Induction of Endoplasmic Reticulum Stress-Mediated Apoptosis by Aminosteroid RM-581 Efficiently Blocks the Growth of PC-3 Cancer Cells and Tumors Resistant or Not to Docetaxel
Abstract
:1. Introduction
2. Results
2.1. RM-581 and RM-581-Fluo Antiproliferative Activities
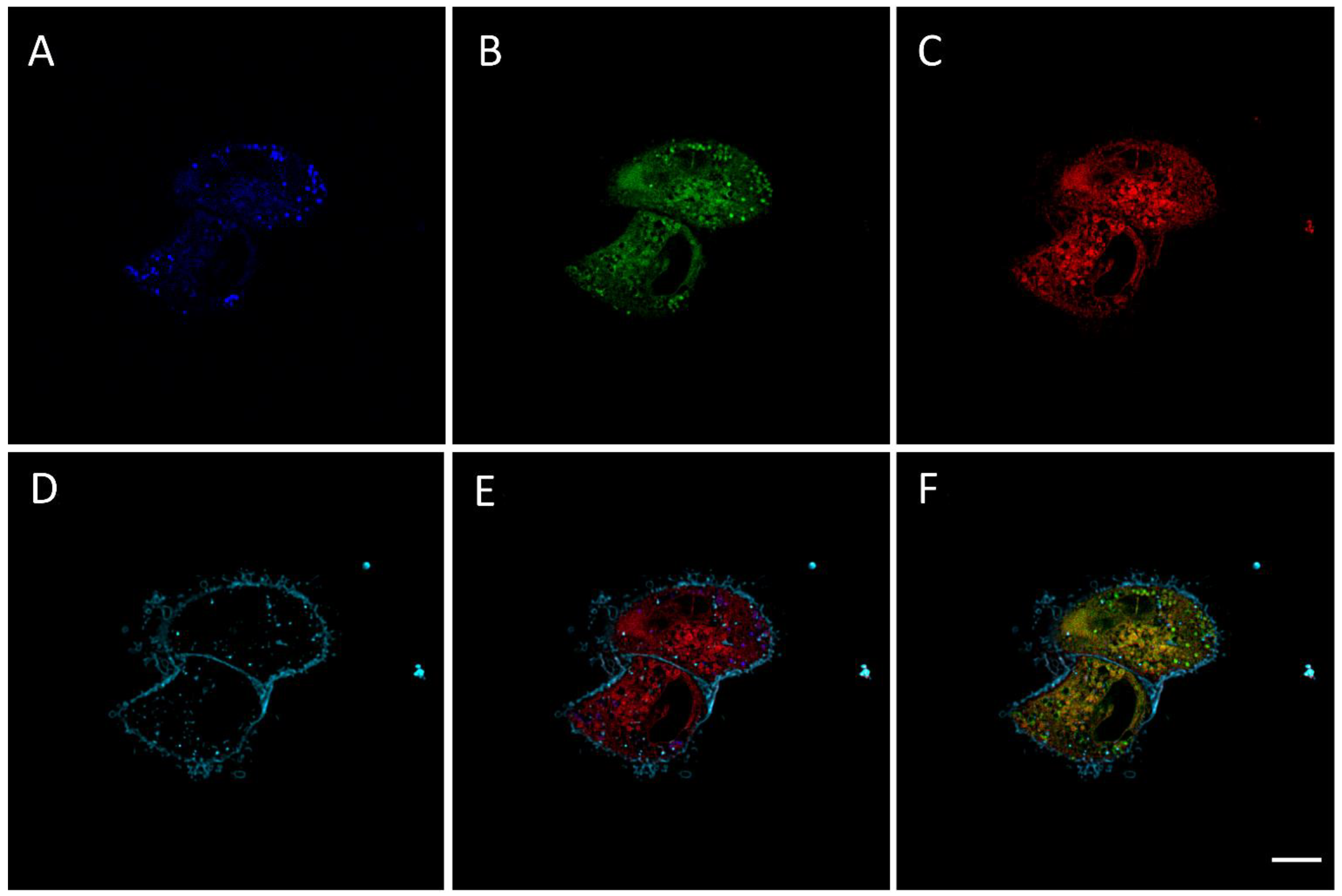
2.2. RM-581-Fluo Accumulates in ER of PC-3 Cells
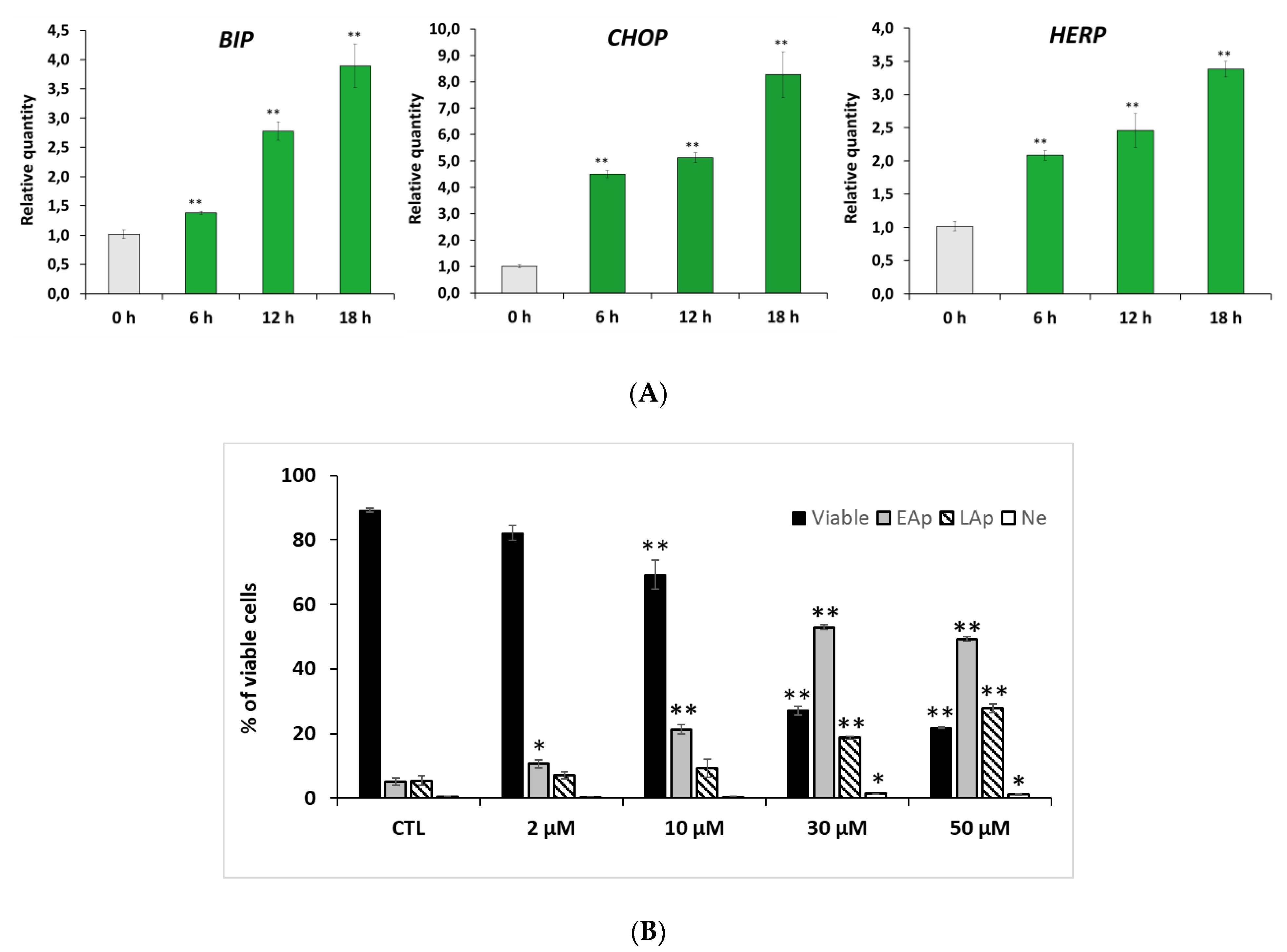
2.3. RM-581 Induces ER-Stress and Apoptosis
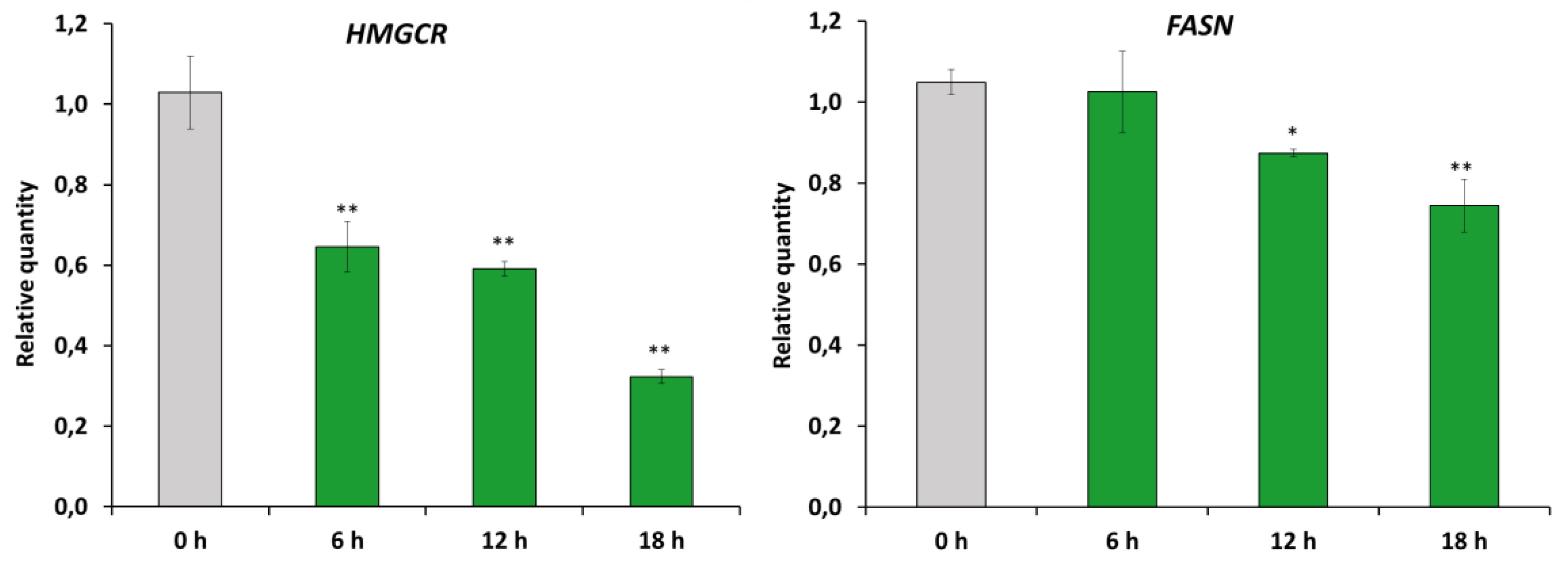
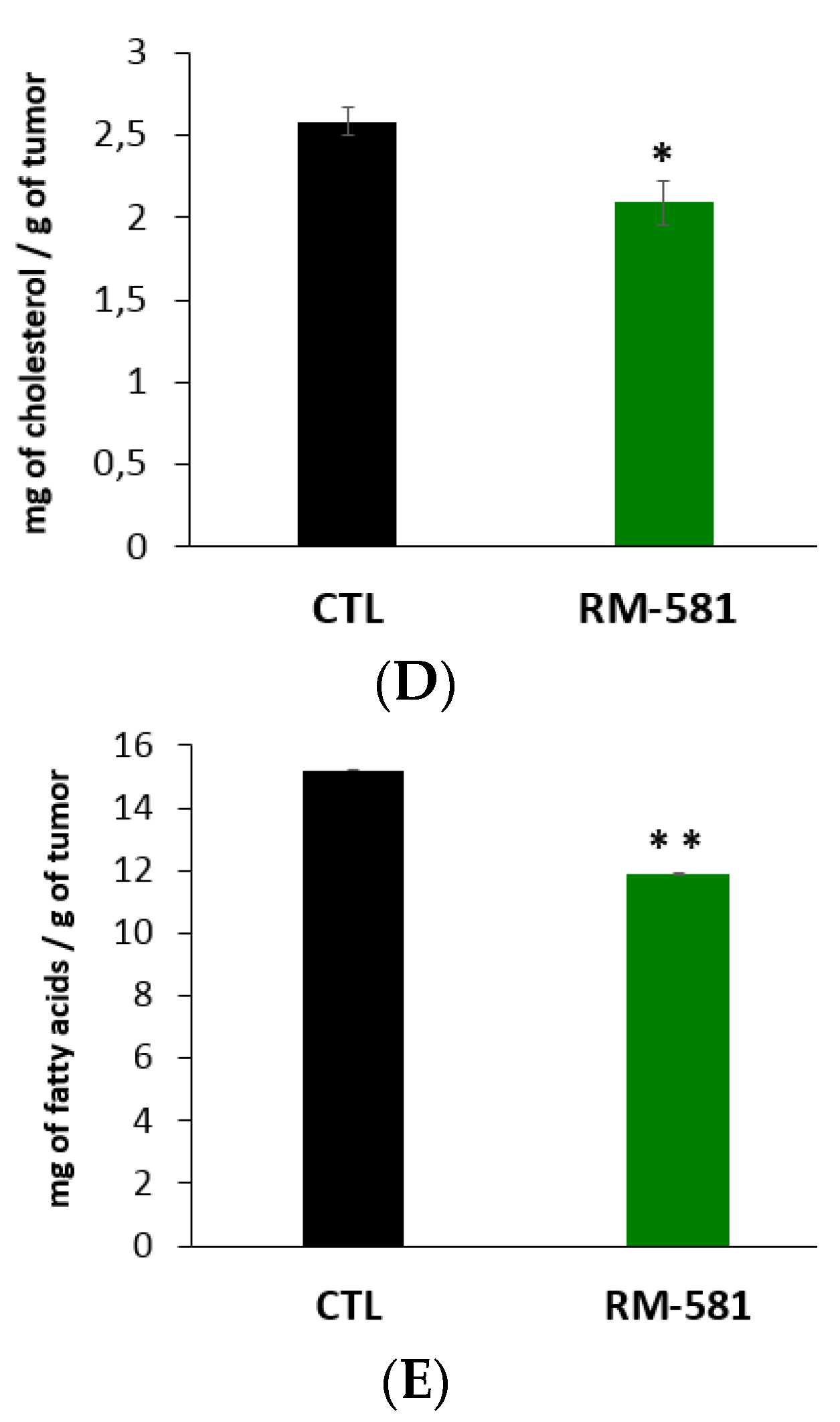
2.4. RM-581 Impairs Lipid Homeostasis
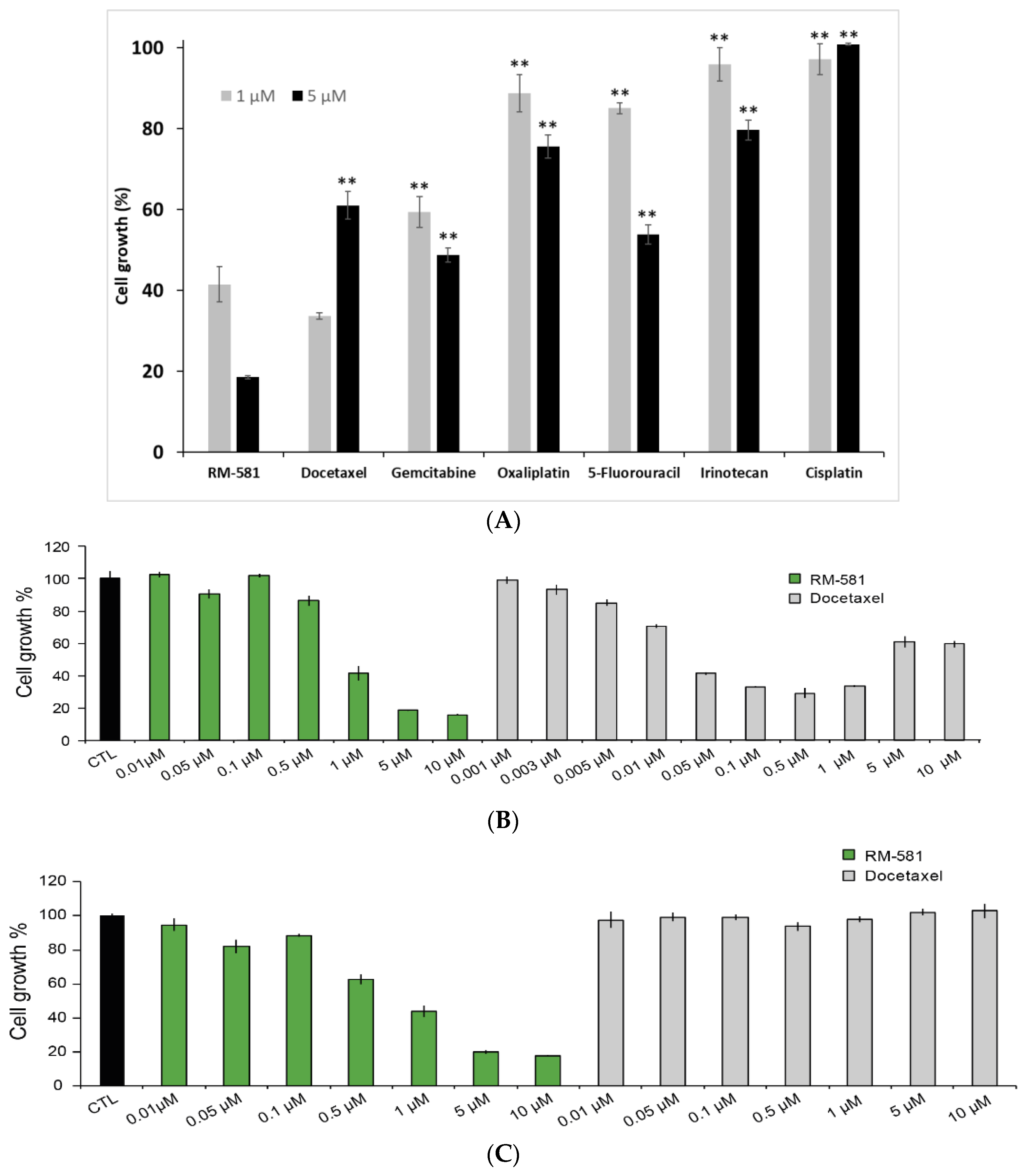
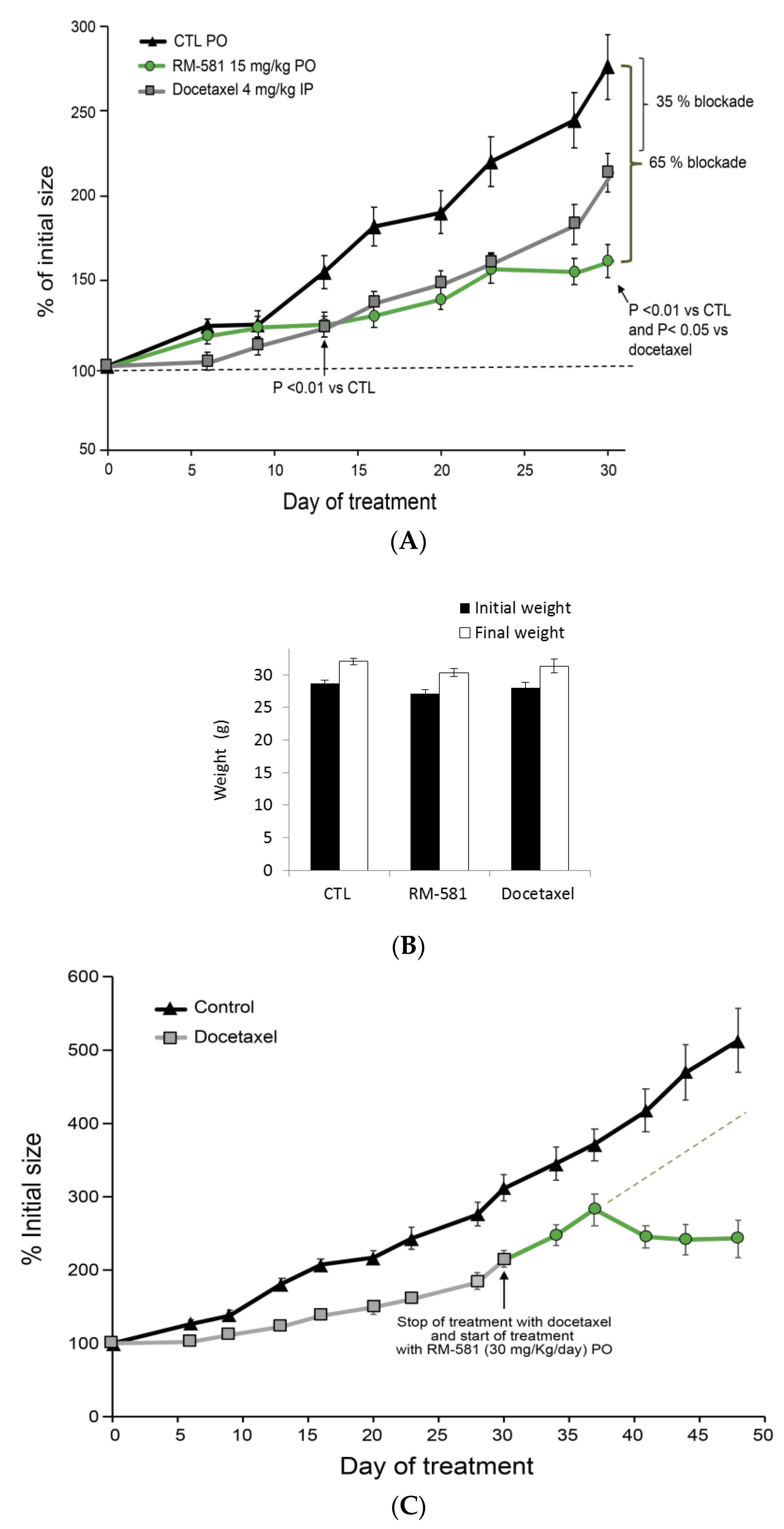
2.5. RM-581 Blocks the Growth of PC-3 Cells and Docetaxel-Resistant PC-3 Cells
2.6. RM-581 Blocks Tumor Growth in PC-3 Xenografts
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis
4.2. Cell Culture
4.3. IC50 Determination of RM-581 vs. RM-581-Fluo in PC-3 Cells
4.4. Confocal Imaging of PC-3 Cells
4.5. Gene Expression Study (Real-Time PCR Analysis)
4.6. Apoptosis Characterization by Flow Cytometry
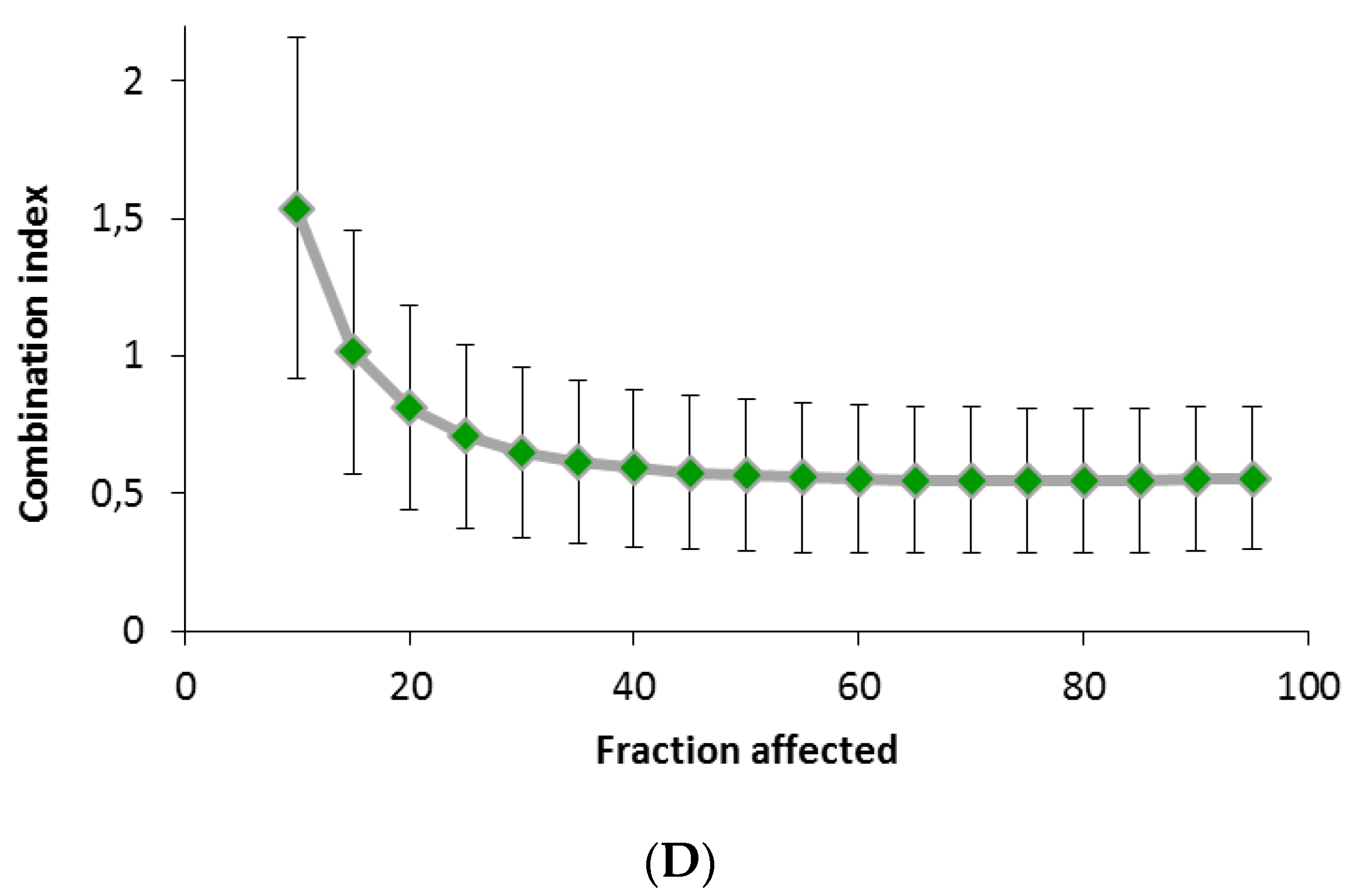
4.7. Cell Viability Proliferation Assays and Combination Index with Docetaxel
4.8. Induction of Docetaxel-Resistant PC-3 Cells
4.9. Effect of RM-581 in PC-3 Xenografts
4.10. Dosage of RM-581 in Tumors and Plasma of Treated Mice
4.11. Fatty Acid Determination in PC-3 Tumors
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel Rebecca, L.; Miller Kimberly, D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.M.; Hoffman, K.E.; Levy, L.B.; Frank, S.J.; Pugh, T.J.; Choi, S.; Nguyen, Q.N.; McGuire, S.E.; Lee, A.K.; Kuban, D.A. Improvement in prostate cancer survival over time: A 