Gut Microbiota-Derived l-Histidine/Imidazole Propionate Axis Fights against the Radiation-Induced Cardiopulmonary Injury
Abstract
:1. Introduction
2. Results
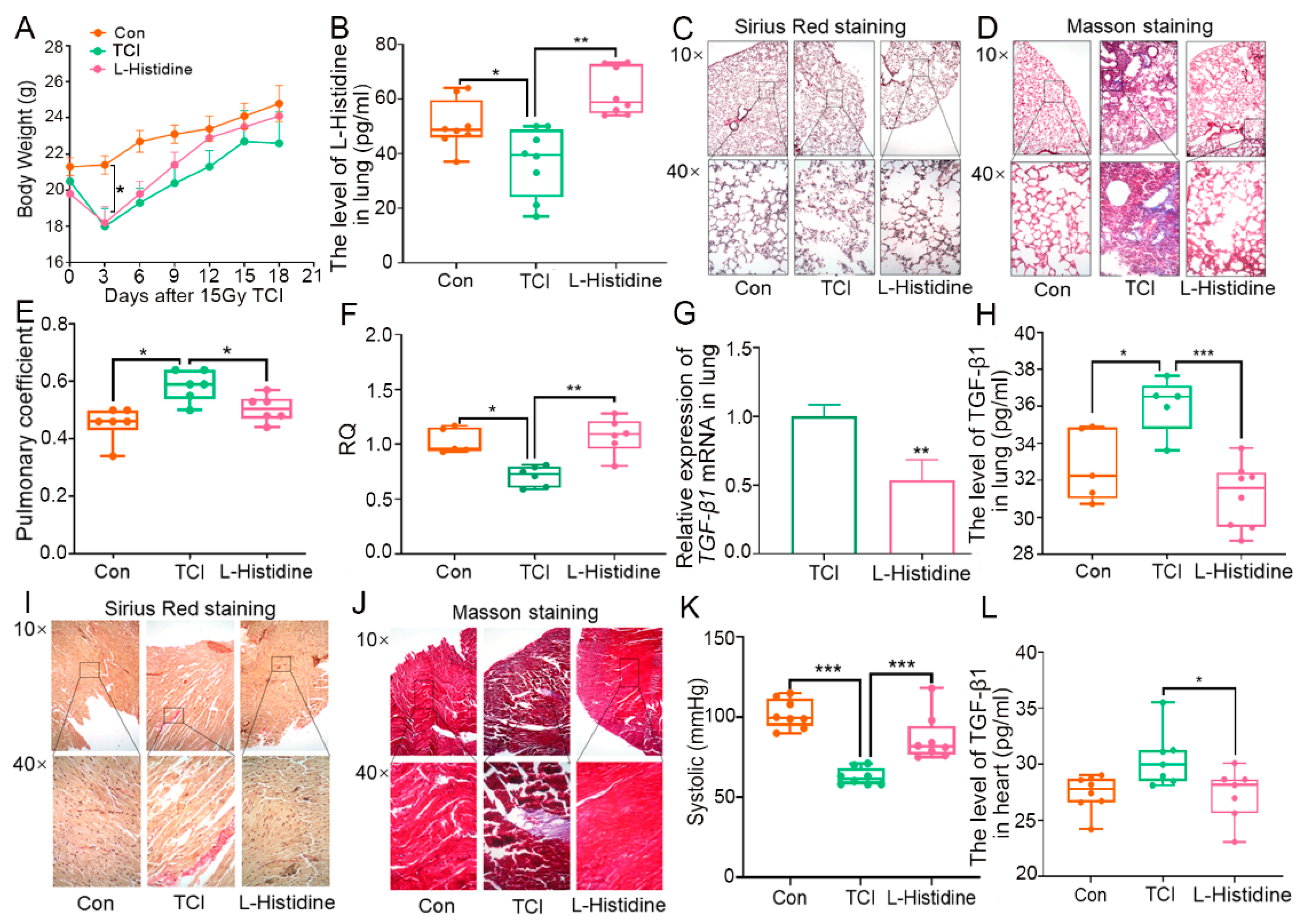
2.1. Gut Microbiota-Derived l-Histidine Improves Radiation Toxicity in Lung and Heart
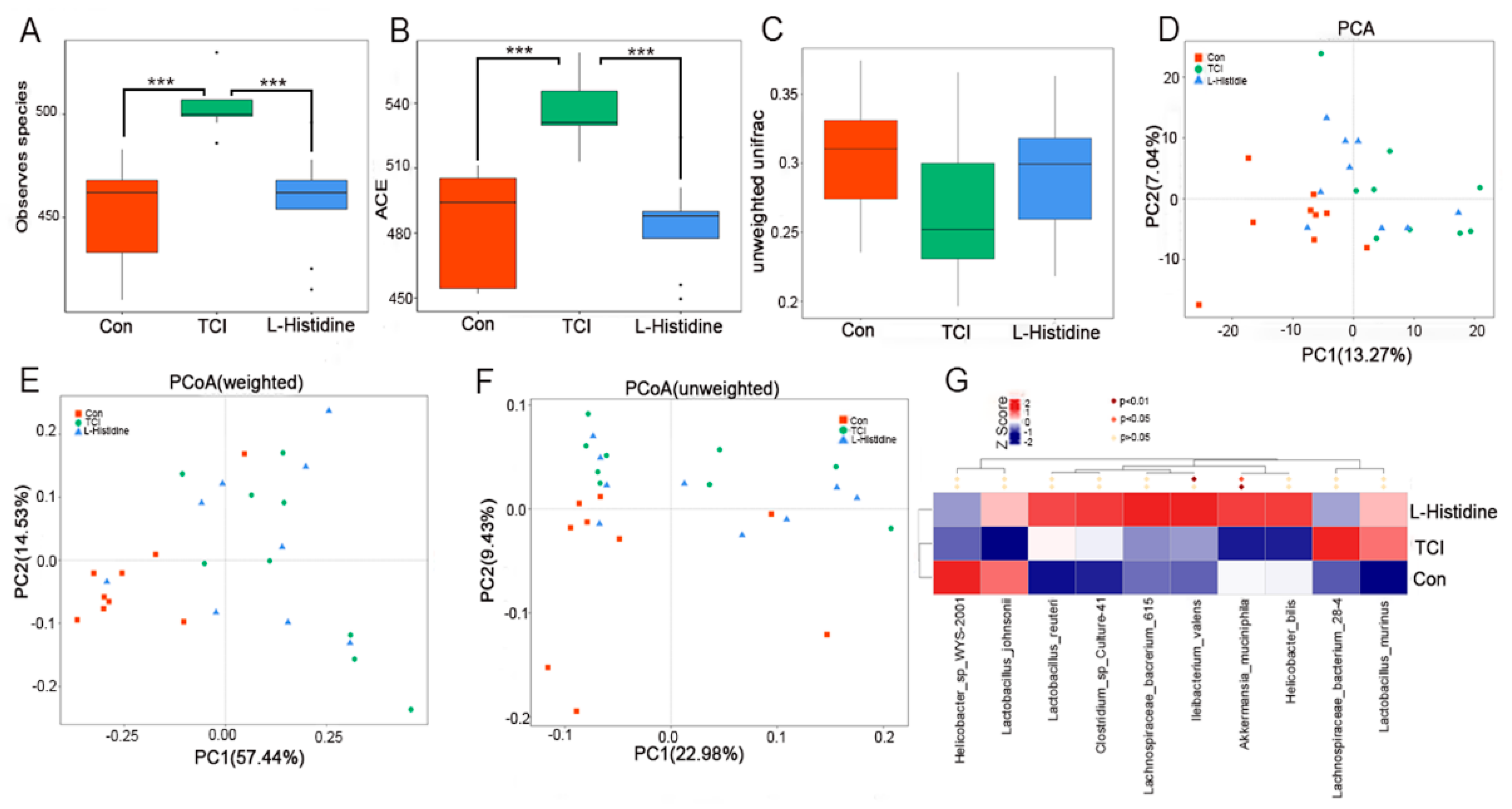
2.2. l-Histidine Shapes the Gut Microbiota Configuration after Chest Local Irradiation
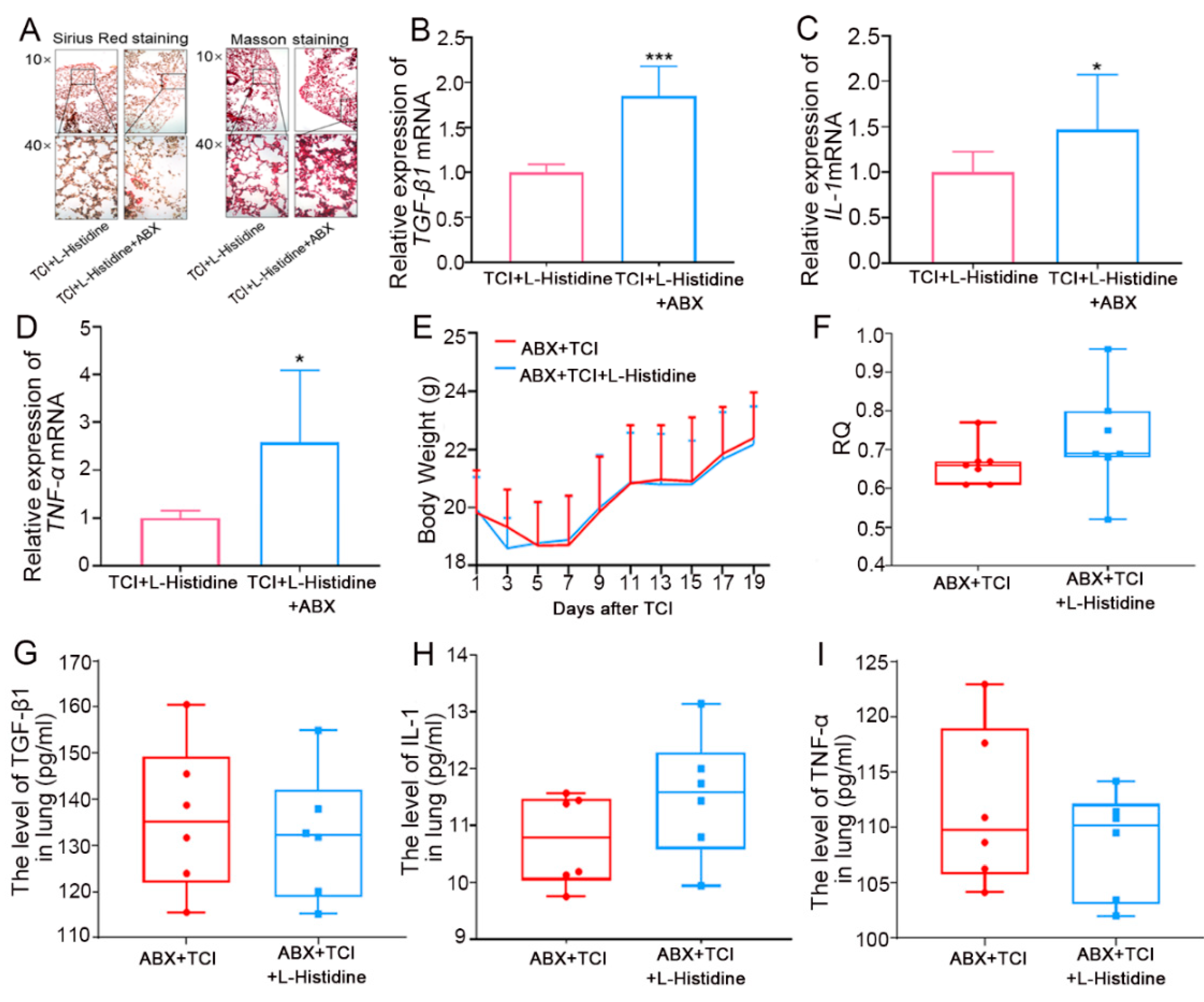
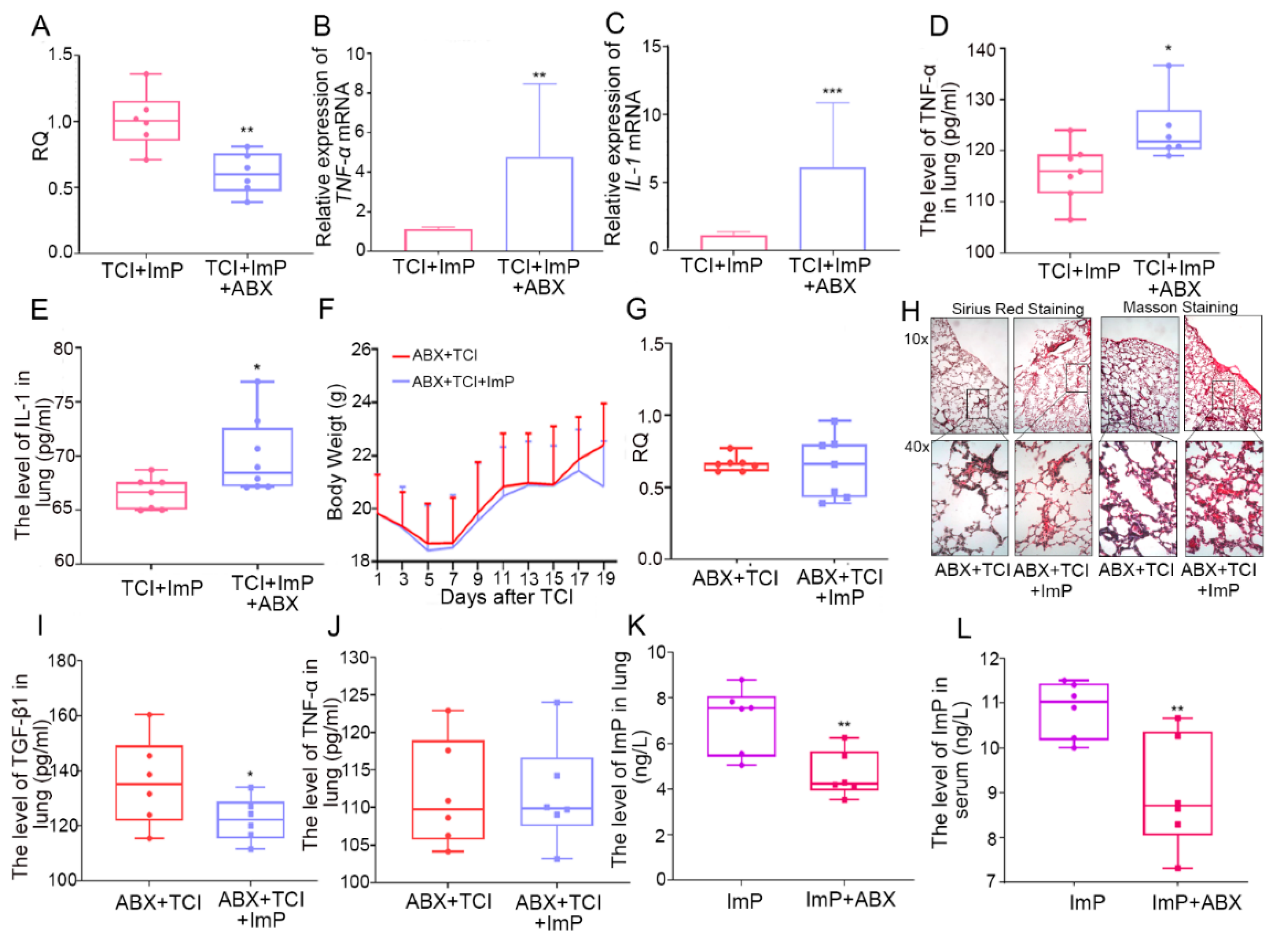
2.3. Gut Microbiota Contributes to l-Histidine-Mediated Radioprotection
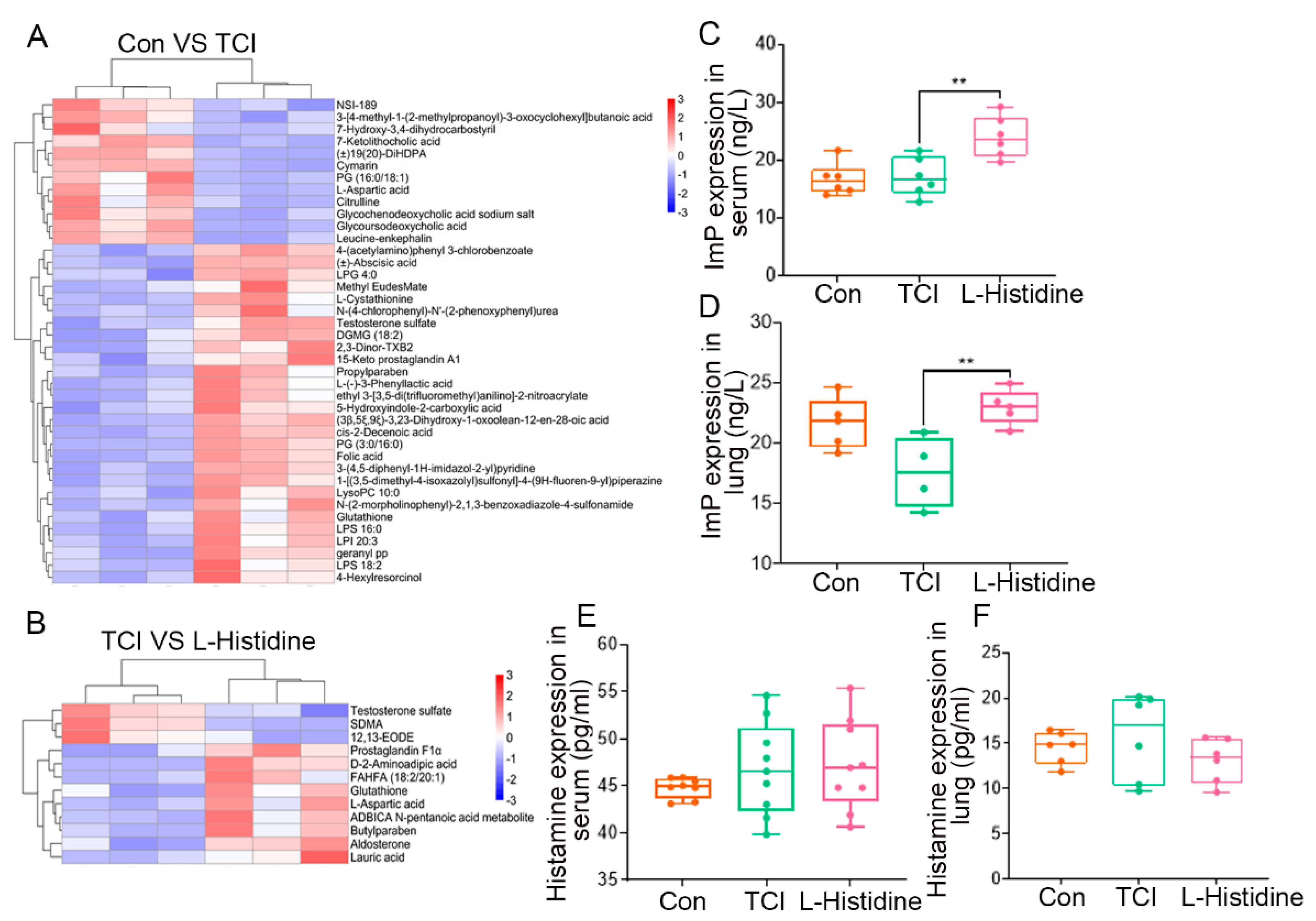
2.4. l-Histidine Remolds the Gut Microbiota Metabolome Fluctuated by Local Chest Irradiation
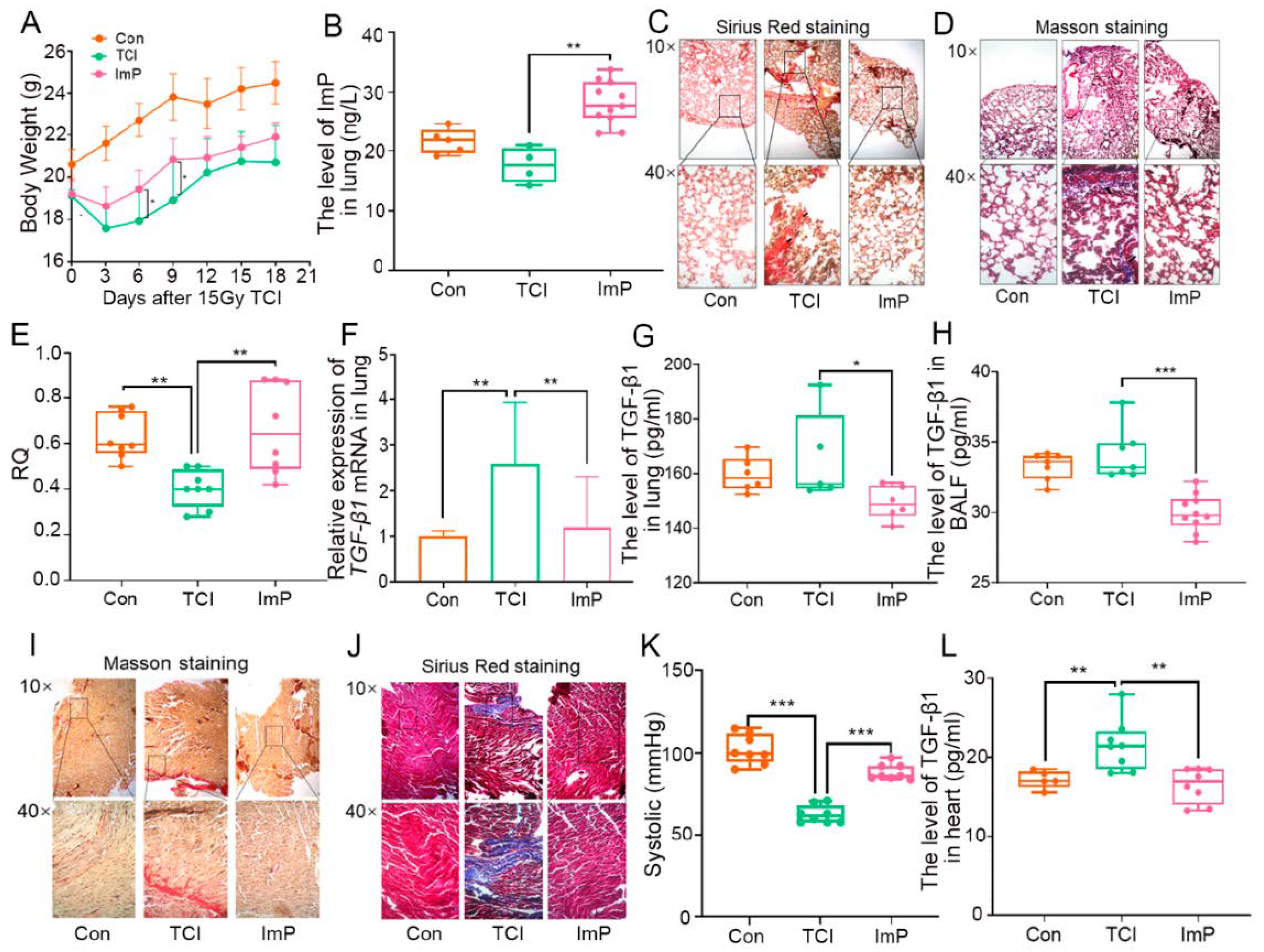
2.5. Imidazole Propionate Ameliorates Chest Local Irradiation-Induced Toxicity
2.6. Gut Microbiota Impacts the Assimilation of Imidazole Propionate
2.7. Imidazole Propionate Inhibits Pyrolysis of Irradiated Lung Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. Irradiation Study
4.4. Fecal Microbiota Transplantation (FMT)
4.5. Antibiotic Cocktail (ABX) Administration
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Immunohistochemistry (IHC)
4.8. Colony Formation Assays
4.9. Bronchoalveolar Lavage Fluid (BALF)
4.10. Respiratory Metabolism
4.11. CODA Noninvasive Blood Pressure System
