Abstract
A major goal of current clinical research in Parkinson’s disease (PD) is the validation and standardization of biomarkers enabling early diagnosis, predicting outcomes, understanding PD pathophysiology, and demonstrating target engagement in clinical trials. Molecular imaging with specific dopamine-related tracers offers a practical indirect imaging biomarker of PD, serving as a powerful tool to assess the status of presynaptic nigrostriatal terminals. In this review we provide an update on the dopamine transporter (DAT) imaging in PD and translate recent findings to potentially valuable clinical practice applications. The role of DAT imaging as diagnostic, preclinical and predictive biomarker is discussed, especially in view of recent evidence questioning the incontrovertible correlation between striatal DAT binding and nigral cell or axon counts.
1. Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disorder characterized primarily by motor symptoms which are attributed mostly to dopaminergic cell loss. However, this is just the “tip of the iceberg” and a range of non-motor disturbances related to a combination of dopaminergic and non-dopaminergic pathways indicates the multi-focal and widespread driven pathology of PD [1,2]. As the diagnosis of PD remains clinical, this heterogeneity can pose diagnostic difficulties in line with clinico-pathologic series reporting suboptimal diagnostic accuracy in the early stages, even at specialized centres [3,4]. A correct diagnosis, however, is important for patient counselling and clinical research purposes. Similarly, rate of disease progression is also heterogeneous so that the identification of markers of progression would be of utmost importance to inform prognosis and timely offer the best therapeutic options for the advanced phases. Dopaminergic medications remain the major therapeutic approach for controlling clinical symptoms of PD and improving the quality of life of patients for many years, but over the years they lose efficacy and are associated with significant complications such as the “wearing off” effect and dyskinesias. There are currently no disease-modifying therapies for PD, heightening the challenges for the public health system posed by the epidemiology of PD, with numbers projected to double in the next few decades [5]. In this context, the validation and standardization of pathophysiological biomarkers which aid clinicians for early detection, prognosis, tracking disease progression and prediction of treatment response, represents an unmet and urgent need to be addressed for improved clinical assessment and management of PD patients.
Positron emission tomography (PET) and single photon emission tomography (SPECT) imaging with specific dopamine-related tracers offer a practical indirect imaging biomarker of PD, serving as a powerful tool to assess the status of presynaptic nigrostriatal terminals. More frequently, the nigrostriatal dopaminergic function is assessed in vivo with several [123I] and 99mTc-labeled SPECT agents that measure the dopamine transporter (DAT) availability. In particular, the radiotracer Ioflupane or [123I]-FP-CIT has been approved by both FDA (DaTscan™) and EMA (DaTSCAN) for clinical use in suspected parkinsonian syndromes, becoming part of the diagnostic guidelines of α-synucleinopathies (PD, multiple system atrophy and dementia with Lewy bodies) [6,7]. In this review, we will discuss the role of DAT imaging as a biomarker in PD for diagnosing, monitoring progression, determining disease severity and predicting clinical outcomes over the course of PD, with particular emphasis on recent updates about the relationship between DAT availability and dopaminergic nigrostriatal neurons.
2. Targets of Dopaminergic Imaging
In clinical setting there are an increasing number of molecular targets for dopaminergic system imaging. The analysis can be performed at a presynaptic level, where we can evaluate the L-aromatic amino acid decarboxylase (AADC), the vesicular monoamine transporter 2 (VMAT2) and the DAT density, or at postsynaptic level estimating dopamine receptors expression [8].
DAT is a transmembrane sodium chloride dependent protein selectively expressed in dopaminergic cells. DAT has a key role in the dopamine (DA) reuptake from the synaptic cleft, and it is critical in the spatial and temporal buffering of released DA levels [9] (Figure 1). Indeed, DAT modulates quantal DA release at endplates regulating also DA storage in synaptic vesicles [10]. DAT expression on presynaptic terminals reflects striatal dopaminergic innervation, implicating a direct relationship between its reduction and nigral cell loss. Imaging of DAT in PD confirms a significant reduction of striatal uptake, with a direct correlation between decreased DAT in the putamen and PD symptoms [11]. [123I]-FP-CIT is the most used radioligand for DAT because of its high affinity, specificity and fast pharmacokinetic profile [12]. Other DAT ligands frequently used to evaluate in vivo the dopaminergic system are [99mTc]TRODAT, [123I]-β-CIT, 18F-FP-CIT and [123I]IPT, but their kinetic properties are slightly different [13,14,15]. Specifically, DAT-SPECT imaging is interpreted qualitatively based on the visual interpretation, but semi-quantitative assessment using striatal region of interests is often commonly performed in the routine clinical setting. Visual assessment is usually sufficient for clinical evaluation, but quantitative analyses of images may provide more objective measurement of striatal binding ratios [16,17]. However, both methods demonstrated an almost perfect agreement rate even though with some challenges and potential inaccurate results due to human error [18]. Besides its high affinity for DAT, it is important to remember that [123I]-FP-CIT also has affinity for other monoamine transporters, such as the serotonin transporter (SERT) and the norepinephrine transporter (NET). Notably, extrastriatal binding of [123I]-FP-CIT seems to derive from serotonergic transporters density, which is found mainly in the midbrain and diencephalon, suggesting DAT-SPECT as a proxy for the integrity the extrastriatal serotonergic system [19].
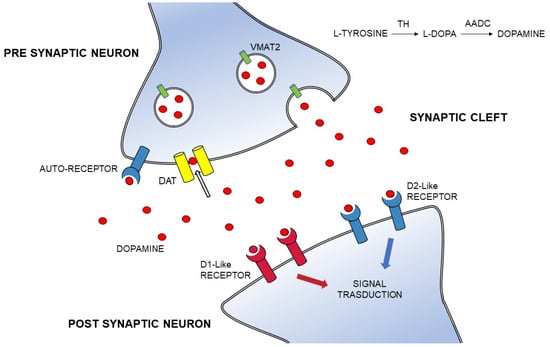
Figure 1.
The dopaminergic synapse. DA is derived from the L-tyrosine metabolism to L-dopa mediated by TH and AADS; the DA transport into synaptic vesicles is operated by VMAT-2; the DA synthesis is regulated via presynaptic auto-receptors (in blue); the presynaptic neuron releases DA (red circles); DA binds two main types of receptors (D1 family formed by D1-D5 receptors and D2 family formed by D2-D3-D4 receptors) on the post-synaptic neuron; presynaptic DAT (in yellow) regulates the DA reuptake from the extracellular fluid. Abbreviations: DA, Dopamine; AADC, aromatic L-amino acid decarboxylase; DAT, dopamine transporter; TH, tyrosine hydroxylase; VMAT-2, vesicular monoamine transporter 2.
3. Confounding Factors on the Interpretation of DAT
Aging and gender can both influence PD incidence, symptoms and treatment response but few studies have examined sex differences and aging effects on DAT in PD patients [20,21].
Progressive decline of DAT with aging is extensively reported in healthy subjects, with an average decline between 4% to 8% per decade [22,23]. Interestingly, experimental and functional data have led to the suggestion that DAT could be down-regulated in intact nerve terminals in response to age-related changes in the basal ganglia, suggesting a possible compensatory phenomenon which contributes to offset a natural decline in motor function observed in older individuals [24,25,26]. In PD there are mixed reports about the influence of aging on DAT, partly because of difficulty standardizing the severity of disease [27,28]. Lee and colleagues demonstrated a smaller-than-normal age effect on DAT ligand binding in the putamen, but not in the caudate of de novo PD patients [29]. The greater disease-related degeneration of putamen in early PD may mask age-related correlations, advocating for a possible floor effect in putaminal DAT binding in PD patients. However, this imbalanced age-decline across striatal subregions could be compatible with compensatory responses in the putamen to further DA losses, superimposed upon even age-related changes [29]. Similarly, in another study on a large sample of PD patients, a significant relationship between the age and DAT uptake was restricted to the caudate nuclei, without differences in the rate of decline between PD patients and controls [20]. In addition the authors described a concurrent decline of [123I]-FP-CIT binding in several extrastriatal regions, except for the SERT-rich midbrain [20].
Sex differences in the dopaminergic system have been similarly reported, with most evidence of higher DAT availability in women than men which could be related to higher dopamine transmission/turnover or greater volume of the striatum for the beneficial influence of estrogens [23,30,31,32]. Accordingly, female patients would exhibit a more rapid age-related decline in DAT activity than male patients as a consequence of lowering levels of estrogen with aging [33]. However, other studies reported equivalent dopaminergic binding between sexes [34,35,36] or a gender effect limited to young-to-middle aged healthy subjects, with no significant differences in the elderly age group [37].
Functional molecular imaging studies in PD patients reported a similar trend with higher DAT binding in women than men at both symptoms onset and during the disease progression [38]. This gender effect seems to be more prominent in the caudate than in the putamen [20].
The influence of dopaminergic drugs on DAT binding has been explored in neuroprotection trials of early PD patients which used striatal DAT-SPECT as an index of disease progression [39,40]. Imaging findings from the CALM-PD-CIT or REAL-PET studies raised the possibility that both dopamine agonists (pramipexole and ropinirole) could exert neuroprotective effects or that levodopa could be toxic for nigral cells in PD [41,42]. Notwithstanding, no corresponding clinical benefit in favor of the dopamine agonists was observed and it is difficult to separate their direct effects on tracers from their effects on disease progression [43]. Actually, another interpretation of these data is that imaging measures may simply reflect different effects of dopamine agonists and levodopa on the regulation of DAT expression [44,45]. Interestingly, a possible mild upregulation of DAT after chronic treatment with rotigotine has been interpreted as the consequence of a different occupancy of D1 versus D2 postsynaptic receptors when compared with the other dopamine agonists [46]. Overall, there is no consensus on the direction of the change in striatal DAT levels caused by dopaminergic drugs and, in the majority of cases, no change has been found [47]. All drugs with high affinity for the DAT and SERT (such as selective serotonin reuptake inhibitors), may result in a higher striatal binding ratio, whereas sertraline may block DAT leading to decreased striatal tracer uptake [48,49]. However, their effects appear to be only around 10%, arguing that it could be therefore not necessary to withdraw these medications for routine clinical studies. Accordingly, patients are currently submitted to DAT-SPECT study without withdrawing dopaminergic medications [50]. Conversely, all kinds of drugs which may lead to a misleading scan should be excluded in scientific studies, when even small differences may be relevant [51]. Among the central nervous system stimulants, cocaine, amphetamines, methylphenidate and modafinil are high-affinity DAT blockers [52]. Other medication and drug abuse that may significantly influence the interpretation of DAT-SPECT by reducing DAT levels include ephedrine, some antidepressants (e.g., bupropion), anticholinergic drugs (e.g., benztropine), opioids (e.g., fentanyl) and anesthetics (e.g., ketamine) [50]. In addition, it has been suggested that drugs increasing plasma levels of the adrenergic agonists may be able to affect striatal binding of DAT [53]. A recent study showed that zonisamide treatment, which has been authorized as add-on treatment for PD patients in Japan, can delay the reduction of striatal DAT levels in relatively early-stage PD patients [54]. Instead, it is unlikely that other common medications, including tricyclic antidepressants, amantadine, neuroleptics and cholinesterase inhibitors, will influence DAT imaging significantly. Finally, it should be noted that other habits, such as tobacco and cannabis addictions, may interfere with dopaminergic brain circuits, decreasing DAT availability [55,56], but large differences between smokers compared to ex-smokers and healthy volunteers have been excluded [57].
Finally, although some studies suggest an association between polymorphisms in the gene coding for DAT (SLC6A3) and in vivo striatal DAT availability in the human brain [58], the findings from SPECT studies concerning its association with striatal DAT availability appear not to be consistent [59].
4. DAT Imaging and Its Relationship to the Pathological Degeneration of the Nigrostriatal Pathway
In line with the characteristic motor asymmetry of PD, it is usual to see asymmetric DAT loss on functional imaging being more marked in the hemisphere contralateral to the most affected side of the body [60]. The asymmetry of dopaminergic depletion, detected by DAT imaging, has been also directly related to the magnitude of dopaminergic response [61]. Some authors suggested increased “left hemisphere susceptibility” in PD, in that the left nigrostriatal pathway is more affected than the right [62], which has been interpreted as an effect of handedness. This is consistent with several studies showing greater proportions of right-handed PD patients presenting with greater motor impairment of their right compared with their left-sided limbs [63]. However, a proportion of PD patients present their primary symptoms on the side of the body ipsilateral to the predominant dopamine deficit, and this appears to be common even in patients with clear motor and DAT asymmetries, arguing against hemispheric dominance as the only factor determining asymmetric nigrostriatal dysfunction in PD [64]. As clinical asymmetry becomes less prominent over the course of the illness, as there is a progressive loss of side-to-side DAT asymmetry with advancing disease [65]. In fact the decline in DAT binding seems to occur at a similar rate across striatal subregions, but maintains the antero-posterior gradient of dopamine dysfunction which is highly preserved during disease course [66]. This may be the result of the different vulnerability of nigrostriatal connections in PD, with an early and prevalent involvement of dopaminergic neurons of the ventrolateral tier of the substantia nigra pars compacta (SNpc) projecting to the dorsal putamen, as suggested by pathological studies [67]. Conversely, a different striatal gradient, with relatively greater caudate involvement, has been observed in patients both with dementia with Lewy bodies (DLB) and Parkinson dementia (PDD) vs. PD, in keeping with the hypothesis that reduced striatal DAT availability in the caudate nucleus is associated with a worse cognitive performance [68,69]. Interestingly, over the course of PD the change appears to be greater in the putamen ipsilateral to the predominant dopaminergic deficit, when compared with the contralateral putamen, suggesting a potential “floor effect” in the latter [70]. However, the putamen remains more affected than the caudate and, according to a PET study with [18F]FP-CIT, the posterior putamen is the subregion exhibiting the fastest annual rate of decline with disease progression [71].
It is worth mentioning that differences in the type of radiotracers, sample sizes, study design, participant characteristics and other methodological differences may be reason for discrepancies in imaging studies. Nevertheless, DAT-SPECT can be an excellent tool to map the spatial and temporal patterns of dopaminergic dysfunction in PD, providing evidence of a negative exponential loss of dopaminergic cells in early disease, which slows down with increasing symptom duration [72]. Clinical features seem to follow a similar exponential model with a faster progression early in the disease than in later years [73]. Accordingly, pathologic data have suggested an early and substantial reduction in dopamine function soon after diagnosis, but with a virtual absence of fibers in the dorsal striatum at 4 years [74]. Yet, imaging studies indicate that approximately 50% of putaminal presynaptic dopamine function is still preserved in PD patients with a mean motor disease duration of 4 to 7 years [75]. Moreover, residual functional dopaminergic nerve terminals have been demonstrated even in the striatum of PD patients with disease durations of more than 15 years [76]. Compensatory mechanisms in the terminal area of the nigrostriatal tract in PD could explain this striking discordance between neuropathology and neuroimaging data. It is acknowledged that motor PD symptoms appear when there is about a 30% loss of total dopaminergic neurons in the SNpc and striatal dopamine content is approximately reduced of 80% [77]. The magnitude of dopaminergic cell loss and striatal dopamine depletion before PD motor symptoms supports the key role of adaptive mechanisms which may effectively counteract nigrostriatal dysfunction at the onset of cellular dysfunction. Among the striatal regulatory changes occurring to partially compensate for the loss of dopaminergic terminals, DAT function has demonstrated to be downregulated to maintain the basal ganglia output within normal limits in response to the reduced levels of synaptic dopamine [78]. Interestingly, extra-striatal processes of neuroplasticity and the increased serotonergic fiber density with its capacity for dopamine release within the denervated striatum may constitute additional adaptive mechanisms of compensation [79]. Additionally, recent evidence on mice models suggests that striatal dopamine is synthesized not only in dopaminergic nigrostriatal axons, but also in the so-called monoenzymatic neurons containing complementary enzymes of DA synthesis, contributing to compensation of the dopamine deficit in the degraded parkinsonian striatum [80]. It is conceivable that compensatory molecular strategies are more pronounced in the pre-symptomatic phase of the disease, when most of the damage to the nigrostriatal dopamine pathway seems to occur [81]. In keeping with this, in asymptomatic mutation LRRK2 carriers, DAT reduction was found to be more pronounced than changes in VMAT2 and L-AADC indicating in vivo a strong involvement of compensatory DAT down-regulation in the pre-symptomatic stage of PD aiming to delay onset of clinical symptoms [82]. A significant reduction in striatal DAT binding but a preserved nigral signal measured with 7T Magnetic Resonance Imaging (MRI) has been observed in a still asymptomatic member of a family carrying the same G2019S LRRK2 gene mutation, supporting possibly compensatory down-regulation of DAT in the premotor disease stage [83]. Still, there are indications that early compensatory mechanisms are more efficient in younger brains [84]. This could argue in favor of a relatively long pre-clinical period in early onset PD patients, but also their greater propensity towards motor response complications, in line with a different time course of regulatory changes as a function of disease progression [85]. To put it another way, compensatory early events could have long-term, deleterious effects on basal ganglia function, ultimately contributing to the development of motor fluctuations and dyskinesias in PD [86]. This could be the effect of an increased dopamine turnover in the presence of reduced DAT levels because of advanced PD, with greater oscillations in L-dopa derived synaptic DA which have been linked to the occurrence of motor response complications [87]. In this regard, we recently found lower putaminal DAT binding and a higher rates of motor complications in early onset de novo PD patients despite a less severe motor phenotype at baseline than those with late onset PD, supporting an age-dependent different functional significance of DAT in early disease [88]. Adaptive striatal changes are greater in the initial stage of disease, especially in the putamen, suggesting that the eventual onset of motor symptoms in PD may reflect a global failure of compensatory mechanisms in nigroputaminal dopaminergic neurons [89]. As a consequence, it is not surprising that motor signs appear between a loss of 56 and 71% of DAT ligand, due to striatal compensatory adjustments as well as high nigrostriatal reserve [90]. In addition, although the severity of cardinal motor symptoms, assessed by the motor section of the unified Parkinson’s disease rating scale (UPDRS), is considered a good predictor of the neuronal loss observed in the substantia nigra (SN) [77], several studies attempting to correlate DAT in vivo imaging outcomes and neuropathological findings in humans have provided conflicting results [91]. Nigral neuronal density was found to be a significant predictor of DAT uptake in the striatum, as measured with [123I]FP-CIT SPECT, in a group of patients comprising of autopsy-confirmed cases with Alzheimer’s disease (AD), DLB and PDD [92]. Again, a high correlation between in vivo striatal DAT binding and dopaminergic cell counts in the SN at autopsy was detected in a heterogeneous cohort of patients with clinically suspected parkinsonism, even in those with moderate to severe dopaminergic degeneration [93].