20-year analysis. Cancer J. 2012, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Solo, K.; Valant, J.; Todd, M.B.; Mehra, M. Prevalence of prostate cancer clinical states and mortality in the United States: Estimates using a dynamic progression model. PLoS ONE 2015, 10, e0139440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Holm, H.V.; Dahl, A.A.; Klepp, O.H.; Fossa, S.D. Modern treatment of metastatic prostate cancer. Tidsskr. Nor. Laegeforen 2017, 137, 803–805. [Google Scholar] [CrossRef] [Green Version]
- Helsen, C.; van den Broeck, T.; Voet, A.; Prekovic, S.; van Poppel, H.; Joniau, S.; Claessens, F. Androgen receptor antagonists for prostate cancer therapy. Endocr. Relat. Cancer 2014, 21, T105–T118. [Google Scholar] [CrossRef] [Green Version]
- Ritch, C.R.; Cookson, M.S. Advances in the management of castration resistant prostate cancer. BMJ 2016, 355, i4405. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Kabbinavar, F.; Saad, F.; Hussain, A.; Gittelman, M.C.; Bilhartz, D.L.; Wynne, C.; Murray, R.; Zinner, N.R.; Schulman, C.; et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J. Clin. Oncol. 2005, 23, 2918–2925. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Soban, F.; de Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N., Jr.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Merseburger, A.S.; Haas, G.P.; von Klot, C.A. An update on enzalutamide in the treatment of prostate cancer. Ther. Adv. Urol. 2015, 7, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, O.; Afonso, J.; Vazquez, S.; Campos, B.; Lazaro, M.; Leon, L.; Anton Aparicio, L.M. Metastatic castration-resistant prostate cancer: Changing landscape with cabazitaxel. Anticancer Drugs 2014, 25, 237–243. [Google Scholar] [CrossRef]
