Mechanisms of Long Non-Coding RNAs in Biological Characteristics and Aerobic Glycolysis of Glioma
Abstract
1. Introduction
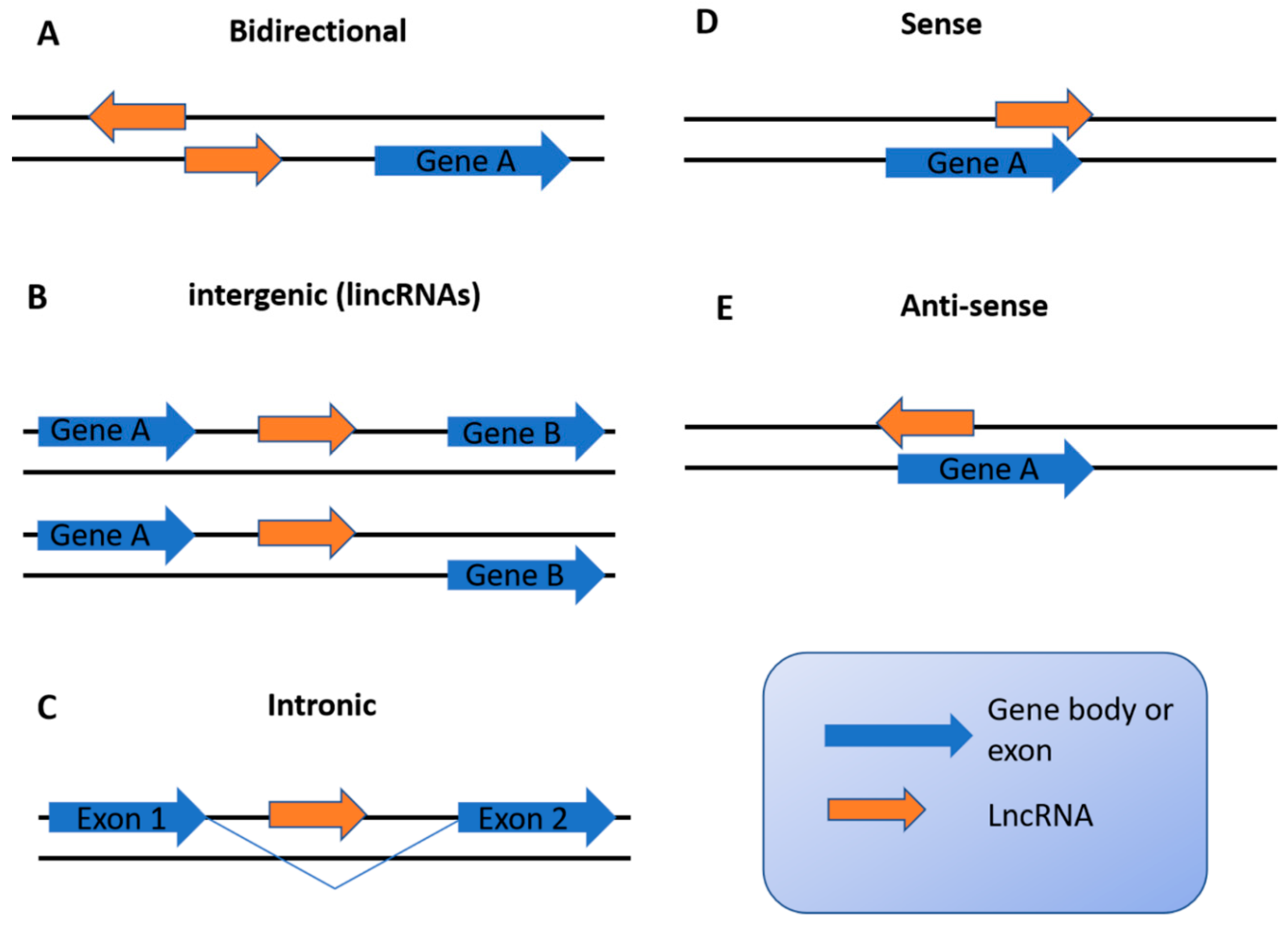
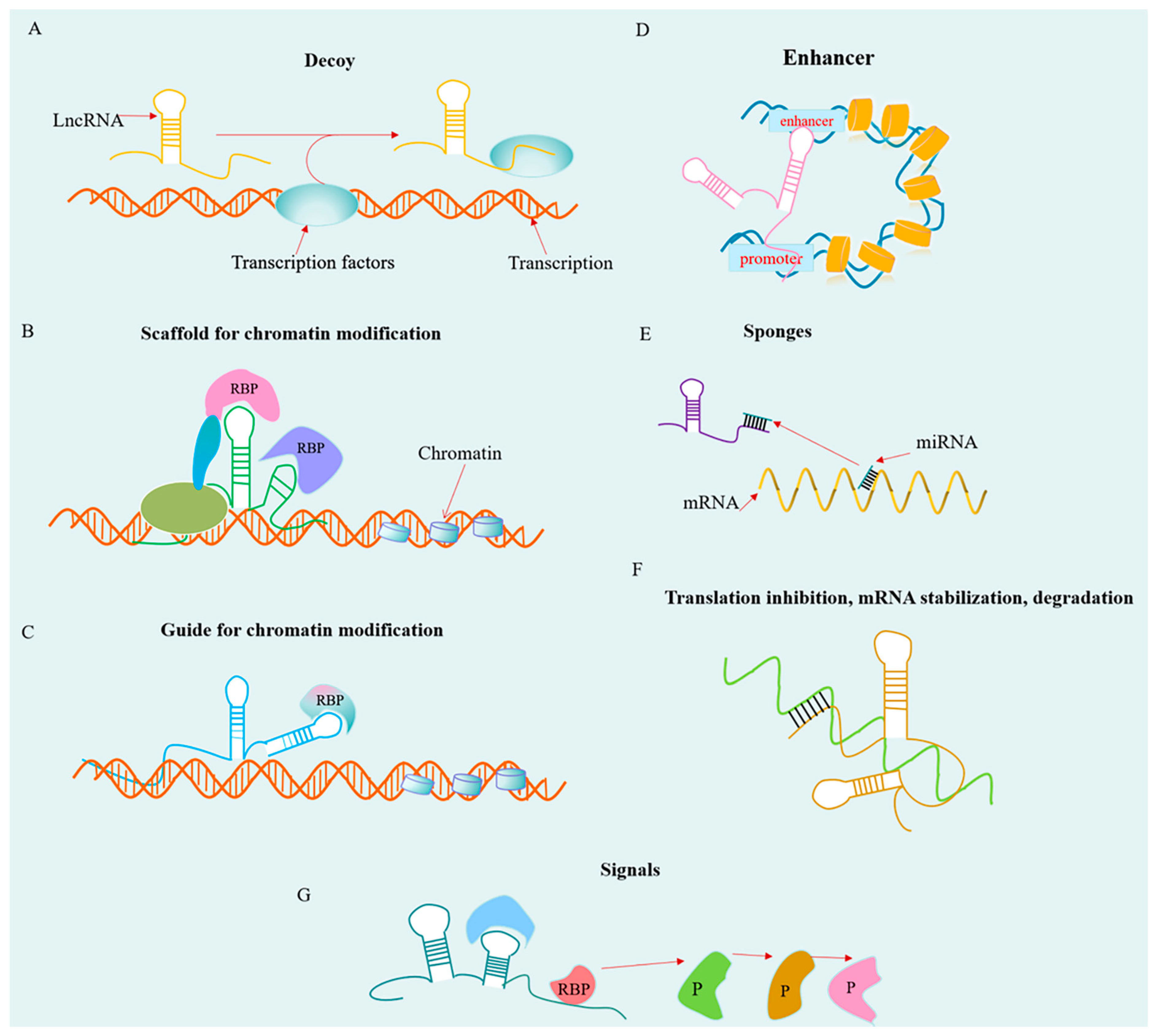
2. Overview of lncRNAs
3. Roles of lncRNAs in Biological Characteristics of Glioma
3.1. lncRNAs Regulate Glioma Cells Stemness
3.2. lncRNAs Regulate Angiogenesis of Glioma Cells
3.3. lncRNAs Contribute to Glioma Drug Resistance
3.4. lncRNAs May Be Implicated in Immune Responses in Glioma
4. Potential Clinical Application of lncRNAs in Glioma
4.1. lncRNAs as Promising Biomarkers for Glioma Diagnosis
4.2. lncRNAs as Biomarkers for Glioma Prognosis
4.3. lncRNAs as Therapeutic Targets
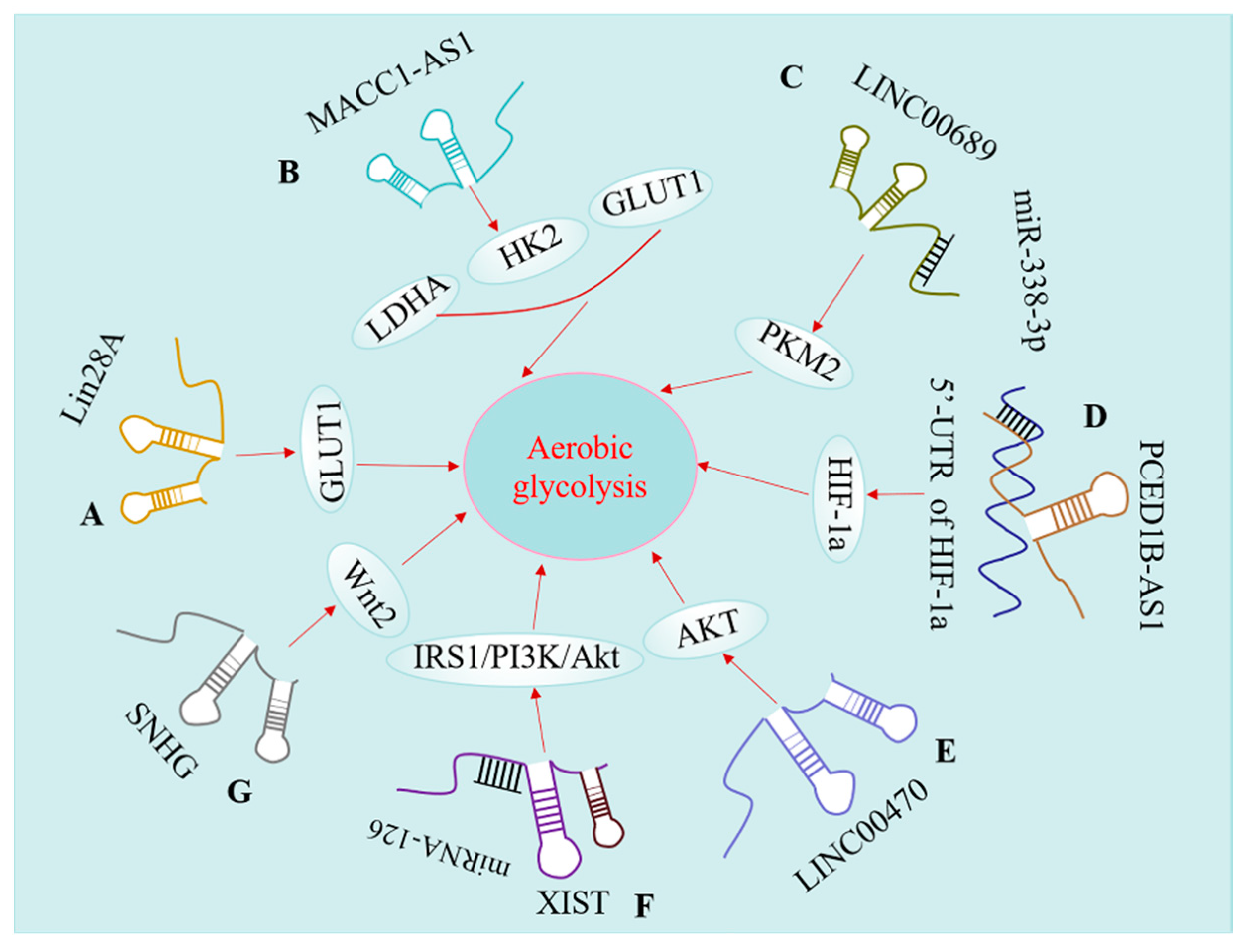
5. lncRNAs Modulate Aerobic Glycolysis of Glioma
5.1. lncRNAs Regulate GLUT Levels in Glioma
5.2. lncRNAs Modulate Key Glycolytic Enzyme
5.3. lncRNA Regulates Glucose Metabolism by Targeting Different Signaling Pathways
5.3.1. HIF-1a Pathway
5.3.2. Phosphoinositide 3-Kinase (PI3K)/Akt Pathway
5.3.3. Wnt Pathway
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santosh, V.; Sravya, P.; Gupta, T.; Muzumdar, D.; Chacko, G.; Suri, V.; Epari, S.; Balasubramaniam, A.; Radotra, B.D.; Chatterjee, S.; et al. ISNO consensus guidelines for practical adaptation of the WHO 2016 classification of adult diffuse gliomas. Neurol. India 2019, 67, 173–182. [Google Scholar]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Exon Publications: Brisbane, QLD, Australia, 2017. [Google Scholar]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef] [PubMed]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef]
- Nicholson, J.G.; Fine, H.A. Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discov. 2021, 11, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.K.; Drummond, K.; Hunn, M.; Williams, D.; O’Brien, T.J.; Monif, M. Potential biomarkers and challenges in glioma diagnosis, therapy and prognosis. BMJ Neurol. Open 2020, 2, e000069. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.C.; Lee, M.H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Lin, Z.; Ko, Y.H.; Goldberg, A.F.; Flomenberg, N.; Wang, C.; Pavlides, S.; Pestell, R.G.; Howell, A.; Sotgia, F.; et al. Understanding the metabolic basis of drug resistance: Therapeutic induction of the Warburg effect kills cancer cells. Cell Cycle 2011, 10, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- San-Millan, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Patel, M.S.; Mahmood, S.; Jung, J.; Rideout, T.C. Reprogramming of aerobic glycolysis in non-transformed mouse liver with pyruvate dehydrogenase complex deficiency. Physiol. Rep. 2021, 9, e14684. [Google Scholar] [CrossRef]
- Ji, L.; Shen, W.; Zhang, F.; Qian, J.; Jiang, J.; Weng, L.; Tan, J.; Li, L.; Chen, Y.; Cheng, H.; et al. Worenine reverses the Warburg effect and inhibits colon cancer cell growth by negatively regulating HIF-1alpha. Cell Mol. Biol. Lett. 2021, 26, 19. [Google Scholar] [CrossRef] [PubMed]
- Pajak, B.; Siwiak, E.; Soltyka, M.; Priebe, A.; Zielinski, R.; Fokt, I.; Ziemniak, M.; Jaskiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef]
- Fan, T.; Sun, G.; Sun, X.; Zhao, L.; Zhong, R.; Peng, Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers 2019, 11, 317. [Google Scholar] [CrossRef]
- Kung, J.T.; Colognori, D.; Lee, J.T. Long noncoding RNAs: Past, present, and future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Deng, L.; Huang, N.; Sun, F. The Biological Roles of lncRNAs and Future Prospects in Clinical Application. Diseases 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhou, Q.; Wang, C.Q.; Zhu, L.; Bi, C.; Zhang, S.; Wang, X.; Jin, H. lncRNAs regulate metabolism in cancer. Int. J. Biol. Sci. 2020, 16, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.F.; Feng, L.; Zhang, X.Q.; Song, L.J.; Liang, H.X.; Li, Z.Q.; Tao, F.B. Role of long non-coding RNAs in gene regulation and oncogenesis. Chin. Med. J. 2011, 124, 2378–2383. [Google Scholar]
- Foroughi, K.; Amini, M.; Atashi, A.; Mahmoodzadeh, H.; Hamann, U.; Manoochehri, M. Tissue-Specific Down-Regulation of the Long Non-Coding RNAs PCAT18 and LINC01133 in Gastric Cancer Development. Int. J. Mol. Sci. 2018, 19, 3881. [Google Scholar] [CrossRef]