Conversely, there is evidence in animal models of PD that striatal DAT uptake strongly correlates with striatal dopamine levels but does not faithfully reflect loss of nigral neurons throughout the full range of neuronal loss [94]. Notably, in a multitracer PET study in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys, striatal uptake correlated with stereological nigral cell counts only with cell loss <50%, with a flooring effect in moderate to severe parkinsonism [95]. In keeping with this, recent functional imaging studies raised important questions about the interpretation of DAT-SPECT in PD demonstrating a lack of correlation between striatal dopaminergic innervation and antemortem DAT imaging [96,97]. If the striatum is the initial site of nigrostriatal damage in PD, we could argue that there could be a positive relationship between striatal DAT binding and the number of axons of the nigrostriatal pathway, considering even the possibility that reduced DAT expression may reflect dysfunctional but viable nigral neurons in PD. However, Honkanen and colleagues failed to show a correlation between putaminal DAT binding and the number of dopaminergic axon counts in 14 patients with neuropathologically confirmed PD or atypical parkinsonism [97]. Taken together, these results tentatively suggest that DAT imaging is not a surrogate marker of PD pathology but rather a functional measurement of nigrostriatal dopaminergic pathway, reflecting axonal activity or DAT expression [98]. However, caution must be applied to such findings, performed in small number of patients and complicated by the time interval between scanning and cell counting which is an intrinsic critical issue of any clinicopathological post-mortem study [99]. Indeed, it is important to note that results might be different in early PD patients in which striatal DAT seems to correlate with the severity of parkinsonism. A potential approach to overcome the limitations associated with the time-interval between the in vivo scan and the post-mortem examination might be to correlate in vivo dopaminergic neuromelanin-rich neurons in SN measured by MRI with striatal DAT levels [100]. Indeed neuromelanin-sensitive MRI (NM-MRI) sequences have demonstrated the ability to be a potential in vivo index of nigral cell death [101]. First multimodal in vivo imaging studies showed a good correlation between NM-MRI SN measures and striatal DAT-SPECT values both in cohorts of patients with PD and mixed parkinsonism [102,103]. However, Martìn-Bastida and colleagues detected a moderate to strong linear association between the two measures across SN and striatum only in the clinically-defined most affected side of moderate stage PD patients, supporting an early asynchrony in the loss of neuromelanin-containing versus tyrosine hydroxylase positive nigral cells [104]. In addition, no correlation was found between NM-MRI in the SN and striatal binding ratio of DAT in another sample of PD patients, supporting the role of DAT-SPECT for early PD diagnosis whereas NM-MRI appears to be useful for determining dopaminergic neuronal degeneration in advanced PD [105]. Hence, the linear correlation between the severity of the parkinsonism and the loss of dopaminergic neurons indicates that molecular imaging markers that reflect residual nigral neuronal counts might provide a more suitable biomarker of clinical progression in advanced parkinsonism [106]. This has been demonstrated in MPTP-treated primates, by comparison of measured binding potential for DAT ligand in the SN with quantitative cell counts in the nigra, confirming that nigral specific binding of a DAT marker clearly reflects the number of dopaminergic neurons [95]. Accordingly, a recent PET study examining in vivo DAT availability along the entire dopaminergic nigrostriatal system using [18F]FE-PE2I, found a 30% of DAT loss at the level of the nigral cell bodies at disease onset, in line with pathologic data about the degree of dopaminergic neuron loss in the SN [107]. A substantial reduction of presynaptic DAT availability in the dorsal putamen and caudate, as opposed to a limited reduction in the SN, was found in another imaging study by Caminiti and colleagues, on patients with early-stage idiopathic PD [108]. This supports the notion that loss of striatal axonal markers of dopamine neurons exceeds the loss of their nigral cell bodies consistent with the apparent retrograde degeneration affecting the nigrostriatal pathway in PD [109,110]. This is not surprising, because axonal loss is now recognized as an early and predominant feature of PD, further corroborated by the evidence that α-synuclein pathology is more abundant in axons and presynaptic terminals [111,112].
5. DAT Imaging as Diagnostic Biomarker
Although several ligands have been produced for DAT visualization through SPECT and PET imaging, Ioflupane or [123I]-FP-CIT is the only approved in vivo diagnostic imaging agent for clinical practice [113]. Notably, DAT-SPECT is indicated in patients with clinically uncertain parkinsonian syndromes, with high sensitivity concerning the diagnosis of PD and a significant effect in influencing both further investigations and therapeutic intervention [114]. The clinical utility of DaTSCAN was investigated in a recent systematic review and meta-analysis demonstrating that the results of DaT imaging are associated both with a change in diagnosis and management of patients with suspected parkinsonian syndromes, even in individuals with long symptom duration [115]. However, despite a rigorous clinical assessment, diagnosing de novo untreated PD can be challenging, even for movement disorders specialist [116]. For example, the clinical presentation of isolated or atypical tremors can be insufficient to allow a precise early-stage diagnosis whereas the detection of abnormal striatal DAT binding in such patients predicts the future development of additional signs and symptoms consistent with clinical criteria for PD [117]. On the other side, patients with essential tremor (ET) were found to have mild striatal DAT abnormalities than healthy controls, but not as low as those with PD and with a pattern of DAT loss different from PD involving both caudate nucleus and putamen [118]. In the three-year follow-up study of the same cohort, no loss of DAT uptake was reported but ET patients continued to exhibit a predominant impairment of DAT in the caudate, suggesting the selective dopaminergic loss in the caudate nucleus as a possible pathophysiological link with PD. However, DAT-SPECT imaging allows differential diagnosis between ET and PD with a specificity comprised between 97 and 100% [119]. Importantly, an Italian study reported an overall decrease of costs for the health care system and an increase of patients’ time on potentially beneficial therapy by implementing the use of [123I]-FP-CIT SPECT in the early differential diagnosis of ET and PD [120].
Additionally, in several studies DAT-SPECT demonstrated a high accuracy in differentiation between PD from non-degenerative parkinsonisms such as those secondary to vascular, toxic, and inflammatory processes, or that are drug induced [121]. However, if DAT imaging in pure vascular parkinsonism is typically normal, other reports have described an involvement of the nigrostriatal system in about two-thirds of patients with parkinsonism and cerebrovascular disease [122]. Striatal asymmetric index is usually significantly higher in PD compared with VP, although occasionally a pattern similar to that described in PD can be found [123]. Vascular lesions that acutely or subacutely affect the basal ganglia may produce a significant pre-synaptic dopaminergic deficit congruent with the focal stroke location [124]. A DAT deficit has been also observed in cases with multiple and diffuse subcortical and periventricular white matter lesions indicating that symptoms of parkinsonism in some patients with diffuse leukoaraiosis can be related to a detectable nigrostriatal degeneration [125]. Thus, striatal DAT assessment may help identifying a comorbid nigrostriatal dopaminergic denervation in doubtful cases with vascular parkinsonism, and this can have therapeutic implications selecting patients who are more likely to response to levodopa [126].
Similarly, DAT imaging is expected to be normal in drug-induced parkinsonism (DIP), a relatively common postsynaptic parkinsonism in people chronically exposed to D2-receptor blocking agents and which can be clinically indistinguishable from PD [127]. Nevertheless, a subtle to severe involvement of the nigrostriatal system is found in up to 50% of patients with DIP, consistent with a progression of motor disability reported in a subset of DIP cases despite withdrawal of the offending agent [128,129]. Accordingly, imaging studies have confirmed DAT abnormalities in a number of patients clinically diagnosed with DIP, in keeping with a concomitant degenerative process, deemed to reflect PD or a subclinical form of the disease unmasked by the anti-dopaminergic drugs [130]. In this regard, a “double hit” hypothesis has been proposed, invoking a primary neurotoxic effect of dopamine blocking agents [131] and autopsy studies have reported changes compatible with an underlying idiopathic PD [132]. Moreover, there are patients with persistent DIP but visually normal DAT imaging in which mechanisms through which the offending drugs lead to parkinsonian symptoms are yet unclear, but a relatively decreased DAT levels within normal values could be indicative of a subtle nigrostriatal dopaminergic dysfunction predicting an early development of DIP [133,134]. This supports the evidence that DIP diagnosis represents a risk factor for idiopathic PD, implying the existence a latent parkinsonism in several patients triggered by the causative drug [135].
It is worth mentioning that DAT imaging has also a recognized role in distinguishing DLB from other forms of dementia, mainly AD, in uncertain cases [136,137], with a sensitivity up to 100% by using the semi-quantitative approach [138]. However, dopaminergic imaging cannot distinguish DLB from PDD, as both are associated with nigrostriatal dopaminergic neurodegeneration [139] or from patients with other dementia types (e.g., frontotemporal dementia), but having variable degrees of parkinsonism [140,141]. Nonetheless, not all patients with DLB have a positive scan, potentially becoming abnormal later in the disease process in a model of rostral-to-caudal progression pattern of Lewy body pathology instead of the reverse, as is usual [142]. Similarly, DAT-SPECT imaging was found to be normal in about 10% of cases of a large cohort of patients with clinical diagnosis of corticobasal syndrome (CBS), despite prominent bilateral extrapyramidal signs [143]. However, if the presynaptic nigrostriatal function may be preserved in the early stages, a progressive decline of striatal binding was demonstrated on follow up imaging [144] as well as confirmed pathologically [145].
Moreover, there are several other clinical scenarios where DAT imaging may be useful, such as in patients with mild or inconsistent parkinsonism despite detailed examination, or patients with parkinsonism but suboptimal response to levodopa [146,147].
Nevertheless, although DAT imaging offers an extraordinarily valuable tool in detecting the presence of a dopaminergic degenerative process, it has to be interpreted just as a useful adjunct to clinical decision making and cannot replace the benefits of thoughtful clinical evaluation [148] Indeed, abnormal DAT scan increases with increasing clinical probability of PD and number of cardinal motor signs present [149]. Older age, motor symptom asymmetry, and shorter disease duration have been identified as possible factors associated with abnormal scans in a large and heterogeneous sample of patients with clinically uncertain parkinsonism or suspected PD [150]
Moreover, it is important to note that an abnormal DAT scan does not necessarily indicate a diagnosis of PD. Indeed, specificity of DAT-SPECT is rather low, and it cannot reliably distinguish PD from atypical parkinsonisms (progressive supranuclear palsy-PSP, multiple system atrophy-MSA and CBS) in which there is a loss of striatonigral dopamine neurons as in PD. Some attempts have been performed to use quantitative DAT-SPECT measures, such as the asymmetric index and the caudate/putamen ratio, for a differential diagnosis between PD and atypical parkinsonisms, but without consistent results for their application in clinical practice [151]. In early PD the DAT signal appears typically most depressed in the posterior putamen contralateral to clinically affected limbs whereas the head of caudate and ventral striatum are relatively preserved [152]. Hence, in visual assessments, egg-shaped pattern has been reportedly more indicative for PD whereas a burst striatum pattern is more common in patients with a clinical diagnosis of atypical parkinsonism.
To further complicate matters, a normal scan could not necessarily exclude a diagnosis of PD. In drug trials of early PD using DAT imaging to enroll patients, 5.7–14.7% of the cases clinically diagnosed as PD had “scans without evidence of dopaminergic deficit” (SWEDD) [41,42,153]. In the majority of these subjects, follow-up scan remained normal in the context of minimal evidence of clinical progression. Thus, it is very likely that most patients with SWEDD, presenting with subtle atypical features, do not have PD but lay mainly between dystonic tremor, ET and other conditions mimicking features of parkinsonism [154,155]. Accordingly, longitudinal observational studies seem to support the notion that SWEDD may simply be a misdiagnosis of other different conditions [156]. It should be also noted that patients could be initially misclassified as SWEDD for an incorrect visual interpretation of DAT imaging [19,157], even though visual assessment of DAT-SPECT images has been established as being highly reliable in the identification of degenerative parkinsonisms [158].
However, some patients with parkinsonism and normal DAT imaging have been reported to both have abnormal scans on follow-up and respond well to dopaminergic therapy [159,160], raising the possibility that DAT-SPECT can be normal in the very early phase of PD [161,162] and challenging the concept of normal DAT-SPECT as an absolute exclusion criterion [6]. Besides, some SWEDD patients may have genetic forms of PD [163], further indicating that a negative DAT-SPECT should not definitively rule out the diagnosis of PD in an individual case [164]. In addition, other data (mainly derived from patients included in the Parkinson’s Progression Marker Initiative study-PPMI) suggest that subjects with SWEDD have an increased burden of non-motor symptoms, both in relation to frequency and severity, compared to PD subjects [165,166,167,168,169]. Conversely, hyposmia is more common in PD but not all patients with SWEDD have normal range in olfactory function [170]. As a consequence, some authors support the notion that patients with SWEDD represent a group distinct from PD but not free from pathology [154,169,171]. Thus, SWEDD remains a heterogeneous and highly debated phenomenon [162].
Use of DAT imaging has been suggested as enrichment biomarker for patient selection in early PD clinical trials for eliminating subjects without apparent striatal dopamine nerve terminal loss and thus allowing reduction of trial size [172]. DAT imaging has confirmed its diagnostic accuracy in a recent paper on PPMI patients, in which the authors found a low rate of diagnostic revision (2%) in early parkinsonism when DAT imaging was utilized, as opposed to a change of 6–13% in diagnosis reported in several PD clinical trials which did not consider DAT binding deficit as an inclusionary criterion [173]. However, while exclusion of SWEDD subjects from trials will improve the chance of determining clinical benefit of disease modifying treatment, some SWEDD subjects who demonstrated to experience a clinically important worsening of the motor scores would be excluded in a DAT-based enriched trial [174].
6. DAT Imaging as Preclinical Biomarker
In PD pathological processes insidiously progress and motor deficits generally appear when 50–60% of dopaminergic neurons in the SN are already lost and striatal dopamine is depleted by 80% [175]. This suggests a potential window for detecting loss of DAT binding in subjects who have subclinical dysfunction, identifying pre-symptomatic nigrostriatal dysfunction in at-risk subjects. Hence, dopamine deficiency has been shown by DAT imaging in individuals with conditions associated with increased risk of future PD such as hyposmia, rapid-eye-movement (REM) sleep behavior disorder (RBD) [176] or particular genetic conditions [177]. Although DTBZ binding provides the earlier and most reliable estimate of dopamine terminal density, actually DAT binding appears to be another sensitive marker of early disease, presumably attributable to a combination of reduced DAT binding sites with loss of dopamine nerve terminals as well as down-regulation of DAT in surviving terminals [178]. Several studies indicate that idiopathic RBD (iRBD) is an early feature of α-synucleinopathy, with the majority of patients who will eventually develop PD and DLB if they live long enough [179].
Reduced striatal DAT signaling has been reported in about 50% of subjects with isolated RBD [180]. Interestingly, in a prospective study on a large iRBD cohort, the authors showed dopaminergic imaging abnormalities in subjects at increased short-term risk for development of PD, similar to those typically seen in PD, with a tracer uptake reduction more pronounced in the putamen than in the caudate nucleus [181]. Additionally, a progressive decline in striatal binding in patients with iRBD further support the notion that RBD is actually an integral prodromal period of a defined neurodegenerative syndrome [182]. Several recent studies confirmed the high sensitivity of abnormal DAT-SPECT to accurately predict the short-term conversion to a clinically-defined α-synucleinopathy [183,184,185,186,187].
In the same vein, dopaminergic imaging could be also used to identify a high-risk group in subjects with hyposmia, which is another common prodromal feature of PD [188,189]. Abnormal DAT findings were found in 11% of hyposmic subjects only [190], but longitudinal studies of striatal DAT imaging in patients with hyposmia have shown a high risk of PD phenoconversion within 4 years [191]. Hyposmic individuals with DAT deficits also converted to PD at a higher rate that hyposmic individuals without a DAT deficit [191]. Recently, long-term follow-up of the PARS (Parkinson Associated Risk Syndrome) cohort confirmed baseline DAT as a strong predictor of conversion to clinical PD for persons with hyposmia and a DAT deficit [192]. Moreover, a progression of DAT deficit was also observed, with an incident DAT deficit in subjects who had either indeterminate or normal DAT status at baseline. Image conversion was also predictive of conversion to motor PD [192].
In support of the role of DAT imaging as marker of nigrostriatal dopamine denervation in the prodromal phase of the disease, Noyce and others investigated whether DAT abnormalities were also present in individuals with other prodromal features of PD [193]. In addition to hyposmia and RBD, they reported an association of striatal binding ratio with subtle motor dysfunction and PREDICT-PD risk estimates, which combine a number of risk and pre-diagnostic features of PD [193] Consistent with this report, elderly individuals with minimal parkinsonism without PD exhibited mild decrease in DAT availability in the striatum, which may be related to the disease process of prodromal PD [194].
