BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury
Abstract
:1. Introduction
2. Results
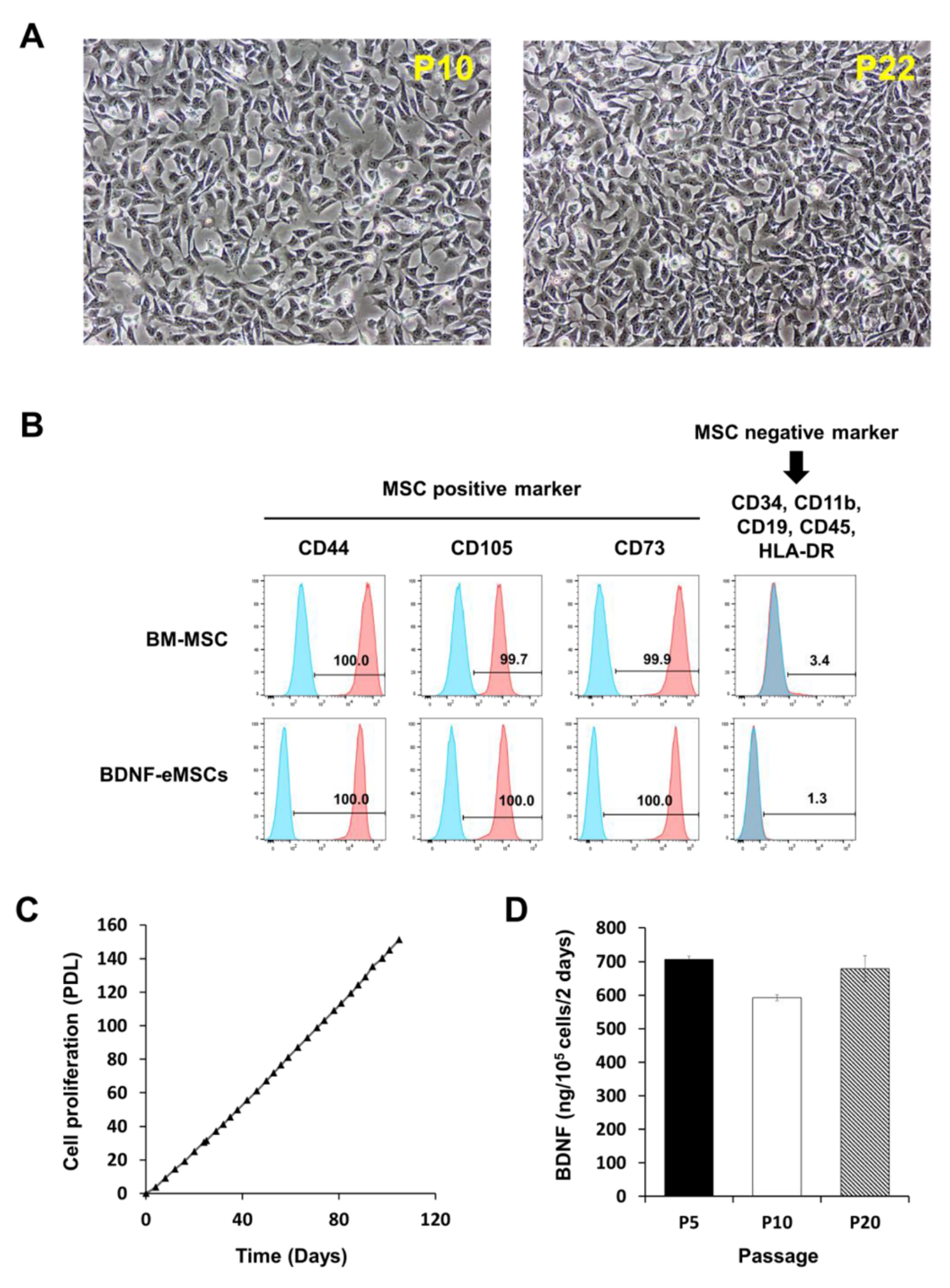
2.1. Cellular Characteristics of BDNF-eMSCs
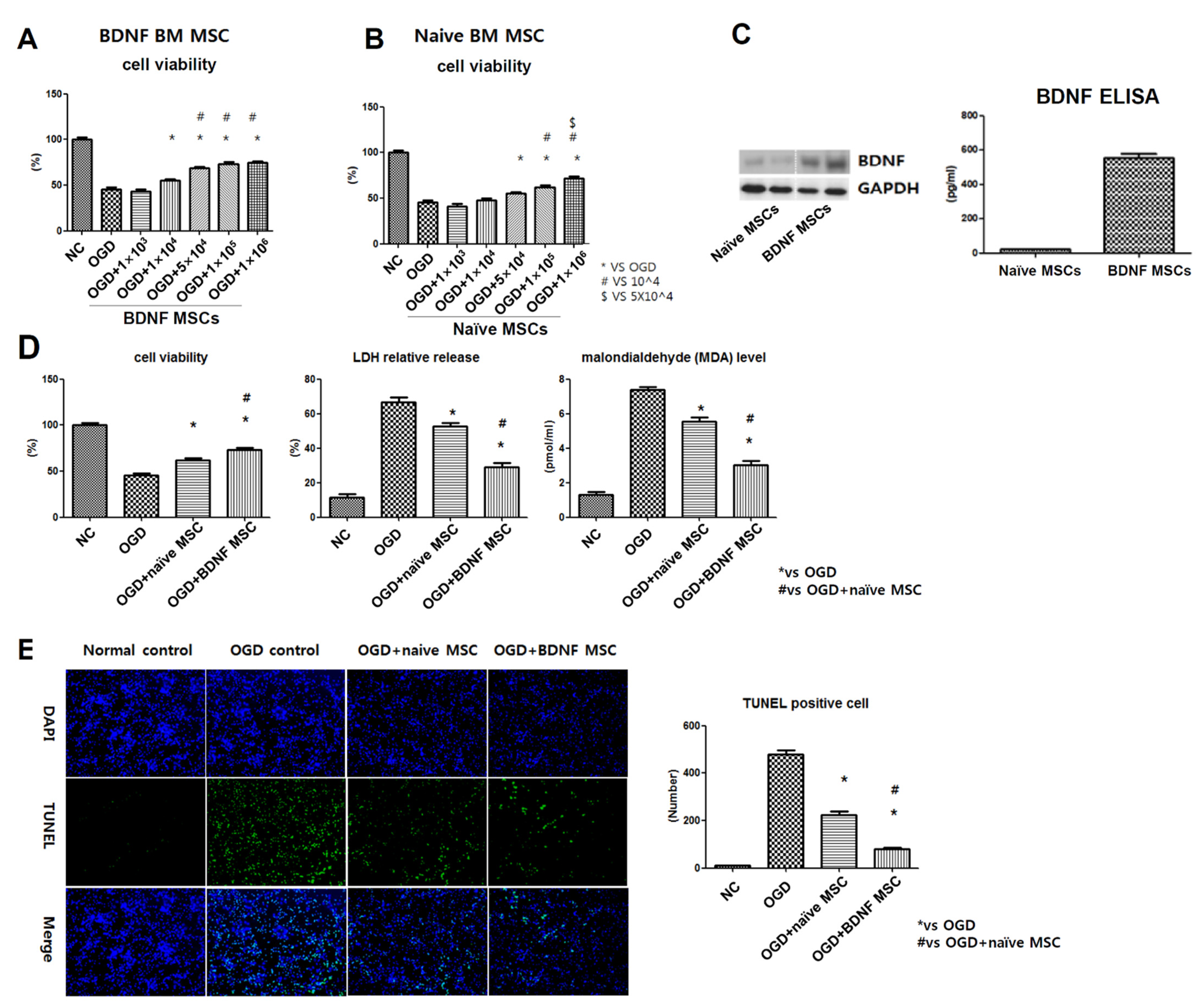
2.2. Cell Viability, Cytotoxicity, Oxidative Stress, and TUNEL Assay after In Vitro OGD
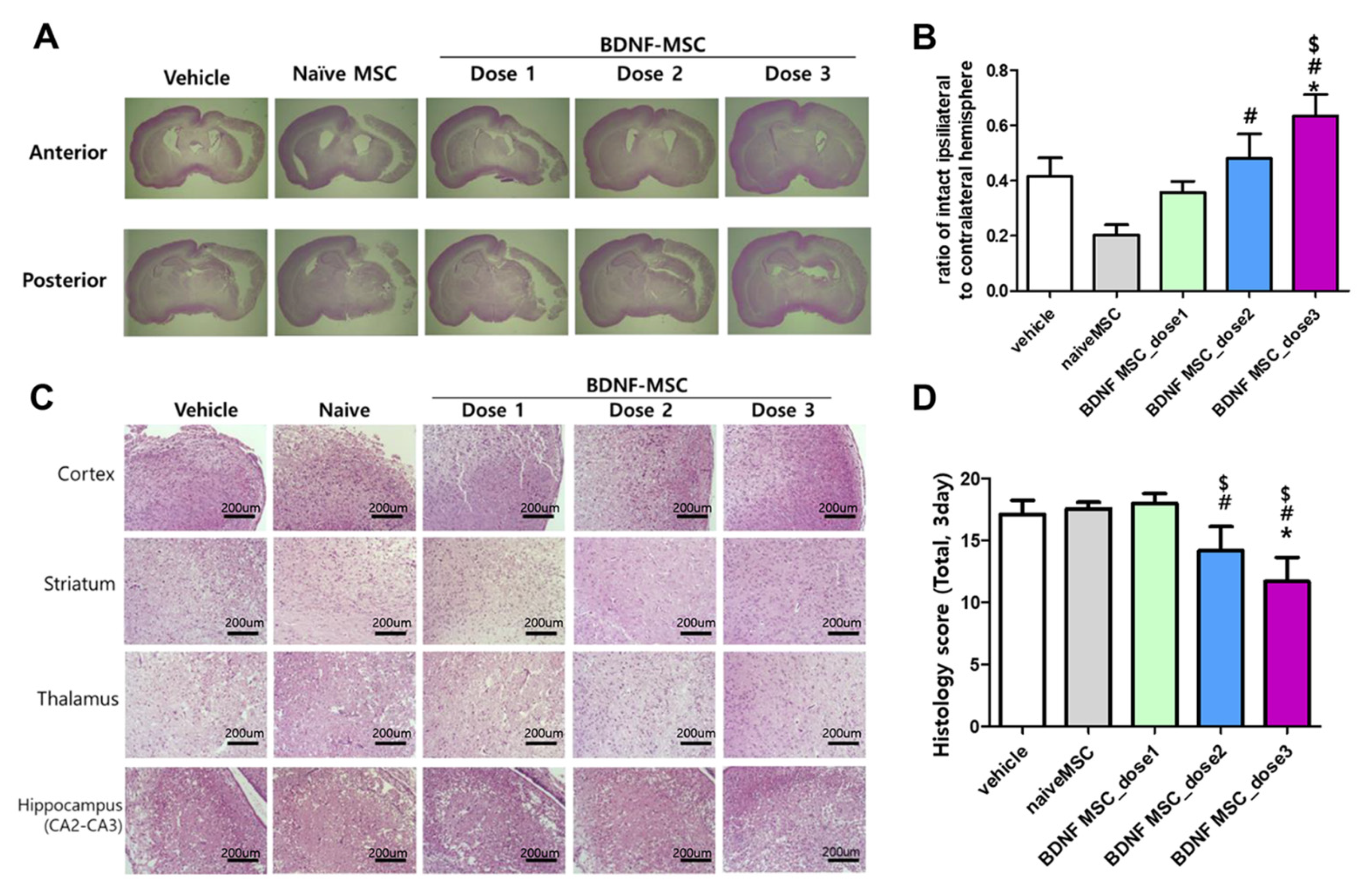
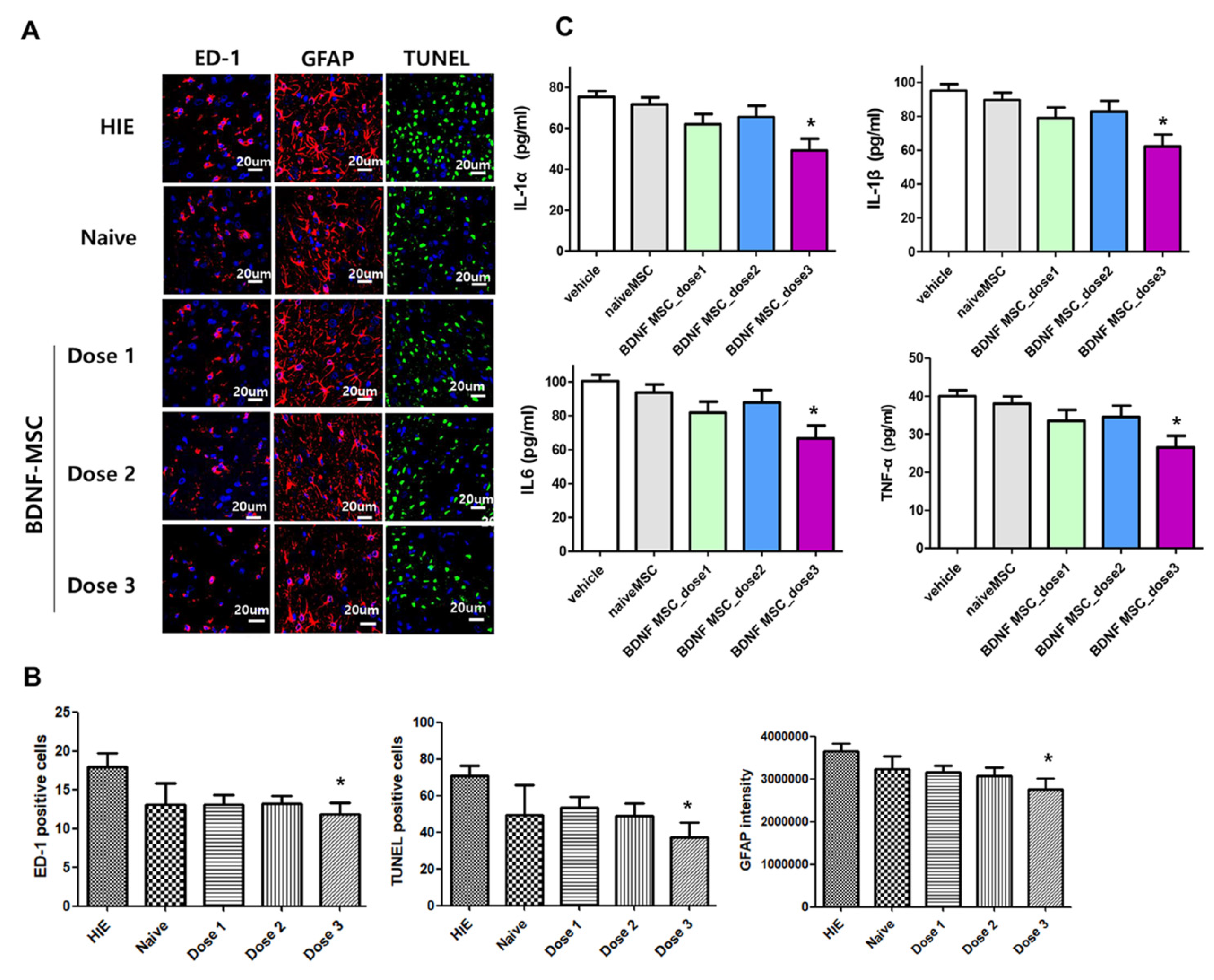
2.3. Short-Term Follow-Up In Vivo Study of HI-Induced Severe Brain Injury
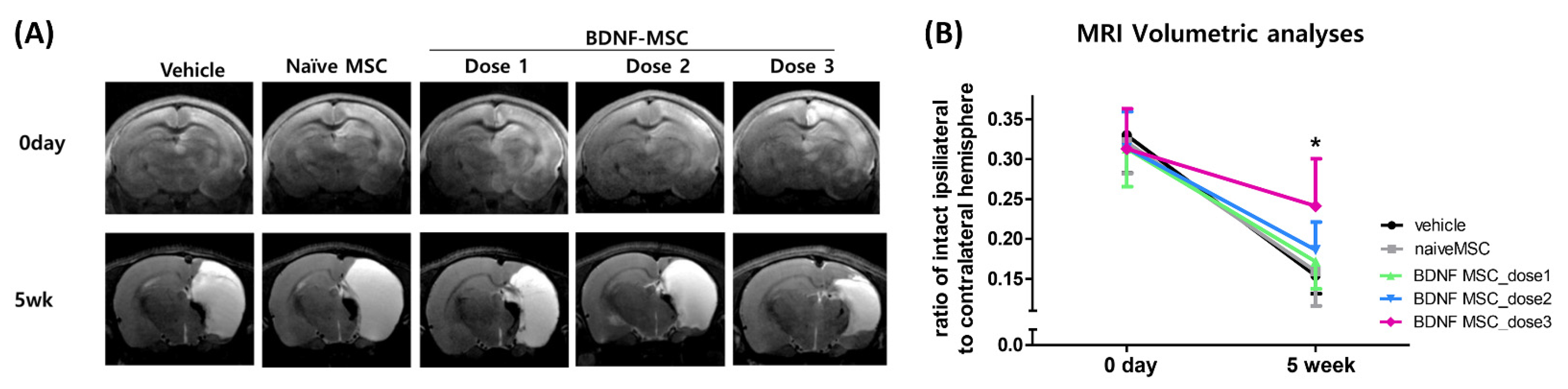
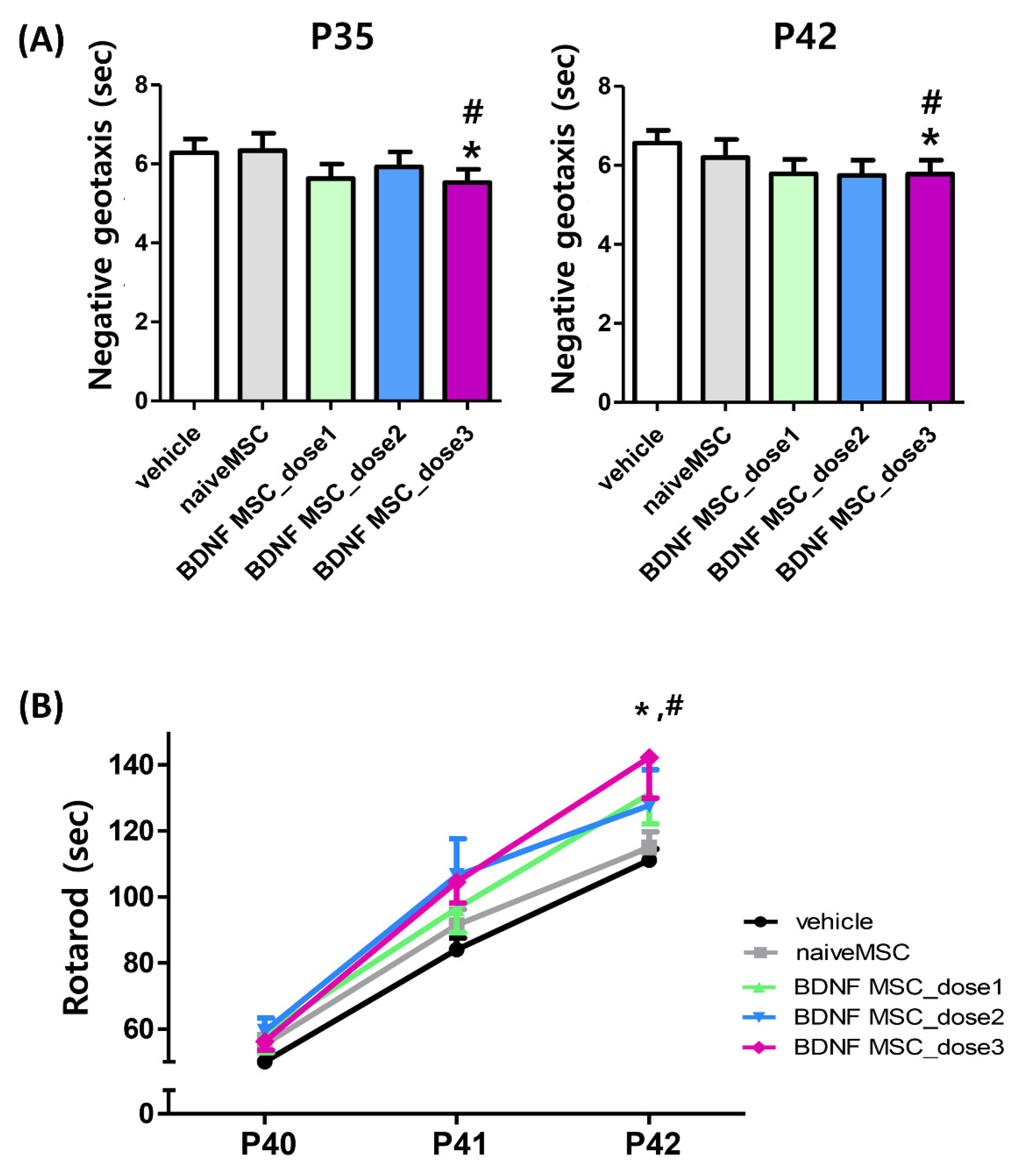
2.4. Long-Term Follow-Up In Vivo Study of Severe HI Induced Brain Injury
3. Discussion
4. Materials and Methods
4.1. Cell Preparation
4.2. MSC Marker Analysis
4.3. Cell Proliferation Analysis
4.4. In Vitro Model of Oxygen–Glucose Deprivation
4.5. In Vitro Cell Viability, Cytotoxicity, and Oxidative Stress Assays
4.6. In Vivo Model of Hypoxic Ischemic Brain Injury
4.7. Tissue Preparation and H&E Staining
4.8. Immunohistochemistry
4.9. TUNEL Assay
4.10. Enzyme Linked Immunosorbent Assay
4.11. Functional Behavioral Tests
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, M.V. Hypoxic and ischemic disorders of infants and children. Lecture for 38th Meeting of Japanese Society of Child Neurology, Tokyo, Japan, July 1996. Brain Dev. 1997, 19, 235–239. [Google Scholar] [CrossRef]