- Fryzek, J.P.; Reichert, H.; Summers, N.; Townes, L.; Deuson, R.; Alexander, D.D.; Vanderpuye-Orgle, J. Indirect treatment comparison of cabazitaxel for patients with metastatic castrate-resistant prostate cancer who have been previously treated with a docetaxel-containing regimen. PLoS ONE 2018, 13, e0195790. [Google Scholar] [CrossRef] [PubMed]
- Brasso, K.; Thomsen, F.B.; Schrader, A.J.; Schmid, S.C.; Lorente, D.; Retz, M.; Merseburger, A.S.; von Klot, C.A.; Boegemann, M.; de Bono, J. Enzalutamide antitumour activity against metastatic castration-resistant prostate cancer previously treated with docetaxel and abiraterone: A multicentre analysis. Eur. Urol. 2015, 68, 317–324. [Google Scholar] [CrossRef]
- Culig, Z. Molecular Mechanisms of Enzalutamide Resistance in Prostate Cancer. Curr. Mol. Biol. Rep. 2017, 3, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Thelen, P.; Gschwend, J.; Wolff, J.M.; Miller, K. Mechanisms of resistance in antihormone therapies of advanced prostate cancer. Aktuelle Urol 2016, 47, 79–85. [Google Scholar]
- Yoo, S.; Choi, S.Y.; You, D.; Kim, C.-S. New drugs in prostate cancer. Prostate Int. 2016, 4, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Perreault, M.; Maltais, R.; Roy, J.; Dutour, R.; Poirier, D. Design of a mestranol 2-N-piperazino-Ssubstituted serivative showing potent and selective in vitro and in vivo activities in MCF-7 breast cancer models. ChemMedChem 2017, 12, 177–182. [Google Scholar] [CrossRef]
- Maltais, R.; Hospital, A.; Delhomme, A.; Roy, J.; Poirier, D. Chemical synthesis, NMR analysis and evaluation on a cancer xenograft model (HL-60) of the aminosteroid derivative RM-133. Steroids 2014, 82, 68–76. [Google Scholar] [CrossRef]