Subclinical nigrostriatal DA dysfunction has been previously demonstrated in subjects with a high genetic risk of PD [195,196]. Decreased putaminal DAT binding has been reported in one unaffected individual heterozygous for the α-synuclein mutation [197]. As mentioned before, clinically unaffected mutation LRRK2 carriers can have reduced DAT binding, in keeping with active compensatory mechanisms at an early preclinical stage [82]. Similarly, in a multitracer PET study undertaken by Wyle and colleagues, DAT binding in LRRK2 mutation carriers without PD was significantly lower than in healthy controls even in younger age groups, by contrast with findings of 18F-FDOPA uptake which was preserved until age 70 years [163]. Converging results were obtained in other studies, with lower DAT levels in asymptomatic carriers of LLRK2 mutation than in noncarriers [83,198,199,200]. The presence of nigrostriatal dopaminergic denervation was found to be larger in the putamen than in the caudate in a cohort of asymptomatic relatives of patients carrying the LRRK2 R1441G mutation, reflecting the pattern of dopaminergic denervation observed in patients with PD. In such subjects reduced putaminal DAT density was even associated with a poorer execution of demanding timed motor tests [201]. It is important to note that penetrance estimations of LRRK2 mutations vary widely and only a proportion of mutation carriers will develop motor symptoms of PD [202]. The baseline DAT binding showed a high sensitivity and specificity in predicting conversion to motor PD within the 4-year period in a recent longitudinal study on 29 carriers of the G2019S LRRK2 mutation [203]. Although few reports are available for non-manifesting carriers of glucocerebrosidase (GBA) mutations, 3% of GBA non-manifesting carriers enrolled in the PPMI cohort showed a DAT deficit at baseline DAT-SPECT [204]. Interestingly, non-manifesting carriers of GBA mutation, but not LRRK2 non-manifest mutation carriers, had increased striatal binding values in all striatal regions when compared with LRRK2 mutant carriers and healthy controls, leading the authors to hypothesize a role of compensatory mechanisms [205]. However, a downregulation of DAT would be the expected response that serve to maintain extracellular levels of dopamine, calling also on alternative explanations [206].
7. DAT Imaging as Predictive Biomarker: Motor Measures
Discovering meaningful endpoints that measure progression is one of the greatest challenges in PD therapeutics. With continuing efforts and advances in the field, imaging strategies hold promise in identifying potential molecular targets of relevance to disease-modifying agents for PD. However, the prognostic validity of DAT-SPECT concerning the clinical progression of PD is unclear and potential confounding factors include a slow disease progression, antiparkinsonian medications, compensatory changes and aging. It should also be taken into account that several clinical patterns of decline exist for different subtypes of PD and the rate of decline is probably slower in young PD patients, as confirmed by longitudinal dopaminergic imaging studies [84]. Furthermore, we need to consider that commonly used radioligands for PET and SPECT also have an affinity for the serotonin (SERT) and noradrenaline (NET) transporters [207]. Nevertheless, in early cross-sectional studies of PD cohorts, a lower baseline DAT binding correlated with the increasing disease duration and motor severity measured by the UPDRS and the Hoehn and Yahr stadium [11,208,209,210] (Table 1). However, a better correlation of DAT binding with bradykinesia and rigidity than with tremor is extensively reported, supporting an independent pathophysiology for tremor which is not assessed by DAT-SPECT techniques [211]. Indeed, PD patients with a tremor-dominant type tend to show a significantly higher putaminal DAT uptake than akinetic-rigid and postural instability-gait disorders phenotypes at the same stage of disease [211,212,213], suggesting different patterns of dopaminergic degeneration in different subtypes of PD [214]. In keeping with this, the visual inspection of DAT-SPECT images can reveal significantly different morphological patterns for tremor-dominant (“eagle-wing shape”) versus akinetic-rigid (“egg-shaped”) PD patients, allowing a reliable differentiation of clinical PD subtypes [215]. Nonetheless, semiquantitative analysis of FP-CIT scan found no differences between the two groups [215]. There is some evidence that dopamine depletion in nuclei other than the striatum (i.e., the retrorubral area) may play a role in parkinsonian tremor [216]. In addition, PD tremor could be related to dysfunction of the serotonergic system in areas related to motor function. Accordingly, reduced [123I]-FP-CIT binding in the brainstem raphe nuclei, reflecting serotonin transporter availability, correlated with tremor scores in a subgroup of early PD patients with a tremulous motor phenotype [217]. Similarly to tremor, postural and gait disturbances were strongly correlated with cholinergic rather than with nigrostriatal dopaminergic activity [218]. Although the mechanisms underlying freezing of gat (FOG) have not yet been identified, recent retrospective studies conducted in early [76] or de novo PD patients [219] have found that the degree of presynaptic dopamine depletion, examined by DAT uptake, predicted the subsequent development of this phenomenon. There are conflicting results about which striatal subregion is more closely linked with FOG, but reduced DAT uptake in the caudate nucleus correlated with the development of FOG in early PD patients [220].

Table 1.
DAT imaging as a predictive biomarker in PD: motor and non-motor outcomes.
Recently, Mäkinen and colleagues investigated the associations of individual parkinsonian motor signs with striatal dopamine deficiency in patients with clinical parkinsonism or tremor of an uncertain origin, finding the highest likelihood of DAT deficiency in upper extremity rigidity and hypomimia [221].
Ravina and colleagues investigated whether baseline DAT levels could predict the development of clinically important long-term motor and nonmotor outcomes. Subjects with lower striatal DAT binding within 2 years of the clinical diagnosis of PD were more likely to develop motor-related disability, as well as cognitive impairment, psychosis, and depression after more than 5 years of follow-up [43]. In this retrospective study, the potential prognostic value of DAT imaging seems to be limited to the baseline scans because only a small association with long-term clinical outcomes was found for the DAT rate changes based on the follow-up scans, in line with a slower rate of nigral degeneration observed over the course of the disease [74].
In contrast, more recent longitudinal studies have failed to demonstrate a correlation between change in the mean striatum uptake and the change in UPDRS motor score [222]. Latourelle and colleagues did not find a predictive effect of DAT-SCAN imaging data on motor progression in their study based on previously established and potential novel markers by using an unbiased machine-learning approach [223]. In a longitudinal study of 44 PD followed-up for a mean of 44 months, baseline ß-CIT SPECT data were not in accordance with the decline of clinical symptoms over the observation period, even though inversely correlated with the UPDRS motor score at baseline [224]. Still, the observed outcome discrepancies between clinical and imaging changes in comparative drug trials of levodopa vs. dopamine agonists using β-CIT SPECT and 18F-dopa PET, could be the effect of a poor significant predictive value of baseline dopaminergic imaging, even though we have to consider potential differential influence of antiparkinsonian drugs on radiotracer uptake [41,42]. Similarly, there was a discordance between dopaminergic PET measures, namely 18F-DOPA, and clinical outcomes in trials of fetal nigral grafting and intraputaminal glial cell line-derived neurotrophic factor (GDNF) in PD patients [225,226]. In a cross-sectional study conducted in a population extrapolated from the PPMI dataset, significant correlation between DAT values and clinical measures, including the UPDRS-III and disease duration, was found only when both healthy controls and PD patients were included in the analysis whereas this relationship failed to remain significant after taking out healthy controls [227]. Similarly, 5-year longitudinal data on the change of clinical and DAT imaging outcomes measures in early, untreated PD patients from the PPMI cohort showed only a weak correlation between MDS-UPDRS-III and DAT binding, most marked at baseline, but no correlation between the rate of change of the 2 variables [70]. In keeping with this, recently no relationship was found between striatal or extrastriatal DAT binding and survival in PD patients [228], but other important neuropathological events and non-dopaminergic mechanisms could be more relevant in predicting also mortality [229]. A recent meta-regression analysis uncovered linear correlation between caudate DAT binding and disease severity in PD as studied cross-sectionally, likely due to earlier and more severe putaminal dopaminergic defect [75]. As described above, the important flooring effect found in the decline pattern of putaminal DAT can explain the lack of correlation with clinical scores, indicating the suboptimal value of striatal presynaptic dopaminergic functional imaging as disease progression marker [94].
Conversely, in a recent PET study using 11C-PE2I (a radioligand with high striatal binding and greater DAT specificity than the others), the authors found not only a high correlation between the reduction in striatal DAT density and PD symptom severity at baseline, but even a significant negative correlation between change in motor severity (both UPDRS-III and bradykinesia/rigidity subscores) and DAT binding in the caudate and posterior putamen [184,230]. Of particular interest is that even the most affected putamen confirmed a relationship at follow-up between changes in 11C-PE2I and in bradykinesia-rigidity scores. This study suggests that DAT quantification using DAT ligands with relatively high DAT-to-SERT selectivity could be more sensitive for investigating disease progression [184]. Furthermore, use of radiomic features extracted from DAT-SPECT images or the combination of multi-parametric and multi-modality solutions, involving different MRI sequences and/or SPECT images, along with non-imaging features, may significantly improve prediction of motor outcomes [231,232].
8. DAT Imaging as Predictive Biomarker: Motor Complications
There is ample evidence to point that striatal dopamine depletion, particularly in the putamen, whether due to neuronal degeneration or functional impairment, predicts the development of motor complications in patients with PD [243,244,245] (Table 1). Specifically, lower levels of baseline DAT activity in the posterior putamen were found to be a strong and independent predictor of the development of levodopa-induced dyskinesia (LID) at the 3-year follow-up in 127 drug-naive de novo patients [87]. Initial DAT activity in the posterior putamen was also significantly associated with the early appearance of LID in another retrospective cohort study by the same authors including a total of 412 consecutive drug-naive patients with PD [233]. Beyond the initial absolute DAT values, baseline and follow-up [I123]-FP-CIT SPECT images from the PPMI population demonstrated that even the deterioration rate of putaminal innervation was significantly higher in PD patients who developed LID during 48 months of follow-up [234]. Similarly, time-related changes in striatal DAT availability have been related to the appearance of future LID in a small longitudinal SPECT study of Piccini’s group [246]. In another large number of patients with de novo PD, subjects with wearing-off (W-O) exhibited lower level of DAT activity in the anterior putamen than did those without W-O [235]. As additional evidence of the regional difference between W-O and LID, a recent study by using a factor analysis based on the DAT availability in striatum, found a relationship between the posterior putamen, which belongs to the sensorimotor striatum, and a higher risk for LID, as well as an association between dopamine depletion in the anterior putamen and both early development of W-O and conversion to dementia [237]. Therefore, patterns of striatal dopaminergic denervation seem to have prognostic implications in patients with early-stage PD [237].
The exact mechanisms of motor response complications are unclear, but DAT reduction would converge to disrupt presynaptic dopamine homeostasis and increase oscillating levels of synaptic dopamine in the parkinsonian striatum, ultimately facilitating the appearance of LID and motor fluctuations [247]. Additionally, repeated administrations of levodopa with chronic pulsatile stimulation of dopamine receptors, and postsynaptic changes including dopamine receptor supersensitivity, may play together an important role in their pathogenesis. While young age at onset is a well-known risk factor for developing LID, the reasons underlying such high risk have not been resolved. In this regard, we recently proposed that a different availability of DAT between PD patients with young and old onset, as the result of age-related differences in early striatal compensatory mechanisms, could account, at least in part, for age-at-onset–dependent risk of motor complications in PD [88]. Therefore, early compensatory DAT downregulation in younger PD patients could be a relevant susceptibility factor to motor complications throughout the course of disease.
Notwithstanding, although there is strong support to the presynaptic hypothesis of motor complications, other studies failed to demonstrate a relationship between DAT binding on SPECT in early-stage PD and their late emergence [76,236]. It is important to note that, alongside these dopaminergic mechanisms, increasing evidence suggests a direct involvement of glutamatergic, serotonergic and cholinergic system in the expression of dyskinesias [248,249]. Notably, striatal serotonin hyperinnervation appears to be a key determinant in the appearance of LID, as revealed by imaging studies demonstrating a strong correlation between the SERT to DAT ratio, as reflected by the 11C-DASB BP to 123I-ioflupane uptake ratio, and LID [45,250].
9. DAT Imaging as Predictive Biomarker: Non-Motor Measures
Several DAT imaging studies revealed an association between decreased striatal DAT availability and non-motor symptoms in PD including anxiety [251], depression [252,253,254], apathy [255], impulsivity [239,256], fatigue, constipation [240], hyposmia [257,258,259,260], autonomic symptoms [261,262,263,264,265,266], sleep disturbances, daytime sleepiness [267], body weight loss [268] and visual hallucinations [241], in support of a partial dopaminergic pathogenesis [269] (Table 1). In a recent study using data from sequential DAT-SPECT imaging in 344 PD patients from the PPMI dataset, Liu and colleagues demonstrated a significant association between DAT binding both at baseline and at follow-up scans and several baseline non-motor symptoms, with RBD showing the strongest association with concurrent and future DAT binding [270]. Moreover, the burden of non-motor symptoms has been associated with distinct patterns of striatal dopamine depletion in a large cohort of patients with de novo PD [271].
However, other findings are dissimilar and there are only few longitudinal data addressing the prognostic impact of striatal DAT on non-motor outcomes, suggesting that dysregulation in other neurotransmitter systems is an important contributor to their pathophysiology [272,273,274,275,276,277,278,279].
Conversely, it is well known the dopaminergic contribution to cognition, mainly in frontal/executive performance, and empirical evidence for this hypothesis comes from several DAT imaging studies reporting a significant relationship between striatal dopaminergic denervation, especially in the caudate, and cognitive functioning [280,281,282,283], both in de novo [284] and advanced PD patients [285]. The occurrence of early caudate dysfunction has been related to later development of non-motor symptoms, such as RBD, depression and cognitive impairment, with overall worse prognosis [286,287]. Furthermore, recent studies targeted the clinical relevance of extrastriatal DAT uptake in early PD, identifying a significant contribution of the DAT uptake within posterior cortical areas to global cognitive status [288]. A recent study from the large PPMI dataset has investigated the role of asymmetric DAT loss as a potential biomarker in drug-naive PD patients followed longitudinally up 4 years revealing a specific influence of hemispheric asymmetry in dopaminergic cell loss on both motor and cognitive outcomes in PD [289].
10. Beyond DAT: Correlation with Other Potential Nigrostriatal Biomarkers in PD
It should be mentioned that other tools and markers have been recognized as useful early diagnostic biomarkers in PD. Novel MRI techniques, able to assess nigral pathology, hold the potential for the differentiation of neurodegenerative from non-neurodegenerative parkinsonian disorders, but may also enhance subtyping and disease severity monitoring in PD [290].
Visual assessment of dorsolateral nigral hyperintensity (DNH) on iron-sensitive MRI sequences yields excellent diagnostic accuracy (pooled sensitivity of 98% and a pooled specificity of 95%) for PD versus healthy controls [291]. Furthermore, absence of DNH on high-field susceptibility weighted imaging (SWI) has also been suggested as a potential MRI biomarker in prodromal degenerative parkinsonism since it has been found in clinically asymptomatic LRKK2 carriers [83] and in at least two-thirds of subjects with iRBD [292]. Accordingly, a good positive correlation between nigrosome imaging and DAT SPECT imaging on both 3- and 7-T MRI has been reported [293]. However, there is some degree of discordance between findings at MRI imaging and [123]I-FP-CIT SPECT, reinforcing the notion that nigrostriatal degeneration occurs before nigral cell loss [294].
Early PD diagnosis (differential diagnosis from healthy controls) using NM-sensitive MRI has a sensitivity of 89% and a specificity of 85% [295]. Again, a significant positive correlation between the NM-positive SN volume on NM-sensitive MRI scans and [123]I-FP-CIT uptake ratios on SPECT scans has been demonstrated [296]. Importantly, multimodal studies employing structural MRI and SPECT imaging showed comparable or better diagnostic performance compared to each parameter of DAT-SPECT and NM-MRI in distinguishing PD from nondegenerative parkinsonian syndrome [297]. It is worth noting that, unlike DAT imaging, NM-MRI imaging seems to be also effective for measuring disease progression demonstrating to closely correlate with clinical scores, including UPDRS-III, in advanced PD patients [105,298].
Quantification of QSM (Quantitative Susceptibility Mapping) values in nigrosomes on 3-T scans is likewise effective in the diagnosis of early-stage PD, showing high diagnostic performance in a recent imaging study in 39 patients vs. 25 controls (area under the curve, 0.73) [299].
Nevertheless, although all these MRI techniques provide suitable markers for the nigral structure, they generally may not differentiate PD and other types of degenerative parkinsonism [116]. Further recent neuroimaging techniques, such as diffusion-tensor imaging and density imaging, might help clinicians to differentiate between these conditions but their unavailability on most scanners and the lack of normative databases challenge their use in clinical practice [300,301].
11. Conclusions
Imaging of DAT binding is useful in early diagnosis for PD and differentiation from other non-degenerative parkinsonian disorders, but it does not differentiate PD from other degenerative parkinsonisms characterized by dopaminergic cell loss. However, it should be noted that dopaminergic imaging cannot replace the benefits of a careful clinical evaluation. DAT deficit is able to identify the risk of PD onset in patients who have prodromal features such as hyposmia, iRBD or carrying a pathogenic mutation of PD. In patients with PD there is generally a good correlation of DAT levels with bradykinesia and rigidity, and less with tremor, but this seems to be limited to the baseline scans because longitudinal evidence suggests that there is no correlation between change in the striatal DAT uptake and the change in UPDRS motor score. DAT imaging may have some prognostic value, especially in the prediction of motor complications and cognitive dysfunction, but this has limited relevance to current clinical practice. Although DAT-SPECT imaging has been suggested as an enrichment biomarker to be used in early PD neuroprotection trials, it should be noted that some patients with normal DAT binding but motor scores worsening over the years are at risk to be excluded. Finally, clinicians should be aware of issues related to the interpretation of DAT imaging results in view of recent studies questioning the actual relationship between DAT levels and nigral cell or axon counts.