- Robertson, C.; Finer, N. Term Infants with Hypoxic-Ischemic Encephalopathy: Outcome At 3.5 Years. Dev. Med. Child Neurol. 1985, 27, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.; Kitagawa, K.; Lyden, P.; Perez-Pinzon, M. Metabolic Downregulation: A Key to Successful Neuroprotection? Stroke 2008, 39, 2910–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankaran, S.; Laptook, A.; Wright, L.L.; Ehrenkranz, R.A.; Donovan, E.F.; Fanaroff, A.A.; Stark, A.R.; Tyson, J.E.; Poole, K.; Carl, W.A.; et al. Whole-Body Hypothermia for Neonatal Encephalopathy: Animal Observations as a Basis for a Randomized, Controlled Pilot Study in Term Infants. Pediatrics 2002, 110, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.V.; Strohm, B.; Edwards, A.D.; Dyet, L.; Halliday, H.; Juszczak, E.; Kapellou, O.; Levene, M.; Marlow, N.; Porter, E.; et al. Moderate Hypothermia to Treat Perinatal Asphyxial Encephalopathy. N. Engl. J. Med. 2009, 361, 1349–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; McDonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-Body Hypothermia for Neonates with Hypoxic–Ischemic Encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Donega, V.; van Velthoven, C.T.J.; Nijboer, C.H.; van Bel, F.; Kas, M.J.H.; Kavelaars, A.; Heijnen, C.J. Intranasal Mesenchymal Stem Cell Treatment for Neonatal Brain Damage: Long-Term Cognitive and Sensorimotor Improvement. PLoS ONE 2013, 8, e51253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal Stem Cell Treatment after Neonatal Hypoxic-Ischemic Brain Injury Improves Behavioral Outcome and Induces Neuronal and Oligodendrocyte Regeneration. Brain Behav. Immun. 