- Cao, Y.P.; Zhou, J.; Li, W.J.; Shao, Y.; Zheng, S.Y.; Tian, T.; Xie, K.P.; Yan, X. Long Non-Coding RNA Expression Profiles for the Characterization of Different Bladder Cancer Grade. Cell Physiol. Biochem. 2018, 50, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Pal, S.; Rubstov, Y.; Goel, A.; Garg, M.; Pavlyukov, M.; Pandey, A.K. lncRNAs associated with glioblastoma: From transcriptional noise to novel regulators with a promising role in therapeutics. Mol. Nucleic Acids 2021, 24, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, S.; Zhao, W. Long Non-Coding RNA NRAD1 and LINC00152 are Highly Expressed and Associated with Prognosis in Patients with Hepatocellular Carcinoma. Oncol. Targets 2020, 13, 10409–10416. [Google Scholar] [CrossRef] [PubMed]
- Stackhouse, C.T.; Gillespie, G.Y.; Willey, C.D. Exploring the Roles of lncRNAs in GBM Pathophysiology and Their Therapeutic Potential. Cells 2020, 9, 2369. [Google Scholar] [CrossRef] [PubMed]
- Kiran, M.; Chatrath, A.; Tang, X.; Keenan, D.M.; Dutta, A. A Prognostic Signature for Lower Grade Gliomas Based on Expression of Long Non-Coding RNAs. Mol. Neurobiol. 2019, 56, 4786–4798. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hao, Q.; Prasanth, K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. 2018, 34, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Nowakowski, T.J.; Pollen, A.A.; Lui, J.H.; Horlbeck, M.A.; Attenello, F.J.; He, D.; Weissman, J.S.; Kriegstein, A.R.; Diaz, A.A.; et al. Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol. 2016, 17, 67. [Google Scholar] [CrossRef]
- Waseem, M.; Liu, Y.; Xia, R. Long Non-Coding RNAs, the Dark Matter: An Emerging Regulatory Component in Plants. Int. J. Mol. Sci. 2020, 22, 86. [Google Scholar] [CrossRef]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Pelechano, V.; Jarvelin, A.I.; Steinmetz, L.M. Functional consequences of bidirectional promoters. Trends Genet. 2011, 27, 267–276. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Krchnakova, Z.; Thakur, P.K.; Krausova, M.; Bieberstein, N.; Haberman, N.; Muller-McNicoll, M.; Stanek, D. Splicing of long non-coding RNAs primarily depends on polypyrimidine tract and 5′ splice-site sequences due to weak interactions with SR proteins. Nucleic Acids Res. 2019, 47, 911–928. [Google Scholar] [CrossRef] [PubMed]
- Latge, G.; Poulet, C.; Bours, V.; Josse, C.; Jerusalem, G. Natural Antisense Transcripts: Molecular Mechanisms and Implications in Breast Cancers. Int. J. Mol. Sci. 2018, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Acuna, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Xi, Y.; McCarthy, R.; Allton, K.; Akdemir, K.C.; Patel, L.R.; Aronow, B.; Lin, C.; Li, W.; Yang, L.; et al. LncPRESS1 Is a p53-Regulated lncRNA that Safeguards Pluripotency by Disrupting SIRT6-Mediated De-acetylation of Histone H3K56. Mol. Cell 2016, 64, 967–981. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, J.; Wang, Q.; Wang, Y.; Liu, M.; Yang, J.; Zhou, J.; Kang, C.; Li, M.; Jiang, C. Long Noncoding RNA NEAT1, Regulated by the EGFR Pathway, Contributes to Glioblastoma Progression Through the WNT/beta-Catenin Pathway by Scaffolding EZH2. Clin. Cancer Res. 2018, 24, 684–695. [Google Scholar] [CrossRef]
- Li, Y.; Ren, Y.; Wang, Y.; Tan, Y.; Wang, Q.; Cai, J.; Zhou, J.; Yang, C.; Zhao, K.; Yi, K.; et al. A Compound AC1Q3QWB Selectively Disrupts HOTAIR-Mediated Recruitment of PRC2 and Enhances Cancer Therapy of DZNep. Theranostics 2019, 9, 4608–4623. [Google Scholar] [CrossRef]
- Xiang, J.F.; Yin, Q.F.; Chen, T.; Zhang, Y.; Zhang, X.O.; Wu, Z.; Zhang, S.; Wang, H.B.; Ge, J.; Lu, X.; et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014, 24, 513–531. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, R.L.; Du, W.; Zhang, L.W.; Du, T.; Geng, Y.D.; Wei, X.T. Long noncoding RNA ENST00000413528 sponges microRNA-593-5p to modulate human glioma growth via polo-like kinase 1. CNS Neurosci. 2019, 25, 842–854. [Google Scholar] [CrossRef]
- Yoon, J.H.; Abdelmohsen, K.; Srikantan, S.; Yang, X.; Martindale, J.L.; De, S.; Huarte, M.; Zhan, M.; Becker, K.G.; Gorospe, M. LincRNA-p21 suppresses target mRNA translation. Mol. Cell 2012, 47, 648–655. [Google Scholar] [CrossRef]