Author Contributions
Conceptualization, G.P. and S.G.; methodology, G.P.; writing—original draft preparation, G.P., S.G. and G.B.; writing—review and editing, G.P. and R.C.; supervision, R.C. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Langston, J.W. The Parkinson’s complex: Parkinsonism is just the tip of the Iceberg. Ann. Neurol. 2006, 59, 591–596. [Google Scholar] [CrossRef]
- Rosa-Grilo, M.; Qamar, M.A.; Taddei, R.N.; Pagonabarraga, J.; Kulisevsky, J.; Sauerbier, A.; Chaudhuri, K.R. Rotigotine transdermal patch and sleep in Parkinson’s disease: Where are we now? NPJ Park. Dis. 2017, 3, 28. [Google Scholar] [CrossRef]
- Adler, C.H.; Beach, T.G.; Hentz, J.G.; Shill, H.A.; Caviness, J.N.; Driver-Dunckley, E.; Sabbagh, M.N.; Sue, L.I.; Jacobson, S.A.; Belden, C.M.; et al. Low clinical diagnostic accuracy of early vs. advanced Parkinson disease: Clinicopathologic study. Neurology 2014, 83, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Copetti, M.; Arcuti, S.; Martino, D.; Fontana, A.; Logroscino, G. Accuracy of clinical diagnosis of Parkinson disease. Neurology 2016, 86, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Ray Dorsey, E.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, V.; Schreglmann, S.R.; Bhatia, K.P. Neuroimaging advances in Parkinson’s disease. Curr. Opin. Neurol. 2018, 31, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Uhl, G.R. Dopamine transporter: Basic science and human variation of a key molecule for dopaminergic function, locomotion, and parkinsonism. Mov. Disord. 2003, 18, 10578. [Google Scholar] [CrossRef]
- Sulzer, D.; Cragg, S.J.; Rice, M.E. Striatal Dopamine Neurotransmission: Regulation of Release and Uptake; Elsevier: Amsterdam, The Netherlands, 2016; Volume 6, ISBN 4418652825. [Google Scholar]
- Benamer, H.T.S.; Patterson, J.; Wyper, D.J.; Hadley, D.M.; Macphee, G.J.A.; Grosset, D.G. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov. Disord. 2000, 15, 692–698. [Google Scholar] [CrossRef]
- Booij, J.; Tissingh, G.; Boer, G.J.; Speelman, J.D.; Stoof, J.C.; Janssen, A.G.M.; Wolters, E.C.; Van Royen, E.A. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 133–140. [Google Scholar] [CrossRef]
- Mozley, P.D.; Schneider, J.S.; Acton, P.D.; Plössl, K.; Stern, M.B.; Siderowf, A.; Leopold, N.A.; Li, P.Y.; Alavi, A.; Kung, H.F. Binding of [99mTc]TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteers. J. Nucl. Med. 2000, 41, 584–589. [Google Scholar]
- Marek, K.; Innis, R.; Van Dyck, C.; Fussell, B.; Early, M.; Eberly, S.; Oakes, D.; Seibyl, J. [123I]β-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology 2001, 57, 2089–2094. [Google Scholar] [CrossRef]
- Kim, H.J.; Im, J.H.; Yang, S.O.; Moon, D.H.; Ryu, J.S.; Bong, J.K.; Nam, K.P.; Cheon, J.H.; Lee, M.C.; Lee, H.K. Imaging and quantitation of dopamine transporters with iodine-123-IPT in normal and Parkinson’s disease subjects. J. Nucl. Med. 1997, 38, 1703–1711. [Google Scholar]
- Bohnen, N.I.; Djang, D.S.W.; Herholz, K.; Anzai, Y.; Minoshima, S. Effectiveness and safety of 18F-FDG PET in the evaluation of dementia: A review of the recent literature. J. Nucl. Med. 2012, 53, 59–71. [Google Scholar] [CrossRef]
- Djang, D.S.W.; Janssen, M.J.R.; Bohnen, N.; Booij, J.; Henderson, T.A.; Herholz, K.; Minoshima, S.; Rowe, C.C.; Sabri, O.; Seibyl, J.; et al. SNM practice guideline for dopamine transporter imaging with 123I-ioflupane SPECT 1.0. J. Nucl. Med. 2012, 53, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Marcus, C.; Sheikhbahaei, S.; Solnes, L.B.; Leal, J.P.; Du, Y.; Rowe, S.P.; Higuchi, T.; Buck, A.K.; Lapa, C.; et al. Visual and semiquantitative accuracy in clinical baseline 123I-Ioflupane SPECT/CT imaging. Clin. Nucl. Med. 2019, 44, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, N.; Garibotto, V.; Burkhard, P.R. Extrastriatal 123I-FP-CIT SPECT impairment in Parkinson’s disease-the PPMI cohort. BMC Neurol. 2020, 20, 192. [Google Scholar] [CrossRef]
- Kaasinen, V.; Joutsa, J.; Noponen, T.; Johansson, J.; Seppänen, M. Effects of aging and gender on striatal and extrastriatal [123I]FP-CIT binding in Parkinson’s disease. Neurobiol. Aging 2015, 36, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Meoni, S.; Macerollo, A.; Moro, E. Sex differences in movement disorders. Nat. Rev. Neurol. 2020, 16, 84–96. [Google Scholar] [CrossRef]
- Van Dyck, C.H.; Seibyl, J.P.; Malison, R.T.; Laruelle, M.; Zoghbi, S.S.; Baldwin, R.M.; Innis, R.B. Age-related decline in dopamine transporters: Analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am. J. Geriatr. Psychiatry 2002, 10, 36–43. [Google Scholar] [CrossRef]
- Varrone, A.; Dickson, J.C.; Tossici-Bolt, L.; Sera, T.; Asenbaum, S.; Booij, J.; Kapucu, O.L.; Kluge, A.; Knudsen, G.M.; Koulibaly, P.M.; et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): Age-related effects, gender differences and evaluation of different methods of analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Apparsundaram, S.; Gerhardt, G.A. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol. Aging 2003, 24, 1147–1154. [Google Scholar] [CrossRef]
- Cruz-Muros, I.; Afonso-Oramas, D.; Abreu, P.; Pérez-Delgado, M.M.; Rodríguez, M.; González-Hernández, T. Aging effects on the dopamine transporter expression and compensatory mechanisms. Neurobiol. Aging 2009, 30, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Troiano, A.R.; Schulzer, M.; De La Fuente-Fernandez, R.; Mak, E.; Mckenzie, J.; Sossi, V.; Mccormick, S.; Ruth, T.J.; Stoessl, A.J. Dopamine transporter PET in normal aging: Dopamine transporter decline and its possible role in preservation of motor function. Synapse 2010, 64, 146–151. [Google Scholar] [CrossRef]
- Tissingh, G.; Bergmans, P.; Booij, J.; Winogrodzka, A.; Stoof, J.C.; Wolters, E.C.; Van Royen, E.A. [123I]β-CIT single-photon emission tomography in Parkinson’s disease reveals a smaller decline in dopamine transporters with age than in controls. Eur. J. Nucl. Med. 1997, 24, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Tissingh, G.; Booij, J.; Bergmans, P.; Winogrodzka, A.; Janssen, A.G.M.; Van Royen, E.A.; Stoof, J.C.; Wolters, E.C. Iodine-123-N-ω-fluoropropyl-2/β-carbomethoxy3β-(4-iodophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease. J. Nucl. Med. 1998, 39, 1143–1148. [Google Scholar] [PubMed]
- Lee, C.S.; Kim, S.J.; Oh, S.J.; Kim, H.O.; Yun, S.C.; Doudet, D.; Kim, J.S. Uneven age effects of [18F]FP-CIT binding in the striatum of Parkinson’s disease. Ann. Nucl. Med. 2014, 28, 874–879. [Google Scholar] [CrossRef]
- Lavalaye, J.; Knol, R.J.J.; De Bruin, K.; Reneman, L.; Janssen, A.G.M.; Booij, J. [123I]FP-CIT binding in rat brain after acute and sub-chronic administration of dopaminergic medication. Eur. J. Nucl. Med. 2000, 27, 346–349. [Google Scholar] [CrossRef]
- Harper Mozley, L.; Gur, R.C.; Mozley, R.D.; Gur, R.E. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am. J. Psychiatry 2001, 158, 1492–1499. [Google Scholar] [CrossRef]
- Staley, J.K.; Krishnan-Sarin, S.; Zoghbi, S.; Tamagnan, G.; Fujita, M.; Seibyl, J.P.; Maciejewski, P.K.; O’Malley, S.; Innis, R.B. Sex differences in [123I]β-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse 2001, 41, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Ham, J.H.; Lee, P.H.; Sohn, Y.H. Gender Differences in Age-Related Striatal Dopamine Depletion in Parkinson’s Disease. J. Mov. Disord. 2015, 8, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ryding, E.; Lindström, M.; Brådvik, B.; Grabowski, M.; Bosson, P.; Träskman-Bendz, L.; Rosén, I. A new model for separation between brain dopamine and serotonin transporters in 123I-β-CIT SPECT measurements: Normal values and sex and age dependence. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Best, S.E.; Sarrel, P.M.; Malison, R.T.; Laruelle, M.; Zoghbi, S.S.; Baldwin, R.M.; Seibyl, J.P.; Innis, R.B.; Van Dyck, C.H. Striatal dopamine transporter availability with [123I]β-CIT SPECT is unrelated to gender or menstrual cycle. Psychopharmacology (Berl). 2005, 183, 181–189. [Google Scholar] [CrossRef]
- Munro, C.A.; McCaul, M.E.; Wong, D.F.; Oswald, L.M.; Zhou, Y.; Brasic, J.; Kuwabara, H.; Kumar, A.; Alexander, M.; Ye, W.; et al. Sex Differences in Striatal Dopamine Release in Healthy Adults. Biol. Psychiatry 2006, 59, 966–974. [Google Scholar] [CrossRef]
- Wong, K.K.; Müller, M.L.T.M.; Kuwabara, H.; Studenski, S.A.; Bohnen, N.I. Gender differences in nigrostriatal dopaminergic innervation are present at young-to-middle but not at older age in normal adults. J. Clin. Neurosci. 2012, 19, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Haaxma, C.A.; Bloem, B.R.; Borm, G.F.; Oyen, W.J.G.; Leenders, K.L.; Eshuis, S.; Booij, J.; Dluzen, D.E.; Horstink, M.W.I.M. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 819–824. [Google Scholar] [CrossRef]
- Innis, R.B.; Marek, K.L.; Sheff, K.; Zoghbi, S.; Castronuovo, J.; Feigin, A.; Seibyl, J.P. Effect of treatment with L-dopa/carbidopa or L-selegiline on striatal dopamine transporter SPECT imaging with [123I]β-CIT. Mov. Disord. 1999, 14, 436–442. [Google Scholar] [CrossRef]
- Nurmi, E.; Bergman, J.; Eskola, O.; Solin, O.; Hinkka, S.M.; Sonninen, P.; Rinne, J.O. Reproducibility and effect of levodopa on dopamine transporter function measurements: A [18F]CFT PET study. J. Cereb. Blood Flow Metab. 2000, 20, 1604–1609. [Google Scholar] [CrossRef]
- Parkinson Study Group Dopamine Transporter Brain Imaging to Assess the Effects of Pramipexole vs. Levodopa on Parkinson Disease Progression. JAMA 2002, 287, 1653. [CrossRef]
- Whone, A.L.; Watts, R.L.; Stoessl, A.J.; Davis, M.; Reske, S.; Nahmias, C.; Lang, A.E.; Rascol, O.; Ribeiro, M.J.; Remy, P.; et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann. Neurol. 2003, 54, 93–101. [Google Scholar] [CrossRef]
- Ravina, B.; Marek, K.; Eberly, S.; Oakes, D.; Kurlan, R.; Ascherio, A.; Beal, F.; Beck, J.; Flagg, E.; Galpern, W.R.; et al. Dopamine transporter imaging is associated with long-term outcomes in Parkinson’s disease. Mov. Disord. 2012, 27, 1392–1397. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Uitti, R.J.; O’Connor, M.K.; Maraganore, D.M.; Matsumoto, J.Y.; Stark, K.F.; Turk, M.F.; Burnett, O.L. The effect of dopamine agonist therapy on dopamine transporter imaging in Parkinson’s disease. Mov. Disord. 1999, 14, 940–946. [Google Scholar] [CrossRef]
- Roussakis, A.A.; Politis, M.; Towey, D.; Piccini, P. Serotonin-to-dopamine transporter ratios in Parkinson disease. Neurology 2016, 86, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Genovesi, D.; Marzullo, P.; Giorgetti, A.; Filidei, E.; Corsini, G.U.; Bonuccelli, U.; Ceravolo, R. Striatal dopamine transporter modulation after rotigotine: Results from a pilot single-photon emission computed tomography study in a group of early stage Parkinson disease patients. Clin. Neuropharmacol. 2017, 40, 34–36. [Google Scholar] [CrossRef]
- Guttman, M.; Stewart, D.; Hussey, D.; Wilson, A.; Houle, S.; Kish, S. Influence of L-dopa and pramipexole on striatal dopamine transporter in early PD. Neurology 2001, 56, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Booij, J.; De Jong, J.; De Bruin, K.; Knol, R.; De Win, M.M.L.; Van Eck-Smit, B.L.F. Quantification of striatal dopamine transporters with123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: A double-blind, placebo-controlled, crossover study in healthy control subjects. J. Nucl. Med. 2007, 48, 359–366. [Google Scholar]
- Ikeda, K.; Ebina, J.; Kawabe, K.; Iwasaki, Y. Dopamine transporter imaging in parkinson disease: Progressive changes and therapeutic modification after anti-parkinsonian medications. Intern. Med. 2019, 58, 1665–1672. [Google Scholar] [CrossRef]
- Morbelli, S.; Esposito, G.; Arbizu, J.; Barthel, H.; Boellaard, R.; Bohnen, N.I. EANM practice guideline / SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1885–1912. [Google Scholar] [CrossRef]
- Booij, J.; Kemp, P. Dopamine transporter imaging with [123I]FP-CIT SPECT: Potential effects of drugs. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 424–438. [Google Scholar] [CrossRef]
- Tatsch, K.; Poepperl, G. Nigrostriatal dopamine terminal imaging with dopamine transporter SPECT: An update. J. Nucl. Med. 2013, 54, 1331–1338. [Google Scholar] [CrossRef]
- Byas-Smith, M.; Votaw, J.; Hua, J.; Voll, R.; Martarello, L.; Levey, A.I.; Goodman, M. Phenylephrine and norepinephrine increase dopamine transporter ligand binding in striatum. Mol. Imaging Biol. 2003, 5, 217–226. [Google Scholar] [CrossRef]
- Ikeda, K.; Yanagihashi, M.; Miura, K.; Ishikawa, Y.; Hirayama, T.; Takazawa, T.; Kano, O.; Kawabe, K.; Mizumura, N.; Iwasaki, Y. Zonisamide cotreatment delays striatal dopamine transporter reduction in Parkinson disease: A retrospective, observational cohort study. J. Neurol. Sci. 2018, 391, 5–9. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Ashok, A.H.; Mizuno, Y.; Howes, O.D. Tobacco smoking and dopaminergic function in humans: A meta-analysis of molecular imaging studies. Psychopharmacology 2019, 236, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, G.; Knudsen, G.M.; Jensen, P.S.; Ziebell, M.; Holst, K.K.; Asenbaum, S.; Booij, J.; Darcourt, J.; Dickson, J.C.; Kapucu, Ö.L.; et al. No difference in striatal dopamine transporter availability between active smokers, ex-smokers and non-smokers using [123I]FP-CIT (DaTSCAN) and SPECT. EJNMMI Res. 2013, 3, 39. [Google Scholar] [CrossRef]
- Van De Giessen, E.M.; De Win, M.M.L.; Tanck, M.W.T.; Van Den Brink, W.; Baas, F.; Booij, J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J. Nucl. Med. 2009, 50, 45–52. [Google Scholar] [CrossRef]
- Costa, A.; Riedel, M.; Müller, U.; Möller, H.J.; Ettinger, U. Relationship between SLC6A3 genotype and striatal dopamine transporter availability: A meta-analysis of human single photon emission computed tomography studies. Synapse 2011, 65, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Manni, C.; Pierantozzi, M.; Brusa, L.; Danieli, R.; Stanzione, P.; Schillaci, O. I-fp-cit semi-quantitative spect detects preclinical bilateral dopaminergic deficit in early parkinson’s disease with unilateral symptoms. Nucl. Med. Commun. 2005, 26, 421–426. [Google Scholar] [CrossRef]
- Contrafatto, D.; Mostile, G.; Nicoletti, A.; Raciti, L.; Luca, A.; Dibilio, V.; Lanzafame, S.; Distefano, A.; Drago, F.; Zappia, M. Single photon emission computed tomography striatal asymmetry index may predict dopaminergic responsiveness in Parkinson disease. Clin. Neuropharmacol. 2011, 34, 71–73. [Google Scholar] [CrossRef]
- Scherfler, C.; Seppi, K.; Mair, K.J.; Donnemiller, E.; Virgolini, I.; Wenning, G.K.; Poewe, W. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson’s disease. Brain 2012, 135, 3348–3354. [Google Scholar] [CrossRef]
- Barrett, M.J.; Wylie, S.A.; Harrison, M.B.; Wooten, G.F. Handedness and motor symptom asymmetry in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1122–1124. [Google Scholar] [CrossRef]