2010, 24, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Ahn, S.Y.; Im, G.H.; Sung, D.K.; Park, Y.R.; Choi, S.H.; Choi, S.J.; Chang, Y.S.; Oh, W.; Lee, J.H.; et al. Human umbilical cord blood–derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr. Res. 2012, 72, 277–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Lee, J.H.; Oh, W.I.; Park, W.S. Mesenchymal Stem Cells Prevent Hydrocephalus After Severe Intraventricular Hemorrhage. Stroke 2013, 44, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Ahn, J.-Y.; Park, W.S. Pivotal Role of Brain-Derived Neurotrophic Factor Secreted by Mesenchymal Stem Cells in Severe Intraventricular Hemorrhage in Newborn Rats. Cell Transplant. 2017, 26, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Park, W.S. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Im, G.H.; Choi, S.J.; Park, W.S. Optimal Route for Mesenchymal Stem Cells Transplantation after Severe Intraventricular Hemorrhage in Newborn Rats. PLoS ONE 2015, 10, e0132919. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, R.; Licht, R.; van Blitterswijk, C.; de Boer, J. Donor Variation and Loss of Multipotency During in Vitro Expansion of Human Mesenchymal Stem Cells for Bone Tissue Engineering. J. Orthop. Res. 2007, 25, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Schipper, B.M.; Marra, K.; Zhang, W.; Donnenberg, A.D.; Rubin, J.P. Regional Anatomic and Age Effects on Cell Function