- Zhang, Y.; Pitchiaya, S.; Cieslik, M.; Niknafs, Y.S.; Tien, J.C.; Hosono, Y.; Iyer, M.K.; Yazdani, S.; Subramaniam, S.; Shukla, S.K.; et al. Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat. Genet. 2018, 50, 814–824. [Google Scholar] [CrossRef]
- Wang, S.J.; Wang, H.; Zhao, C.D.; Li, R. Long noncoding RNA LINC01426 promotes glioma progression through PI3K/AKT signaling pathway and serves as a prognostic biomarker. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6358–6368. [Google Scholar]
- Chen, G.; Cao, Y.; Zhang, L.; Ma, H.; Shen, C.; Zhao, J. Analysis of long non-coding RNA expression profiles identifies novel lncRNA biomarkers in the tumorigenesis and malignant progression of gliomas. Oncotarget 2017, 8, 67744–67753. [Google Scholar] [CrossRef]
- Zottel, A.; Samec, N.; Videtic Paska, A.; Jovcevska, I. Coding of Glioblastoma Progression and Therapy Resistance through Long Noncoding RNAs. Cancers 2020, 12, 1842. [Google Scholar] [CrossRef]
- Chen, X.; Guo, G.; Lu, Y.; Wang, S.; Zhang, Y.; Huang, Q. Mechanisms and functions of long noncoding RNAs in glioma (Review). Oncol. Rep. 2021, 45, 1. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.K.; Lin, J.W.; Shih, J.W.; Chuang, H.Y.; Fong, I.H.; Yeh, C.T.; Lin, C.M. Targeting BC200/miR218-5p Signaling Axis for Overcoming Temozolomide Resistance and Suppressing Glioma Stemness. Cells 2020, 9, 1859. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Fu, C.; Wang, C.; Duan, X.; Zou, W.; Zhao, T. Knockdown of long non-coding RNA PCAT1 in glioma stem cells promotes radiation sensitivity. Med. Mol. Morphol. 2019, 52, 114–122. [Google Scholar] [CrossRef]
- Yu, M.; Xue, Y.; Zheng, J.; Liu, X.; Yu, H.; Liu, L.; Li, Z.; Liu, Y. Linc00152 promotes malignant progression of glioma stem cells by regulating miR-103a-3p/FEZF1/CDC25A pathway. Mol. Cancer 2017, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Endler, A.; Shibasaki, F. Hypoxia and angiogenesis: Regulation of hypoxia-inducible factors via novel binding factors. Exp. Mol. Med. 2009, 41, 849–857. [Google Scholar] [CrossRef]
- Zhu, X.; Pan, H.; Liu, L. Long noncoding RNA network: Novel insight into hepatocellular carcinoma metastasis (Review). Int. J. Mol. Med. 2021, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Niu, W.; Zhang, X.; Hu, S.; Niu, C. lncRNA BCYRN1 inhibits glioma tumorigenesis by competitively binding with miR-619-5p to regulate CUEDC2 expression and the PTEN/AKT/p21 pathway. Oncogene 2020, 39, 6879–6892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, Z.Z.; Wu, H.; Kuang, W.L. lncRNA H19 Promotes Cell Proliferation, Migration, and Angiogenesis of Glioma by Regulating Wnt5a/beta-Catenin Pathway via Targeting miR-342. Cell Mol. Neurobiol. 2020, 1–13. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Z.; Ma, K.; Li, X.; Tian, N.; Duan, J.; Xiao, X.; Wang, Y. Long Non-coding RNA XIST Promotes Glioma Tumorigenicity and Angiogenesis by Acting as a Molecular Sponge of miR-429. J. Cancer 2017, 8, 4106–4116. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Costa, A.; Osorio, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Exon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Shergalis, A.; Bankhead, A., 3rd; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Ding, L.; Wang, R.; Shen, D.; Cheng, S.; Wang, H.; Lu, Z.; Zheng, Q.; Wang, L.; Xia, L.; Li, G. Role of noncoding RNA in drug resistance of prostate cancer. Cell Death Dis. 2021, 12, 590. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, D.; Wang, J.; Yu, W.; Yang, J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells 2019, 8, 1015. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, N.; Liu, Y.; Liu, B.; Yang, Z.; Fu, Z.; Lian, C.; Guo, H. Genomic profiling of long non-coding RNA and mRNA expression associated with acquired temozolomide resistance in glioblastoma cells. Int. J. Oncol. 2017, 51, 445–455. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, J.; Wang, C.; Chi, Y.; Wei, Q.; Fu, Z.; Lian, C.; Huang, Q.; Liao, C.; Yang, Z.; et al. lncRNA SOX2OT promotes temozolomide resistance by elevating SOX2 expression via ALKBH5-mediated epigenetic regulation in glioblastoma. Cell Death Dis. 