- Kaasinen, V. Ipsilateral deficits of dopaminergic neurotransmission in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2016, 3, 21–26. [Google Scholar] [CrossRef]
- Shigekiyo, T.; Arawaka, S. Laterality of specific binding ratios on DAT-SPECT for differential diagnosis of degenerative parkinsonian syndromes. Sci. Rep. 2020, 10, 15761. [Google Scholar] [CrossRef]
- Nandhagopal, R.; Kuramoto, L.; Schulzer, M.; Mak, E.; Cragg, J.; Lee, C.S.; McKenzie, J.; McCormick, S.; Samii, A.; Troiano, A.; et al. Longitudinal progression of sporadic Parkinson’s disease: A multi-tracer positron emission tomography study. Brain 2009, 132, 2970–2979. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, T.; Pagano, G.; Niccolini, F.; Politis, M. Predicting cognitive decline with non-clinical markers in Parkinson’s disease (PRECODE-2). J. Neurol. 2019, 266, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Colloby, S.J.; Williams, E.D.; Burn, D.J.; Lloyd, J.J.; McKeith, I.G.; O’Brien, J.T. Progression of dopaminergic degeneration in dementia with Lewy bodies and Parkinson’s disease with and without dementia assessed using 123I-FP-CIT SPECT. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1176–1185. [Google Scholar] [CrossRef]
- Simuni, T.; Siderowf, A.; Lasch, S.; Coffey, C.S.; Caspell-Garcia, C.; Jennings, D.; Tanner, C.M.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; et al. Longitudinal Change of Clinical and Biological Measures in Early Parkinson’s Disease: Parkinson’s Progression Markers Initiative Cohort. Mov. Disord. 2018, 33, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.; Lee, J.H.; Oh, J.S.; Oh, M.; Lee, S.J.; Oh, S.J.; Chung, S.J.; Lee, C.S.; Kim, J.S. Longitudinal Decline of Striatal Subregional [18F]FP-CIT Uptake in Parkinson’s Disease. Nucl. Med. Mol. Imaging 2017, 51, 304–313. [Google Scholar] [CrossRef]
- Hilker, R.; Schweitzer, K.; Coburger, S.; Ghaemi, M.; Weisenbach, S.; Jacobs, A.H.; Rudolf, J.; Herholz, K.; Heiss, W.D. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch. Neurol. 2005, 62, 378–382. [Google Scholar] [CrossRef]
- Vu, T.C.; Nutt, J.G.; Holford, N.H.G. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br. J. Clin. Pharmacol. 2012, 74, 267–283. [Google Scholar] [CrossRef]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef]
- Kaasinen, V.; Vahlberg, T. Striatal dopamine in Parkinson disease: A meta-analysis of imaging studies. Ann. Neurol. 2017, 82, 873–882. [Google Scholar] [CrossRef]
- Djaldetti, R.; Lorberboym, M.; Karmon, Y.; Treves, T.A.; Ziv, I.; Melamed, E. Residual striatal dopaminergic nerve terminals in very long-standing Parkinson’s disease: A single photon emission computed tomography imaging study. Mov. Disord. 2011, 26, 327–330. [Google Scholar] [CrossRef]
- Greffard, S.; Verny, M.; Bonnet, A.M.; Beinis, J.Y.; Gallinari, C.; Meaume, S.; Piette, F.; Hauw, J.J.; Duyckaerts, C. Motor score of the unified Parkinson disease rating scale as a good predictor of lewy body-associated neuronal loss in the substantia nigra. Arch. Neurol. 2006, 63, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Samii, A.; Sossi, V.; Ruth, T.J.; Schulzer, M.; Holden, J.E.; Wudel, J.; Pal, P.K.; De La Fuente-Fernandez, R.; Calne, D.B.; et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann. Neurol. 2000, 47, 493–503. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, L.; Blesa, J.; Del Rey, N.L.; Monje, M.H.G.; Obeso, J.A.; Cavada, C. Serotonergic innervation of the striatum in a nonhuman primate model of Parkinson’s disease. Neuropharmacology 2020, 170, 107806. [Google Scholar] [CrossRef] [PubMed]
- Kozina, E.A.; Kim, A.R.; Kurina, A.Y.; Ugrumov, M.V. Cooperative synthesis of dopamine by non-dopaminergic neurons as a compensatory mechanism in the striatum of mice with MPTP-induced Parkinsonism. Neurobiol. Dis. 2017, 98, 108–121. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Dileone, M.; del Rey, N.L.G.; Hernandez, L.F.; Obeso, J.A. Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification. Exp. Neurol. 2017, 298, 148–161. [Google Scholar] [CrossRef]
- Adams, J.R.; Van Netten, H.; Schulzer, M.; Mak, E.; Mckenzie, J.; Strongosky, A.; Sossi, V.; Ruth, T.J.; Lee, C.S.; Farrer, M.; et al. PET in LRRK2 mutations: Comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain 2005, 128, 2777–2785. [Google Scholar] [CrossRef]
- Ceravolo, R.; Antonini, A.; Frosini, D.; De Iuliis, A.; Weis, L.; Cecchin, D.; Tosetti, M.; Bonuccelli, U.; Cosottini, M. Nigral anatomy and striatal denervation in genetic Parkinsonism: A family report. Mov. Disord. 2015, 30, 1148–1149. [Google Scholar] [CrossRef]
- De La Fuente-Fernández, R.; Schulzer, M.; Kuramoto, L.; Cragg, J.; Ramachandiran, N.; Au, W.L.; Mak, E.; McKenzie, J.; McCormick, S.; Sossi, V.; et al. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann. Neurol. 2011, 69, 803–810. [Google Scholar] [CrossRef]
- Sossi, V.; De La Fuente-Fernández, R.; Schulzer, M.; Adams, J.; Stoessl, J. Age-related differences in levodopa dynamics in Parkinson’s: Implications for motor complications. Brain 2006, 129, 1050–1058. [Google Scholar] [CrossRef]
- Sossi, V.; De La Fuente-Fernández, R.; Schulzer, M.; Troiano, A.R.; Ruth, T.J.; Stoessl, A.J. Dopamine transporter relation to dopamine turnover in Parkinson’s disease: A positron emission tomography study. Ann. Neurol. 2007, 62, 468–474. [Google Scholar] [CrossRef]
- Hong, J.Y.; Oh, J.S.; Lee, I.; Sunwoo, M.K.; Ham, J.H.; Lee, J.E.; Sohn, Y.H.; Kim, J.S.; Lee, P.H. Presynaptic dopamine depletion predicts levodopa-induced dyskinesia in de novo Parkinson disease. Neurology 2014, 82, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Giannoni, S.; Frosini, D.; Morganti, R.; Volterrani, D.; Bonuccelli, U.; Pavese, N.; Ceravolo, R. Dopamine Transporter, Age, and Motor Complications in Parkinson’s Disease: A Clinical and Single-Photon Emission Computed Tomography Study. Mov. Disord. 2020, 35, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Schulzer, M.; De La Fuente-Fernandez, R.; Mak, E.; Kuramoto, L.; Sossi, V.; Ruth, T.J.; Calne, D.B.; Stoessl, A.J. Lack of regional selectivity during the progression of Parkinson disease: Implications for pathogenesis. Arch. Neurol. 2004, 61, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.J. Molecular imaging of dopamine transporters. Ageing Res. Rev. 2016, 30, 114–121. [Google Scholar] [CrossRef]
- Palermo, G.; Ceravolo, R. Molecular Imaging of the Dopamine Transporter. Cells 2019, 8, 872. [Google Scholar] [CrossRef] [PubMed]
- Colloby, S.J.; McParland, S.; O’Brien, J.T.; Attems, J. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain 2012, 135, 2798–2808. [Google Scholar] [CrossRef]
- Kraemmer, J.; Kovacs, G.G.; Perju-Dumbrava, L.; Pirker, S.; Traub-Weidinger, T.; Pirker, W. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov. Disord. 2014, 29, 1767–1773. [Google Scholar] [CrossRef]
- Karimi, M.; Tian, L.; Brown, C.A.; Flores, H.P.; Loftin, S.K.; Videen, T.O.; Moerlein, S.M.; Perlmutter, J.S. Validation of nigrostriatal positron emission tomography measures: Critical limits. Ann. Neurol. 2013, 73, 390–396. [Google Scholar] [CrossRef]
- Brown, C.A.; Karimi, M.K.; Tian, L.; Flores, H.; Su, Y.; Tabbal, S.D.; Loftin, S.K.; Moerlein, S.M.; Perlmutter, J.S. Validation of midbrain positron emission tomography measures for nigrostriatal neurons in macaques. Ann. Neurol. 2013, 74, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Saari, L.; Kivinen, K.; Gardberg, M.; Joutsa, J.; Noponen, T.; Kaasinen, V. Dopamine transporter imaging does not predict the number of nigral neurons in Parkinson disease. Neurology 2017, 88, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, E.A.; Saari, L.; Orte, K.; Gardberg, M.; Noponen, T.; Joutsa, J.; Kaasinen, V. No link between striatal dopaminergic axons and dopamine transporter imaging in Parkinson’s disease. Mov. Disord. 2019, 34, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, J.S.; Stoessl, A.J. Striatal DAT SPECT: Caveat Emptor! Mov. Disord. 2019, 34, 1430–1432. [Google Scholar] [CrossRef]
- Pifl, C. The time delay between in vivo imaging and postmortem data poses a caveat on “no link” findings. Mov. Disord. 2019, 34, 1579–1580. [Google Scholar] [CrossRef]
- Frosini, D.; Cosottini, M.; Volterrani, D.; Ceravolo, R. Neuroimaging in Parkinson’s disease: Focus on substantia nigra and nigro-striatal projection. Curr. Opin. Neurol. 2017, 30, 416–426. [Google Scholar] [CrossRef]
- Fabbri, M.; Reimão, S.; Carvalho, M.; Nunes, R.G.; Abreu, D.; Guedes, L.C.; Bouça, R.; Lobo, P.P.; Godinho, C.; Coelho, M.; et al. Substantia Nigra Neuromelanin as an Imaging Biomarker of Disease Progression in Parkinson’s Disease. J. Park. Dis. 2017, 7, 491–501. [Google Scholar] [CrossRef]
- Isaias, I.U.; Trujillo, P.; Summers, P.; Marotta, G.; Mainardi, L.; Pezzoli, G.; Zecca, L.; Costa, A. Neuromelanin imaging and dopaminergic loss in parkinson’s disease. Front. Aging Neurosci. 2016, 8, 196. [Google Scholar] [CrossRef]
- Kuya, K.; Shinohara, Y.; Miyoshi, F.; Fujii, S.; Tanabe, Y.; Ogawa, T. Correlation between neuromelanin-sensitive MR imaging and 123I-FP-CIT SPECT in patients with parkinsonism. Neuroradiology 2016, 58, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Martín-Bastida, A.; Lao-Kaim, N.P.; Roussakis, A.A.; Searle, G.E.; Xing, Y.; Gunn, R.N.; Schwarz, S.T.; Barker, R.A.; Auer, D.P.; Piccini, P. Relationship between neuromelanin and dopamine terminals within the Parkinson’s nigrostriatal system. Brain 2019, 142, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Okuzumi, A.; Hatano, T.; Kamagata, K.; Hori, M.; Mori, A.; Oji, Y.; Taniguchi, D.; Daida, K.; Shimo, Y.; Yanagisawa, N.; et al. Neuromelanin or DaT-SPECT: Which is the better marker for discriminating advanced Parkinson’s disease? Eur. J. Neurol. 2019, 26, 1408–1416. [Google Scholar] [CrossRef]
- Tabbal, S.D.; Tian, L.L.; Karimi, M.; Brown, C.A.; Loftin, S.K.; Perlmutter, J.S. Low nigrostriatal reserve for motor parkinsonism in nonhuman primates. Exp. Neurol. 2012, 237, 355–362. [Google Scholar] [CrossRef]
- Fazio, P.; Svenningsson, P.; Cselényi, Z.; Halldin, C.; Farde, L.; Varrone, A. Nigrostriatal dopamine transporter availability in early Parkinson’s disease. Mov. Disord. 2018, 33, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, S.P.; Presotto, L.; Baroncini, D.; Garibotto, V.; Moresco, R.M.; Gianolli, L.; Volonté, M.A.; Antonini, A.; Perani, D. Axonal damage and loss of connectivity in nigrostriatal and mesolimbic dopamine pathways in early Parkinson’s disease. NeuroImage Clin. 2017, 14, 734–740. [Google Scholar] [CrossRef]
- Hsiao, I.T.; Weng, Y.H.; Hsieh, C.J.; Lin, W.Y.; Wey, S.P.; Kung, M.P.; Yen, T.C.; Lu, C.S.; Lin, K.J. Correlation of parkinson disease severity and 18F-DTBZ positron emission tomography. JAMA Neurol. 2014, 71, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Fazio, P.; Svenningsson, P.; Forsberg, A.; Jönsson, E.G.; Amini, N.; Nakao, R.; Nag, S.; Halldin, C.; Farde, L.; Varrone, A. Quantitative analysis of 18F-(E)-N-(3-iodoprop-2-enyl)-2β-carbofluoroethoxy-3β-(4′-methyl-phenyl) nortropane binding to the dopamine transporter in Parkinson disease. J. Nucl. Med. 2015, 56, 714–720. [Google Scholar] [CrossRef]
- Tagliaferro, P.; Burke, R.E. Retrograde Axonal Degeneration in Parkinson Disease. J. Park. Dis. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Luk, K.; Purtell, K.; Burke Nanni, S.; Stoessl, A.J.; Trudeau, L.E.; Yue, Z.; Krainc, D.; Oertel, W.; Obeso, J.A.; et al. Neuronal vulnerability in Parkinson disease: Should the focus be on axons and synaptic terminals? Mov. Disord. 2019, 34, 1406–1422. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, N.; Hauser, R.A.; Grachev, I.D. Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1288–1295. [Google Scholar] [CrossRef]
- Catafau, A.M.; Tolosa, E.; Laloux, P.; Vander Borght, T.; Van Zandijcke, M.; De Geeter, F.; Destee, A.; Steinling, M.; Lacomblez, L.; Habert, M.O.; et al. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain parkinsonian syndromes. Mov. Disord. 2004, 19, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Bega, D.; Kuo, P.H.; Chalkidou, A.; Grzeda, M.T.; Macmillan, T.; Brand, C.; Sheikh, Z.H.; Antonini, A. Clinical utility of DaTscan in patients with suspected Parkinsonian syndrome: A systematic review and meta-analysis. NPJ Park. Dis. 2021, 7. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Ceravolo, R.; Antonini, A.; Volterrani, D.; Rossi, C.; Kiferle, L.; Frosini, D.; Lucetti, C.; Isaias, I.U.; Benti, R.; Murri, L.; et al. Predictive value of nigrostriatal dysfunction in isolated tremor: A clinical and SPECT study. Mov. Disord. 2008, 23, 2049–2054. [Google Scholar] [CrossRef]
- Isaias, I.U.; Canesi, M.; Benti, R.; Gerundini, P.; Cilia, R.; Pezzoli, G.; Antonini, A. Striatal dopamine transporter abnormalities in patients with essential tremor. Nucl. Med. Commun. 2008, 29, 349–353. [Google Scholar] [CrossRef]
- Benamer, H.T.S.; Patterson, J.; Grosset, D.G.; Booij, J.; De Bruin, K.; Van Royen, E.; Speelman, J.D.; Horstink, M.H.I.M.; Sips, H.J.W.A.; Dierckx, R.A.; et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: The [123I]-FP-CIT study group. Mov. Disord. 2000, 15, 503–510. [Google Scholar] [CrossRef]
- Antonini, A.; Berto, P.; Lopatriello, S.; Tamma, F.; Annemans, L.; Chambers, M. Cost-effectiveness of 123I-FP-CIT SPECT in the differential diagnosis of essential tremor and Parkinson’s disease in Italy. Mov. Disord. 2008, 23, 2202–2209. [Google Scholar] [CrossRef]
- Thobois, S.; Prange, S.; Scheiber, C.; Broussolle, E. What a neurologist should know about PET and SPECT functional imaging for parkinsonism: A practical perspective. Park. Relat. Disord. 2019, 59, 93–100. [Google Scholar] [CrossRef]
- Benítez-Rivero, S.; Marín-Oyaga, V.A.; García-Solís, D.; Huertas-Fernández, I.; García-Gomez, F.J.; Jesus, S.; Caceres, M.T.; Carrillo, F.; Ortiz, A.M.; Carballo, M.; et al. Clinical features and 123I-FP-CIT SPECT imaging in vascular parkinsonism and Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2013, 84, 122–129. [Google Scholar] [CrossRef]
- Ma, K.K.Y.; Lin, S.; Mok, V.C.T. Neuroimaging in Vascular Parkinsonism. Curr. Neurol. Neurosci. Rep. 2019, 19. [Google Scholar] [CrossRef]
- Kalra, S.; Grosset, D.G.; Benamer, H.T.S. Differentiating vascular parkinsonism from idiopathic Parkinson’s disease: A systematic review. Mov. Disord. 2010, 25, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Funke, E.; Kupsch, A.; Buchert, R.; Brenner, W.; Plotkin, M. Impact of subcortical white matter lesions on dopamine transporter SPECT. J. Neural Transm. 2013, 120, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Vitale, C.; Barone, P.; Cilia, R.; Righini, A.; Bonuccelli, U.; Abbruzzese, G.; Ramat, S.; Petrone, A.; Quatrale, R.; et al. The relationship between cerebral vascular disease and parkinsonism: The VADO study. Park. Relat. Disord. 2012, 18, 775–780. [Google Scholar] [CrossRef]