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jairam, V.; Lee, V.; Park, H.S.; Thomas, C.J.; Melnick, E.R.; Gross, C.P.; Presley, C.J.; Adelson, K.B.; Yu, J.B. Treatment-Related Complications of Systemic Therapy and Radiotherapy. JAMA Oncol. 2019, 5, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.; Lawrence, Y.R.; Appel, S.; Weiss, I.; Ben, A.M.; Akiva, B.M.; Peled, N.; Goldstein, J.D.; Weizman, N.; Galper, S.; et al. Benefits of Continuous Positive Airway Pressure (CPAP) During Radiation Therapy: A Prospective Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1466–1472. [Google Scholar] [CrossRef]
- Giridhar, P.; Mallick, S.; Rath, G.K.; Julka, P.K. Radiation induced lung injury: Prediction, assessment and management. Asian Pac. J. Cancer Prev. 2015, 16, 2613–2617. [Google Scholar] [CrossRef] [Green Version]
- Yap, M.L.; Zubizarreta, E.; Bray, F.; Ferlay, J.; Barton, M. Global Access to Radiotherapy Services: Have We Made Progress During the Past Decade? J. Glob. Oncol. 2016, 2, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, E.X.; Hope, A.J.; Lindsay, P.E.; Trovo, M.; El, N.I.; Deasy, J.O.; Bradley, J.D. Heart irradiation as a risk factor for radiation pneumonitis. ACTA Oncol. 2011, 50, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Myers, C.J.; Lu, B. Decreased Survival After Combining Thoracic Irradiation and an Anti-PD-1 Antibody Correlated With Increased T-cell Infiltration Into Cardiac and Lung Tissues. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1129–1136. [Google Scholar] [CrossRef]