- Perreault, M.; Maltais, R.; Dutour, R.; Poirier, D. Explorative study on the anticancer activity, selectivity and metabolic stability of related analogs of aminosteroid RM-133. Steroids 2016, 115, 105–113. [Google Scholar] [CrossRef]
- Kenmogne, L.C.; Ayan, D.; Roy, J.; Maltais, R.; Poirier, D. The aminosteroid derivative RM-133 shows in vitro and in vivo antitumor activity in human ovarian and pancreatic cancers. PLoS ONE 2015, 10, e0144890. [Google Scholar] [CrossRef] [Green Version]
- Jegham, H.; Maltais, R.; Dufour, P.; Roy, J.; Poirier, D. Solid-phase chemical synthesis and in vitro biological evaluation of novel 2β-piperazino-(20R)-5α-pregnane-3α,20-diol N-derivatives as anti-leukemic agents. Steroids 2012, 77, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Jegham, H.; Maltais, R.; Roy, J.; Doillon, C.; Poirier, D. Biological evaluation of a new family of aminosteroids that display a selective toxicity for various malignant cell lines. Anticancer Drugs 2012, 23, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.; Maltais, R.; Kenmogne, L.K.; Létourneau, D.; Gobeil, S.; LeHoux, J.-G.; Poirier, D. Implication of STARD5 and cholesterol homeostasis disturbance in the endoplasmic reticulum stress-related response induced by pro-apoptotic aminosteroid RM-133. Pharmacol. Res. 2018, 128, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.; Maltais, R.; Roy, J.; Picard, S.; Popa, I.; Bertrand, N.; Poirier, D. Induction of endoplasmic reticulum stress by aminosteroid derivative RM-581 leads to tumor regression in PANC-1 xenograft model. Investig. New Drugs 2019, 37, 431–440. [Google Scholar] [CrossRef]