- Factor, S.A.; Burkhard, P.R.; Caroff, S.; Friedman, J.H.; Marras, C.; Tinazzi, M.; Comella, C.L. Recent developments in drug-induced movement disorders: A mixed picture. Lancet Neurol. 2019, 18, 880–890. [Google Scholar] [CrossRef]
- Tinazzi, M.; Cipriani, A.; Matinella, A.; Cannas, A.; Solla, P.; Nicoletti, A.; Zappia, M.; Morgante, L.; Morgante, F.; Pacchetti, C.; et al. [123I]FP-CIT single photon emission computed tomography findings in drug-induced Parkinsonism. Schizophr. Res. 2012, 139, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, M.; Morgante, F.; Matinella, A.; Bovi, T.; Cannas, A.; Solla, P.; Marrosu, F.; Nicoletti, A.; Zappia, M.; Luca, A.; et al. Imaging of the dopamine transporter predicts pattern of disease progression and response to levodopa in patients with schizophrenia and parkinsonism: A 2-year follow-up multicenter study. Schizophr. Res. 2014, 152, 344–349. [Google Scholar] [CrossRef]
- Brigo, F.; Erro, R.; Marangi, A.; Bhatia, K.; Tinazzi, M. Differentiating drug-induced parkinsonism from Parkinson’s disease: An update on non-motor symptoms and investigations. Park. Relat. Disord. 2014, 20, 808–814. [Google Scholar] [CrossRef]
- Erro, R.; Bhatia, K.P.; Tinazzi, M. Parkinsonism following neuroleptic exposure: A double-hit hypothesis? Mov. Disord. 2015, 30, 780–785. [Google Scholar] [CrossRef]
- Shuaib, U.A.; Rajput, A.H.; Robinson, C.A.; Rajput, A. Neuroleptic-induced Parkinsonism: Clinicopathological study. Mov. Disord. 2016, 31, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Sunwoo, M.K.; Oh, J.S.; Kim, J.S.; Sohn, Y.H.; Lee, P.H. Persistent drug-induced parkinsonism in patients with normal dopamine transporter imaging. PLoS ONE 2016, 11, e157410. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Yoo, H.S.; Moon, H.; Oh, J.S.; Kim, J.S.; Park, Y.H.; Hong, J.Y.; Ye, B.S.; Sohn, Y.H.; Lee, P.H. Early-onset drug-induced parkinsonism after exposure to offenders implies nigrostriatal dopaminergic dysfunction. J. Neurol. Neurosurg. Psychiatry 2018, 89, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Cho, H.; Kim, Y.J.; Ma, H.I.; Jang, S. Drug-induced Parkinsonism: A strong predictor of idiopathic Parkinson’s disease. PLoS ONE 2021, 16, e247354. [Google Scholar] [CrossRef]
- O’Brien, J.T.; McKeith, I.G.; Walker, Z.; Tatsch, K.; Booij, J.; Darcourt, J.; Marquardt, M.; Reininger, C. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br. J. Psychiatry 2009, 194, 34–39. [Google Scholar] [CrossRef]
- Oliveira, F.P.M.; Walker, Z.; Walker, R.W.H.; Attems, J.; Castanheira, J.C.; Silva, Â.; Oliveira, C.; Vaz, S.; Silva, M.; Costa, D.C. 123I-FP-CIT SPECT in dementia with Lewy bodies, Parkinson’s disease and Alzheimer’s disease: A new quantitative analysis of autopsy confirmed cases. J. Neurol. Neurosurg. Psychiatry 2021, 92, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Mccleery, J.; Morgan, S.; Hyde, C.; Bradley, K.; Ansorge, O. Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst. Rev. 2015, 1, CD010633. [Google Scholar] [CrossRef]
- Saeed, U.; Compagnone, J.; Aviv, R.I.; Strafella, A.P.; Black, S.E.; Lang, A.E.; Masellis, M. Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: Current and emerging concepts. Transl. Neurodegener. 2017, 6, 8. [Google Scholar] [CrossRef]
- Morgan, S.; Kemp, P.; Booij, J.; Costa, D.C.; Padayachee, S.; Lee, L.; Barber, C.; Carter, J.; Walker, Z. Differentiation of frontotemporal dementia from dementia with Lewy bodies using FP-CIT SPECT. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1063–1070. [Google Scholar] [CrossRef]
- Sestini, S.; Alongi, P.; Berti, V.; Calcagni, M.L.; Cecchin, D.; Chiaravalloti, A.; Chincarini, A.; Cistaro, A.; Guerra, U.P.; Pappatà, S.; et al. Correction to: The role of molecular imaging in the frame of the revised dementia with Lewy body criteria (Clinical and Translational Imaging, (2019), 7, 2, (83-98), 10.1007/s40336-019-00321-8). Clin. Transl. Imaging 2019, 7, 231. [Google Scholar] [CrossRef]
- van der Zande, J.J.; Booij, J.; Scheltens, P.; Raijmakers, P.G.H.M.; Lemstra, A.W. [123]FP-CIT SPECT scans initially rated as normal became abnormal over time in patients with probable dementia with Lewy bodies. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1060–1066. [Google Scholar] [CrossRef]
- Cilia, R.; Rossi, C.; Frosini, D.; Volterrani, D.; Siri, C.; Pagni, C.; Benti, R.; Pezzoli, G.; Bonuccelli, U.; Antonini, A.; et al. Dopamine transporter spect imaging in corticobasal syndrome. PLoS ONE 2011, 6, e018301. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, R.; Rossi, C.; Cilia, R.; Tognoni, G.; Antonini, A.; Volterrani, D.; Bonuccelli, U. Evidence of delayed nigrostriatal dysfunction in corticobasal syndrome: A SPECT follow-up study. Park. Relat. Disord. 2013, 19, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Pirker, S.; Perju-Dumbrava, L.; Kovacs, G.G.; Traub-Weidinger, T.; Pirker, W. Progressive dopamine transporter binding loss in autopsy-confirmed Corticobasal Degeneration. J. Park. Dis. 2015, 5, 907–912. [Google Scholar] [CrossRef]
- Rodriguez-Porcel, F.; Jamali, S.; Duker, A.P.; Espay, A.J. Dopamine transporter scanning in the evaluation of patients with suspected Parkinsonism: A case-based user’s guide. Expert Rev. Neurother. 2016, 16, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, S.H.; Fisher, S.; Gupta, F.; Hermanowicz, N.; Kremens, D.E.; Lew, M.F.; Marek, K.; Pahwa, R.; Russell, D.S.; Seibyl, J. Clinical utility of DaTscanTM imaging in the evaluation of patients with parkinsonism: A US perspective. Expert Rev. Neurother. 2017, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ba, F.; Martin, W.R.W. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Park. Relat. Disord. 2015, 21, 87–94. [Google Scholar] [CrossRef]
- Isaacson, J.R.; Brillman, S.; Chhabria, N.; Isaacson, S.H. Impact of DaTscan Imaging on Clinical Decision Making in Clinically Uncertain Parkinson’s Disease. J. Park. Dis. 2021, 11, 885–889. [Google Scholar] [CrossRef]
- Jaakkola, E.; Joutsa, J.; Kaasinen, V. Predictors of normal and abnormal outcome in clinical brain dopamine transporter imaging. J. Neural Transm. 2016, 123, 205–209. [Google Scholar] [CrossRef]
- Perlmutter, J.S.; Norris, S.A. Neuroimaging biomarkers for Parkinson disease: Facts and fantasy. Ann. Neurol. 2014, 76, 769–783. [Google Scholar] [CrossRef]
- Marek, K.L.; Seibyl, J.P.; Zoghbi, S.S.; Zea-Ponce, Y.; Baldwin, R.M.; Fussell, B.; Charney, D.S.; Van Dyck, C.; Hoffer, P.B.; Innis, R.B. [123I]β-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology 1996, 46, 231–237. [Google Scholar] [CrossRef]
- Oakes, D.; Shoulson, I.; Kieburtz, K.; Rudolph, A.; Lang, A.; Olanow, C.W.; Tanner, C.; Marek, K.; Parkinson Study Group. F.S. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004, 9, 2498–2508. [Google Scholar] [CrossRef]
- Marshall, V.L.; Patterson, J.; Hadley, D.M.; Grosset, K.A.; Grosset, D.G. Two-year follow-up in 150 consecutive cases with normal dopamine transporter imaging. Nucl. Med. Commun. 2006, 27, 933–937. [Google Scholar] [CrossRef]
- Schneider, S.A.; Edwards, M.J.; Mir, P.; Cordivari, C.; Hooker, J.; Dickson, J.; Quinn, N.; Bhatia, K.P. Patients with adult-onset dystonic tremor resembling Parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov. Disord. 2007, 22, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.; Seibyl, J.; Eberly, S.; Oakes, D.; Shoulson, I.; Lang, A.E.; Hyson, C.; Jennings, D. Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology 2014, 82, 1791–1797. [Google Scholar] [CrossRef]
- Nicastro, N.; Garibotto, V.; Badoud, S.; Burkhard, P.R. Scan without evidence of dopaminergic deficit: A 10-year retrospective study. Park. Relat. Disord. 2016, 31, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Suwijn, S.R.; Verschuur, C.V.M.; Slim, M.A.; Booij, J.; de Bie, R.M.A. Reliability of visual assessment by non-expert nuclear medicine physicians and appropriateness of indications of [123I]FP-CIT SPECT imaging by neurologists in patients with early drug-naive Parkinson’s disease. EJNMMI Res. 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Sixel-Döring, F.; Liepe, K.; Mollenhauer, B.; Trautmann, E.; Trenkwalder, C. The role of 123I-FP-CIT-SPECT in the differential diagnosis of Parkinson and tremor syndromes: A critical assessment of 125 cases. J. Neurol. 2011, 258, 2147–2154. [Google Scholar] [CrossRef]
- Batla, A.; Erro, R.; Stamelou, M.; Schneider, S.A.; Schwingenschuh, P.; Ganos, C.; Bhatia, K.P. Patients with scans without evidence of dopaminergic deficit: A long-term follow-up study. Mov. Disord. 2014, 29, 1820–1825. [Google Scholar] [CrossRef]
- Menéndez-González, M.; Tavares, F.; Zeidan, N.; Salas-Pacheco, J.M.; Arias-Carrión, O. Diagnoses behind patients with hard-to-classify tremor and normal DaT-SPECT: A clinical follow up study. Front. Aging Neurosci. 2014, 6, 56. [Google Scholar] [CrossRef]
- Erro, R.; Schneider, S.A.; Stamelou, M.; Quinn, N.P.; Bhatia, K.P. What do patients with scans without evidence of dopaminergic deficit (SWEDD) have? New evidence and continuing controversies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Wile, D.J.; Agarwal, P.A.; Schulzer, M.; Mak, E.; Dinelle, K.; Shahinfard, E.; Vafai, N.; Hasegawa, K.; Zhang, J.; McKenzie, J.; et al. Serotonin and dopamine transporter PET changes in the premotor phase of LRRK2 parkinsonism: Cross-sectional studies. Lancet Neurol. 2017, 16, 351–359. [Google Scholar] [CrossRef]
- Erro, R.; Bhatia, K.P. Novel movement disorder society-Parkinson’s disease criteria: What about SWEDD and genetic forms? Mov. Disord. 2016, 31, 431. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, F.S.; Seppi, K.; Djamshidian, A.; Reiter, E.; Nocker, M.; Mair, K.; Göbel, G.; Poewe, W. Nonmotor Symptoms in Subjects Without Evidence of Dopaminergic Deficits. Mov. Disord. 2015, 30, 976–981. [Google Scholar] [CrossRef]
- Taylor, S.; Gafton, J.; Shah, B.; Pagano, G.; Chaudhuri, K.R.; Brooks, D.J.; Pavese, N. Progression of nonmotor symptoms in subgroups of patients with non-dopamine-deficient Parkinsonism. Mov. Disord. 2016, 31, 344–351. [Google Scholar] [CrossRef]
- Wyman-Chick, K.A.; Martin, P.K.; Minár, M.; Schroeder, R.W. Cognition in Patients with a Clinical Diagnosis of Parkinson Disease and Scans Without Evidence of Dopaminergic Deficit (SWEDD): 2-Year Follow-Up. Cogn. Behav. Neurol. 2016, 29, 190–196. [Google Scholar] [CrossRef]
- Jackson, L.; Turcano, P.; Stitt, D.; Coon, E.; Savica, R. Autonomic Testing Profiles in Scans without Evidence of Dopaminergic Deficit (SWEDD). J. Park. Dis. 2020, 10, 945–949. [Google Scholar] [CrossRef]
- Lee, J.W.; Song, Y.S.; Kim, H.; Ku, B.D.; Lee, W.W. Patients with scans without evidence of dopaminergic deficit (SWEDD) do not have early Parkinson’s disease: Analysis of the PPMI data. PLoS ONE 2021, 16, e246881. [Google Scholar] [CrossRef]
- Oh, Y.S.; Choi, J.H.; Kwon, D.Y. Classification of Scans Without Evidence of Dopamine Deficit (SWEDD) According to the Olfactory Function. J. Park. Dis. 2016, 6, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Silveira-Moriyama, L.; Birchall, J.; Bain, P.; Lees, A.J.; Bajaj, N.P.S. Hyposmia in SWEDD. Mov. Disord. 2015, 30, 1436–1437. [Google Scholar] [CrossRef]
- Conrado, D.J.; Nicholas, T.; Tsai, K.; Macha, S.; Sinha, V.; Stone, J.; Corrigan, B.; Bani, M.; Muglia, P.; Watson, I.A.; et al. Dopamine Transporter Neuroimaging as an Enrichment Biomarker in Early Parkinson’s Disease Clinical Trials: A Disease Progression Modeling Analysis. Clin. Transl. Sci. 2018, 11, 63–70. [Google Scholar] [CrossRef]
- Massa, J.; Chahine, L.M. Revision of Diagnosis in Early Parkinsonism with Abnormal Dopamine Transporter Imaging. J. Park. Dis. 2019, 9, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Romero, K.; Conrado, D.; Burton, J.; Nicholas, T.; Sinha, V.; Macha, S.; Ahamadi, M.; Cedarbaum, J.; Seibyl, J.; Marek, K.; et al. Molecular Neuroimaging of the Dopamine Transporter as a Patient Enrichment Biomarker for Clinical Trials for Early Parkinson’s Disease. Clin. Transl. Sci. 2019, 12, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, J.M.; Lees, A.J. Ageing and parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Stiasny-Kolster, K.; Doerr, Y.; Möller, J.C.; Höffken, H.; Behr, T.M.; Oertel, W.H.; Mayer, G. Combination of “idiopathic” REM sleep behaviour disorder and olfactory dysfunction as possible indicator for α-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 2005, 128, 126–137. [Google Scholar] [CrossRef]
- Bonifati, V. Genetics of Parkinson’s disease—State of the art, 2013. Park. Relat. Disord. 2014, 20, S23–S28. [Google Scholar] [CrossRef]
- Stoessl, A.J. Positron emission tomography in premotor Parkinson’s disease. Park. Relat. Disord. 2007, 13, 421–424. [Google Scholar] [CrossRef]
- Iranzo, A.; Tolosa, E.; Gelpi, E.; Molinuevo, J.L.; Valldeoriola, F.; Serradell, M.; Sanchez-Valle, R.; Vilaseca, I.; Lomeña, F.; Vilas, D.; et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol. 2013, 12, 443–453. [Google Scholar] [CrossRef]
- Bauckneht, M.; Chincarini, A.; De Carli, F.; Terzaghi, M.; Morbelli, S.; Nobili, F.; Arnaldi, D. Presynaptic dopaminergic neuroimaging in REM sleep behavior disorder: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 41, 266–274. [Google Scholar] [CrossRef]
- Iranzo, A.; Lomeña, F.; Stockner, H.; Valldeoriola, F.; Vilaseca, I.; Salamero, M.; Molinuevo, J.L.; Serradell, M.; Duch, J.; Pavía, J.; et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: A prospective study. Lancet Neurol. 2010, 9, 1070–1077. [Google Scholar] [CrossRef]
- Iranzo, A.; Valldeoriola, F.; Lomeña, F.; Molinuevo, J.L.; Serradell, M.; Salamero, M.; Cot, A.; Ros, D.; Pavía, J.; Santamaria, J.; et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: A prospective study. Lancet Neurol. 2011, 10, 797–805. [Google Scholar] [CrossRef]
- Iranzo, A.; Santamaría, J.; Valldeoriola, F.; Serradell, M.; Salamero, M.; Gaig, C.; Niñerola-Baizán, A.; Sánchez-Valle, R.; Lladó, A.; De Marzi, R.; et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann. Neurol. 2017, 82, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lao-Kaim, N.P.; Roussakis, A.A.; Martín-Bastida, A.; Valle-Guzman, N.; Paul, G.; Loane, C.; Widner, H.; Politis, M.; Foltynie, T.; et al. 11 C-PE2I and 18 F-Dopa PET for assessing progression rate in Parkinson’s: A longitudinal study. Mov. Disord. 2018, 33, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Chahine, L.M.; Brumm, M.C.; Caspell-Garcia, C.; Oertel, W.; Mollenhauer, B.; Amara, A.; Fernandez-Arcos, A.; Tolosa, E.; Simonet, C.; Hogl, B.; et al. Dopamine transporter imaging predicts clinically-defined α-synucleinopathy in REM sleep behavior disorder. Ann. Clin. Transl. Neurol. 2021, 8, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Arnaldi, D.; Chincarini, A.; Hu, M.T.; Sonka, K.; Boeve, B.; Miyamoto, T.; Puligheddu, M.; De Cock, V.C.; Terzaghi, M.; Plazzi, G.; et al. Dopaminergic imaging and clinical predictors for phenoconversion of REM sleep behaviour disorder. Brain 2021, 144, 278–287. [Google Scholar] [CrossRef]