- Speirs, C.K.; DeWees, T.A.; Rehman, S.; Molotievschi, A.; Velez, M.A.; Mullen, D.; Fergus, S.; Trovo, M.; Bradley, J.D.; Robinson, C.G. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Eblan, M.J.; Deal, A.M.; Lipner, M.; Zagar, T.M.; Wang, Y.; Mavroidis, P.; Lee, C.B.; Jensen, B.C.; Rosenman, J.G.; et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J. Clin. Oncol. 2017, 35, 1387–1394. [Google Scholar] [CrossRef]
- Ghobadi, G.; van der Veen, S.; Bartelds, B.; de Boer, R.A.; Dickinson, M.G.; de Jong, J.R.; Faber, H.; Niemantsverdriet, M.; Brandenburg, S.; Berger, R.M.; et al. Physiological interaction of heart and lung in thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e639–e646. [Google Scholar] [CrossRef] [Green Version]
- van Nimwegen, F.A.; Ntentas, G.; Darby, S.C.; Schaapveld, M.; Hauptmann, M.; Lugtenburg, P.J.; Janus, C.; Daniels, L.; van Leeuwen, F.E.; Cutter, D.J.; et al. Risk of heart failure in survivors of Hodgkin lymphoma: Effects of cardiac exposure to radiation and anthracyclines. Blood 2017, 129, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Wang, W.; Cao, X.; Piao, M.; Khan, S.; Yan, F.; Cao, H.; Wang, B. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS ONE 2016, 11, e0161174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, K.A.; Stewart, J.D.; Ambalavanan, N. Recent Advances in Understanding the Ecology of the Lung Microbiota and Deciphering the Gut-Lung Axis. Am. J. Physiol. Lung. Cell Mol. Physiol. 