BolTLP1, a Thaumatin-like Protein Gene, Confers Tolerance to Salt and Drought Stresses in Broccoli (Brassica oleracea L. var. Italica)
Abstract
1. Introduction
2. Results
2.1. BolTLP1 Was Identified in Broccoli
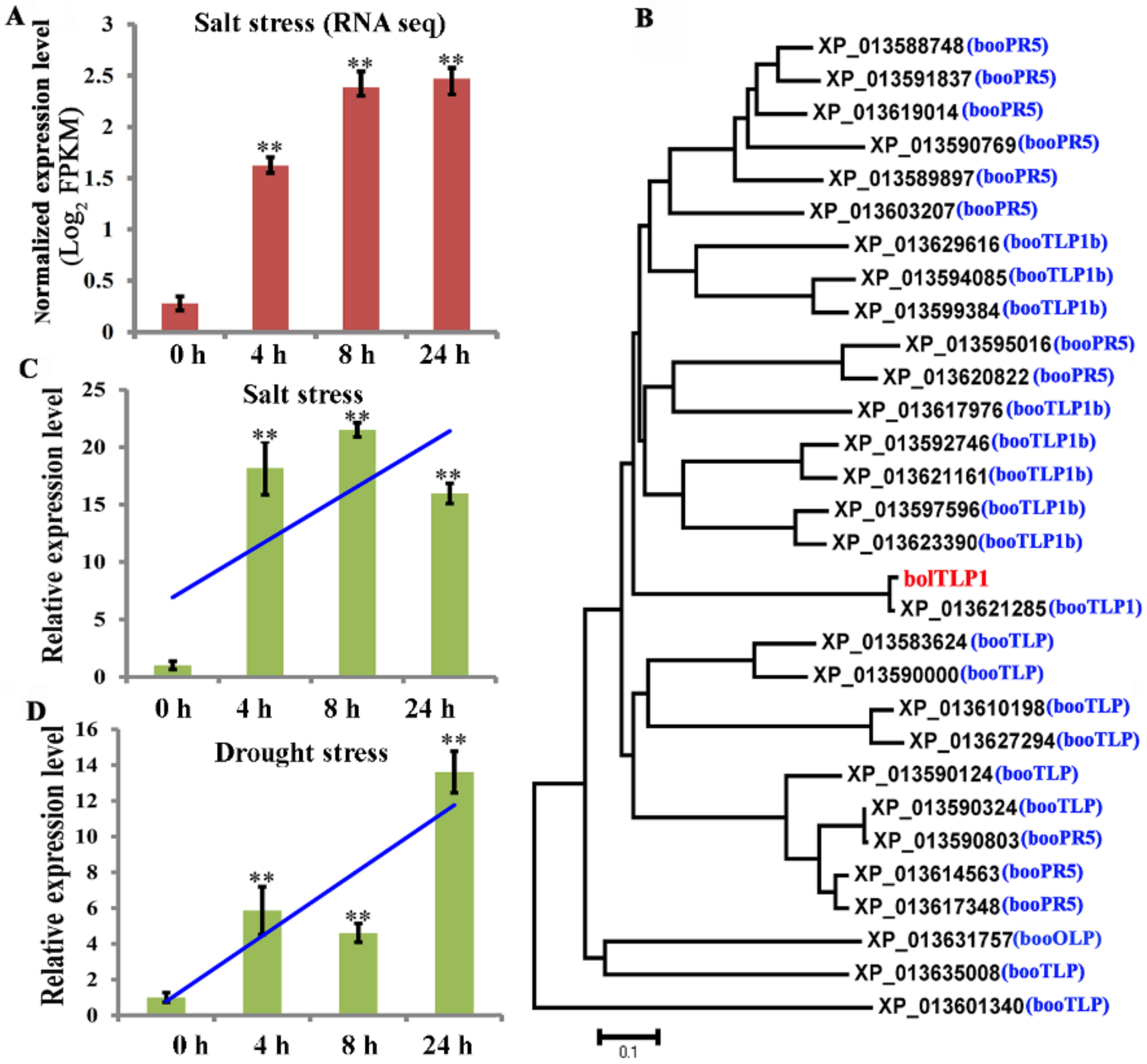
2.2. BolTLP1 Positively Responded to Salt and Drought Stresses in Broccoli
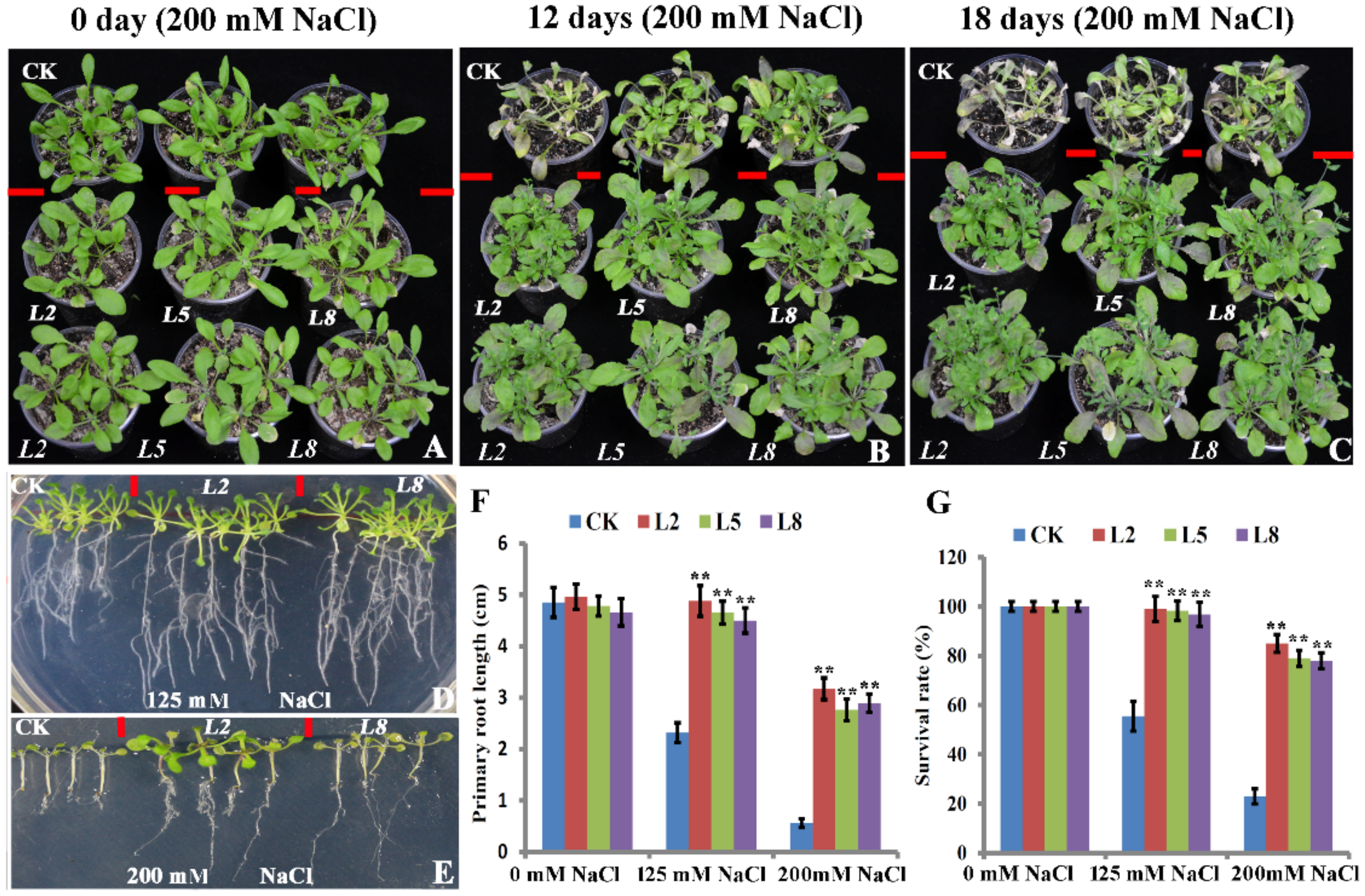
2.3. Overexpression of bolTLP1 Increased Resistance to Salt and Drought Stresses in Arabidopsis
2.4. Transgenic Broccoli Overexpressing bolTLP1 Exhibited High Salt and Drought Tolerance
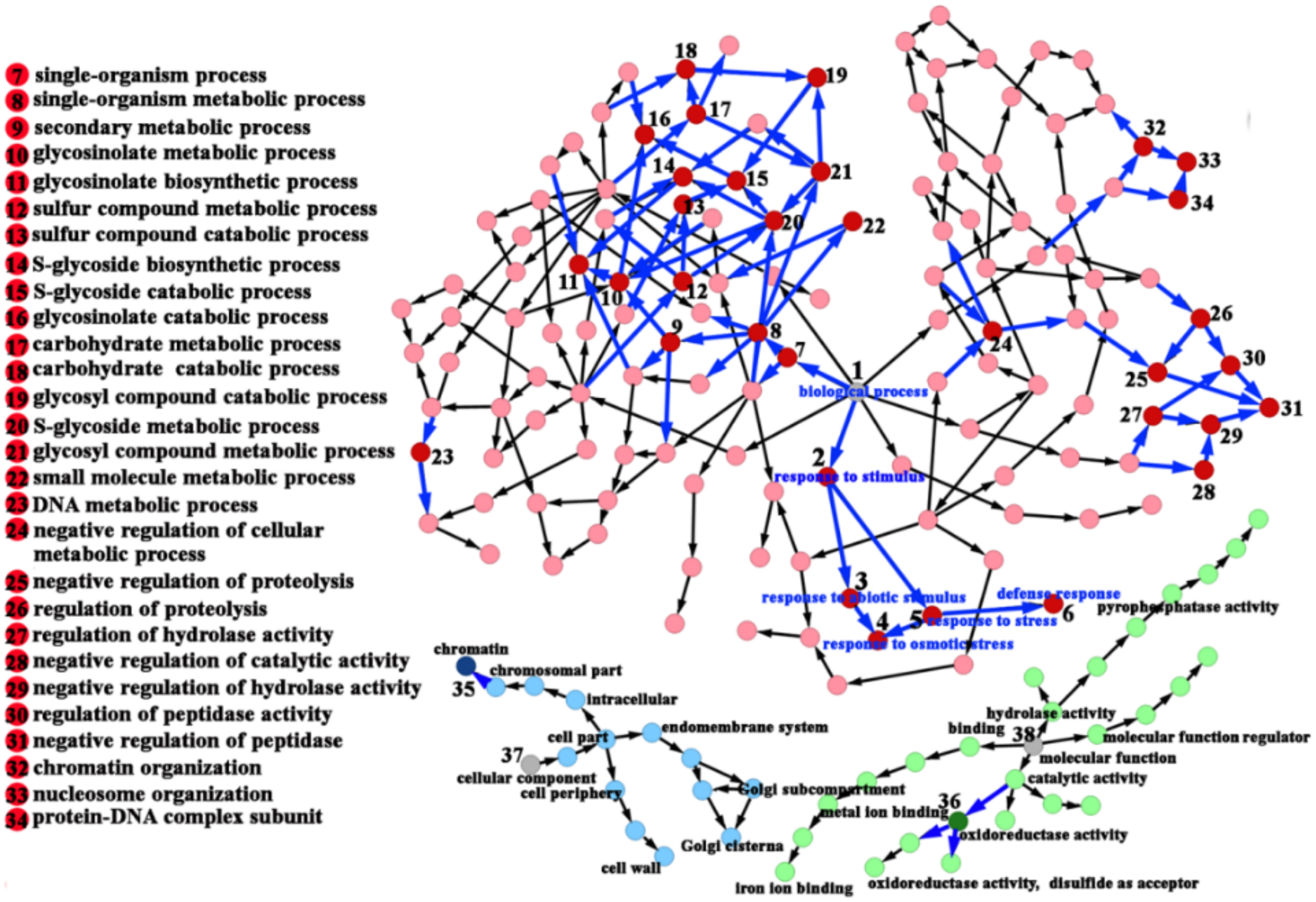
2.5. DEGs Were Confirmed between the 35S::bolTLP1 Broccoli and Vector Control
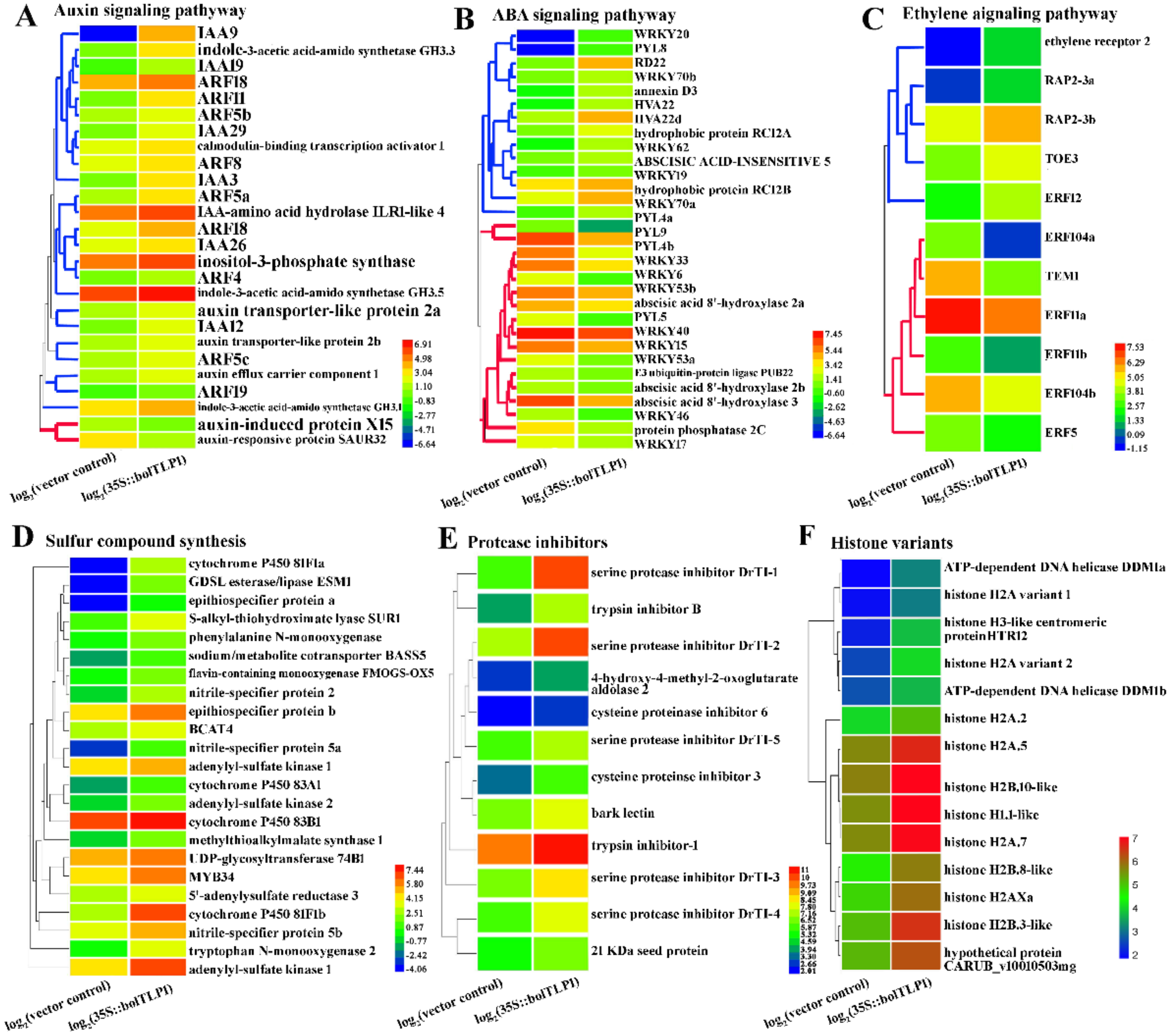
2.6. Expression Profiles of Genes Involved in ABA, Ethylene and Auxin-Mediated Signaling Pathways Were Significantly Altered by Overexpression of bolTLP1
2.7. Genes Involved in Sulfur Compound Synthesis and Hydrolase/Oxidoreductase Activity Were Significantly Inductively Expressed by the Overexpression of bolTLP1
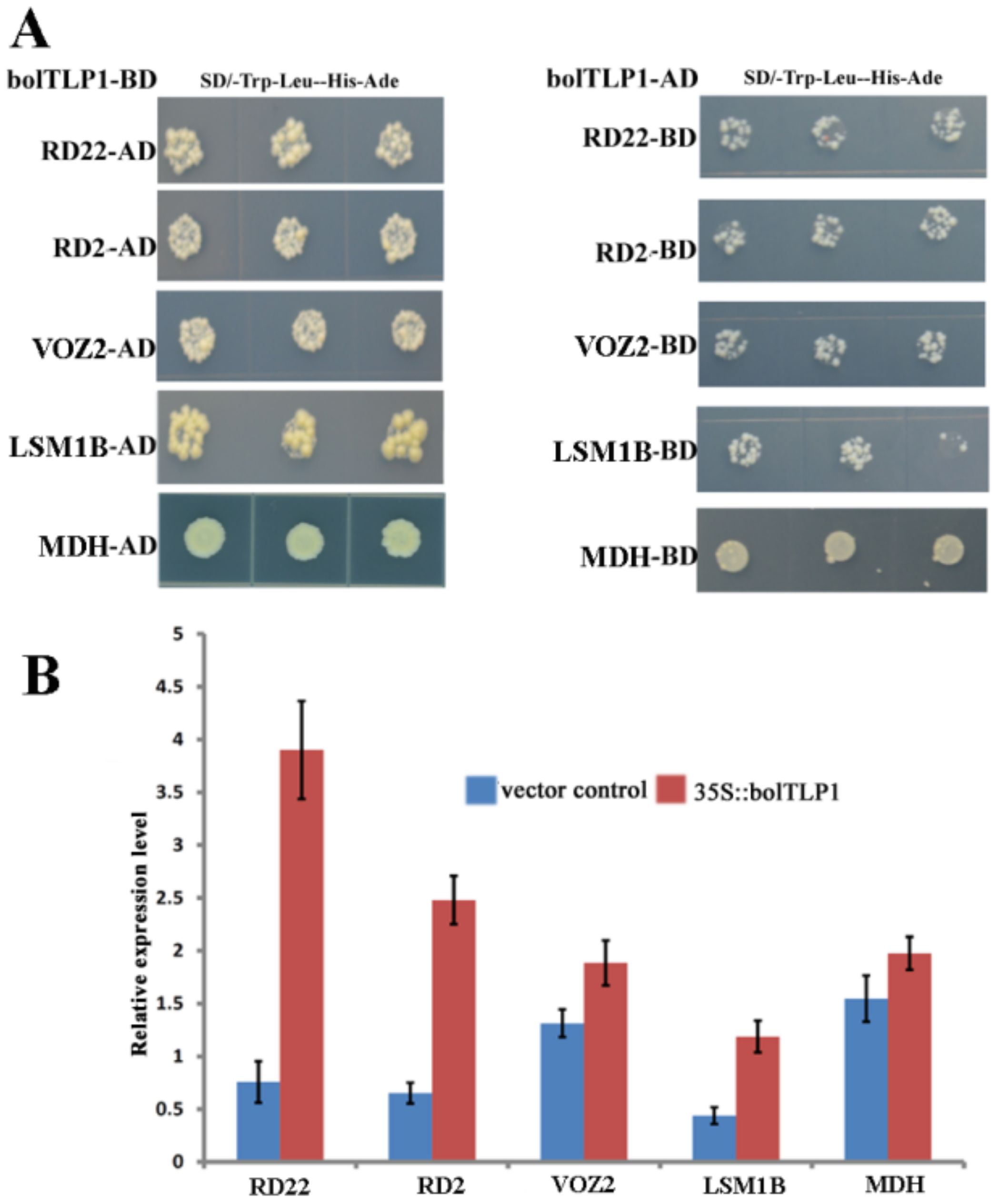
2.8. Five Proteins Involved in Abiotic Stress Responses Were Confirmed to Interact with bolTLP1
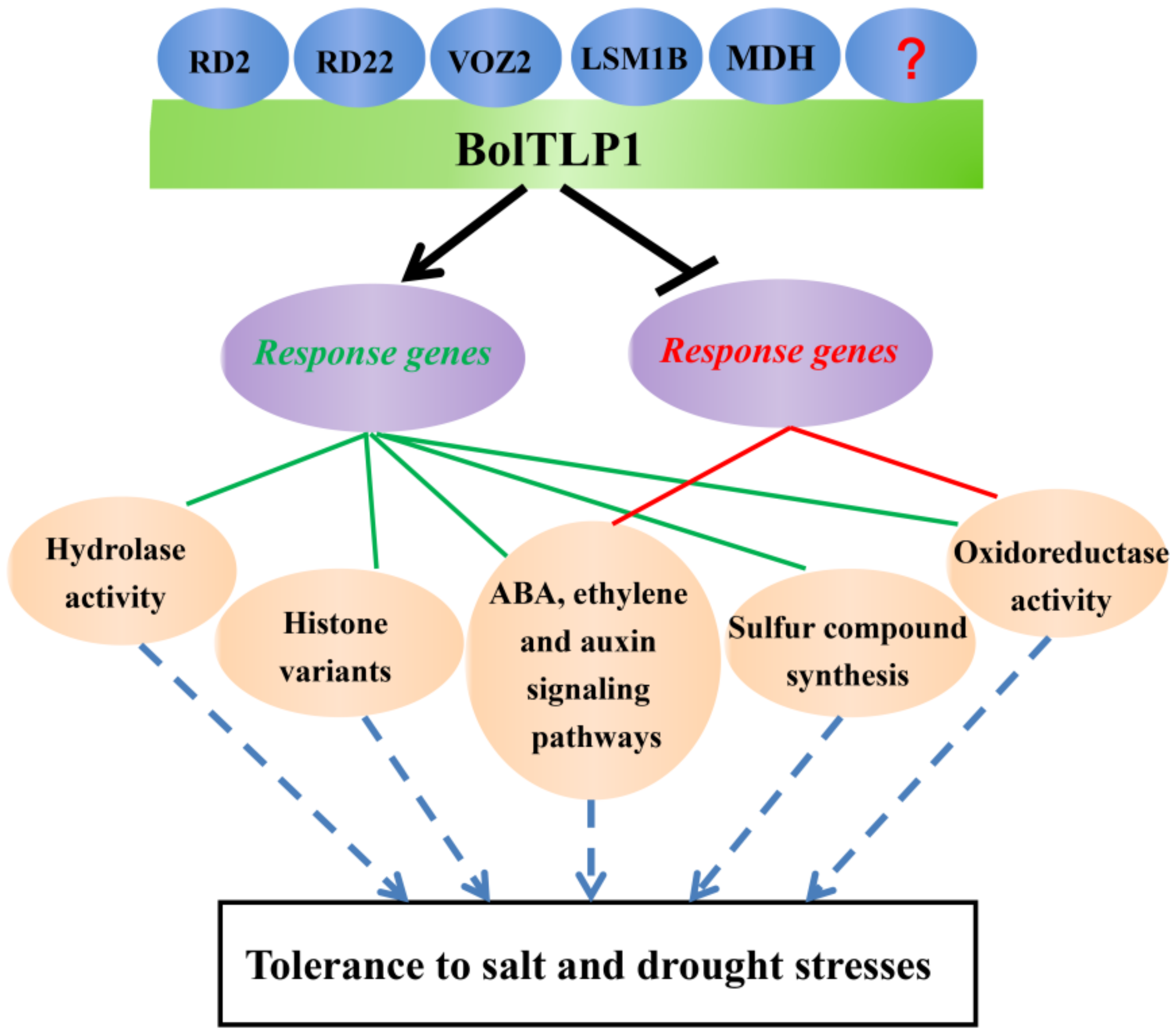
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Stress Treatment
4.2. BolTLP1 Cloning and Phylogenetic Analysis
4.3. BolTLP1 Expression Profile Analysis
4.4. Expression Vector Construction and Genetic Transformation
4.5. Transcriptome Sequencing and Data Analysis
4.6. Differentially Expressed Gene Identification by qRT-PCR
4.7. Yeast Two-Hybrid Screening and Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.J.; Sturrock, R.; Ekramoddoullah, A.K. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, B.J.; Hooft, V.; Bol, J.F. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-testing protein thaumatin. Nature 1986, 32, 1531–1532. [Google Scholar]
- Ghosh, R.; Chakrabarti, C. Crystal structure analysis of NP24-I, A thaumatin-like protein. Planta 2008, 228, 883–890. [Google Scholar] [CrossRef]