- Berendse, H.W.; Booij, J.; Francot, C.M.J.E.; Bergmans, P.L.M.; Hijman, R.; Stoof, J.C.; Wolters, E.C. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann. Neurol. 2001, 50, 34–41. [Google Scholar] [CrossRef]
- Ponsen, M.M.; Stoffers, D.; Booij, J.; Van Eck-Smit, B.L.F.; Wolters, E.C.; Berendse, H.W. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann. Neurol. 2004, 56, 173–181. [Google Scholar] [CrossRef]
- Jennings, D.; Siderowf, A.; Stern, M.; Seibyl, J.; Eberly, S.; Oakes, D.; Marek, K. Imaging prodromal Parkinson disease: The Parkinson associated risk syndrome study. Neurology 2014, 83, 1739–1746. [Google Scholar] [CrossRef]
- Jennings, D.; Siderowf, A.; Stern, M.; Seibyl, J.; Eberly, S.; Oakes, D.; Marek, K. Conversion to Parkinson disease in the PARS hyposmic and dopamine transporter-deficit prodromal cohort. JAMA Neurol. 2017, 74, 933–940. [Google Scholar] [CrossRef]
- Siderowf, A.; Jennings, D.; Stern, M.; Seibyl, J.; Eberly, S.; Oakes, D.; Marek, K.; Jennings, D.; Marek, K.; Seibyl, J.; et al. Clinical and Imaging Progression in the PARS Cohort: Long-Term Follow-up. Mov. Disord. 2020, 35, 1550–1557. [Google Scholar] [CrossRef]
- Noyce, A.J.; Dickson, J.; Rees, R.N.; Bestwick, J.P.; Isaias, I.U.; Politis, M.; Giovannoni, G.; Warner, T.T.; Lees, A.J.; Schrag, A. Dopamine reuptake transporter–single-photon emission computed tomography and transcranial sonography as imaging markers of prediagnostic Parkinson’s disease. Mov. Disord. 2018, 33, 478–482. [Google Scholar] [CrossRef]
- Chung, S.J.; Yoo, H.S.; Lee, Y.H.; Sohn, Y.H.; Ye, B.S.; Cha, J.; Lee, P.H. Minimal parkinsonism in the elderly is associated with striatal dopamine loss and pontine structural damage. Park. Relat. Disord. 2020, 81, 140–143. [Google Scholar] [CrossRef]
- Piccini, P.; Burn, D.J.; Ceravolo, R.; Maraganore, D.; Brooks, D.J. The role of inheritance in sporadic Parkinson’s disease: Evidence from a longitudinal study of dopaminergic function in twins. Ann. Neurol. 1999, 45, 577–582. [Google Scholar] [CrossRef]
- Varrone, A.; Pellecchia, M.T. SPECT Molecular Imaging in Familial Parkinson’s Disease, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 142, ISBN 9780128151419. [Google Scholar]
- Ricciardi, L.; Petrucci, S.; Di Giuda, D.; Serra, L.; Spanò, B.; Sensi, M.; Ginevrino, M.; Cocciolillo, F.; Bozzali, M.; Valente, E.M.; et al. The Contursi Family 20 Years Later: Intrafamilial Phenotypic Variability of the SNCA p.A53T Mutation. Mov. Disord. 2016, 31, 257–258. [Google Scholar] [CrossRef]
- Sierra, M.; Sánchez-Juan, P.; Martínez-Rodríguez, M.I.; González-Aramburu, I.; García-Gorostiaga, I.; Quirce, M.R.; Infante, J.C. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson disease. Neurology 2013, 80, 621–626. [Google Scholar] [CrossRef]
- Vilas, D.; Ispierto, L.; Álvarez, R.; Pont-Sunyer, C.; Martí, M.J.; Valldeoriola, F.; Compta, Y.; de Fabregues, O.; Hernández-Vara, J.; Puente, V.; et al. Clinical and imaging markers in premotor LRRK2 G2019S mutation carriers. Park. Relat. Disord. 2015, 21, 1170–1176. [Google Scholar] [CrossRef]
- Artzi, M.; Even-Sapir, E.; Shacham, H.L.; Thaler, A.; Urterger, A.O.; Bressman, S.; Marder, K.; Hendler, T.; Giladi, N.; Bashat, D.B.; et al. DaT-SPECT assessment depicts Dopamine depletion among asymptomatic G2019S LRRK2 mutation carriers. PLoS ONE 2017, 12, e175424. [Google Scholar] [CrossRef] [PubMed]
- Bergareche, A.; Rodríguez-Oroz, M.C.; Estanga, A.; Gorostidi, A.; López de Munain, A.; Castillo-Triviño, T.; Ruiz-Martínez, J.; Mondragón, E.; Gaig, C.; Lomeña, F.; et al. DAT imaging and clinical biomarkers in relatives at genetic risk for LRRK2R1441G Parkinson’s disease. Mov. Disord. 2016, 31, 335–343. [Google Scholar] [CrossRef]
- Sierra, M.; González-Aramburu, I.; Sánchez-Juan, P.; Sánchez-Quintana, C.; Polo, J.M.; Berciano, J.; Combarros, O.; Infante, J. High frequency and reduced penetrance of lRRK2 g2019S mutation among Parkinson’s disease patients in Cantabria (Spain). Mov. Disord. 2011, 26, 2343–2346. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.; Martínez-Rodríguez, I.; Sánchez-Juan, P.; González-Aramburu, I.; Jiménez-Alonso, M.; Sánchez-Rodríguez, A.; Berciano, J.; Banzo, I.; Infante, J. Prospective clinical and DaT-SPECT imaging in premotor LRRK2 G2019S-Associated Parkinson disease. Neurology 2017, 89, 439–444. [Google Scholar] [CrossRef]
- Simuni, T.; Uribe, L.; Cho, H.R.; Caspell-Garcia, C.; Coffey, C.S.; Siderowf, A.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; Singleton, A.; et al. Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson’s Progression Markers Initiative (PPMI): A cross-sectional study. Lancet Neurol. 2020, 19, 71–80. [Google Scholar] [CrossRef]
- Simuni, T.; Brumm, M.C.; Uribe, L.; Caspell-Garcia, C.; Coffey, C.S.; Siderowf, A.; Alcalay, R.N.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; et al. Clinical and Dopamine Transporter Imaging Characteristics of Leucine Rich Repeat Kinase 2 (LRRK2) and Glucosylceramidase Beta (GBA) Parkinson’s Disease Participants in the Parkinson’s Progression Markers Initiative: A Cross-Sectional Study. Mov. Disord. 2020, 35, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Albin, R.L. Increased striatal dopamine in carriers of GBA mutations: Compensation or epiphenomenon? Lancet Neurol. 2020, 19, 27–29. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liu, F.T.; Zuo, C.T.; Koprich, J.B.; Wang, J. Update on Molecular Imaging in Parkinson’s Disease. Neurosci. Bull. 2018, 34, 330–340. [Google Scholar] [CrossRef]
- Nissen, T.; Malek, N.; Grosset, K.A.; Newman, E.J.; Patterson, J.; Hadley, D.; Grosset, D.G. Baseline [123I]FP-CIT SPECT (DaTSCAN) severity correlates with medication use at 3 years in Parkinson’s disease. Acta Neurol. Scand. 2014, 129, 204–208. [Google Scholar] [CrossRef]
- Tissingh, G.; Bergmans, P.; Booij, J.; Winogrodzka, A.; Van Royen, E.A.; Stoof, J.C.; Wolters, E.C. Drug-naive patients with Parkinson’s disease in Hoehn and Yahr stages I and II show a bilateral decrease in striatal dopamine transporters as revealed by [123I]β-CIT SPECT. J. Neurol. 1998, 245, 14–20. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, C.T.; Jiang, Y.P.; Guan, Y.H.; Chen, Z.P.; Xiang, J.D.; Yang, L.Q.; Ding, Z.T.; Wu, J.J.; Su, H.L. 18F-FP-CIT PET imaging and SPM analysis of dopamine transporters in Parkinson’s disease in various Hoehn & Yahr stages. J. Neurol. 2007, 254, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.; Hellwig, D.; Samnick, S.; Jost, W.; Möllers, M.O.; Fassbender, K.; Kirsch, C.M.; Dillmann, U. Striatal FP-CIT uptake differs in the subtypes of early Parkinson’s disease. J. Neural Transm. 2007, 114, 331–335. [Google Scholar] [CrossRef]
- Rossi, C.; Frosini, D.; Volterrani, D.; De Feo, P.; Unti, E.; Nicoletti, V.; Kiferle, L.; Bonuccelli, U.; Ceravolo, R. Differences in nigro-striatal impairment in clinical variants of early Parkinson’s disease: Evidence from a FP-CIT SPECT study. Eur. J. Neurol. 2010, 17, 626–630. [Google Scholar] [CrossRef]
- Moccia, M.; Pappatà, S.; Picillo, M.; Erro, R.; Coda, A.R.D.; Longo, K.; Vitale, C.; Amboni, M.; Brunetti, A.; Capo, G.; et al. Dopamine transporter availability in motor subtypes of de novo drug-naïve Parkinson’s disease. J. Neurol. 2014, 261, 2112–2118. [Google Scholar] [CrossRef]
- Jellinger, K.A. Neuropathology of sporadic Parkinson’s disease: Evaluation and changes of concepts. Mov. Disord. 2012, 27, 8–30. [Google Scholar] [CrossRef]
- Eggers, C.; Kahraman, D.; Fink, G.R.; Schmidt, M.; Timmermann, L. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT Single photon emission computed tomography. Mov. Disord. 2011, 26, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Helmich, R.C. The cerebral basis of Parkinsonian tremor: A network perspective. Mov. Disord. 2018, 33, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Qamhawi, Z.; Towey, D.; Shah, B.; Pagano, G.; Seibyl, J.; Marek, K.; Borghammer, P.; Brooks, D.J.; Pavese, N. Clinical correlates of raphe serotonergic dysfunction in early Parkinson’s disease. Brain 2015, 138, 2964–2973. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Müller, M.L.T.M.; Koeppe, R.A.; Studenski, S.A.; Kilbourn, M.A.; Frey, K.A.; Albin, R.L. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 2009, 73, 1670–1676. [Google Scholar] [CrossRef]
- Kim, R.; Lee, J.; Kim, Y.; Kim, A.; Jang, M.; Kim, H.J.; Jeon, B.; Kang, U.J.; Fahn, S. Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson’s disease: An analysis of the PPMI cohort. Park. Relat. Disord. 2018, 51, 49–54. [Google Scholar] [CrossRef]
- Kim, R.; Lee, J.; Kim, H.J.; Kim, A.; Jang, M.; Jeon, B.; Kang, U.J. CSF β-amyloid42 and risk of freezing of gait in early Parkinson disease. Neurology 2019, 92, E40–E47. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, E.; Joutsa, J.; Jaakkola, E.; Noponen, T.; Johansson, J.; Pitkonen, M.; Levo, R.; Mertsalmi, T.; Scheperjans, F.; Kaasinen, V. Individual parkinsonian motor signs and striatal dopamine transporter deficiency: A study with [I-123]FP-CIT SPECT. J. Neurol. 2019, 266, 826–834. [Google Scholar] [CrossRef]
- Djaldetti, R.; Rigbi, A.; Greenbaum, L.; Reiner, J.; Lorberboym, M. Can early dopamine transporter imaging serve as a predictor of Parkinson’s disease progression and late motor complications? J. Neurol. Sci. 2018, 390, 255–260. [Google Scholar] [CrossRef]
- Latourelle, J.C.; Beste, M.T.; Hadzi, T.C.; Miller, R.E.; Oppenheim, J.N.; Valko, M.P.; Wuest, D.M.; Church, B.W.; Khalil, I.G.; Hayete, B.; et al. Large-scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson’s disease: A longitudinal cohort study and validation. Lancet Neurol. 2017, 16, 908–916. [Google Scholar] [CrossRef]
- Vogt, T.; Kramer, K.; Gartenschlaeger, M.; Schreckenberger, M. Estimation of further disease progression of Parkinson’s disease by dopamin transporter scan vs. clinical rating. Park. Relat. Disord. 2011, 17, 459–463. [Google Scholar] [CrossRef]
- Whone, A.; Luz, M.; Boca, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 2019, 142, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Goetz, C.G.; Chu, Y.; Halliday, G.M.; Nicholson, D.A.; Musial, T.F.; Marmion, D.J.; Stoessl, A.J.; Sossi, V.; Freeman, T.B.; et al. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann. Neurol. 2017, 81, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Salimpour, Y.; Jain, S.; Blinder, S.A.L.; Klyuzhin, I.S.; Smith, G.S.; Mari, Z.; Sossi, V. Application of texture analysis to DAT SPECT imaging: Relationship to clinical assessments. NeuroImage Clin. 2016, 12, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, E.; Joutsa, J.; Vahlberg, T.; Kaasinen, V. Survival in Parkinson’s disease in relation to striatal dopamine transporter binding. Park. Relat. Disord. 2017, 42, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Grossman, M.; Weintraub, D.; Hurtig, H.I.; Duda, J.E.; Xie, S.X.; Lee, E.B.; Van Deerlin, V.M.; Lopez, O.L.; Kofler, J.K.; et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol. 2017, 16, 55. [Google Scholar] [CrossRef]
- Pagano, G.; Niccolini, F.; Wilson, H.; Yousaf, T.; Khan, N.L.; Martino, D.; Plisson, C.; Gunn, R.N.; Rabiner, E.A.; Piccini, P.; et al. Comparison of phosphodiesterase 10A and dopamine transporter levels as markers of disease burden in early Parkinson’s disease. Mov. Disord. 2019, 34, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Betrouni, N.; Moreau, C.; Rolland, A.S.; Carrière, N.; Chupin, M.; Kuchcinski, G.; Lopes, R.; Viard, R.; Defebvre, L.; Devos, D. Texture-based markers from structural imaging correlate with motor handicap in Parkinson’s disease. Sci. Rep. 2021, 11, 2724. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Huang, P.; Shenkov, N.; Fotouhi, S.; Davoodi-Bojd, E.; Lu, L.; Mari, Z.; Soltanian-Zadeh, H.; Sossi, V. Improved prediction of outcome in Parkinson’s disease using radiomics analysis of longitudinal DAT SPECT images. NeuroImage Clin. 2017, 16, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Chung, S.J.; Chung, S.J.; Moon, H.; Oh, J.S.; Kim, J.S.; Hong, J.Y.; Ye, B.S.; Sohn, Y.H.; Lee, P.H. Presynaptic dopamine depletion determines the timing of levodopa-induced dyskinesia onset in Parkinson’s disease. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 423–431. [Google Scholar] [CrossRef]
- Jeong, E.H.; Sunwoo, M.K.; Song, Y.S. Serial I-123-FP-CIT SPECT Image Findings of Parkinson’s Disease Patients With Levodopa-Induced Dyskinesia. Front. Neurol. 2018, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Lee, Y.; Oh, J.S.; Kim, J.S.; Lee, P.H.; Sohn, Y.H. Putaminal dopamine depletion in de novo Parkinson’s disease predicts future development of wearing-off. Park. Relat. Disord. 2018, 53, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Roussakis, A.-A.; Gennaro, M.; Lao-Kaim, N.P.; Towey, D.; Piccini, P. Dopamine Transporter Density in de novo Parkinson’s Disease Does Not Relate to the Development of Levodopa-Induced Dyskinesias. J. Neuroinflamm. Neurodegener. Dis. 2019, 3, 10000. [Google Scholar] [PubMed]
- Chung, S.J.; Lee, H.S.; Yoo, H.S.; Lee, Y.H.; Lee, P.H.; Sohn, Y.H. Patterns of striatal dopamine depletion in early Parkinson disease: Prognostic relevance. Neurology 2020, 95, E280–E290. [Google Scholar] [CrossRef]
- Pak, K.; Kim, H.; Seok, J.W.; Lee, M.J.; Shin, S.; Kim, K.; Lee, J.M.; Seo, Y.; Kim, B.S.; Jun, S.; et al. Prediction of future weight change with dopamine transporter in patients with Parkinson’s disease. J. Neural Transm. 2019, 126, 723–729. [Google Scholar] [CrossRef]
- La Torre, G.; Herman, A.M.; Jessop, M.; Abdula, N.; Crawshaw, A.; Begley, P.; Wroe, E.; Saha, R.A.; Duka, T.; Dizdarevic, S. 123I-Ioflupane dopamine transporter imaging (DaTSCAN) appearances in relation to emotional responsiveness, impulsivity and olfaction in suspected Parkinsonian syndrome. Nucl. Med. Commun. 2020, 41, 1117–1127. [Google Scholar] [CrossRef]
- Hinkle, J.T.; Perepezko, K.; Mills, K.A.; Mari, Z.; Butala, A.; Dawson, T.M.; Pantelyat, A.; Rosenthal, L.S.; Pontone, G.M.; Program, S.T.; et al. Dopamine transporter availability reflects gastrointestinal dysautonomia in early Parkinson disease. Park. Relat. Disord. 2018, 55, 8–14. [Google Scholar] [CrossRef]
- Jaakkola, E.; Joutsa, J.; Mäkinen, E.; Johansson, J.; Kaasinen, V. Ventral striatal dopaminergic defect is associated with hallucinations in Parkinson’s disease. Eur. J. Neurol. 2017, 24, 1341–1347. [Google Scholar] [CrossRef]
- Arnaldi, D.; De Carli, F.; Famà, F.; Brugnolo, A.; Girtler, N.; Picco, A.; Pardini, M.; Accardo, J.; Proietti, L.; Massa, F.; et al. Prediction of cognitive worsening in de novo Parkinson’s disease: Clinical use of biomarkers. Mov. Disord. 2017, 32, 1738–1747. [Google Scholar] [CrossRef]
- Lohle, M.; Mende, J.; Wolz, M.; Beuthien-Baumann, B.; Oehme, L.; Van Den Hoff, J.; Kotzerke, J.; Reichmann, H.; Storch, A. Putaminal dopamine turnover predicts levodopa-induced motor complications in de novo parkinson’s disease (I3-1A). Neurology 2015, 84, 14. [Google Scholar]