of Human Adipose-Derived Stem Cells. Ann. Plast. Surg. 2008, 60, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Binato, R.; de Souza Fernandez, T.; Lazzarotto-Silva, C.; Du Rocher, B.; Mencalha, A.; Pizzatti, L.; Bouzas, L.F.; Abdelhay, E. Stability of Human Mesenchymal Stem Cells During in vitro Culture: Considerations for Cell Therapy. Cell Prolif. 2013, 46, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L.; Blasi, A.; Saldarelli, M.; Soleti, A.; Pessina, A.; Bonomi, A.; Coccè, V.; Dossena, M.; Tosetti, V.; Ceserani, V.; et al. Immortalization of Human Adipose-Derived Stromal Cells: Production of Cell Lines with High Growth Rate, Mesenchymal Marker Expression and Capability to Secrete High Levels of Angiogenic Factors. Stem Cell Res. Ther. 2014, 5, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skårn, M.; Noordhuis, P.; Wang, M.-Y.; Veuger, M.; Kresse, S.H.; Egeland, E.V.; Micci, F.; Namløs, H.M.; Håkelien, A.-M.; Olafsrud, S.M.; et al. Generation and Characterization of an Immortalized Human Mesenchymal Stromal Cell Line. Stem Cells Dev. 2014, 23, 2377–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckenkamp, L.R.; Da Fontoura, D.M.S.; Korb, V.G.; De Campos, R.P.; Onzi, G.R.; Iser, I.C.; Bertoni, A.; Sévigny, J.; Lenz, G.; Wink, M.R. Immortalization of Mesenchymal Stromal Cells by TERT Affects Adenosine Metabolism and Impairs their Immunosuppressive Capacity. Stem Cell Rev. Rep. 2020, 16, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Ahn, S.Y.; Sung, S.I.; Ahn, J.