Abstract
One of the most common chronic liver disorders, affecting mainly people in Western countries, is nonalcoholic fatty liver disease (NAFLD). Unfortunately, its pathophysiological mechanism is not fully understood, and no dedicated treatment is available. Simple steatosis can lead to nonalcoholic steatohepatitis and even to fibrosis, cancer, and cirrhosis of the liver. NAFLD very often occurs in parallel with type 2 diabetes mellitus and in obese people. Furthermore, it is much more likely to develop in patients with metabolic syndrome (MS), whose criteria include abdominal obesity, elevated blood triacylglycerol level, reduced high-density lipoprotein cholesterol level, increased blood pressure, and high fasting glucose. An important phenomenon in MS is also insulin resistance (IR), which is very common in NAFLD. Liver IR and NAFLD development are linked through an interaction between the accumulation of free fatty acids, hepatic inflammation, and increased oxidative stress. The liver is particularly exposed to elevated levels of reactive oxygen species due to a large number of mitochondria in hepatocytes. In these organelles, the main DNA repair pathway is base excision repair (BER). The present article will illustrate how impairment of BER may be related to the development of NAFLD.
1. Introduction
One of the most common liver disorders is nonalcoholic fatty liver disease (NAFLD). Approximately 30% of the global population suffer from this condition [1]. Although it is a common ailment, the molecular mechanism responsible for its development remains elusive, and hence the only treatment is based on diets and medications administered to relieve the symptoms. A key problem is that NAFLD does not present any signs throughout the course of its development, and only non-specific symptoms such as abdominal pain or weakness are typically observed in its advanced state [2].
NAFLD is mainly associated with type 2 diabetes (T2DM) but is also related to metabolic syndrome (MS) [3]. The criteria for determining MS overlap with the symptoms of NAFLD, such as abdominal obesity, elevated triacylglycerols (TG) level in the blood, decreased high-density lipoprotein (HDL) cholesterol, increased blood pressure, and high fasting glucose. These elevated biochemical parameters are most often associated with an inadequate diet and lifestyle, which can result in liver damage, as well as other diseases such as obesity [4].
The main common denominator of NAFLD and MS symptoms is insulin resistance (IR) [5]. It is a major component in the two-hit hypothesis explaining the mechanism of the progression of simple steatosis (NAFL) to more aggressive nonalcoholic steatohepatitis (NASH), in which the increasing level of fibrosis causes cirrhosis and even liver cancer. The first hit is based on the combination of IR with fat accumulation in hepatocytes, while the second hit comprises inflammation, damage to liver cells, and consequent fibrosis. However, the two-hit hypothesis is not a sufficient explanation of the progression mechanism, as genetic and epigenetic factors may also be involved, as well as the intestinal microflora [6].
The key aspects in the development of NAFLD are the presence of liver inflammation, increased oxidative stress, and fat accumulation; these are linked through IR. For example, oxidative stress is known to enhance inflammation and play an important role in the IR process [7]. High levels of reactive oxygen species (ROS) are formed in mitochondria due to the functioning of the electron transport chain (ETC). This elevates the level of oxidative stress near the mitochondrial DNA (mtDNA), thus increasing the likelihood of DNA damage [8]. If the DNA repair pathways become impaired, this would lead to more stress and, consequently, to an increased IR. The main DNA repair system in mitochondria is base excision repair (BER): a complex process whose activity involves a range of proteins, and failure to perform even one of its steps results in a reduction in the efficiency of the process [9].
The exact molecular mechanisms of the development and progression of NAFLD remain elusive, particularly the impact of BER on IR. The present article provides an overview of the current state of knowledge regarding the relationship between IR, inflammation, oxidative stress, mitochondrial dysfunction, BER, and metabolic syndrome in NAFLD. We suspect that the DNA repair pathway may play an important role in the pathomechanism of NAFLD, and it is closely associated with the molecular background of the disease. A search was made of PubMed to identify papers focusing on the molecular background of NAFLD in animal models and human subjects. The following keywords were applied: NAFLD, fatty liver, nonalcoholic fatty liver, nonalcoholic steatohepatitis, Kupffer cells, insulin resistance, metabolic syndrome, T2DM, obesity, liver inflammation, mitochondrial dysfunction, oxidative stress, high-fat diet, methionine and choline deficient diet, fat accumulation, free fatty acids, BER, and DNA repair.
2. Development of NAFLD
The development of NAFLD is influenced by various factors, which are also responsible for its progression and conversion to NASH, characterized by chronic inflammation in the liver. The most important factors of NAFLD have increased lipolysis of triglycerides (TG) and the release of free fatty acids (FFA), which are taken up by the liver [1]. Elevated FFA levels also indirectly contribute to the development of inflammation and are known to exacerbate IR. In addition, oxidative stress may also be a critical factor in the development of inflammation and IR. These disturbances are reminiscent of a metabolic disorder. Due to this fact, NAFLD is called the hepatic manifestation of a MS [10].
2.1. Epidemiology and Etiology
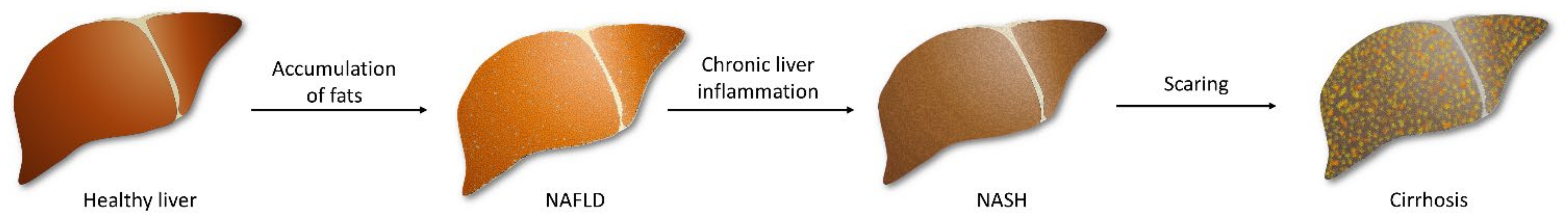
NAFLD is characterized by lipid accumulation in hepatocytes and abnormal liver enzyme levels. Steatosis is diagnosed when at least 5% of liver cells contain excess fat [11], particularly in people who do not drink alcohol or limit their consumption to 30 g of alcohol (men) and 20 g (women) per day [12]. Furthermore, about one-fifth of NAFLD patients will develop NASH as a result of chronic liver inflammation [13], characterized by hepatocellular injury and the characteristic ballooning of hepatocytes [14]. Small lesions could easily be regenerated by the liver, but prolonged inflammation causes fibrosis, resulting in scarring of the liver, which can lead to cirrhosis and liver failure. Unfortunately, progressive lesions are also able to trigger hepatocellular carcinoma (Figure 1) [15].