- De La Fuente-Fernández, R.; Sossi, V.; McCormick, S.; Schulzer, M.; Ruth, T.J.; Stoessl, A.J. Visualizing vesicular dopamine dynamics in Parkinson’s disease. Synapse 2009, 63, 713–716. [Google Scholar] [CrossRef]
- Troiano, A.R.; De La Fuente-Fernandez, R.; Sossi, V.; Schulzer, M.; Mak, E.; Ruth, T.J.; Stoessl, A.J. PET demonstrates reduced dopamine transporter expression in PD with dyskinesias. Neurology 2009, 72, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Roussakis, A.; Towey, D.; Gennaro, M.; Lao–Kaim, N.P.; Piccini, P. Parkinson’s Disease Dyskinesias Possibly Relate to Greater Dopamine Transporter Losses in the Putamen Over Time. J. Neurol. Exp. Neurosci. 2019, 5, 6–11. [Google Scholar] [CrossRef]
- Angela Cenci, M. Presynaptic mechanisms of L-DOPA-induced dyskinesia: The findings, the debate, the therapeutic implications. Front. Neurol. 2014, 5, 242. [Google Scholar] [CrossRef]
- Lee, J.Y.; Seo, S.; Lee, J.S.; Kim, H.J.; Kim, Y.K.; Jeon, B.S. Putaminal serotonergic innervation. Neurology 2015, 85, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, J.; Küsters, S.; Al-Momani, E.; Marotta, G.; Cosgrove, K.P.; van Dyck, C.H.; Herrmann, K.; Homola, G.A.; Pezzoli, G.; Buck, A.K.; et al. Cholinergic activity and levodopa-induced dyskinesia: A multitracer molecular imaging study. Ann. Clin. Transl. Neurol. 2017, 4, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Niccolini, F.; Politis, M. The serotonergic system in Parkinson’s patients with dyskinesia: Evidence from imaging studies. J. Neural Transm. 2018, 125, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Santangelo, G.; Erro, R.; Cozzolino, A.; Amboni, M.; Vitale, C.; Barone, P.; Pellecchia, M.T. Association between dopaminergic dysfunction and anxiety in de novo Parkinson’s disease. Park. Relat. Disord. 2017, 37, 106–110. [Google Scholar] [CrossRef]
- Hesse, S.; Meyer, P.M.; Strecker, K.; Barthel, H.; Wegner, F.; Oehlwein, C.; Isaias, I.U.; Schwarz, J.; Sabri, O. Monoamine transporter availability in Parkinson’s disease patients with or without depression. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 428–435. [Google Scholar] [CrossRef]
- Vriend, C.; Raijmakers, P.; Veltman, D.J.; Van Dijk, K.D.; Van Der Werf, Y.D.; Foncke, E.M.J.; Smit, J.H.; Berendse, H.W.; Van Den Heuvel, O.A. Depressive symptoms in Parkinson’s disease are related to reduced [123I]FP-CIT binding in the caudate nucleus. J. Neurol. Neurosurg. Psychiatry 2014, 85, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.W.; Oh, Y.S.; Hwang, E.J.; Ryu, D.W.; Lee, K.S.; Lyoo, C.H.; Kim, J.S. “Depressed” caudate and ventral striatum dopamine transporter availability in de novo Depressed Parkinson’s disease. Neurobiol. Dis. 2019, 132, 104563. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Vitale, C.; Picillo, M.; Cuoco, S.; Moccia, M.; Pezzella, D.; Erro, R.; Longo, K.; Vicidomini, C.; Pellecchia, M.T.; et al. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Park. Relat. Disord. 2015, 21, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Cilia, R.; Ko, J.H.; Cho, S.S.; van Eimeren, T.; Marotta, G.; Pellecchia, G.; Pezzoli, G.; Antonini, A.; Strafella, A.P. Reduced dopamine transporter density in the ventral striatum of patients with Parkinson’s disease and pathological gambling. Neurobiol. Dis. 2010, 39, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Siderowf, A.; Newberg, A.; Chou, K.L.; Lloyd, M.; Colcher, A.; Hurtig, H.I.; Stern, M.B.; Doty, R.L.; Mozley, P.D.; Wintering, N.; et al. [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology 2005, 64, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Berendse, H.W.; Roos, D.S.; Raijmakers, P.; Doty, R.L. Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J. Neurol. Sci. 2011, 310, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Deeb, J.; Shah, M.; Muhammed, N.; Gunasekera, R.; Gannon, K.; Findley, L.J.; Hawkes, C.H. A basic smell test is as sensitive as a dopamine transporter scan: Comparison of olfaction, taste and DaTSCAN in the diagnosis of Parkinson’s disease. QJM 2010, 103, 941–952. [Google Scholar] [CrossRef]
- Roos, D.S.; Twisk, J.W.R.; Raijmakers, P.G.H.M.; Doty, R.L.; Berendse, H.W. Hyposmia as a marker of (non-)motor disease severity in Parkinson’s disease. J. Neural Transm. 2019, 126, 1471–1478. [Google Scholar] [CrossRef]
- Sakakibara, R.; Shinotoh, H.; Uchiyama, T.; Yoshiyama, M.; Hattori, T.; Yamanishi, T. SPECT imaging of the dopamine transporter with [123I]-β-CIT reveals marked decline of nigrostriatal dopaminergic function in Parkinson’s disease with urinary dysfunction. J. Neurol. Sci. 2001, 187, 55–59. [Google Scholar] [CrossRef]
- Pagano, G.; Niccolini, F.; Yousaf, T.; Wilson, H.; Polychronis, S.; Chaudhuri, K.R.; Politis, M. Urinary dysfunction in early de novo patients with Parkinson’s disease. Mov. Disord. 2017, 32, 939–940. [Google Scholar] [CrossRef]
- van Deursen, D.N.; van den Heuvel, O.A.; Booij, J.; Berendse, H.W.; Vriend, C. Autonomic failure in Parkinson’s disease is associated with striatal dopamine deficiencies. J. Neurol. 2020, 267, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Mito, Y.; Yabe, I.; Yaguchi, H.; Takei, T.; Terae, S.; Tajima, Y. Relation of overactive bladder with motor symptoms and dopamine transporter imaging in drug-naïve Parkinson’s disease. Park. Relat. Disord. 2018, 50, 37–41. [Google Scholar] [CrossRef]
- Kim, R.; Jun, J.S. Association of autonomic symptoms with presynaptic striatal dopamine depletion in drug-naive Parkinson’s disease: An analysis of the PPMI data. Auton. Neurosci. Basic Clin. 2019, 216, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, R.; Huang, T.; Liu, C.; Fan, Y. Urinary dysfunction is associated with nigrostriatal dopaminergic degeneration in early and untreated patients with Parkinson’s disease. Park. Dis. 2020, 2020, 4981647. [Google Scholar] [CrossRef]
- Yousaf, T.; Pagano, G.; Niccolini, F.; Politis, M. Excessive daytime sleepiness may be associated with caudate denervation in Parkinson disease. J. Neurol. Sci. 2018, 387, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Oh, J.S.; Ham, J.H.; Lee, D.H.; Lee, I.; Sohn, Y.H.; Kim, J.S.; Lee, P.H. Association of body mass index and the depletion of nigrostriatal dopamine in Parkinson’s disease. Neurobiol. Aging 2016, 38, 197–204. [Google Scholar] [CrossRef]
- Qamar, M.A.; Sauerbier, A.; Politis, M.; Carr, H.; Loehrer, P.; Chaudhuri, K.R. Presynaptic dopaminergic terminal imaging & non-motor symptoms assessment of Parkinson’s disease: Evidence for dopaminergic basis? Park. Dis. 2017, 3, 5. [Google Scholar] [CrossRef]
- Liu, R.; Umbach, D.M.; Tröster, A.I.; Huang, X.; Chen, H. Non-motor symptoms and striatal dopamine transporter binding in early Parkinson’s disease. Park. Relat. Disord. 2020, 72, 23–30. [Google Scholar] [CrossRef]
- Chung, S.J.; Lee, S.; Yoo, H.S.; Lee, Y.H.; Lee, H.S.; Choi, Y.; Lee, P.H.; Yun, M.; Sohn, Y.H. Association of the Non-Motor Burden with Patterns of Striatal Dopamine Loss in de novo Parkinson’s Disease. J. Park. Dis. 2020, 10, 1541–1549. [Google Scholar] [CrossRef]
- Schifitto, G.; Friedman, J.H.; Oakes, D.; Shulman, L.; Comella, C.L.; Marek, K.; Fahn, S. Fatigue in levodopa-naïve subjects with Parkinson disease. Neurology 2008, 71, 481–485. [Google Scholar] [CrossRef]
- Moriyama, T.S.; Felicio, A.C.; Chagas, M.H.N.; Tardelli, V.S.; Ferraz, H.B.; Tumas, V.; Amaro-Junior, E.; Andrade, L.A.F.; Crippa, J.A.; Bressan, R.A. Increased dopamine transporter density in Parkinson’s disease patients with social anxiety disorder. J. Neurol. Sci. 2011, 310, 53–57. [Google Scholar] [CrossRef]
- Ceravolo, R.; Frosini, D.; Poletti, M.; Kiferle, L.; Pagni, C.; Mazzucchi, S.; Volterrani, D.; Bonuccelli, U. Mild affective symptoms in de novo Parkinson’s disease patients: Relationship with dopaminergic dysfunction. Eur. J. Neurol. 2013, 20, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Lee, J.J.; Ham, J.H.; Lee, P.H.; Sohn, Y.H. Apathy and striatal dopamine defects in non-demented patients with Parkinson’s disease. Park. Relat. Disord. 2016, 23, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Lee, J.J.; Ham, J.H.; Ye, B.S.; Lee, P.H.; Sohn, Y.H. Striatal dopamine depletion patterns and early non-motor burden in Parkinsons disease. PLoS ONE 2016, 11, e161316. [Google Scholar] [CrossRef] [PubMed]
- Moccia, M.; Erro, R.; Picillo, M.; Santangelo, G.; Spina, E.; Allocca, R.; Longo, K.; Amboni, M.; Palladino, R.; Assante, R.; et al. A four-year longitudinal study on restless legs syndrome in Parkinson disease. Sleep 2016, 39, 405–412. [Google Scholar] [CrossRef]
- Jaakkola, E.; Joutsa, J.; Mäkinen, E.; Noponen, T.; Pitkonen, M.; Levo, R.; Mertsalmi, T.; Scheperjans, F.; Kaasinen, V. Burden of non-motor symptoms in unclear parkinsonism and tremor: A study with [123I]FP-CIT SPECT. J. Neurol. Sci. 2019, 404, 124–127. [Google Scholar] [CrossRef]
- Ye, B.S.; Jeon, S.; Yoon, S.; Kang, S.W.; Baik, K.W.; Lee, Y.; Chung, S.J.; Oh, J.S.; Moon, H.; Kim, J.S.; et al. Effects of dopaminergic depletion and brain atrophy on neuropsychiatric symptoms in de novo Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2018, 89, 197–204. [Google Scholar] [CrossRef]
- Nobili, F.; Campus, C.; Arnaldi, D.; De Carli, F.; Cabassi, G.; Brugnolo, A.; Dessi, B.; Morbelli, S.; Sambuceti, G.; Abbruzzese, G.; et al. Cognitive-nigrostriatal relationships in de novo, drug-naïve Parkinson’s disease patients: A [I-123]FP-CIT SPECT Study. Mov. Disord. 2010, 25, 35–43. [Google Scholar] [CrossRef]
- Siepel, F.J.; Brønnick, K.S.; Booij, J.; Ravina, B.M.; Lebedev, A.V.; Pereira, J.B.; Grüner, R.; Aarsland, D. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson’s disease. Mov. Disord. 2014, 29, 1802–1808. [Google Scholar] [CrossRef]
- Pellecchia, M.T.; Picillo, M.; Santangelo, G.; Longo, K.; Moccia, M.; Erro, R.; Amboni, M.; Vitale, C.; Vicidomini, C.; Salvatore, M.; et al. Cognitive performances and DAT imaging in early Parkinson’s disease with mild cognitive impairment: A preliminary study. Acta Neurol. Scand. 2015, 131, 275–281. [Google Scholar] [CrossRef]
- Kübler, D.; Schroll, H.; Buchert, R.; Kühn, A.A. Cognitive performance correlates with the degree of dopaminergic degeneration in the associative part of the striatum in non-demented Parkinson’s patients. J. Neural Transm. 2017, 124, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Yoo, H.S.; Oh, J.S.; Kim, J.S.; Ye, B.S.; Sohn, Y.H.; Lee, P.H. Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Park. Relat. Disord. 2018, 51, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Oh, M.; Oh, J.S.; Moon, H.; Chung, S.J.; Lee, C.S.; Kim, J.S. Association of striatal dopaminergic neuronal integrity with cognitive dysfunction and cerebral cortical metabolism in Parkinson’s disease with mild cognitive impairment. Nucl. Med. Commun. 2019, 40, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Arnaldi, D.; De Carli, F.; Picco, A.; Ferrara, M.; Accardo, J.; Bossert, I.; Famà, F.; Girtler, N.; Morbelli, S.; Sambuceti, G.; et al. Nigro-caudate dopaminergic deafferentation: A marker of REM sleep behavior disorder? Neurobiol. Aging 2015, 36, 3300–3305. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, J.; Durcan, R.; Wiblin, L.; Gersel Stokholm, M.; Rochester, L.; Brooks, D.J.; Burn, D.; Pavese, N. Clinical implications of early caudate dysfunction in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2019, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, F.; Marín-Lahoz, J.; Martínez-Horta, S.; Camacho, V.; Lopez-Mora, D.A.; Pagonabarraga, J.; Kulisevsky, J. Extrastriatal SPECT-DAT uptake correlates with clinical and biological features of de novo Parkinson’s disease. Neurobiol. Aging 2021, 97, 120–128. [Google Scholar] [CrossRef]
- Fiorenzato, E.; Antonini, A.; Bisiacchi, P.; Weis, L.; Biundo, R. Asymmetric Dopamine Transporter Loss Affects Cognitive and Motor Progression in Parkinson’s Disease. Mov. Disord. 2021, 2021, 28682. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kim, J.M.; Sohn, C.H.; Choi, J.H.; Choi, B.S.; Song, Y.S.; Nam, Y.; Cho, S.J.; Jeon, B.; Kim, J.H. Imaging the substantia nigra in Parkinson disease and other Parkinsonian syndromes. Radiology 2021, 300, 260–278. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Krismer, F.; Poewe, W.; Seppi, K. Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson’s disease. Mov. Disord. 2017, 32, 619–623. [Google Scholar] [CrossRef]
- De Marzi, R.; Seppi, K.; Högl, B.; Müller, C.; Scherfler, C.; Stefani, A.; Iranzo, A.; Tolosa, E.; Santamarìa, J.; Gizewski, E.; et al. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann. Neurol. 2016, 79, 1026–1030. [Google Scholar] [CrossRef]
- Kim, J.M.; Jeong, H.J.; Bae, Y.J.; Park, S.Y.; Kim, E.; Kang, S.Y.; Oh, E.S.; Kim, K.J.; Jeon, B.; Kim, S.E.; et al. Loss of substantia nigra hyperintensity on 7 Tesla MRI of Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Park. Relat. Disord. 2016, 26, 47–54. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kim, J.M.; Kim, E.; Lee, K.M.; Kang, S.Y.; Park, H.S.; Kim, K.J.; Kim, Y.E.; Oh, E.S.; Yun, J.Y.; et al. Loss of Nigral Hyperintensity on 3 Tesla MRI of Parkinsonism: Comparison with 123I-FP-CIT SPECT. Mov. Disord. 2016, 31, 684–692. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhu, C.; Li, G.; Kang, J.; Chen, F.; Yang, L. The diagnostic value of SNpc using NM-MRI in Parkinson’s disease: Meta-analysis. Neurol. Sci. 2019, 40, 2479–2489. [Google Scholar] [CrossRef]
- Kuya, K.; Ogawa, T.; Shinohara, Y.; Ishibashi, M.; Fujii, S.; Mukuda, N.; Tanabe, Y. Evaluation of Parkinson’s disease by neuromelanin-sensitive magnetic resonance imaging and 123I-FP-CIT SPECT. Acta Radiol. 2018, 59, 593–598. [Google Scholar] [CrossRef]
- Matsusue, E.; Fujihara, Y.; Tanaka, K.; Aozasa, Y.; Shimoda, M.; Nakayasu, H.; Nakamura, K.; Ogawa, T. The utility of the combined use of 123 I-FP-CIT SPECT and neuromelanin MRI in differentiating Parkinson’s disease from other parkinsonian syndromes. Acta Radiol. 2019, 60, 230–238. [Google Scholar] [CrossRef]
- Gaurav, R.; Yahia-Cherif, L.; Pyatigorskaya, N.; Mangone, G.; Biondetti, E.; Valabrègue, R.; Ewenczyk, C.; Hutchison, R.M.; Cedarbaum, J.M.; Corvol, J.C.; et al. Longitudinal Changes in Neuromelanin MRI Signal in Parkinson’s Disease: A Progression Marker. Mov. Disord. 2021, 36, 1592–1602. [Google Scholar] [CrossRef]
- Takahashi, H.; Watanabe, Y.; Tanaka, H.; Mihara, M.; Mochizuki, H.; Takahashi, K.; Yamamoto, K.; Liu, T.; Wang, Y.; Tomiyama, N. Comprehensive MRI quantification of the substantia nigra pars compacta in Parkinson’s disease. Eur. J. Radiol. 2018, 109, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Heim, B.; Krismer, F.; De Marzi, R.; Seppi, K. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J. Neural Transm. 2017, 124, 915–964. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.; Lehéricy, S. Illuminating basal ganglia and beyond in Parkinson’s disease. Mov. Disord. 2018, 33, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).