-Y.; Chang, Y.S. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr. Res. 2017, 83, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doorn, J.; Moll, G.; Le Blanc, K.; van Blitterswijk, C.; de Boer, J. Therapeutic Applications of Mesenchymal Stromal Cells: Paracrine Effects and Potential Improvements. Tissue Eng. Part B Rev. 2012, 18, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, N.; de Theije, C.; De Vries, L.S.; Groenendaal, F.; Benders, M.J.N.L.; Nijboer, C.H.A. Promoting neuroregeneration after perinatal arterial ischemic stroke: Neurotrophic factors and mesenchymal stem cells. Pediatr. Res. 2017, 83, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, K.; Nakamura, K.; Tamiya, T.; Kawano, Y.; Kobune, M.; Hirai, S.; Uchida, H.; Sasaki, K.; Ito, Y.; Kato, K.; et al. BDNF Gene-Modified Mesenchymal Stem Cells Promote Functional Recovery and Reduce Infarct Size in the Rat Middle Cerebral Artery Occlusion Model. Mol. Ther. 2004, 9, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Van Velthoven, C.T.J.; Sheldon, R.A.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.L.D.M.; Maas, M.C.; Heijnen, C.J.; Ferriero, D.M. Mesenchymal Stem Cell Transplantation Attenuates Brain Injury After Neonatal Stroke. Stroke 2013, 44, 1426–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The Influence of Immaturity on Hypoxic-Ischemic Brain Damage in the Rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnar, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokaia, Z.; Andsberg, G.; Yan, Q.; Lindvall, O. Rapid Alterations of BDNF Protein Levels in the Rat Brain after Focal Ischemia: Evidence for Increased Synthesis and Anterograde Axonal Transport. Exp. Neurol. 1998, 154, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Ernfors, P.; Bengzon, J.; Kokaia, Z.; Smith, M.L.; Siesjo, B.K.; Persson, H. Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc. Natl. Acad. Sci. USA 1992, 89, 648–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Shui, Q.-X.; Zhao, Z.-Y. Regulation of Brain-Derived Neurotrophic Factor (BDNF) Expression Following Antibiotic Treatment of Experimental Bacterial Meningitis. J. Child Neurol. 2003, 18, 828–834. [Google Scholar] [PubMed]
- Park, B.W.; Jung, S.H.; Das, S.; Lee, S.M.; Park, J.H.; Kim, H.; Hwang, J.W.; Lee, S.; Kim, H.J.; Kim, H.Y.; et al. In Vivo Priming of Human Mesenchymal Stem Cells with Hepatocyte Growth Factor-Engineered Mesenchymal Stem Cells Promotes Therapeutic Potential for Cardiac Repair. Sci. Adv. 2020, 6, eaay6994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nold, P.; Hackstein, H.; Riedlinger, T.; Kasper, C.; Neumann, A.; Mernberger, M.; Fölsch, C.; Schmitt, J.; Fuchs-Winkelmann, S.; Barckhausen, C.; et al. Immunosuppressive capabilities of mesenchymal stromal cells are maintained under hypoxic growth conditions and after gamma irradiation. Cytotherapy 2015, 17, 152–162. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, A.V.G.; Riewaldt, J.; Wehner, R.; Schmitz, M.; Odendahl, M.; Bornhäuser, M.; Tonn, T. Gamma irradiation preserves immunosuppressive potential and inhibits clonogenic capacity of human bone marrow-derived mesenchymal stromal cells. J. Cell. Mol. Med. 2014, 18, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.R.; Ahn, S.Y.; Chang, Y.S.; Hwang, I.; Yun, T.; Sung, D.K.; Sung, S.I.; Park, W.S.; Ahn, J.-Y. Human UCB-MSCs treatment upon intraventricular hemorrhage contributes to attenuate hippocampal neuron loss and circuit damage through BDNF-CREB signaling. Stem Cell Res. Ther. 2018, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Park, W.S. Cell type–dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy 2015, 17, 1025–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Kim, S.Y.; Sung, D.K.; Chang, Y.S.; Park, W.S. Neuroprotective effects of L-carnitine against oxygen-glucose deprivation in rat primary cortical neurons. Korean J. Pediatr. 2012, 55, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Sung, S.I.; Chang, Y.S.; Ahn, S.Y.; Sung, D.K.; Park, W.S. Thrombin Preconditioning Enhances Therapeutic Efficacy of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells in Severe Neonatal Hypoxic Ischemic Encephalopathy. Int. J. Mol. Sci. 2019, 20, 2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedtjärn, M.; Leverin, A.-L.; Eriksson, K.; Blomgren, K.; Mallard, C.; Hagberg, H. Interleukin-18 Involvement in Hypoxic–Ischemic Brain Injury. J. Neurosci. 