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Chen, H.J.; Hou, G.Q.; Zhang, X.H.; Ge, J.W. LINC01198 promotes proliferation and temozolomide resistance in a NEDD4-1-dependent manner, repressing PTEN expression in glioma. Aging 2019, 11, 6053–6068. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, S.; Chen, L. MSC-AS1 knockdown inhibits cell growth and temozolomide resistance by regulating miR-373-3p/CPEB4 axis in glioma through PI3K/Akt pathway. Mol. Cell Biochem. 2021, 476, 699–713. [Google Scholar] [CrossRef]
- Cheng, J.; Meng, J.; Zhu, L.; Peng, Y. Exosomal noncoding RNAs in Glioma: Biological functions and potential clinical applications. Mol. Cancer 2020, 19, 66. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Vega, E.A.; Graner, M.W.; Sampson, J.H. Combating immunosuppression in glioma. Future Oncol. 2008, 4, 433–442. [Google Scholar] [CrossRef]
- Wang, X.; Gao, M.; Ye, J.; Jiang, Q.; Yang, Q.; Zhang, C.; Wang, S.; Zhang, J.; Wang, L.; Wu, J.; et al. An Immune Gene-Related Five-lncRNA Signature for to Predict Glioma Prognosis. Front. Genet. 2020, 11, 612037. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, T.; Zhou, W.; Li, J.; Li, X.; Wang, Q.; Jin, X.; Yin, J.; Chen, L.; Zhang, Y.; et al. Pan-cancer characterization of immune-related lncRNAs identifies potential oncogenic biomarkers. Nat. Commun. 2020, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Li, Q.; Wu, G.; Huang, Y. An Immune-Related lncRNA Signature to Predict Survival in Glioma Patients. Cell Mol. Neurobiol. 2021, 41, 365–375. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhong, S.; Wang, Z.N.; Xie, T.; Duan, H.; Zhang, J.Y.; Zhang, G.H.; Liang, L.; Cui, R.; Hu, H.R.; et al. Immunogenomic Profiling Demonstrate AC003092.1 as an Immune-Related eRNA in Glioblastoma Multiforme. Front. Genet. 2021, 12, 633812. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.J.; Li, Y.; Ying, X.W.; Chen, L. Prognostic Value of Immune-Related lncRNA SBF2-AS1 in Diffuse Lower-Grade Glioma. Technol. Cancer Res. Treat. 2021, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cao, L.; Zhou, R.; Yang, X.; Wu, M. The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nat. Commun. 2019, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Gong, A.Y.; Wang, Y.; Ma, S.; Chen, X.; Chen, J.; Su, C.J.; Shibata, A.; Strauss-Soukup, J.K.; Drescher, K.M.; et al. LincRNA-Cox2 Promotes Late Inflammatory Gene Transcription in Macrophages through Modulating SWI/SNF-Mediated Chromatin Remodeling. J. Immunol. 2016, 196, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.K.; Yu, J.C. Circulating microRNAs and long non-coding RNAs in gastric cancer diagnosis: An update and review. World J. Gastroenterol. 2015, 21, 9863–9886. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.B.; Zhu, Y.; Zhang, Q.W.; Zhang, C.H.; Shao, A.; Zhang, J. Prognostic and Predictive Value of a Long Non-coding RNA Signature in Glioma: A lncRNA Expression Analysis. Front. Oncol. 2020, 10, 1057. [Google Scholar] [CrossRef]
- Rezaei, O.; Tamizkar, K.H.; Sharifi, G.; Taheri, M.; Ghafouri-Fard, S. Emerging Role of Long Non-Coding RNAs in the Pathobiology of Glioblastoma. Front. Oncol. 2020, 10, 625884. [Google Scholar] [CrossRef]
- Mahinfar, P.; Baradaran, B.; Davoudian, S.; Vahidian, F.; Cho, W.C.; Mansoori, B. Long Non-Coding RNAs in Multidrug Resistance of Glioblastoma. Genes 2021, 12, 455. [Google Scholar] [CrossRef]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef]
- Min, W.; Dai, D.; Wang, J.; Zhang, D.; Zhang, Y.; Han, G.; Zhang, L.; Chen, C.; Li, X.; Li, Y.; et al. Long Noncoding RNA miR210HG as a Potential Biomarker for the Diagnosis of Glioma. PLoS ONE 2016, 11, e0160451. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, S.; Duan, C.; Ma, S.; Hussain, S.; Wei, L.; Chu, M. Genome-wide identification of lncRNAs as novel prognosis biomarkers of glioma. J. Cell Biochem. 2019, 120, 19518–19528. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, Z.G.; Dai, L.Z. Long non-coding RNA LINC01503 predicts worse prognosis in glioma and promotes tumorigenesis and progression through activation of Wnt/beta-catenin signaling. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1600–1609. [Google Scholar]