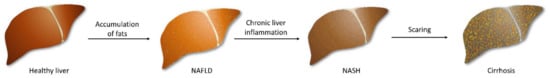
Figure 1.
The development of fatty liver beginning with the accumulation of fats in primarily healthy hepatocytes. Prolonged inflammation in the fatty liver leads to steatosis and fibrosis of tissue. The following scaring of the liver triggers irreversible damage, and cirrhosis occurs.
NAFLD is more common in older people [16], and men tend to demonstrate greater frequency and severity than women. However, the incidence increases among postmenopausal women [2]. Moreover, the condition is the most widespread in people living in Western countries and is more prevalent in people of Asian, Hispanic, or Native American ancestry than in Eastern Europeans or Africans [17]. It is speculated that this might be associated with the diet of various populations.
As mentioned above, NAFLD does not show noticeable symptoms for a long time, and these become visible only when the liver is significantly enlarged; furthermore, many of these symptoms are non-specific, such as abdominal discomfort, pain, or fatigue [18]. NAFLD is often diagnosed during laboratory tests when increased levels of the liver enzymes alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (GGTP) are present. Furthermore, the diagnostic picture of the disease resembles that of metabolic syndrome (MS), i.e., low level of HDL cholesterol, increased level of TG in the blood, hypertension, hyperglycemia, and IR [19]. However, the gold standard of NAFLD diagnosis is liver biopsy. Nevertheless, the other effective methods should be searched and applied, as the surgery is invasive and may trigger complications [20].
NAFLD is closely associated with MS and hence is often referred to as metabolic-associated fatty liver disease (MAFLD) [21]. This relationship is also reflected by the similarity of the conditions that occur in both disorders. However, equally important is the fact that NAFLD very often coexists with T2DM [22]. The key common denominator linking NAFLD and T2DM is IR. However, apart from MS and T2DM, other risk factors are obesity (playing an important role in the MS), physical inactivity, and a Western diet rich in fats and carbohydrates, eaten irregularly and too abundantly. The first factor contributes the most to the disease. Obese people demonstrate excess accumulation of fats in adipocytes, which is the essence of steatosis, and even up to 74% of obese individuals have fatty livers. Moreover, obesity is also associated with NAFLD risk factors such as lack of exercise and unhealthy nutrition. A sedentary lifestyle along with an inadequate high-calorie diet, including high fructose and saturated fat intake, can trigger the development of fatty liver [2].
Studies indicate that NAFLD also has a genetic basis. The first gene found to be related to NAFLD was patatin-like phospholipase domain-containing 3 (PNPLA3). It was shown that the rs738409 (c.444C>G) missense variant I148M in this gene, i.e., the replacement of isoleucine with methionine, occurring in the catalytic patatin domain (position 148), a highly-conserved location in vertebrates, was strongly associated with hepatic inflammation and increased liver fat levels [23]. Patatin catalyzes the cleavage of fatty acids from membrane lipids [24]. PNPLA3 encodes triacylglycerol lipase, also known as adiponutrin, belonging to the patatin-like family. The function of this molecule is not well understood, but it is related to the metabolism of TG in adipocytes. The I148M variant present in NAFLD reduces lipolytic activity against TG, possibly due to limited substrate availability [25]. This leads to decreased lipolysis of fats, and their increased production, in the liver [26]. The I148M variant is also associated with an increased likelihood of hepatocellular carcinoma occurrence [27]. Recent studies have also found other genes to influence NAFLD, e.g., TM6SF2, GCKR, or HSD17B13. Their products are involved in the synthesis of cholesterol, lipogenesis by regulating the influx of glucose into hepatocytes, and modulation of serum liver enzyme levels, respectively [28]. Moreover, the E167K variant (Glu167Lys) in TM6SF2 enhances hepatic steatosis and the progression of fibrosis [27]. Increasingly, new research studies indicate a link between NAFLD and the products of various genes.
2.2. FFA Accumulation
A Western diet, rich in high-fat and high-sucrose products, may lead to increased FFA accumulation and, in consequence, cause hepatic disorders such as NAFLD [29,30]. Accordingly, a case-control study showed that patients suffering from NAFLD had significantly higher serum FFA profiles compared to healthy controls [31]. There are several possible mechanisms of fatty accumulation in NAFLD, including (i) elevated de novo synthesis of fatty acids in hepatocytes, (ii) β-oxidation of fatty acids, (iii) excess dietary fat and carbohydrate intake, and (iv) retention of lipids due to impaired hepatocyte apolipoprotein [30].
Lipogenesis enables the liver to synthesizes new fatty acids from non-lipid substrates, like sucrose and glucose. Briefly, carbohydrates are converted to acetyl-CoA, which is a precursor for new fatty acids, which subsequently can be esterified and stored as TG. Acetyl-CoA carboxylase (ACC) and fatty acid synthase (FASN) are the main enzymes involved in this pathway. Patients suffering from NAFLD were found to have higher non-esterified fatty acid (NEFA) concentrations but normal NEFA turnover rates. Moreover, an increase in the contribution of hepatic lipogenesis to TG secretion was observed in patient plasma [32]. Additionally, patients with NAFLD have a higher synthesis of fatty acids as compared to healthy controls [33].
De novo lipogenesis is mainly regulated by the sterol regulatory element-binding transcription factor 1c (SREBP1c) and the carbohydrate response element-binding protein (ChREBP) [34,35,36]. SREBP1c is activated by insulin and induces the expression of genes involved in glucose utilization, while ChREBP, which is activated by glucose, is mostly expressed in active sites of de novo lipogenesis, such as hepatocytes and adipocytes [37]. In vivo studies indicate that SREBP1c knockout mice have decreased expression of lipogenic enzymes, whereas transgenic mice overexpressing SREBP1c have higher hepatic TG levels and insulin levels, indicating IR [38,39]. Interestingly, patients with NAFLD have been found to demonstrate elevated SREBP1 expression [40]. Furthermore, mice with ChREBP knockout have shown fructose, glucose as well as sucrose intolerance. Such fructose intolerance can be associated with the reduction in fructokinase and triose kinase expression [41]. Moreover, a connection has been noted between sucrose intolerance and decreased expression of intestinal sucrose-isomaltase (SI), the glucose transporters 5 (GLUT5) and 2 (GLUT2), as well ketohexokinase (Khk) [42]. Furthermore, de novo lipogenesis, and as a result, NAFLD are frequently associated with the IR state, like obesity and type 2 diabetes [43,44].