2020, 319, L710–L716. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, J.W.; Kim, R.Y.; Robertson, A.; Hirota, J.A.; Wood, L.G.; Knight, D.A.; Cooper, M.A.; O’Neill, L.; Horvat, J.C.; Hansbro, P.M. Inflammasomes in the lung. Mol. Immunol. 2017, 86, 44–55. [Google Scholar] [CrossRef]
- Zeng, C.; Tan, H. Gut Microbiota and Heart, Vascular Injury. Adv. Exp. Med. Biol. 2020, 1238, 107–141. [Google Scholar]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieers, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, T.; Yoshida, N.; Emoto, T.; Saito, Y.; Hirata, K.I. Two Gut Microbiota-Derived Toxins Are Closely Associated with Cardiovascular Diseases: A Review. Toxins 2021, 13, 297. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Li, Y.; Hu, Y.; Franke, A.A.; Liang, L.; Hu, F.B.; Chan, A.T.; Mukamal, K.J.; Rimm, E.B.; et al. Gut microbiota-derived metabolites and risk of coronary artery disease: A prospective study among US men and women. Am. J. Clin. Nutr. 2021, 114, 238–247. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A.; Stahlman, M.; Khan, M.T.; Schmidt, C.; Manneras-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Xu, J.; Zhang, Y.; Zhu, X.; Liu, J.; Tan, Y. Ligustrazine Alleviate Acute Lung Injury Through Suppressing Pyroptosis and Apoptosis of Alveolar Macrophages. Front. Pharmacol. 2021, 12, 680512. [Google Scholar] [CrossRef]
