 2016, 106, 56–61. [Google Scholar] [CrossRef]
- Kobayashi, M.; Saito, A.; Tanaka, Y.; Michishita, M.; Kobayashi, M.; Irimajiri, M.; Kaneda, T.; Ochiai, K.; Bonkobara, M.; Takahashi, K.; et al. MicroRNA expression profiling in canine prostate cancer. J. Veter. Med Sci. 2017, 79, 719–725. [Google Scholar] [CrossRef]
- Calderón, L.G.R.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Vasconcelos, R.; Laufer-Amorim, R. p-mTOR, p-4EBP-1 and eIF4E expression in canine prostatic carcinoma. Res. Veter. Sci. 2018, 122, 86–92. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Laufer-Amorim, R. Evaluation of NKX3.1 and C-MYC expression in canine prostatic cancer. Res. Veter. Sci. 2018, 118, 365–370. [Google Scholar] [CrossRef]
- Gobello, C.; Castex, G.; Corrada, Y. Serum and seminal markers in the diagnosis of disorders of the genital tract of the dog: A mini-review. Theriogenology 2002, 57, 1285–1291. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Yoneyama, T.; Tobisawa, Y.; Ohyama, C. Recent progress and perspectives on prostate cancer biomarkers. Int. J. Clin. Oncol. 2016, 22, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.B.; Irwin, D.M.; Diamandis, E.P. In silico identification and Bayesian phylogenetic analysis of multiple new mammalian kallikrein gene families. Genomics 2006, 88, 591–599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chapdelaine, P.; Gauthier, E.; Ho-Kim, M.A.; Bissonnette, L.; Tremblay, R.R.; Dubé, J.Y. Characterization and Expression of the Prostatic Arginine Esterase Gene, a Canine Glandular Kallikrein. DNA Cell Biol. 1991, 10, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Alonge, S.; Melandri, M.; Aiudi, G.; Lacalandra, G.M. Advances in prostatic diagnostics in dogs: The role of Canine Prostatic Specific Esterase (CPSE) in the early diagnosis of prostatic disorders. Top. Companion Anim. Med. 2018, 33, 105–108. [Google Scholar] [CrossRef]
- Bell, F.W.; Klausner, J.S.; Hayden, D.W.; Lund, E.M.; Liebenstein, B.B.; Feeney, D.A.; Johnston, S.D.; Shivers, J.L.; Ewing, C.M.; Isaacs, W.B. Evaluation of Serum and Seminal Plasma Markers in the Diagnosis of Canine Prostatic Disorders. J. Veter. Intern. Med. 1995, 9, 149–153. [Google Scholar] [CrossRef]
- Melandri, M.; Alonge, S. Highlights on the Canine Prostatic Specific Esterase (CPSE): A diagnostic and screening tool in veterinary andrology. Veter. Med. Sci. 2020, 7, 35–40. [Google Scholar] [CrossRef]
- Palmieri, C.; Grieco, V. Proposal of Gleason-like grading system of canine prostate carcinoma in veterinary pathology practice. Res. Veter. Sci. 2015, 103, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Tolkach, Y.; Kristiansen, G. The Heterogeneity of Prostate Cancer: A Practical Approach. Pathobiology 2018, 85, 108–116. [Google Scholar] [CrossRef]
- Salami, S.S.; Hovelson, D.H.; Kaplan, J.B.; Mathieu, R.; Udager, A.M.; Curci, N.E.; Lee, M.; Plouffe, K.R.; De La Vega, L.L.; Susani, M.; et al. Transcriptomic heterogeneity in multifocal prostate cancer. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Katogiritis, A.; Khanna, C. Towards the Delivery of Precision Veterinary Cancer Medicine. Veter. Clin. N. Am. Small Anim. Pr. 2019, 49, 809–818. [Google Scholar] [CrossRef]
- Laufer-Amorim, R.; Fonseca-Alves, C.E.; Villacis, R.; Linde, S.A.D.; Carvalho, M.; Larsen, S.J.; Marchi, F.A.; Rogatto, S.R. Comprehensive Genomic Profiling of Androgen-Receptor-Negative Canine Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1555. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Gruber, A.D. Transcriptome and proteome research in veterinary science: What is possible and what questions can be asked? Sci. World J. 2012, 2012, 254962. [Google Scholar] [CrossRef]