Obese subjects demonstrate an increased ratio of hepatic lipogenesis to the circulating TG pool. Furthermore, energy restriction in obese patients was found to decrease plasma insulin and leptin levels and inhibit normalized hepatic lipogenesis [43]. The increase in hepatic lipogenesis is responsible for the phenotype of IR in the lipid-deficient (ob/ob) mice. Liver-specific inhibition of ChREBP in ob/ob mice decreased lipogenic rates, which was correlated with reduction in hepatic levels of TG and NEFA, and, as a consequence, improved hepatic steatosis [45]. Liver biopsies from patients with NASH demonstrated increased ChREBP expression, where steatosis was greater than 50% and decreased expression in the presence of severe IR [46]. Moreover, mice overexpressing ChREBP fed standard diet remained insulin sensitive, despite increased expression of lipogenesis genes, whereas mice overexpressing ChREBP fed a high-fat diet presented normal insulin levels and improved glucose tolerance as compared to controls, despite having greater hepatic steatosis [46]. Furthermore, mice with liver-specific deletion of acetyl-CoA carboxylase isoform 1 (ACC1) had 40%–70% less TG accumulation; however, after receiving a fat-free diet, the levels of lipogenic enzymes, including FASN, were upregulated [47]. To summarize, many studies indicate that elevated de novo lipogenesis is a major mechanism involved in NAFLD pathophysiology.
Another mechanism of fatty acid accumulation involves β-oxidation. Fatty acid oxidation (FAO) is mainly regulated by peroxisome proliferator-activated receptor γ (PPARγ) and reduces intrahepatic fat levels by utilizing lipids as an energy source [48]. Mice with a high-fat and methionine- and choline-deficient (MCD) diet develop steatohepatitis, which is histologically similar to human metabolic steatohepatitis. Administration of the PPARγ agonist Wy-14,643 significantly decreased the levels of ALT and hepatic lipoperoxides, and improved steatohepatitis [49]. Furthermore, PPARγ knockout mice fed the MCD diet developed more severe steatohepatitis than wild-type (WT) mice and were unaffected by Wy-14,643. The results also indicate that activation of PPARγ correlates with hepatic lipid turnover [50], suggesting that MCD diet-induced fibrosing steatohepatitis can be reversed by PPARγ agonist treatment. Additionally, pharmacological PPARγ activation improves the metabolic milieu and steatosis; it also suppresses NF-κB and c-Jun N-terminal kinase (JNK) activation, neutrophil, and F4/80 macrophage recruitment in diabetes-related NASH [51]. PPARα also plays an important role in insulin sensitivity in liver disorders. Three selective PPARα agonists reduced IR without having harmful effects on body weight and adipose tissue mass in animal models of high-fat diet-induced and genetic IR [52]. Additionally, PPARα-null and apoE-null mice fed a high-fat diet had increased suppression of endogenous glucose production in hyperinsulinemic clamp experiments, reflecting less IR in the absence of PPARα [53].
Finally, excess dietary fat and carbohydrate intake also take part in the NAFLD pathophysiology [54,55]. Many studies confirmed that a long-term high-fat diet promotes the development of NAFLD [56,57,58]. Patients with NAFLD consume less n-3 polyunsaturated fatty acids (PUFAs) and a higher n-6/n-3 PUFA ratio than healthy controls [59,60]. It is important to note that n-3 PUFAs have an anti-inflammatory effect, regulate hepatic lipid consumption, and improve insulin sensitivity [61], while n-6 PUFAs are considered pro-inflammatory molecules and contribute to hepatic steatosis [62]. Furthermore, fructose, which is the main component in sucrose and high fructose corn syrup, is a major mediator of NAFLD [63,64]. Two meta-analyses have identified a strong association between sugar-sweetened beverage consumption and increased risk of NAFLD [65,66]. Even a short-term carbohydrate overfeeding had a significant lipogenic effect on the liver [67]. Another study showed that fructose participates in both increased lipogenesis and impaired fat oxidation [63]. Moreover, a linkage has been found between the occurrence of fatty liver and the metabolism of fructose by fructokinase C, resulting in ATP consumption, nucleotide turnover, and the generation of uric acid that mediates fat accumulation [63]. In conclusion, there is strong evidence suggesting that the limitation of fat and sugar from daily diet may have a positive effect on reducing hepatic fat accumulation.
2.3. Inflammation
Obesity, the leading cause of NAFLD, is characterized by increased lipid accumulation in the adipose and liver tissues. The resulting elevated FFA levels are responsible for lipotoxicity and dysfunction in adipose tissue [1]. Disturbances in lipid metabolism, consequent IR, and the presence of gut-derived endotoxins contribute to the production and release of proinflammatory TNF-α, IL-1b, and IL-6 [68]. These cytokines inhibit the signaling of insulin receptors and lower insulin sensitivity in the liver, which leads to the induction of steatosis and fibrosis [69].
In the liver, about 10% of all hepatic cells are Kupffer cells, which are macrophages characterized by increased production of proinflammatory cytokines [70]. They mediate the activation of T cells and the regulation of hepatocyte apoptosis [71]. Due to their direct contribution to inducing inflammation, they play an important role in the development of NASH. Kupffer cells appear as proinflammatory M1 cells and anti-inflammatory M2 cells. The former promotes liver steatosis, fibrogenesis, and elevation of hepatic lipid accumulation, while the latter are resident macrophages of the liver and are responsible for activating M1 apoptosis in a caspase-3-dependent manner [72,73].
Under the influence of proinflammatory resident factors, i.e., interferon-gamma (IFNγ) and ligands of toll-like receptor (TLR), M2 macrophages undergo classical activation to M1 [74]. This follows the production of inflammatory cytokines, including TNF-α and chemokines [74,75]. Chemokines, as a family of cytokines activating leukocyte chemotaxis, contribute to the development of inflammation and secondarily IR. The attachment of the chemokine ligand 2 (CCL-2) to the chemokine receptor 2 (CCR2) results in the migration of the bone marrow-derived macrophages to the liver or adipose tissue [76]. CCL-2 expression is elevated in the adipose tissues of obese patients [77]. Other chemokines important in liver steatosis, RENTES/CCL5, CXCL8, CXCL9, and CXCL10, are significantly elevated in the case of NASH. Additionally, they are involved in cases of increased hepatic fat accumulation, which results in dysregulation of the lipid metabolism [78,79,80].
To relieve inflammation, M1 is stimulated by Il-4 and IL-13 to induce M2 activation [74]. M2 neutralizes proinflammatory cells by apoptosis. This process is critical to the attenuation of the inflammatory response present in NAFLD. The M1/M2 balance is important for regulating inflammation in the liver [73]; however, the M1/M2 ratio is increased during NAFLD progression [81].
Another transcription factor responsible for inducing inflammation is NF-κB. In the liver of the animal models of NAFLD and NASH patients, the NF-κB signaling pathway is continuously active, and its IKK2 subunit is upregulated [82,83]. This upregulation results in the occurrence of chronic inflammation and IR and promotes the development of hepatocellular carcinoma [6].