- Menezes, K.M.; Wang, H.; Hada, M.; Saganti, P.B. Radiation Matters of the Heart: A Mini Review. Front. Cardiovasc. Med. 2018, 5, 83. [Google Scholar] [CrossRef]
- Stewart, F.A.; Seemann, I.; Hoving, S.; Russell, N.S. Understanding radiation-induced cardiovascular damage and strategies for intervention. Clin. Oncol. 2013, 25, 617–624. [Google Scholar] [CrossRef]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Tesei, A. The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis. Int. J. Mol. Sci. 2019, 20, 3876. [Google Scholar] [CrossRef] [Green Version]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Faivre, F.C. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Nie, X.; Li, L.; Yi, M.; Qin, W.; Zhao, W.; Li, F.; Wu, B.; Yuan, X. The Intestinal Microbiota Plays as a Protective Regulator Against Radiation Pneumonitis. Radiat. Res. 2020, 194, 52–60. [Google Scholar] [CrossRef]
- Zhang, Q.; Ran, X.; He, Y.; Ai, Q.; Shi, Y. Acetate Downregulates the Activation of NLRP3 Inflammasomes and Attenuates Lung Injury in Neonatal Mice With Bronchopulmonary Dysplasia. Front. Pediatr. 2020, 8, 595157. [Google Scholar] [CrossRef]