- Li, J.; Liao, T.; Liu, H.; Yuan, H.; Ouyang, T.; Wang, J.; Chai, S.; Li, J.; Chen, J.; Li, X.; et al. Hypoxic Glioma Stem Cell-Derived Exosomes Containing Linc01060 Promote Progression of Glioma by Regulating the MZF1/c-Myc/HIF1alpha Axis. Cancer Res. 2021, 81, 114–128. [Google Scholar]
- Niu, X.; Sun, J.; Meng, L.; Fang, T.; Zhang, T.; Jiang, J.; Li, H. A Five-lncRNAs Signature-Derived Risk Score Based on TCGA and CGGA for Glioblastoma: Potential Prospects for Treatment Evaluation and Prognostic Prediction. Front. Oncol. 2020, 10, 590352. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Y.; Chen, H.; Ma, F. Long Non-coding RNA Expression Profiling Identifies a Four-Long Non-coding RNA Prognostic Signature for Isocitrate Dehydrogenase Mutant Glioma. Front. Neurol. 2020, 11, 573264. [Google Scholar] [CrossRef]
- Arun, G.; Diermeier, S.D.; Spector, D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018, 24, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Malatesta, M.; Lien, B.V.; Saha, P.; Thombare, S.S.; Hong, S.J.; Pedraza, L.; Koontz, M.; Seo, K.; Horlbeck, M.A.; et al. CRISPRi-based radiation modifier screen identifies long non-coding RNA therapeutic targets in glioma. Genome Biol. 2020, 21, 83. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Han, B.; Wang, Q.; Zhang, B.; Qi, C.; Liu, F. lncRNA GAS5-AS1 inhibits glioma proliferation, migration, and invasion via miR-106b-5p/TUSC2 axis. Hum. Cell 2020, 33, 416–426. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Y.; Shi, J.; Wang, H.; Sheng, Y.; Jiang, Q.; Chen, H.; Li, X.; Dong, J. The long non-coding RNA SNHG1 promotes glioma progression by competitively binding to miR-194 to regulate PHLDA1 expression. Cell Death Dis. 2019, 10, 463. [Google Scholar] [CrossRef]
- Sheng, J.; He, X.; Yu, W.; Chen, Y.; Long, Y.; Wang, K.; Zhu, S.; Liu, Q. p53-targeted lncRNA ST7-AS1 acts as a tumour suppressor by interacting with PTBP1 to suppress the Wnt/beta-catenin signalling pathway in glioma. Cancer Lett. 2021, 503, 54–68. [Google Scholar] [CrossRef]
- Xiao, M.; Feng, Y.; Liu, C.; Zhang, Z. Prognostic values of long noncoding RNA PVT1 in various carcinomas: An updated systematic review and meta-analysis. Cell Prolif. 2018, 51, e12519. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Chen, J.; Liu, X.; Gong, W.; Zheng, J.; Guo, X.; Liu, Y.; Liu, L.; Ma, J.; Wang, P.; et al. PVT1 regulates the malignant behaviors of human glioma cells by targeting miR-190a-5p and miR-488-3p. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt A, 1783–1794. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 612393. [Google Scholar] [CrossRef]
- Fu, D.; Shi, Y.; Liu, J.B.; Wu, T.M.; Jia, C.Y.; Yang, H.Q.; Zhang, D.D.; Yang, X.L.; Wang, H.M.; Ma, Y.S. Targeting Long Non-coding RNA to Therapeutically Regulate Gene Expression in Cancer. Mol. Nucleic Acids 2020, 21, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Mucka, P.; Kern, J.G.; Feng, H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 2018, 9, 216–237. [Google Scholar] [CrossRef]
- Strickland, M.; Stoll, E.A. Metabolic Reprogramming in Glioma. Front. Cell Dev. Biol. 2017, 5, 43. [Google Scholar] [CrossRef]
- Labak, C.M.; Wang, P.Y.; Arora, R.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. Glucose transport: Meeting the metabolic demands of cancer, and applications in glioblastoma treatment. Am. J. Cancer Res. 2016, 6, 1599–1608. [Google Scholar]
- Lu, W.; Cao, F.; Wang, S.; Sheng, X.; Ma, J. lncRNAs: The Regulator of Glucose and Lipid Metabolism in Tumor Cells. Front. Oncol. 2019, 9, 1099. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Zheng, J.; Song, J.; Liu, Y.; Ruan, X.; Shen, S.; Shao, L.; Yang, C.; Wang, D.; et al. Lin28A promotes IRF6-regulated aerobic glycolysis in glioma cells by stabilizing SNHG14. Cell Death Dis. 2020, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Black, K.L.; Pardridge, W.M. Gene expression of GLUT3 and GLUT1 glucose transporters in human brain tumors. Brain Res. Mol. Brain Res. 1994, 27, 51–57. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Moshtaghie, A.A.; Daneshpoor, M.; Hedayati, M. Regulators of glucose uptake in thyroid cancer cell lines. Cell Commun. Signal. 2020, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Marayati, R.; Moffitt, R.; Yeh, J.J. Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget 2017, 8, 56081–56094. [Google Scholar] [CrossRef]