- Petre, B.; Major, I.; Rouhier, N.; Duplessis, S. Genome-wide analysis of eukaryote thaumatin-like proteins (TLPs) with an emphasis on poplar. BMC Plant Biol. 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.B.; Cho, B.H.; Næsby, M.; Gregersen, P.L.; Brandt, J.; Madriz-Ordeñana, K.; Collinge, D.B.; Thordal-Christensen, H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 2002, 3, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Iqbal, I.; Tripathi, R.K.; Wilkins, O.; Singh, J. Thaumatin-Like Protein (TLP) gene family in barley: Genome-wide exploration and expression analysis during germination. Genes 2020, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Wang, H.X.; Ng, T.B. First chromatographic isolation of an antifungal thaumatin-like protein from French bean legumes and demonstration of its antifungal activity. Biochem. Biophys. Res. Commun. 1999, 263, 130–134. [Google Scholar] [CrossRef]
- Vitali, A.; Pacini, L.; Bordi, E.; De Mori, P.; Pucillo, L.; Maras, B.; Botta, B.; Brancaccio, A.; Giardina, B. Purification and characterization of an antifungal thaumatin-like protein from Cassia didymobotrya cell culture. Plant Physiol. Biochem. 2006, 44, 604–610. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Liang, F.; Zhang, Y.; Ma, L.; Wang, H.; Liu, D. Functional analysis of a pathogenesis-related thaumatin-like protein gene TaLr35PR5 from wheat induced by leaf rust fungus. BMC Plant Biol. 2018, 18, 76. [Google Scholar] [CrossRef]
- Krebitz, M.; Wagner, B.; Ferreira, F.; Peterbauer, C.; Campillo, N.; Witty, M.; Kolarich, D.; Steinkellner, H.; Scheiner, O.; Breiteneder, H. Plant-based heterologous expression of Mald2, a thaumatin-like protein and allergen of apple (Malus domestica), and its characterization as an antifungal protein. J. Mol. Biol. 2003, 329, 721–730. [Google Scholar] [CrossRef]
- Liu, D.Q.; He, X.; Li, W.X.; Chen, C.Y.; Ge, F. Molecular cloning of a thaumatin-like protein gene from Pyrus pyrifolia and overexpression of this gene in tobacco increased resistance to pathogenic fungi. Plant Cell Tissue Organ Cult. 2012, 111, 29–39. [Google Scholar] [CrossRef]
- Singh, N.K.; Kumar, K.R.; Kumar, D.; Shukla, P.; Kirti, P.B. Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachis diogoi, a wild peanut. PLoS ONE 2013, 8, e83963. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, R.; Zamani, M.; Motallebi, M.; Moradyar, M. Agrobacterium-mediated transformation of the Oryza sativa thaumatin-like protein to canola (R Line Hyola308) for enhancing resistance to Sclerotinia sclerotiorum. Iran. J. Biotechnol. 