2.4. ER Stress
Hepatocytes are rich in the endoplasmic reticulum (ER) [84], which plays a crucial role in protein and lipid biosynthesis. Any disturbances in its functioning may lead to the occurrence of ER stress. This kind of stress stimulates the signaling pathways that induce lipotoxicity, inflammation, and apoptosis [85]. Presumably, as ER stress appears to have an influence on alterations in lipogenesis, it may also contribute to the occurrence of IR. Thus, one can assume that the presence of ER stress has an impact on NAFLD and NASH.
An important process relevant to NAFLD is the unfolded protein response (UPR) [86], i.e., the physiological response of the cell to inappropriate protein maturation, folding, or transfer, caused by a high-fat diet, among others [87]. The aim of the UPR is to increase protein folding and thus stabilize the functioning of the cell. It also contributes to a reduction in protein synthesis, increased degradation of unfolded ones, and upregulation of chaperones, which are responsible for the folding of proteins [88,89]. Moreover, it is responsible for the phosphorylation of Nrf2, by which it activates the transcription of antioxidant enzymes to lower the ROS level [90,91]. However, prolonged UPR activity has a negative effect on energy metabolism and results in the induction of proinflammatory and proapoptotic pathways [12]. Moreover, UPR is also responsible for the induction of ER stress due to the initiation of UPR signal proteins: activating transcription factor 6 (ATF6), pancreatic ER kinase (PKR)-like ER kinase (PERK), and inositol-requiring enzyme 1 (IRE1) [92]. PERK activation blocks the synthesis of proteins, whereas ATF6, together with IRE1, upregulates the expression of ER chaperones genes. IRE1 promotes the mRNA splicing of X box-binding protein 1 (XBP1), and the spliced variant of XBP1 and ATF6 acts as transcription factors. In hepatocytes, XBP1 regulates lipogenesis in the liver and contributes to VLDL. In NAFLD patients, the level of XBP1 is elevated. Mice fed with fructose and with depletion of XBP1 have reduced hepatic lipid accumulation and IR due to JNK signaling activation resulting from IRE1 interactions. XBP1 level is also elevated in patients with T2DM [84].
Recent studies confirm the association between ER stress and IR. ER stress is characterized by the activation of JNK and NF-κB, which play important roles in the inflammation process and IR. JNK induces the phosphorylation of serine (Ser307) in the insulin receptor substrate 1 (IRS1), which impairs insulin signaling [93,94]. In addition, ER stress activates the tribbles 3 (TRB3) protein, by which the Akt/PKB signaling pathway is inhibited, and then the insulin-stimulated glucose uptake is reduced, which may lead to IR [95].
ER stress has been detected in animal models of obesity and animals fed with MCD diet [85,96,97]. Moreover, rapid onset and progression of steatohepatitis were observed in animal models with Nrf2 (factor promoting antioxidant effect) deletion and in those fed with MCD or high-fat diets [84]. Furthermore, increased UPR component activity was noticed in the liver of NAFLD patients [98]. Clearly, ER stress plays an important role in inflammation, insulin signaling, and lipid homeostasis, all of which are crucial in the NAFLD pathogenesis.
2.5. Oxidative Stress
Oxidative stress is one of the main factors associated with obesity and related disorders, such as cardiovascular diseases, T2DM, MS, as well as cancer, and neurodegenerative disorders [99,100]. Oxidative stress arises from the imbalance of ROS generation and neutralization with antioxidants [101]. ROS such as superoxide, hydroxyl radicals, and hydrogen peroxide are physiologically produced in low concentrations by peroxisomes and mitochondria [102,103] and are involved in signaling pathways as factors controlling transcription and the cell cycle [102,104]. However, at higher concentrations, ROS cause DNA damage, alterations in gene expression, chromosomal instability, and lipid oxidation [101]. Oxidative stress is also associated with the mechanism of insulin formation. Recent studies confirm the presence of oxidative stress biomarkers, i.e., malondialdehyde, 7,8-dihydro-8-oxoguanine (8-oxo-dG), or other markers of oxidative damage in cases of IR [105]. Additionally, the presence of elevated oxidative stress is also directly involved in the development and progression of NAFLD [101]. It contributes to impaired mitochondrial, peroxisomal FFA oxidation and altered cytokine release, and consequently results in increased lipid peroxidation and liver inflammation, leading to the formation of stellate cells and fibrogenesis [106].
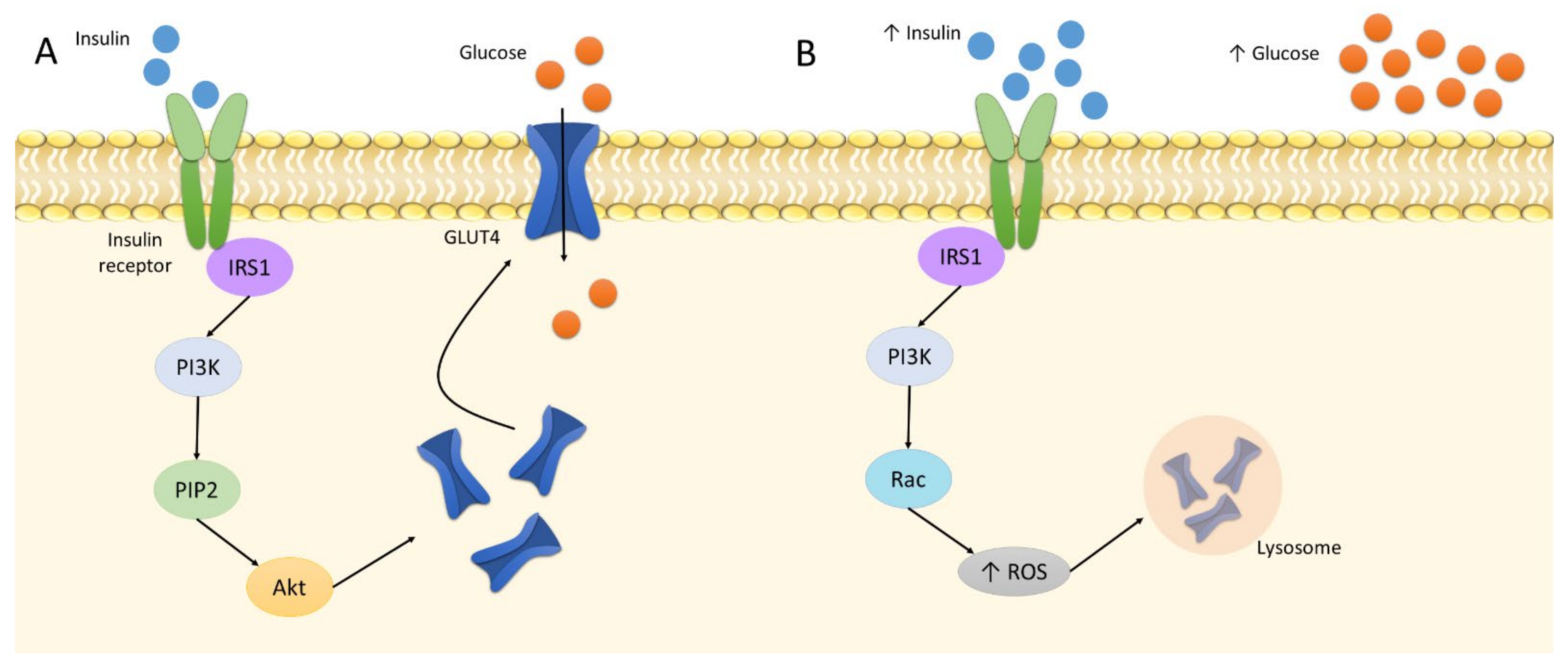