- Cieślik, M.; Chinnaiyan, A.M. Cancer transcriptome profiling at the juncture of clinical translation. Nat. Rev. Genet. 2017, 19, 93–109. [Google Scholar] [CrossRef]
- Rajan, P.; Stockley, J.; Sudbery, I.M.; Fleming, J.T.; Hedley, A.; Kalna, G.; Sims, D.; Ponting, C.P.; Heger, A.; Robson, C.N.; et al. Identification of a candidate prognostic gene signature by transcriptome analysis of matched pre- and post-treatment prostatic biopsies from patients with advanced prostate cancer. BMC Cancer 2014, 14, 977. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.D.; Kulp, K.S.; Loots, G. Prostate cancer invasion and metastasis: Insights from mining genomic data. Briefings Funct. Genom. 2013, 12, 397–410. [Google Scholar] [CrossRef]
- Na, R.; Wu, Y.; Ding, Q.; Xu, J. Clinically available RNA profiling tests of prostate tumors: Utility and comparison. Asian J. Androl. 2016, 18, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Thiemeyer, H.; Taher, L.; Schille, J.T.; Harder, L.; Hungerbuehler, S.O.; Mischke, R.; Hewicker-Trautwein, M.; Kiełbowicz, Z.; Brenig, B.; Schütz, E.; et al. Suitability of ultrasound-guided fine-needle aspiration biopsy for transcriptome sequencing of the canine prostate. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar]
- Supek, F.; Bošnjak, M.; Škunca, N.; Smuc, T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Luo, W.; Pant, G.; Bhavnasi, Y.K.; Blanchard, S.G., Jr.; Brouwer, C. Pathview Web: User friendly pathway visualization and data integration. Nucleic Acids Res. 2017, 45, W501–W508. [Google Scholar] [CrossRef]
- Vindrieux, D.; Escobar, P.; Lazennec, G. Emerging roles of chemokines in prostate cancer. Endocrine-Related Cancer 2009, 16, 663–673. [Google Scholar] [CrossRef]
- Raposo, T.; Beirão, B.; Pang, L.; Queiroga, F.; Argyle, D. Inflammation and cancer: Till death tears them apart. Veter. J. 2015, 205, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Corn, P.G. The tumor microenvironment in prostate cancer: Elucidating molecular pathways for therapy development. Cancer Manag. Res. 2012, 4, 183–193. [Google Scholar] [CrossRef]
- Shiao, S.L.; Chu, G.C.-Y.; Chung, L.W. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016, 380, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Calderón, L.G.R.; Kobayashi, P.E.; Vasconcelos, R.O.; Fonseca-Alves, C.E.; Laufer-Amorim, R. Characterization of collagens fibers (I, III, IV) and elastin in the extracellular matrix of normal and neoplastic canine prostate. Vet Sci. 2019, 6. [Google Scholar]
- Ruetten, H.; Wegner, K.A.; Romero, M.F.; Wood, M.W.; Marker, P.C.; Strand, U.; Colopy, S.A.; Vezina, C.M. Prostatic collagen architecture in neutered and intact canines. Prostate 2018, 78, 839–848. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Rybak, A.P.; Bristow, R.; Kapoor, A. Prostate cancer stem cells: Deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget 2014, 6, 1900–1919. [Google Scholar] [CrossRef]
- Saad, F.; Shore, N.; Zhang, T.; Sharma, S.; Cho, H.K.; Jacobs, I.A. Emerging therapeutic targets for patients with advanced prostate cancer. Cancer Treat. Rev. 2019, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Braglia, L.; Zavatti, M.; Vinceti, M.; Martelli, A.M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: Still a potential druggable target? Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2020, 1867, 118731. [Google Scholar] [CrossRef]
- Pienta, K.J.; Abate-Shen, C.; Agus, D.B.; Attar, R.M.; Chung, L.W.; Greenberg, N.M.; Hahn, W.C.; Isaacs, J.T.; Navone, N.M.; Peehl, D.M.; et al. The current state of preclinical prostate cancer animal models. Prostate 2008, 68, 629–639. [Google Scholar] [CrossRef]
- Yu, C.; Hu, K.; Nguyen, D.; Wang, Z.A. From genomics to functions: Preclinical mouse models for understanding oncogenic pathways in prostate cancer. Am. J. Cancer Res. 2019, 9, 2079. [Google Scholar] [PubMed]