2017, 15, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Chen, P.D.; Liu, D.J.; Kynast, R.; Friebe, B.; Velazhahan, R.; Muthukrishnan, S.; Gil, B.S. Development of wheat scab symptoms is delayed in transgenic wheat plants that constitutively express a rice thaumatin-like protein gene. Theor. Appl. Genet. 1999, 99, 755–760. [Google Scholar] [CrossRef]
- Mahdavi, F.; Sariah, M.; Maziah, M. Expression of rice thaumatin-like protein gene in transgenic banana plants enhances resistance to fusarium wilt. Appl. Biochem. Biotechnol. 2012, 166, 1008–1019. [Google Scholar] [CrossRef]
- Misra, R.C.; Sandeep; Kamthan, M.; Kumar, S.; Ghosh, S. A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis. Sci. Rep. 2016, 6, 25340. [Google Scholar] [CrossRef]
- Jung, Y.C.; Lee, H.J.; Yum, S.S.; Soh, W.Y.; Cho, D.Y.; Auh, C.K.; Lee, T.K.; Soh, H.C.; Kim, Y.S.; Lee, S.C. Drought-inducible-but ABA-independent-thaumatin-like protein from carrot (Daucus carota L.). Plant Cell Rep. 2005, 24, 366–373. [Google Scholar] [CrossRef]
- Muoki, R.C.; Paul, A.; Kumar, S. A shared response of thaumatin like protein, chitinase, and late embryogenesis abundant protein 3 to environmental stresses in tea Camellia sinensis (L.) O. Kuntze. Funct. Integr. Genom. 2012, 12, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Salzman, R.A.; Tikhonova, I.; Bordelon, B.P.; Hasegawa, P.M.; Bressan, R.A. Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defense response during fruit ripening in grape. Plant Physiol. 1998, 117, 465–472. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Watanabe, H.; Nagai, M.; Nakade, K.; Takahashi, M.; Sato, T. Lentinula edodes tlg1 encodes a thaumatin-like protein that is involved in lentinan degradation and fruiting body senescence. Plant Physiol. 2006, 141, 793–801. [Google Scholar] [CrossRef]
- Damme, E.; Charels, D.; Menu-Bouaouiche, L.; Proost, P.; Barre, A.; Rougé, P.; Peumans, W.J. Biochemical, molecular and structural analysis of multiple thaumatin-like proteins from the elderberry tree (Sambucus nigra L.). Planta 2002, 214, 853–862. [Google Scholar] [CrossRef]