2002, 22, 5910–5919. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, O.; Revuelta, M.; Urigüen, L.; Álvarez, A.; Montalvo, H.; Hilario, E. Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats. PLoS ONE 2015, 10, e0142424. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.Y.; Sung, D.K.; Chang, Y.S.; Sung, S.I.; Kim, Y.E.; Kim, H.-J.; Lee, S.M.; Park, W.S. BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury. Int. J. Mol. Sci. 2021, 22, 11395. https://doi.org/10.3390/ijms222111395
Ahn SY, Sung DK, Chang YS, Sung SI, Kim YE, Kim H-J, Lee SM, Park WS. BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury. International Journal of Molecular Sciences. 2021; 22(21):11395. https://doi.org/10.3390/ijms222111395
Chicago/Turabian StyleAhn, So Yoon, Dong Kyung Sung, Yun Sil Chang, Se In Sung, Young Eun Kim, Hyo-Jin Kim, Soon Min Lee, and Won Soon Park. 2021. "BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury" International Journal of Molecular Sciences 22, no. 21: 11395. https://doi.org/10.3390/ijms222111395
APA StyleAhn, S. Y., Sung, D. K., Chang, Y. S., Sung, S. I., Kim, Y. E., Kim, H.-J., Lee, S. M., & Park, W. S. (2021). BDNF-Overexpressing Engineered Mesenchymal Stem Cells Enhances Their Therapeutic Efficacy against Severe Neonatal Hypoxic Ischemic Brain Injury. International Journal of Molecular Sciences, 22(21), 11395. https://doi.org/10.3390/ijms222111395