- Dutour, R.; Maltais, R.; Perreault, M.; Roy, J.; Poirier, D. Parallel solid-phase synthesis using a new diethylsilylacetylenic linker and leading to mestranol derivatives with potent antiproliferative activities on multiple cancer cell lines. Anticancer Agents Med. Chem. 2018, 18, 1469–1481. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chae, S.W.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and cancer. J. Cancer Prev. 2014, 19, 75–88. [Google Scholar] [CrossRef]
- Storm, M.; Sheng, X.; Arnoldussen, Y.J.; Saatcioglu, F. Prostate cancer and the unfolded protein response. Oncotarget 2016, 7, 54051–54066. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef] [Green Version]
- Atala, A. Re: PC3 is a cell line characteristic of prostatic small cell carcinoma. J. Urol. 2012, 188, 325. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 1979, 17, 16–23. [Google Scholar]
- Maltais, R.; Roy, J.; Poirier, D. Turning a quinoline-based steroidal anticancer agent into fluorescent dye for its tracking by cell imaging. ACS Med. Chem. Lett. 2021, 12, 822–826. [Google Scholar] [CrossRef]
- Fribley, A.; Zhang, K.; Kaufman, R.J. Regulation of apoptosis by the unfolded protein response. Methods Mol. Biol. 2009, 559, 191–204. [Google Scholar]
- Nagelkerke, A.; Bussink, J.; Sweep, F.C.G.J.; Span, P.N. The unfolded protein response as a target for cancer therapy. Biochim. Biophys. Acta BBA Rev. Cancer 2014, 1846, 277–284. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 2014, 14, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug Combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykes, D.J.; Bissery, M.C.; Harrison, S.D.; Waud, W.R. Response of human tumor xenografts in athymic nude mice to docetaxel (RP 56976, Taxotere®). Invest. New Drugs 1995, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pineau, L.; Colas, J.; Dupont, S.; Beney, L.; Fleurat-Lessard, P.; Berjeaud, J.M.; Bergès, T.; Ferreira, T. Lipid-induced ER stress: Synergistic effects of sterols and saturated fatty acids. Traffic 2009, 10, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, F.H.; Zhang, W.T.; Guo, Y.D.; Ye, L.; Yao, X.D. Mesenchymal stem cells desensitize castration-resistant prostate cancer to docetaxel chemotherapy via inducing TGF-β1-mediated cell autophagy. Cell Biosci. 2021, 11, 7. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Et Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deep, G.; Schlaepfer, I.R. Aberrant lipid metabolism promotes prostate cancer: Role in cell survival under hypoxia and extracellular vesicles biogenesis. Int. J. Mol. Sci. 2016, 17, 1061. [Google Scholar] [CrossRef] [Green Version]
- Colgan, S.M.; Hashimi, A.A.; Austin, R.C. Endoplasmic reticulum stress and lipid dysregulation. Expert Rev. Mol. Med. 2011, 13, e4. [Google Scholar] [CrossRef]
- Cheng, X.; Li, J.; Guo, D. SCAP/SREBPs are central players in lipid metabolism and novel metabolic targets in cancer therapy. Curr. Top. Med. Chem. 2018, 18, 484–493. [Google Scholar] [CrossRef] [PubMed]
- DeBose-Boyd, R.A.; Brown, M.S.; Li, W.P.; Nohturfft, A.; Goldstein, J.L.; Espenshade, P.J. Transport-dependent proteolysis of SREBP: Relocation of site-1 protease from Golgi to ER obviates the need for SREBP transport to Golgi. Cell 1999, 99, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Krycer, J.R.; Kristiana, I.; Brown, A.J. Cholesterol homeostasis in two ommonly used human prostate cancer cell-lines, LNCaP and PC-3. PLoS ONE 2010, 4, e8496. [Google Scholar]