- Skadsen, R.W.; Sathish, P.; Kaeppler, H.F. Expression of thaumatin-like permatin PR-5 genes switches from the ovary wall to the aleurone in developing barley and oat seeds. Plant Sci. 2000, 156, 11–22. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, R.K.; Lemaux, P.G.; Buchanan, B.B.; Singh, J. Redox-dependent interaction between thaumatin-like protein and β-glucan influences malting quality of barley. Proc. Natl. Acad. Sci. USA 2017, 114, 7725–7730. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and Post-harvest Factors Affecting Glucosinolate Content in Broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Molina, J.; Proietti, S.; Pollier, J.; Orozco-Freire, W.; Ramirez-Villacis, D.; Leon-Reyes, A. Induced tolerance to abiotic and biotic stresses of broccoli and Arabidopsis after treatment with elicitor molecules. Sci. Rep. 2020, 10, 10319. [Google Scholar] [CrossRef] [PubMed]
- Chevilly, S.; Dolz-Edo, L.; López-Nicolás, J.M.; Morcillo, L.; Vilagrosa, A.; Yenush, L.; Mulet, J.M. Physiological and molecular characterization of the differential response of broccoli (Brassica oleracea var. Italica) cultivars reveals limiting factors for broccoli tolerance to drought stress. J. Agric. Food Chem. 2021, 69, 10394–10404. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, Y.; Luo, X.; Zhou, T.; Huang, Z.; Liu, Y.; Qiu, Y.; Hou, B.; Sun, D.; Deng, H.; et al. Identification and characterization of microRNAs related to salt stress in broccoli, using high-throughput sequencing and bioinformatics analysis. BMC Plant Biol. 2014, 14, 226. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wu, M.; Li, L.H.; Li, C.; Han, Z.P.; Yuan, J.Y.; Chen, C.B.; Song, W.Q.; Wang, C.G. Genome-wide identification of AP2/ERF transcription factors in cauliflower and expression profiling of the erf family under salt and drought stresses. Front. Plant Sci. 2017, 8, 946. [Google Scholar] [CrossRef]
- Muthusamy, M.; Kim, J.Y.; Yoon, E.K.; Kim, J.A.; Lee, S.I. BrEXLB1, a Brassica rapa expansin-like B1 gene is associated with root development, drought stress response, and seed germination. Genes 2020, 11, 404. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Hu, Y.F.; Li, J.J.; Yao, X.; Liu, K.D. BnaABF2, a bZIP transcription factor from rapeseed (Brassica napus L.), enhances drought and salt tolerance in transgenic Arabidopsis. Bot. Stud. 2016, 57, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, F.; Hao, H.; Zhang, Y.; Zhao, H.; Guo, A.; Hu, J.; Zhou, X.; Xie, C.G. BolOST1, an ortholog of Open Stomata 1 with alternative splicing products in Brassica oleracea, positively modulates drought responses in plants. Biochem. Biophys. Res. Commun. 2013, 442, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Samota, M.K.; Sasi, M.; Awana, M.; Yadav, O.P.; Amitha Mithra, S.V.; Tyagi, A.; Kumar, S.; Singh, A. Elicitor-induced biochemical and molecular manifestations to improve drought tolerance in rice (Oryza Sativa, L.) through seed-priming. Front. Plant Sci. 2017, 8, 934. [Google Scholar] [CrossRef] [PubMed]
- Harshavardhan, V.T.; Van Son, L.; Seiler, C.; Junker, A.; Weigelt-Fischer, K.; Klukas, C.; Altmann, T.; Sreenivasulu, N.; Bäumlein, H.; Kuhlmann, M. AtRD22 and AtUSPL1, members of the plant-specific BURP domain family involved in Arabidopsis thaliana drought tolerance. PLoS ONE 2014, 9, e110065. [Google Scholar] [CrossRef]
- Ijaz, R.; Ejaz, J.; Gao, S.; Liu, T.; Imtiaz, M.; Ye, Z.; Wang, T. Overexpression of annexin gene AnnSp2, enhances drought and salt tolerance through modulation of ABA synthesis and scavenging ROS in tomato. Sci. Rep. 2017, 7, 12087. [Google Scholar] [CrossRef]
- Luo, X.; Bai, X.; Sun, X.; Zhu, D.; Liu, B.; Ji, W.; Cai, H.; Cao, L.; Wu, J.; Hu, M.; et al. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signaling. J. Exp. Bot. 2013, 64, 2155–2169. [Google Scholar] [CrossRef]
- Takeuchi, J.; Okamoto, M.; Mega, R.; Kanno, Y.; Ohnishi, T.; Seo, M.; Todoroki, Y. Abscinazole-E3M, a practical inhibitor of abscisic acid 8’-hydroxylase for improving drought tolerance. Sci. Rep. 2016, 6, 37060. [Google Scholar] [CrossRef]
- Ju, Y.L.; Yue, X.F.; Min, Z.; Wang, X.H.; Fang, Y.L.; Zhang, J.X. VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 146, 98–111. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Fu, Y.; Cheung, M.Y.; Wong, F.L.; Phang, T.H.; Sun, Z.; Lam, H.M. Expression of an apoplast-localized BURP-domain protein from soybean (GmRD22) enhances tolerance towards abiotic stress. Plant Cell Environ. 2012, 35, 1932–1947. [Google Scholar] [CrossRef]