- Wypych, T.P.; Pattaroni, C.; Perdijk, O.; Yap, C.; Trompette, A.; Anderson, D.; Creek, D.J.; Harris, N.L.; Marsland, B.J. Microbial metabolism of L-tyrosine protects against allergic airway inflammation. Nat. Immunol. 2021, 22, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, L.; Liu, B.; Qin, S. Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed. Pharmacother. 2020, 132, 110826. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, N.K. l-Histidine Supplementation in Adults and Young Children with Atopic Dermatitis (Eczema). J. Nutr. 2020, 150 (Suppl. S1), 2576S–2579S. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, F. Systematic review of preservation solutions for allografts for liver transplantation based on a network meta-analysis. Int. J. Surg. 2018, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.A.; Ramos, F.M.; Almeida, S.M.; Vizioli, M.R.; Boscolo, F.N. Evaluation of radioprotective effect of carnosine (beta-alanyl-1-histidine) on the wound healing in rats. J. Appl. Oral Sci. 2005, 13, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guney, Y. Carnosine may reduce lung injury caused by radiation therapy. Med. Hypotheses 2006, 66, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Peng, S.; Shan, X.; Deng, G.; Shen, L.; Sun, J.; Jiang, C.; Yang, X.; Chang, Z.; Sun, X.; et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis. 2019, 10, 957. [Google Scholar] [CrossRef] [Green Version]