- Ittmann, M.; Huang, J.; Radaelli, E.; Martin, P.; Signoretti, S.; Sullivan, R.; Simons, B.; Ward, J.M.; Robinson, B.D.; Chu, G.C.; et al. Animal Models of Human Prostate Cancer: The Consensus Report of the New York Meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res. 2013, 73, 2718–2736. [Google Scholar] [CrossRef]
- Balk, S.P.; Knudsen, K.E. AR, the cell cycle, and prostate cancer. Nucl. Recept. Signal. 2008, 6, 6001. [Google Scholar] [CrossRef]
- Packer, J.R.; Maitland, N.J. The molecular and cellular origin of human prostate cancer. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 1238–1260. [Google Scholar] [CrossRef] [PubMed]
- Erho, N.; Crisan, A.; Vergara, I.; Mitra, A.P.; Ghadessi, M.; Buerki, C.; Bergstralh, E.J.; Kollmeyer, T.; Fink, S.; Haddad, Z.; et al. Discovery and Validation of a Prostate Cancer Genomic Classifier that Predicts Early Metastasis Following Radical Prostatectomy. PLoS ONE 2013, 8, e66855. [Google Scholar] [CrossRef]
- Cuzick, J.; Swanson, G.; Fisher, G.; Brothman, A.R.; Berney, D.; Reid, J.E.; Mesher, D.; Speights, V.; Stankiewicz, E.; Foster, C.S.; et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011, 12, 245–255. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, X.; Liang, X.; Jiang, G. Molecular and cellular mechanisms of castration resistant prostate cancer (Review). Oncol. Lett. 2018, 15, 6063–6076. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.; Gupta, A.; Savage, C.J.; Pettersson, K.; Dahlin, A.; Bjartell, A.; Manjer, J.; Scardino, P.T.; Ulmert, D.; Lilja, H. A Panel of Kallikrein Marker Predicts Prostate Cancer in a Large, Population-Based Cohort Followed for 15 Years without Screening. Cancer Epidemiol. Biomarkers Prev. 2010, 20, 255–261. [Google Scholar] [CrossRef]
- Mavridis, K.; Avgeris, M.; Scorilas, A. Targeting kallikrein-related peptidases in prostate cancer. Expert Opin. Ther. Targets 2014, 18, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, I.; Stephan, C.; Jung, K.; Dietel, M.; Rieger, A.; Tolkach, Y.; Kristiansen, G. Sensitivity of HOXB13 as a Diagnostic Immunohistochemical Marker of Prostatic Origin in Prostate Cancer Metastases: Comparison to PSA, Prostein, Androgen Receptor, ERG, NKX3.1, PSAP, and PSMA. Int. J. Mol. Sci. 2017, 18, 1151. [Google Scholar] [CrossRef]
- Kristiansen, G. Markers of clinical utility in the differential diagnosis and prognosis of prostate cancer. Mod. Pathol. 2018, 31, 143–155. [Google Scholar] [CrossRef]
- Shtivelman, E.; Beer, T.M.; Evans, C.P. Molecular pathways and targets in prostate cancer. Oncotarget 2014, 5, 7217–7259. [Google Scholar] [CrossRef]
- Drake, J.; Graham, N.A.; Stoyanova, T.; Sedghi, A.; Goldstein, A.; Cai, H.; Smith, D.A.; Zhang, H.; Komisopoulou, E.; Huang, J.; et al. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc. Natl. Acad. Sci. USA 2012, 109, 1643–1648. [Google Scholar] [CrossRef]
- Bavcar, S.; Argyle, D.J. Receptor tyrosine kinase inhibitors: Molecularly targeted drugs for veterinary cancer therapy. Veter. Comp. Oncol. 2012, 10, 163–173. [Google Scholar] [CrossRef]
- London, C.A.; Hannah, A.L.; Zadovoskaya, R.; Chien, M.B.; Kollias-Baker, C.; Rosenberg, M.; Downing, S.; Post, G.; Boucher, J.; Shenoy, N.; et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin. Cancer Res. 2003, 9, 2755–2768. [Google Scholar] [PubMed]
- London, C.A.; Malpas, P.B.; Wood-Follis, S.L.; Boucher, J.F.; Rusk, A.W.; Rosenberg, M.P.; Henry, C.J.; Mitchener, K.L.; Klein, M.K.; Hintermeister, J.G.; et al. Multi-center, Placebo-controlled, Double-blind, Randomized Study of Oral Toceranib Phosphate (SU11654), a Receptor Tyrosine Kinase Inhibitor, for the Treatment of Dogs with Recurrent (Either Local or Distant) Mast Cell Tumor Following Surgical Excision. Clin. Cancer Res. 2009, 15, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.; Ahn, A. Masitinib–the efficacy of targeted therapy in veterinary medicine. Vet. Cancer Soc. Newsl. Summer 2010, 34, 6–11. [Google Scholar]