Oxidative stress is related to the etiology of IR. In its physiological state, insulin binds to the insulin receptor to phosphorylate IRS1. This triggers the phosphorylation of PI3 kinases and subsequent PIP2. The cascade activates the Akt pathway [107]. It is followed by the translocation of GLUT4 to the plasma membrane in tissues sensitive to insulin, i.e., adipocytes and skeletal muscles. It increases the cellular absorption of glucose and reduces its concentration in the blood [108]. Insulin elevates GLUT4 expression to increase glucose uptake; however, at increased insulin levels, GLUT4 is downregulated. Four-hour treatment of 1 nM insulin on 3T3-L1 adipocytes was found to decrease the protein level of GLUT4 by approximately 20% [109]. Following this downregulation and the buildup of glucose in the bloodstream, the pancreas produces more insulin due to the positive feedback loop; as a result, the cells become insulin resistant, i.e., insensitive to insulin. In such cases, GLUT4 demonstrates continually lower expression, which results in hyperinsulinemia, hyperglycemia, and increased oxidative stress, leading directly to inflammation [7,109]. In addition, a higher insulin level also alters the action of PI3, which phosphorylates Rac instead of PIP2, and, thus, activates NADPH oxidase 4, producing large amounts of ROS [110]. This involves the activation of the retromer, i.e., a group of proteins engaged in endosomal transport; as a result, GLUT4 is transported to lysosomes for degradation and cannot perform its function (Figure 2) [7].
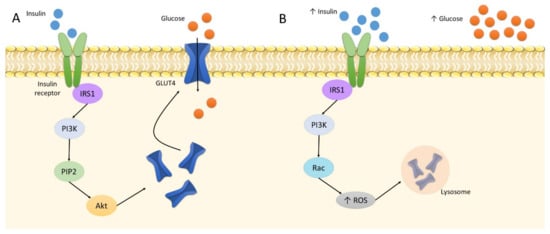
Figure 2.
The insulin signaling: A: normal insulin level; B: increased insulin level. Abbreviations: Akt: protein kinase B; IRS1: insulin receptor substrate 1; PI3K: phosphoinositide 3-kinase; PIP2: phosphatidylinositol biphosphate; ROS: reactive oxygen species.
When the diet is full of glucose-rich meals, the mitochondria produce more ATP. Due to the rise in their activity, the level of ROS also increases [111]. To repair the associated damage, the mitochondrial response stimulates the NF-κB and JNK pathways, both of which influence the pathophysiology of NAFLD [112].
2.6. Mitochondrial Dysfunction
Mitochondria are the main locations for energy storage and fatty acid metabolism in cells [108]. They maintain the energy homeostasis of the cell, i.e., β-oxidation, ETC, as well as the production of ATP and ROS [113]. Thus, it is not surprising that mitochondria play a significant role in diseases associated with metabolic pathways. More than two decades ago, a relationship between disturbed mitochondrial function and NAFLD, i.e., ETC and beta-oxidation dysfunction, was confirmed [114,115]. Now, it is obvious that mitochondrial dysfunction is associated with insufficient oxidation of fatty acids, leading to elevated ROS levels and, consequently, increased oxidative stress [116]. Free radicals produced in mitochondria stimulate the activity of signaling pathways capable of inducing necroinflammation in liver cells, as well as mitochondrial damage [117]. Thus, mitochondrial dysfunction contributes to the accumulation of FFA in hepatocytes and the development of IR and NAFLD, especially in the case of a fat-rich diet intake [118].
Mitochondrial dysfunction can be triggered by disturbances in the functioning of various families of proteins. One of these families is sirtuins, which are a group of NAD+-dependent deacetylases [119]. Sirtuin 1 (SIRT1) mediates the regulation of oxidative stress by upregulating antioxidant enzymes [120], while sirtuin 3 (SIRT3) enhances the level of fatty acid oxidation by stimulating long-chain acyl-CoA dehydrogenases. SIRT3 level was found to be reduced in animals with fatty livers [121]. Additionally, since sirtuins act with NAD+, its depletion may result in mitochondrial dysfunction and an increase in FFA level in hepatocytes [122].
Slc25a1, a citrate carrier accessing the inner mitochondrial membrane, which is associated with FFA metabolic processes and glycolytic pathways, is also involved in the proper functioning of mitochondria. The inhibition of Slc25a1 decreases steatosis, protects against steatohepatitis, and reduces the inflammation in adipose tissue [123]. Another mitochondrial transporting molecule, carnitine, is responsible for transferring long-chain fatty acids from cytoplasm to matrix. It inhibits oxidative stress, enhances β-oxidation, and reduces IR. The supplementation of carnitine improves the AST, ALT, TG, and HOMA-IR (indicator of IR) parameters in NAFLD patients [124].
In the case of NAFLD, the main challenge faced by the mitochondria is managing a large amount of fat flowing into the hepatocytes. These organelles are responsible for the transformation of lipotoxic FFA into intracellular TG stores, which limits the increase in oxidative stress [125]. Mice fed an MCD diet with depletion of diacylglycerol O-acyltransferase 2 (DGAT2), a protein involved in the final step of FFA to TG conversion, demonstrate elevated levels of markers of lipid oxidation stress, necroinflammation, and fibrosis [126]. In addition, attenuated β-oxidation leads to the accumulation of lipotoxic intermediates, which also causes inflammation and alters insulin signaling [127].
Recent studies have identified several mechanisms that link mitochondrial dysfunction with obesity and IR [128]. Insulin is important to the mitochondria for two key reasons: firstly, insulin maintains an appropriate NAD+/NADH ratio in them, and secondly, free radicals derived from mitochondria modulate insulin sensitivity, while the ROS excess disrupts insulin signaling and induces IR [129].