- Fink, S.L.; Bergsbaken, T.; Cookson, B.T. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl. Acad. Sci. USA 2008, 105, 4312–4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 2020, 180, 941–955.e20. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Z.; Magupalli, V.G.; Pablo, J.L.; Dong, Y.; Vora, S.M.; Wang, L.; Fu, T.M.; Jacobson, M.P.; Greka, A.; et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 2021, 593, 607–611. [Google Scholar] [CrossRef]
- Zhou, B.; Abbott, D.W. Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep. 2021, 35, 108998. [Google Scholar] [CrossRef]
- Karmakar, M.; Minns, M.; Greenberg, E.N.; Diaz-Aponte, J.; Pestonjamasp, K.; Johnson, J.L.; Rathkey, J.K.; Abbott, D.W.; Wang, K.; Shao, F.; et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1beta release independently of plasma membrane pores and pyroptosis. Nat. Commun. 2020, 11, 2212. [Google Scholar] [CrossRef] [PubMed]
- Maelfait, J.; Vercammen, E.; Janssens, S.; Schotte, P.; Haegman, M.; Magez, S.; Beyaert, R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J. Exp. Med. 2008, 205, 1967–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappaB/GSDMD signal in mice adipose tissue. J. Pineal. Res. 2017, 63, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiao, X.; Zhang, C.; Yu, W.; Guo, W.; Zhang, Z.; Li, Z.; Feng, X.; Hao, J.; Zhang, K.; et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J. Pineal Res. 2017, 62, e12380. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Perez, S.; Kane, N.; Kurmis, A.A.; Yang, F.; Kummer, N.T.; Dervan, P.B.; Nickols, N.G. Interference with DNA repair after ionizing radiation by a pyrrole-imidazole polyamide. PLoS ONE 2018, 13, e196803. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wang, B.; Dong, J.; Li, Y.; Zhang, S.; Zeng, X.; Xiao, H.; Fan, S.; Cui, M. Gut Microbiota-Derived l-Histidine/Imidazole Propionate Axis Fights against the Radiation-Induced Cardiopulmonary Injury. Int. J. Mol. Sci. 2021, 22, 11436. https://doi.org/10.3390/ijms222111436
Chen Z, Wang B, Dong J, Li Y, Zhang S, Zeng X, Xiao H, Fan S, Cui M. Gut Microbiota-Derived l-Histidine/Imidazole Propionate Axis Fights against the Radiation-Induced Cardiopulmonary Injury. International Journal of Molecular Sciences. 2021; 22(21):11436. https://doi.org/10.3390/ijms222111436
Chicago/Turabian StyleChen, Zhiyuan, Bin Wang, Jiali Dong, Yuan Li, Shuqin Zhang, Xiaozhou Zeng, Huiwen Xiao, Saijun Fan, and Ming Cui. 2021. "Gut Microbiota-Derived l-Histidine/Imidazole Propionate Axis Fights against the Radiation-Induced Cardiopulmonary Injury" International Journal of Molecular Sciences 22, no. 21: 11436. https://doi.org/10.3390/ijms222111436
APA StyleChen, Z., Wang, B., Dong, J., Li, Y., Zhang, S., Zeng, X., Xiao, H., Fan, S., & Cui, M. (2021). Gut Microbiota-Derived l-Histidine/Imidazole Propionate Axis Fights against the Radiation-Induced Cardiopulmonary Injury. International Journal of Molecular Sciences, 22(21), 11436. https://doi.org/10.3390/ijms222111436