- Ciscato, F.; Ferrone, L.; Masgras, I.; Laquatra, C.; Rasola, A. Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters. Int. J. Mol. Sci. 2021, 22, 4716. [Google Scholar] [CrossRef]
- Sheikh, T.; Gupta, P.; Gowda, P.; Patrick, S.; Sen, E. Hexokinase 2 and nuclear factor erythroid 2-related factor 2 transcriptionally coactivate xanthine oxidoreductase expression in stressed glioma cells. J. Biol. Chem. 2018, 293, 4767–4777. [Google Scholar] [CrossRef]
- Zheng, D.; Che, D.; Lin, F.; Wang, X.; Lu, L.; Chen, J.; Xu, X. lncRNA MACC1-AS1/MACC1 enhances the progression of glioma via regulating metabolic plasticity. Cell Cycle 2020, 19, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Phillips, J.J.; Zheng, S.; Wiencke, J.; Ronen, S.M.; Pieper, R.O. Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PLoS ONE 2013, 8, e57610. [Google Scholar] [CrossRef]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef]
- Mirzaei, H.; Hamblin, M.R. Regulation of Glycolysis by Non-coding RNAs in Cancer: Switching on the Warburg Effect. Mol. Oncolytics 2020, 19, 218–239. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Shen, Y.; He, A. Long non-coding RNA-based glycolysis-targeted cancer therapy: Feasibility, progression and limitations. Mol. Biol. Rep. 2021, 48, 2713–2727. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Yan, S.; Wang, H.; Shao, X.; Xiao, M.; Yang, B.; Qin, G.; Kong, R.; Chen, R.; et al. Interactome analysis reveals that lncRNA HULC promotes aerobic glycolysis through LDHA and PKM2. Nat. Commun. 2020, 11, 3162. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, J.; Li, M.; Feng, Y.; Wang, X.; Zhang, J.; Yao, S.; Jin, G.; Du, J.; Han, W.; et al. lncRNA-FEZF1-AS1 Promotes Tumor Proliferation and Metastasis in Colorectal Cancer by Regulating PKM2 Signaling. Clin. Cancer Res. 2018, 24, 4808–4819. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Q.; Guo, Y.; Xiao, Z.; Hu, L.; Xu, Q. lncRNA LINC00689 promotes the growth, metastasis and glycolysis of glioma cells by targeting miR-338-3p/PKM2 axis. Biomed. Pharmacol. 2019, 117, 109069. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F. Inhibitors of Hypoxia-Inducible Factors as Treatment for Cancer. ACS Med. Chem. Lett. 2020, 11, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar] [PubMed]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.T.; Harada, H. HIF-1-Dependent Reprogramming of Glucose Metabolic Pathway of Cancer Cells and Its Therapeutic Significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, Q.; Guo, F.; Guo, S.; Yang, B.; Liu, B.; Li, P.; Li, J.; Guan, S.; Liu, X. Long Noncoding RNA PCED1B-AS1 Promotes the Warburg Effect and Tumorigenesis by Upregulating HIF-1alpha in Glioblastoma. Cell Transpl. 2020, 29, 1–11. [Google Scholar] [CrossRef]
- Barth, D.A.; Prinz, F.; Teppan, J.; Jonas, K.; Klec, C.; Pichler, M. Long-Noncoding RNA (lncRNA) in the Regulation of Hypoxia-Inducible Factor (HIF) in Cancer. Noncoding RNA 2020, 6, 27. [Google Scholar] [CrossRef]
- Choudhry, H.; Mole, D.R. Hypoxic regulation of the noncoding genome and NEAT1. Brief. Funct. Genom. 2016, 15, 174–185. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Xie, H.; Hu, Y. Interplay of long non-coding RNAs and HIF-1alpha: A new dimension to understanding hypoxia-regulated tumor growth and metastasis. Cancer Lett. 2021, 499, 49–59. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, H.; Hong, C.; Chen, Z.; Deng, X.; Wang, A.; Yang, F.; Yang, L.; Chen, C.; Qin, X. PDGF Promotes the Warburg Effect in Pulmonary Arterial Smooth Muscle Cells via Activation of the PI3K/AKT/mTOR/HIF-1alpha Signaling Pathway. Cell Physiol. Biochem. 2017, 42, 1603–1613. [Google Scholar] [CrossRef]