- da Costa, R.M.G. C-kit as a prognostic and therapeutic marker in canine cutaneous mast cell tumours: From laboratory to clinic. Vet. J. 2015, 205, 5–10. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Kohn, B.; Gruber, A. Mechanisms of tumour resistance against chemotherapeutic agents in veterinary oncology. Veter. J. 2016, 207, 63–72. [Google Scholar] [CrossRef]
- Packeiser, E.-M.; Hewicker-Trautwein, M.; Thiemeyer, H.; Mohr, A.; Junginger, J.; Schille, J.T.; Escobar, H.M.; Nolte, I. Characterization of six canine prostate adenocarcinoma and three transitional cell carcinoma cell lines derived from primary tumor tissues as well as metastasis. PLoS ONE 2020, 15, e0230272. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Consortium, U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Griffith, M.; Griffith, O.L.; Coffman, A.C.; Weible, J.V.; McMichael, J.F.; Spies, N.C.; Koval, J.; Das, I.; Callaway, M.B.; Eldred, J.M.; et al. DGIdb: Mining the druggable genome. Nat. Methods 2013, 10, 1209–1210. [Google Scholar] [CrossRef]
- Klein, E.A.; Cooperberg, M.R.; Magi-Galluzzi, C.; Simko, J.P.; Falzarano, S.M.; Maddala, T.; Chan, J.; Li, J.; Cowan, J.E.; Tsiatis, A.C.; et al. A 17-gene Assay to Predict Prostate Cancer Aggressiveness in the Context of Gleason Grade Heterogeneity, Tumor Multifocality, and Biopsy Undersampling. Eur. Urol. 2014, 66, 550–560. [Google Scholar] [CrossRef]
- Pagliarone, S.; Frattone, L.; Pirocchi, V.; Della Salda, L.; Palmieri, C. Altered expression of p53, but not Rb, is involved in canine prostatic carcinogenesis. Res. Veter. Sci. 2016, 105, 195–199. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Rodrigues, M.M.; de Moura, V.M.; Rogatto, S.; Laufer-Amorim, R. Alterations of C-MYC, NKX3.1, and E-cadherin expression in canine prostate carcinogenesis. Microsc. Res. Tech. 2013, 76, 1250–1256. [Google Scholar] [CrossRef]
- Sun, F.; Báez-Díaz, C.; Sánchez-Margallo, F.M. Canine prostate models in preclinical studies of minimally invasive interventions: Part II, benign prostatic hyperplasia models. Transl. Androl. Urol. 2017, 6, 547. [Google Scholar] [CrossRef]
- Gallardo, F.; Mogas, T.; Baró, T.; Rabanal, R.; Morote, J.; Abal, M.; Reventós, J.; Lloreta, J. Expression of Androgen, Oestrogen α and β, and Progesterone Receptors in the Canine Prostate: Differences between Normal, Inflamed, Hyperplastic and Neoplastic Glands. J. Comp. Pathol. 2007, 136, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; Depinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef]
- Johnston, S.; Kamolpatana, K.; Kustritz, M.R.; Johnston, G. Prostatic disorders in the dog. Anim. Reprod. Sci. 2000, 60–61, 405–415. [Google Scholar] [CrossRef]
- Kobayashi, P.E.; Rodrigues, M.M.; Gartner, F.; Rema, A.; Fonseca-Alves, C.E.; Laufer-Amorim, R. Association between decreased expression of estrogen receptor alpha, androgen receptor and phosphatase and tensin homolog immunoexpression in the canine prostate. Pesqui. Veterinária Bras. 2019, 39, 40–46. [Google Scholar] [CrossRef]
- Shidaifat, F. Age-Dependent Expression of 5α-Reductase and Androgen Receptors mRNA by the Canine Prostate. Physiol. Res. 2009, 58, 155–158. [Google Scholar] [CrossRef]
- Amin, M.B.; Epstein, J.I.; Ulbright, T.M.; Humphrey, P.A.; Egevad, L.; Montironi, R.; Grignon, D.; Trpkov, K.; Lopez-Beltran, A.; Zhou, M. Best practices recommendations in the application of immunohistochemistry in urologic pathology: Report from the International Society of Urological Pathology consensus conference. Am. J. Surg. Pathol. 2014, 38, 1017–1022. [Google Scholar] [CrossRef]