- Galon, Y.; Aloni, R.; Nachmias, D.; Snir, O.; Feldmesser, E.; Scrase-Field, S.; Boyce, J.M.; Bouché, N.; Knight, M.R.; Fromm, H. Calmodulin-binding transcription activator 1 mediates auxin signaling and responds to stresses in Arabidopsis. Planta 2010, 232, 165–178. [Google Scholar] [CrossRef]
- Pandey, N.; Ranjan, A.; Pant, P.; Tripathi, R.K.; Ateek, F.; Pandey, H.P.; Patre, U.V.; Sawant, S.V. CAMTA 1 regulates drought responses in Arabidopsis thaliana. BMC Genom. 2013, 14, 216. [Google Scholar] [CrossRef]
- Kusuda, H.; Koga, W.; Kusano, M.; Oikawa, A.; Saito, K.; Hirai, M.Y.; Yoshida, K.T. Ectopic expression of myo-inositol 3-phosphate synthase induces a wide range of metabolic changes and confers salt tolerance in rice. Plant Sci. 2015, 232, 49–56. [Google Scholar] [CrossRef]
- Das-Chatterjee, A.; Goswami, L.; Maitra, S.; Dastidar, K.G.; Ray, S.; Majumder, A.L. Introgression of a novel salt-tolerant L-myo-inositol 1-phosphate synthase from Porteresia coarctata (Roxb.) Tateoka (PcINO1) confers salt tolerance to evolutionary diverse organisms. FEBS. Lett. 2006, 580, 3980–3988. [Google Scholar] [CrossRef] [PubMed]
- Papdi, C.; Pérez-Salamó, I.; Joseph, M.P.; Giuntoli, B.; Bögre, L.; Koncz, C.; Szabados, L. The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor VII genes RAP2.12, RAP2.2 and RAP2.3. Plant J. 2015, 82, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Skirycz, A.; Claeys, H.; Maleux, K.; Dhondt, S.; De Bodt, S.; Vanden Bossche, R.; De Milde, L.; Yoshizumi, T.; Matsui, M.; et al. Ethylene Response Factor 6 acts as a central regulator of leaf growth under water-limiting conditions in Arabidopsis. Plant Physiol. 2013, 162, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Xing, D.; Reddy, A. Vascular plant one-zinc-finger (VOZ) transcription factors are positive regulators of salt tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 3731. [Google Scholar] [CrossRef]
- Wawer, I.; Golisz, A.; Sulkowska, A.; Kawa, D.; Kulik, A.; Kufel, J. mRNA Decapping and 5’-3’ decay contribute to the regulation of aba signaling in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 312. [Google Scholar] [CrossRef]
- Kandoi, D.; Mohanty, S.; Tripathy, B.C. Overexpression of plastidic maize NADP-malate dehydrogenase (ZmNADP-MDH) in Arabidopsis thaliana confers tolerance to salt stress. Protoplasma 2018, 255, 547–563. [Google Scholar] [CrossRef]