- Adams, C.M.; Reitz, J.; De Brabander, J.K.; Feramisco, J.D.; Li, L.; Brown, M.S.; Goldstein, J.L. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 2004, 279, 52772–52780. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Saha, S.T.; Thomas, J.; Kaur, M. Targeting cellular cholesterol for anticancer therapy. FEBS J. 2019, 286, 4192–4208. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Cortés-Benítez, F.; Roy, J.; Perreault, M.; Maltais, R.; Poirier, D. A- and D-ring structural modifications of an androsterone derivative inhibiting 17β-hydroxysteroid dehydrogenase type 3: Chemical synthesis and structure-activity relationships. J. Med. Chem. 2019, 62, 7070–7088. [Google Scholar] [CrossRef] [PubMed]
- Moreel, X.; Allaire, J.; Leger, C.; Caron, A.; Labonte, M.E.; Lamarche, B.; Julien, P.; Desmeules, P.; Tetu, B.; Fradet, V. Prostatic and dietary omega-3 fatty acids and prostate cancer progression during active surveillance. Cancer Prev. Res. 2014, 7, 766–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gevariya, N.; Besancon, M.; Robitaille, K.; Picard, V.; Diabate, L.; Alesawi, A.; Julien, P.; Fradet, Y.; Bergeron, A.; Fradet, V. Omega-3 fatty acids decrease prostate cancer progression associated with an anti-tumor immune response in eugonadal and castrated mice. Prostate 2019, 79, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.Y. Extension of multiple range tests to roup means with unequal numbers of replications. Biometrics 1956, 12, 307–310. [Google Scholar] [CrossRef]