Moreover, mutations in mtDNA are associated with MS. For instance, a mutation in the tRNA(Leu)(UUR) gene causes impairment of insulin secretion from pancreatic β cells [130]. Cytochrome P4502E1 (CYP2E1), which is the source of ROS, is also involved in the metabolism of fatty acids. Its level is increased in the animal models of NASH and NASH patients [131,132]. In addition, some polymorphisms found in CYP2E1 are associated with the occurrence of NASH in obese T2DM patients [133].
Due to the fact that mitochondria produce ROS, antioxidant activity is required. An animal model of NASH was found to demonstrate decreased glutathione peroxidase (GPx) activity, probably caused by the depletion of glutathione and its poor transport to the mitochondrial matrix [134]. Moreover, the occurrence of the C47T polymorphism in the gene encoding superoxide dismutase 2 (SOD2) not only lowers its activity and contributes to an increase in ROS levels but also increases the likelihood of NASH development and advanced fibrosis in NAFLD [135]. These findings demonstrate a clear link between mitochondrial dysfunction and fatty liver disease.
3. The Interplay between NAFLD, MS, IR, and DNA Repair
There are many features in the development of NAFLD and MS that play crucial roles in both diseases. For example, both are characterized by altered insulin signaling and insulin sensitivity of cells. In this chapter, we present studies that confirm the link not only between IR, MS, and its associated obesity and T2DM but also the role of DNA repair in the development of IR. This body of evidence highlights the possible relationship between DNA repair, especially BER, and NAFLD itself.
3.1. Common Features of NAFLD and MS
Metabolic syndrome (MS) is a cluster of features that emerges from disturbances in the metabolism of fats, sugars, amino acids, or iron. MS is a significant factor that increases the risk of T2DM, cardiovascular diseases, or chronic kidney disorders [136]. To diagnose MS, at least three out of the following five criteria should be confirmed: abdominal obesity, elevated TG level, reduced HDL, hypertension, and disturbances in fasting glucose [137]. It is estimated that over 23% of the general population suffers from MS, and 10% of these patients demonstrate the presence of all five criteria [1].
The key factors in the development of MS are IR and hyperinsulinemia [138]. These features are associated with obesity, which also appears to be a fundamental problem in this condition. This is due to the presence of an increased level of very-low-density lipoproteins (VLDL), functioning as TG carriers, which mediates the hypertriglyceridemia [139]. In addition, elevated FFA flux to hepatic and other tissues may be observed in MS, which also results in an increase in TG levels, and thus contributes to the development of IR [140]. Additionally, fat accumulation in visceral adipose tissue stimulates the production of adipocytokines, e.g., TNF-α and IL-6; this not only leads to inflammation but can also induce hypertension [141]. Hypertension may be caused due to hyperinsulinemia resulting from increased intake of fats. In such cases, insulin stimulates endothelin-1 to increase the proliferation of smooth muscle cells, leading to vasoconstriction [142]. Thus, it is not surprising that due to the IR, the vasodilation of the blood vessels in the liver can be observed. In fatty livers, there is an increased diameter of the portal vein along with a decreased flow velocity. Moreover, the diameter of the portal vein is positively correlated with the severity of NAFLD [143].
The above processes immediately bring to mind the mechanisms occurring in NAFLD, where insulin signaling and disturbances in lipid metabolism are also important factors in the development of the disease [144]. Moreover, 90% of cases of nonalcoholic fatty livers are accompanied by the presence of at least one diagnostic criterium of MS, while 33% have all [1]. Interestingly, fatty livers are much more common in people with MS than in ones without this disorder [145]. A study based on 4401 subjects confirms that the presence of MS increases the likelihood of NAFLD onset almost 10-fold and significantly reduces the chances of disease regression [146]. This suggests that MS is a risk factor for NAFLD.
Due to the numerous similarities and relationships between NAFLD and MS, in 2020, a new concept of the disease, called MAFLD, was proposed [147]. However, the criteria for the diagnosis of this disease are different, as they do not exclude the consumption of alcohol or chronic liver diseases [148]. Moreover, the condition requires the presence of metabolic disorders [149]. Thus, people with MAFLD tend to be older and have a higher body-mass index (BMI), blood pressure, and levels of lipids and hepatic enzymes than NAFLD patients [21].
Despite the strong association of NAFLD with obesity, the disease also occurs in non-obese patients; however, it is less prevalent. Interestingly, it is more common for lean patients to demonstrate metabolic problems [10,150]. Even in non-obese individuals, a relationship can be found between NAFLD and visceral fat tissue: a greater thickness of the visceral fat is related to a greater incidence of MS and severity of NAFLD [151]. This could indicate that visceral fat is the link between these two diseases.
In both cases, the disorder may be caused by inadequate nutrition and lack of physical activity. This promotes an increase in fat accumulation, which correlates positively with hepatic IR, leading to enhanced lipolysis and a rise in circulating FFA levels [125,152]. However, the problem with IR is not restricted to hepatic cells but is also found in other tissues. It has been shown that in human skeletal muscle cells with IR, glucose, stored in muscle as glycogen, is directed to the de novo lipogenesis pathway in the liver [153,154]. This may result in the occurrence of NAFLD. Nevertheless, peripheral IR alters the lipid metabolism, leading to the development of liver steatosis. Moreover, the elevated level of lipolysis in white adipose tissue indirectly contributes to the accumulation of lipids in the other tissues, facilitating the formation of hepatic IR [155]. However, both visceral or peripheral fat content induces IR in hepatocytes [10].
The prevalence of MS and NAFLD increases with age. The reason is that fat moves from subcutaneous to visceral adipose tissue with age [10,156,157]. This results in the production of pro-inflammatory cytokines; the level of production increases in adipose tissue with higher fat content, resulting in higher NF-κB activity [158,159,160]. Indeed, inflammation is an essential aspect of the development of both diseases. MS has elevated levels of TNF-α, IL-6, and c-reactive protein (CRP) [161]. In addition, FFA has an indirect impact on toll-like receptor 4 (TLR4), which triggers the NF-κB signaling pathway [162]. TLR4 knockout mice are protected from inflammation caused by IR and lipid influx [162,163]. In humans with T2DM, the observed increase in TLR4 expression in skeletal muscle correlates positively with IR [164,165].
Recent studies indicate that NAFLD and MS are not only related to the metabolism of lipids and carbohydrates; the occurrence of MS, T2DM, and NASH is also associated with uric acid, a natural waste product of purine metabolism. In a study of NAFLD patients, 53% of whom had NASH, the highest serum uric acid levels were found in the older participants and those with higher BMI, blood pressure, TG levels, and total cholesterol, and reduced HDL. In addition, those with the highest uric acid levels also had advanced liver steatosis [166]. On the other hand, fatty liver is not only related to purine metabolism but also associated with dysregulation of liver aminotransferase expression, which results from disturbances in amino acid metabolism [167].