- Muniyan, S.; Ingersoll, M.A.; Batra, S.K.; Lin, M.-F. Cellular prostatic acid phosphatase, a PTEN-functional homologue in prostate epithelia, functions as a prostate-specific tumor suppressor. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2014, 1846, 88–98. [Google Scholar] [CrossRef]
- Kong, H.Y.; Byun, J. Emerging Roles of Human Prostatic Acid Phosphatase. Biomol. Ther. 2013, 21, 10–20. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Goldschmidt, M.; Shofer, F.; Goldkamp, C.; Ferracone, J. Immunohistochemical characterization of canine prostatic carcinoma and correlation with castration status and castration time. Veter. Comp. Oncol. 2003, 1, 48–56. [Google Scholar] [CrossRef]
- Vaz, S.; Hadaschik, B.; Gabriel, M.; Herrmann, K.; Eiber, M.; Costa, D. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 9–15. [Google Scholar] [CrossRef]
- Wu, L.Y.; Johnson, J.M.; Simmons, J.K.; Mendes, D.E.; Geruntho, J.J.; Liu, T.; Dirksen, W.P.; Rosol, T.; Davis, W.C.; Berkman, C.E. Biochemical characterization of prostate-specific membrane antigen from canine prostate carcinoma cells. Prostate 2014, 74, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2012, 41, D991–D995. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 18 October 2021).
- Yates, A.D.; Achuthan, P.; Akanni, W.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R. Ensembl 2020. Nucleic Acids Res. 2020, 48, D682–D688. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2012, 29, 15–21. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology 2014, 15, 550. [Google Scholar] [CrossRef]
- Aken, B.L.; Ayling, S.C.; Barrell, D.; Clarke, L.; Curwen, V.; Fairley, S.; Banet, J.F.; Billis, K.; Giron, C.G.; Hourlier, T.; et al. The Ensembl gene annotation system. Database 2016, 2016. [Google Scholar] [CrossRef]
- Kinsella, R.J.; Kähäri, A.; Haider, S.; Zamora, J.; Proctor, G.; Spudich, G.; Almeida-King, J.; Staines, D.; Derwent, P.; Kerhornou, A.; et al. Ensembl BioMarts: A hub for data retrieval across taxonomic space. Database 2011, 2011, bar030. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2016, gkw937. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiemeyer, H.; Taher, L.; Schille, J.T.; Packeiser, E.-M.; Harder, L.K.; Hewicker-Trautwein, M.; Brenig, B.; Schütz, E.; Beck, J.; Nolte, I.; et al. An RNA-Seq-Based Framework for Characterizing Canine Prostate Cancer and Prioritizing Clinically Relevant Biomarker Candidate Genes. Int. J. Mol. Sci. 2021, 22, 11481. https://doi.org/10.3390/ijms222111481
Thiemeyer H, Taher L, Schille JT, Packeiser E-M, Harder LK, Hewicker-Trautwein M, Brenig B, Schütz E, Beck J, Nolte I, et al. An RNA-Seq-Based Framework for Characterizing Canine Prostate Cancer and Prioritizing Clinically Relevant Biomarker Candidate Genes. International Journal of Molecular Sciences. 2021; 22(21):11481. https://doi.org/10.3390/ijms222111481
Chicago/Turabian StyleThiemeyer, Heike, Leila Taher, Jan Torben Schille, Eva-Maria Packeiser, Lisa K. Harder, Marion Hewicker-Trautwein, Bertram Brenig, Ekkehard Schütz, Julia Beck, Ingo Nolte, and et al. 2021. "An RNA-Seq-Based Framework for Characterizing Canine Prostate Cancer and Prioritizing Clinically Relevant Biomarker Candidate Genes" International Journal of Molecular Sciences 22, no. 21: 11481. https://doi.org/10.3390/ijms222111481
APA StyleThiemeyer, H., Taher, L., Schille, J. T., Packeiser, E.-M., Harder, L. K., Hewicker-Trautwein, M., Brenig, B., Schütz, E., Beck, J., Nolte, I., & Murua Escobar, H. (2021). An RNA-Seq-Based Framework for Characterizing Canine Prostate Cancer and Prioritizing Clinically Relevant Biomarker Candidate Genes. International Journal of Molecular Sciences, 22(21), 11481. https://doi.org/10.3390/ijms222111481