- Koiwa, H.; Bressan, R.A.; Hasegawa, P.M. Regulation of protease inhibitors and plant defense. Trends. Plant. Sci. 1997, 2, 379–384. [Google Scholar] [CrossRef]
- Breitenbach Barroso Coelho, L.C.; Marcelino Dos Santos Silva, P.; Felix de Oliveira, W.; de Moura, M.C.; Viana Pontual, E.; Soares Gomes, F.; Guedes Paiva, P.M.; Napoleão, T.H.; Dos Santos Correia, M.T. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018, 125, 1238–1252. [Google Scholar] [CrossRef]
- Srinivasan, T.; Kumar, K.R.; Kirti, P.B. Constitutive expression of a trypsin protease inhibitor confers multiple stress tolerance in transgenic tobacco. Plant Cell Physiol. 2009, 50, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Quain, M.D.; Makgopa, M.E.; Márquez-García, B.; Comadira, G.; Fernandez-Garcia, N.; Olmos, E.; Schnaubelt, D.; Kunert, K.J.; Foyer, C.H. Ectopic phytocystatin expression leads to enhanced drought stress tolerance in soybean (Glycine max) and Arabidopsis thaliana through effects on strigolactone pathways and can also result in improved seed traits. Plant Biotechnol. J. 2014, 12, 903–913. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Takano, T. Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol. Biol. 2008, 68, 131–143. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Cheng, Z.; Xia, G.; Wang, M. A wheat histone variant gene TaH2A.7 enhances drought tolerance and promotes stomatal closure in Arabidopsis. Plant Cell Rep. 2016, 35, 1853–1862. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Xu, T.; Srivastava, A.K.; Wang, D.; Zeng, L.; Yang, L.; He, L.; Zhang, H.; Zheng, Z.; et al. The chromatin remodeler DDM1 promotes hybrid vigor by regulating salicylic acid metabolism. Cell Discov. 2016, 2, 16027. [Google Scholar] [CrossRef]
- Yao, Y.; Bilichak, A.; Golubov, A.; Kovalchuk, I. ddm1 plants are sensitive to methyl methane sulfonate and NaCl stresses and are deficient in DNA repair. Plant Cell Rep. 2012, 31, 1549–1561. [Google Scholar] [CrossRef]