In addition to dysfunctional uric acid or amino acid metabolisms, NAFLD and NASH patients often demonstrate changes in iron metabolism. NASH patients demonstrate elevated levels of ferritin and transferrin, which are responsible for the storage and transport of iron in the organism [168]. Increased ferritin levels are also noticeable in half of NAFLD patients [8]. Interestingly, hyperferritinemia is a predictor of advanced liver fibrosis and an increased risk of death [169]. Disturbances in iron metabolism may contribute to the oxidative stress and IR present in MS and NAFLD. Excess iron enhances the Fenton reaction, which converts hydrogen peroxide to hydroxyl radicals. Moreover, mice with higher dietary iron intake demonstrate increased levels of 15-lipoxygenase. Normally, it is associated with the physiological leakage of the peroxisomal membrane. Nevertheless, with increased iron concentration, this enzyme is more active, resulting in elevated levels of hydrogen peroxide, thus in lipid peroxidation [170]. This consequently leads to the production of MDA, which activates stellate cells in the liver and fibrogenesis [171]. In rats, a carbonyl-iron diet leads to the activation of NF-κB and a reduction in antioxidant capacity [172]. Thus, rats fed a diet depleted of iron exhibit lower oxidative stress due to decreased production of free radicals in the liver and reduced lipid peroxidation [173].
Recent studies indicate that the development of NAFLD is influenced by the gut, specifically the gut microbiota, which are associated with obesity and IR [174]. Different microbiota is observed in the feces of obese and lean people. For instance, obese people and mice have reduced levels of Bacteroidetes, i.e., the main phylum of beneficial bacteria indirectly involved in various metabolic pathways [175]. Men suffering from MS and IR who received intestinal microbiota infusions from non-obese people demonstrate improved insulin sensitivity [176], and the subjects with MS demonstrated higher numbers of bacteria that produced butyrate after treatment [176]. Oral administration of butyrate to mice reduces IR, while sodium butyrate administered to rats protects against NAFLD, even when fed with a high-fat diet [177,178].
3.1.1. Obesity
As it was mentioned, obesity is a major contributor to the development of MS but is also strongly associated with the prevalence of NAFLD. Therefore, it can be assumed that this is the common denominator between these two diseases [179]. Nevertheless, despite the presence of fatty livers also in lean individuals, IR is much more related to higher fat accumulation than lower fat body content. The relationship between obesity, MS, and NAFLD is noticeable because each disease derives mainly from a sedentary lifestyle and a high-fat and high-carbohydrate diet [179].
Over 95% of morbidly obese patients undergoing bariatric surgery are diagnosed with NAFLD [180]. Up to 12% of these people have severe fibrosis or cirrhosis [2]. Interestingly, simple fatty liver disease only converts into NASH in 3% of lean patients, but in 20% of obese patients [18]. As the prevalence of obesity increases, so does the frequency of MS. A multi-cohort study of more than 160,000 obese Europeans found that MS occurred in 43%–78% of men and 24%–65% of women [181]. Although MS and obesity are linked with NAFLD, even 29% of patients with fatty liver are non-obese [182]. Moreover, obesity and NAFLD, including fibrogenesis and cirrhosis, occur in children as often as in adults [18].
Comprehensively, it has been confirmed that the degree of steatosis correlates positively with BMI [183,184]. However, while these patients appear to have normal BMI, they show lower fat content in the legs but higher fat accumulation in the midsection, as abdominal obesity; a similar phenomenon is encountered in MS [185]. Lipodystrophy, the non-uniform distribution of body fats, is often present at healthy BMI; it is believed to be important in the mechanism of MS and is a risk factor in NAFLD [186,187]. In general, lipodystrophy, obesity, NAFLD, and MS demonstrate disturbances in visceral fat tissue, which together with subcutaneous tissue, form white adipose tissue [188]. Often, the visceral adipose tissue/subcutaneous adipose tissue ratio is increased in NAFLD [189]. Excessive production of FFAs by visceral fat tissue in a pathological situation impairs the PI3K/Akt signaling pathway, increases oxidative stress, and reduces insulin sensitivity [5].
Obviously, since adipose tissue influences inflammatory pathways through the production of hormones, adipokines, cytokines, and chemokines [190], obesity is considered a condition of low-grade chronic inflammation [18]. The cytokines secreted by adipose tissue have not only proinflammatory properties but also produce protective proteins, such as IL10 and adiponectin [191]. TNF-α is the first cytokine to link obesity with IR [192]. Low levels of TNF-α are secreted physiologically by adipose tissue, but its expression was found to be increased in obese rodents [193], and its mRNA levels are also elevated in obese IR patients [194]. Moreover, obese mice with depletion of TNF-α demonstrated significantly improved insulin sensitivity compared to WT mice [195].
In contrast, IL6, a proinflammatory cytokine, can prevent damage to the liver. It is believed to protect the liver against hepatic fibrosis by attenuating oxidative stress and mitochondrial dysfunction and enhancing hepatocyte proliferation [190]. Although inhibition of IL6 activity triggered IR in mice, studies using both animal models and NAFLD patients have shown that the severity of disease correlates with IL6 level [196]. Indeed, although IL6 protects against damage, it may also cause steatosis [197]. In addition, the elevated levels of TNF-α and IL6 observed in obese patients activate JNK signaling and IR [10]. Besides producing the cytokines, adipose tissue also secretes leptin, which stops hunger. In animal models of NAFLD, a higher level of leptin activates fibrogenesis, and in humans with this disease, its rise contributes to an increase in the severity of steatosis [198,199,200]. Although obesity is not required to diagnose NAFLD, studies show that it is a serious risk factor of fatty liver disease.
3.1.2. T2DM
Diabetes mellitus can be classified into type 1 diabetes (T1DM), i.e., non-insulin-dependent type, IR-related insulin-dependent T2DM (most occurring cases of diabetes), and other less common diabetes [201]. T2DM is a multifactorial disease in which the organism is unable to respond appropriately to insulin concentrations due to abundant nutrition and ensuing obesity [202]. It belongs to the diagnostic criteria cluster for MS and, along with obesity and IR, is very closely related to NAFLD [203]. Most patients with both NAFLD and T2DM fulfill all criteria for MS, so it can be assumed that the diseases are dependent on each other [187]. NAFLD patients suffering from T2DM concurrently have a much more severe disease course than non-diabetic patients [204].