| RM-581 Tumor (ng/g) | RM-581 Plasma (ng/mL) | Concentration Index * Tumor/Plasma |
|---|---|---|
| 150 ± 71 | 50.5 ± 6.3 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltais, R.; Roy, J.; Perreault, M.; Sato, S.; Lévesque, J.-C.; Poirier, D. Induction of Endoplasmic Reticulum Stress-Mediated Apoptosis by Aminosteroid RM-581 Efficiently Blocks the Growth of PC-3 Cancer Cells and Tumors Resistant or Not to Docetaxel. Int. J. Mol. Sci. 2021, 22, 11181. https://doi.org/10.3390/ijms222011181
Maltais R, Roy J, Perreault M, Sato S, Lévesque J-C, Poirier D. Induction of Endoplasmic Reticulum Stress-Mediated Apoptosis by Aminosteroid RM-581 Efficiently Blocks the Growth of PC-3 Cancer Cells and Tumors Resistant or Not to Docetaxel. International Journal of Molecular Sciences. 2021; 22(20):11181. https://doi.org/10.3390/ijms222011181
Chicago/Turabian StyleMaltais, René, Jenny Roy, Martin Perreault, Sachiko Sato, Julie-Christine Lévesque, and Donald Poirier. 2021. "Induction of Endoplasmic Reticulum Stress-Mediated Apoptosis by Aminosteroid RM-581 Efficiently Blocks the Growth of PC-3 Cancer Cells and Tumors Resistant or Not to Docetaxel" International Journal of Molecular Sciences 22, no. 20: 11181. https://doi.org/10.3390/ijms222011181
APA StyleMaltais, R., Roy, J., Perreault, M., Sato, S., Lévesque, J.-C., & Poirier, D. (2021). Induction of Endoplasmic Reticulum Stress-Mediated Apoptosis by Aminosteroid RM-581 Efficiently Blocks the Growth of PC-3 Cancer Cells and Tumors Resistant or Not to Docetaxel. International Journal of Molecular Sciences, 22(20), 11181. https://doi.org/10.3390/ijms222011181