- Bednarek, P.; Osbourn, A. Plant–microbe interactions: Chemical diversity in plant defense. Science 2009, 324, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kensler, T.W.; Cho, C.G.; Posner, G.H.; Talalay, P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.; Moreno-Fernández, D.A.; Castejón, D.; Ochando, C.; Morandini, P.A.; Carvajal, M. The impact of the absence of aliphatic glucosinolates on water transport under salt stress in Arabidopsis thaliana. Front. Plant. Sci. 2015, 6, 524. [Google Scholar] [CrossRef]
- Seo, M.S.; Jin, M.; Sohn, S.H.; Kim, J.S. Expression profiles of BrMYB transcription factors related to glucosinolate biosynthesis and stress response in eight subspecies of Brassica rapa. FEBS Open. Bio. 2017, 7, 1646–1659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, C.; Zhu, F.; Li, Y. Mild osmotic stress promotes 4-methoxy indolyl-3-methyl glucosinolate biosynthesis mediated by the MKK9-MPK3/MPK6 cascade in Arabidopsis. Plant Cell Rep. 2017, 36, 543–555. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.D.C.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Li, L.; Zhu, Y.; Pan, Y.; Zhang, X.; Han, X.; Li, M.; Chen, C.; Li, H.; Wang, C. BolTLP1, a Thaumatin-like Protein Gene, Confers Tolerance to Salt and Drought Stresses in Broccoli (Brassica oleracea L. var. Italica). Int. J. Mol. Sci. 2021, 22, 11132. https://doi.org/10.3390/ijms222011132
He L, Li L, Zhu Y, Pan Y, Zhang X, Han X, Li M, Chen C, Li H, Wang C. BolTLP1, a Thaumatin-like Protein Gene, Confers Tolerance to Salt and Drought Stresses in Broccoli (Brassica oleracea L. var. Italica). International Journal of Molecular Sciences. 2021; 22(20):11132. https://doi.org/10.3390/ijms222011132
Chicago/Turabian StyleHe, Lixia, Lihong Li, Yinxia Zhu, Yu Pan, Xiuwen Zhang, Xue Han, Muzi Li, Chengbin Chen, Hui Li, and Chunguo Wang. 2021. "BolTLP1, a Thaumatin-like Protein Gene, Confers Tolerance to Salt and Drought Stresses in Broccoli (Brassica oleracea L. var. Italica)" International Journal of Molecular Sciences 22, no. 20: 11132. https://doi.org/10.3390/ijms222011132
APA StyleHe, L., Li, L., Zhu, Y., Pan, Y., Zhang, X., Han, X., Li, M., Chen, C., Li, H., & Wang, C. (2021). BolTLP1, a Thaumatin-like Protein Gene, Confers Tolerance to Salt and Drought Stresses in Broccoli (Brassica oleracea L. var. Italica). International Journal of Molecular Sciences, 22(20), 11132. https://doi.org/10.3390/ijms222011132