- Jean, S.; Kiger, A.A. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014, 127 Pt 5, 923–928. [Google Scholar] [CrossRef]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kuramitsu, Y.; Baron, B.; Kitagawa, T.; Tokuda, K.; Akada, J.; Maehara, S.I.; Maehara, Y.; Nakamura, K. PI3K inhibitor LY294002, as opposed to wortmannin, enhances AKT phosphorylation in gemcitabine-resistant pancreatic cancer cells. Int. J. Oncol. 2017, 50, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, X.; Sun, X.; Wang, L.; Chen, S. The Glycolytic Switch in Tumors: How Many Players Are Involved? J. Cancer 2017, 8, 3430–3440. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Wu, L.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 126. [Google Scholar] [CrossRef] [PubMed]
- Aboudehen, K. Regulation of mTOR signaling by long non-coding RNA. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194449. [Google Scholar] [CrossRef]
- Liu, C.H.; Zhang, Y.; She, X.L.; Fan, L.; Li, P.Y.; Feng, J.B.; Fu, H.J.; Liu, Q.; Liu, Q.; Zhao, C.H.; et al. A cytoplasmic long noncoding RNA LINC00470 as a new AKT activator to mediate glioblastoma cell autophagy. J. Hematol. Oncol. 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, C.; Guo, Z. lncRNA-XIST/microRNA-126 sponge mediates cell proliferation and glucose metabolism through the IRS1/PI3K/Akt pathway in glioma. J. Cell Biochem. 2020, 121, 2170–2183. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.W.; Liu, G.Z.; Li, C.X.; Song, Y.R.; Cao, Z.W.; Li, W.; Hu, J.H.; Lu, C.; Liu, Y.Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Caspi, M.; Wittenstein, A.; Kazelnik, M.; Shor-Nareznoy, Y.; Rosin-Arbesfeld, R. Therapeutic targeting of the oncogenic Wnt signaling pathway for treating colorectal cancer and other colonic disorders. Adv. Drug Deliv. Rev. 2021, 169, 118–136. [Google Scholar] [CrossRef]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT Signaling in Tumors: The Way to Evade Drugs and Immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Vidal-Puig, A. Wnt signalling and the control of cellular metabolism. Biochem. J. 2010, 427, 1–17. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Zhang, L.; Yang, L.; Zhou, M.; Li, X.; Li, Y.; Li, G.; Zeng, Z.; Xiong, W.; et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J. Cancer 2019, 10, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, V. WNT signaling: An emerging mediator of cancer cell metabolism? Mol. Cell Biol. 2015, 35, 2–10. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, D.; Jiang, Z.; Zhang, J. SNHG9/miR-199a-5p/Wnt2 Axis Regulates Cell Growth and Aerobic Glycolysis in Glioblastoma. J. Neuropathol. Exp. Neurol. 2019, 78, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hu, G.; Yang, Q.; Zhang, P.; Kuang, W.; Zhu, X.; Wu, L. Knockdown of long non-coding RNA CCAT2 suppressed proliferation and migration of glioma cells. Oncotarget 2016, 7, 81806–81814. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhu, G.; Zeng, W.; Wang, J.C.; Li, Z.H.; Wang, B.; Tian, B.; Lu, D.; Zhang, X.Y.; Gao, G.D.; et al. Long noncoding RNA AB073614 promotes the malignance of glioma by activating Wnt/beta-catenin signaling through downregulating SOX7. Oncotarget 2017, 8, 65577–65587. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, D.L.; Wan, L.; Yan, S.F.; Sun, Y.H. Highly expressed lncRNA CCND2-AS1 promotes glioma cell proliferation through Wnt/beta-catenin signaling. Biochem. Biophys. Res. Commun. 2017, 482, 1219–1225. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L. Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/beta-catenin signaling. Biomed. Pharmacol. 2018, 102, 602–607. [Google Scholar] [CrossRef]
- El-Sahli, S.; Xie, Y.; Wang, L.; Liu, S. Wnt Signaling in Cancer Metabolism and Immunity. Cancers 2019, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.R.; Cai, G.; Li, Q. Exosomes as Actively Targeted Nanocarriers for Cancer Therapy. Int. J. Nanomed. 2020, 15, 4257–4273. [Google Scholar] [CrossRef] [PubMed]
 represents the oncogene,
represents the oncogene,  represents the tumor suppressor gene.
represents the tumor suppressor gene.
 represents the oncogene,
represents the oncogene,  represents the tumor suppressor gene.
represents the tumor suppressor gene.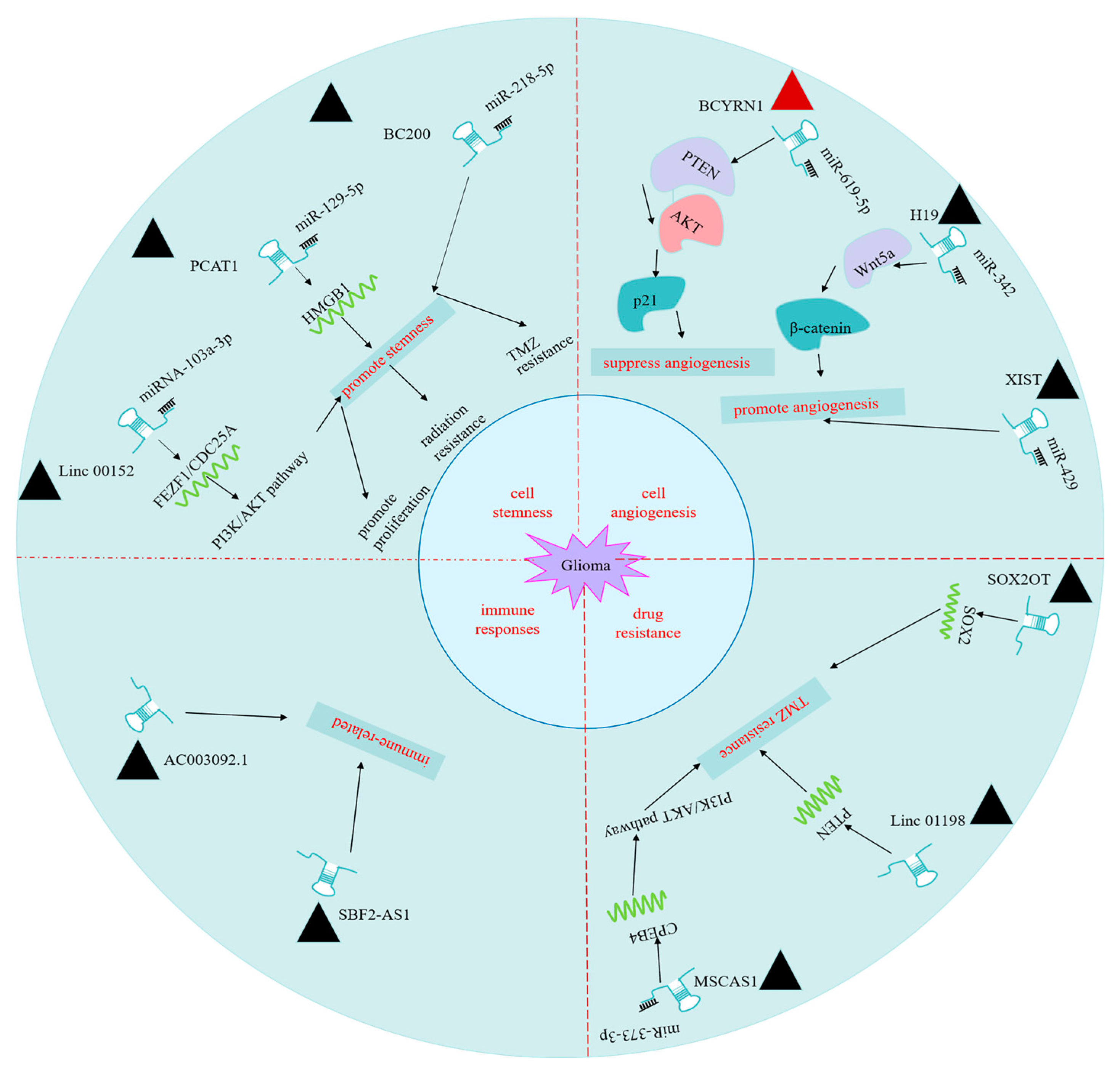
| Expression Level of lncRNAs | lncRNAs | Mechanism | Reported Potential Clinical Application | References |
|---|---|---|---|---|
| Upregulated | HOTAIR | Diagnosis and prognosis | [81] | |
| Upregulated | miR210HG | Diagnosis | [82] | |
| Downregulated | BCYRN1 | Sponge miR-619-5p and interact with the CUEDC2/PTEN/AKT/p21 pathway | Diagnosis, therapeutic target, and prognosis | [55] |
| Upregulated | AC064875.2 | Neoplasm grade and prognosis | [83] | |
| Upregulated | HOTAIRM1 | Neoplasm grade and prognosis | [83] | |
| Upregulated | LINC00908 | Neoplasm grade and prognosis | [83] | |
| Upregulated | RP11-84A19.3 | Neoplasm grade and prognosis | [83] | |
| Upregulated | LINC 01503 | Interact with Wnt/β-catenin pathway | Tumorigenesis, progression, and prognosis | [84] |
| Upregulated | LINC 01060 | Interact with MZF1/c-Myc/HIF-1α axis | Prognosis and progression | [85] |
| Downregulated | TBX5-AS1 | therapeutic target and prognosis | [86] | |
| Upregulated | LNC01545 | therapeutic target and prognosis | [86] | |
| Upregulated | WDR11-AS1 | therapeutic target and prognosis | [86] | |
| Upregulated | NDUFA6-DT | therapeutic target and prognosis | [86] | |
| Upregulated | FRY-AS1 | therapeutic target and prognosis | [86] | |
| Upregulated | H19 | prognosis | [87] | |
| Downregulated | HAR1A | prognosis | [87] | |
| Upregulated | NEAT1 | prognosis | [88] | |
| Upregulated | lncGRS-1 | Therapeutic target | [89] | |
| Downregulated | GAS5-AS1 | Interact with miR-106b-5p/TUSC2 axis | Proliferation, therapeutic target | [90] |
| Upregulated | SNHG1 | Progression, therapeutic target | [91] | |
| Downregulated | ST7-AS1 | Interact with p53/ST7-AS1/PTBP1 feedback loop | Therapeutic target | [92] |
| Upregulated | PVT1 | Pan-cancer therapeutic target and prognosis | [87,93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Zhang, J.; Zhao, Q.; Chen, C.; Wang, H. Mechanisms of Long Non-Coding RNAs in Biological Characteristics and Aerobic Glycolysis of Glioma. Int. J. Mol. Sci. 2021, 22, 11197. https://doi.org/10.3390/ijms222011197
Zhao N, Zhang J, Zhao Q, Chen C, Wang H. Mechanisms of Long Non-Coding RNAs in Biological Characteristics and Aerobic Glycolysis of Glioma. International Journal of Molecular Sciences. 2021; 22(20):11197. https://doi.org/10.3390/ijms222011197
Chicago/Turabian StyleZhao, Ningning, Jiajie Zhang, Qian Zhao, Chao Chen, and Huijuan Wang. 2021. "Mechanisms of Long Non-Coding RNAs in Biological Characteristics and Aerobic Glycolysis of Glioma" International Journal of Molecular Sciences 22, no. 20: 11197. https://doi.org/10.3390/ijms222011197
APA StyleZhao, N., Zhang, J., Zhao, Q., Chen, C., & Wang, H. (2021). Mechanisms of Long Non-Coding RNAs in Biological Characteristics and Aerobic Glycolysis of Glioma. International Journal of Molecular Sciences, 22(20), 11197. https://doi.org/10.3390/ijms222011197