More than 70% of T2DM patients also suffer from NAFLD [179,205,206]. Among the hospitalized ones with T2DM, 45%–75% also had fatty livers, and according to the population study, 30%–70% of diabetics had NAFLD [207]. In a meta-analysis examining 35,599 cases of T2DM, 59.67% of cases had NAFLD, while 77.87% were simultaneously obese with fatty livers [208]. In another meta-analysis of 49,419 people with T2DM, 55.5% of them had NAFLD [22]. In general, advanced liver fibrosis is a common occurrence in patients with T2DM [209]. Studies with magnetic resonance imaging, magnetic resonance elastography, FibroScan, and biopsy were used to evaluate the steatosis and stiffness of the liver; the results indicate that 37%–70% of T2DM patients had NASH and 7%–50% had advanced fibrosis [207,209,210,211,212]. While NAFLD can be observed in the occurrence of T2DM, the opposite is also true: T2DM or dysregulation of fasting glucose is observed in 18%–33% of NAFLD patients [213]. In a study of 88 NAFLD patients, 69 had T2DM or impaired glucose tolerance, and patients with advanced fibrosis showed a higher IR [214].
While T2DM often precedes the onset of NAFLD, NAFLD is also considered a predictor of T2DM [215]. Based on the research, it is not possible to conclude unambiguously whether NAFLD is the cause or effect of T2DM since NAFLD increases the risk of diabetes and vice versa [216]. It is estimated that diabetics are 5.36 times more likely to develop NAFLD than healthy people [217]. The results of numerous biopsies have shown that T2DM is a predictor of NASH and advanced fibrosis [218]. A study of 108 double biopsies with a 6.6-year interval found that the group of people who demonstrated deteriorated fibrosis after this time had a higher percentage of diabetes than those without progression of fibrosis [219]. T2DM also increases the risk of cirrhosis, liver failure, and hepatocellular carcinoma, as well as hospitalization and death, as a consequence of NAFLD [218]. Other data show NAFLD to be a significant risk factor in the development of T2DM [220,221,222]. IR almost always accompanies NAFLD, as it facilitates the course of diabetes [205]. The presence of above 10% of hepatic liver fat content significantly increases systemic IR and the risk of T2DM [223]. The chance also rises in the case of the co-occurrence of NAFLD and MS [215]. Moreover, the presence of the PNPLA3 I148M variant, related to NAFLD, elevates the risk of T2DM and IR [224,225].
Visceral adipose tissue plays an important role in the development of T2DM, as well as NAFLD, by producing cytokines and activating the NF-κB pathway [226,227]. Increased gluconeogenesis and attenuation of insulin sensitivity are observed due to the activity of protein kinase Cε, fetuin A/B, retinol-binding protein 4, or selenoprotein P. T2DM patients typically demonstrate higher hepatic fat content and inflammation. In addition, lipogenesis is continuously active due to IR, and dyslipidemia occurs. Thus, patients demonstrate elevated blood pressure, and low-density lipoprotein (LDL) and TG levels, as well as decreased HDL. T2DM contributes to an increased influx of FFA into the liver, which induces the onset and progression of NAFLD. Obesity is exacerbated, and the disease worsens in response to oxidative stress, ER stress, lipotoxicity, and the activity of proinflammatory cytokines released by Kupffer cells and adipocytes, leading to apoptosis or necrosis [202,228,229].
T2DM contributes to the dysregulation of metabolic and inflammatory pathways. Furthermore, cases with both T2DM and NAFLD demonstrate more severe hyperinsulinemia, dyslipidemia, and hepatic IR than those with T2DM alone [221,230]. Higher levels of ALT and GGTP indicate an increased risk of T2DM. However, even NAFLD patients with the proper ATL serum levels, but with T2DM and IR, have a greater chance of higher grade steatosis and a more severe course of the disease [205]. Studies show that in patients with T2DM, a high-fat diet rich in pathogen-associated molecular patterns and altering gut microbiota may stimulate TLR2 and TLR4 and lead to elevated intestinal permeability and inflammation. T2DM is also related to mitochondrial dysfunction since reduced ATP production levels have been detected in diabetics after fasting or fructose administration [231,232]. To summarize, T2DM is closely associated with the development and progression of NAFLD.
3.2. The Role of BER in NAFLD
Due to the fact that oxidative stress plays a considerable role in NAFLD development and progression, the mechanisms that are responsible for the repair of oxidative injure must function efficiently enough to handle the problem. Free radicals and mitochondrial dysfunction can promote damage to genetic material. Therefore, it is important that DNA repair pathways are not impaired and that lesions are eliminated effectively.
3.2.1. BER and Adipose Tissue Metabolism
Mitochondria play an important role in the pathogenesis of NAFLD and are closely associated with IR through FFA oxidation and lipotoxicity [233]. The huge impact of mitochondria is exemplified by the relationship between NAFLD and MS, both of which are linked to mitochondrial dysfunction and oxidative stress [234,235]. Mitochondrial dysfunction and the intracellular accumulation of diacylglycerol and ceramide disrupt insulin signaling pathways. Furthermore, a significant role is played by the relationship between IR and ROS generated as by-products of the energy metabolism in the mitochondria. The increased supply of nutrients and the catabolic reactions present in obesity, NAFLD, or T2DM, increases the number of electrons provided to the ETC, thus elevating the proton gradient across the inner mitochondrial membrane. Instead of increasing the synthesis of ATP, this results in overproduction of ROS, which not only induces oxidative damage to the DNA, proteins, and lipids, but also act as signaling molecules modulating insulin signaling [236].
In addition to the damage to nuclear DNA, IR also causes damage to mtDNA. The most important DNA repair mechanism in mitochondria is BER, which is able to eliminate oxidative lesions like 8-oxo-dG adducts from mtDNA, as in its nuclear counterpart [237]. BER recognizes damage as oxidative, alkylation, deamination, and AP sites. The process consists of four steps: (i) recognition, (ii) excision, (iii) synthesis, and (iv) ligation of DNA. The recognition and removal of lesions are performed by glycosylases, including oxoguanine glycosylase (OGG1), nei-like DNA glycosylase 1 (NEIL1), or MutY DNA glycosylase (MUTYH). The prepared structures are then processed by apurinic/apyrimidinic endodeoxyribonuclease 1 (APEX1), polynucleotide kinase 3’-phosphatase (PNKP) or 5’-deoxyribose phosphate (dRP)/AP lyase, respectively, which are able to cleave the phosphodiester bonds [238]. The synthesis stage is performed by DNA polymerase, which inserts correct nucleotides or nucleotides in the generated gap. Single nucleotides are repaired by short patch BER (SP-BER), while groups are replaced by long patch BER (LP-BER) in which a flap structure-specific endonuclease 1 (FEN1) acts to create and remove 5 ‘overhanging flaps [238]. In the final step of BER, DNA strands are sealed by DNA ligase 1 (LIG1) or 3 (LIG3), along with interacting proteins Poly(ADP-ribose) polymerase 1 (PARP1) and X-ray repair cross-complementing protein 1 (XRCC1) [239,240].
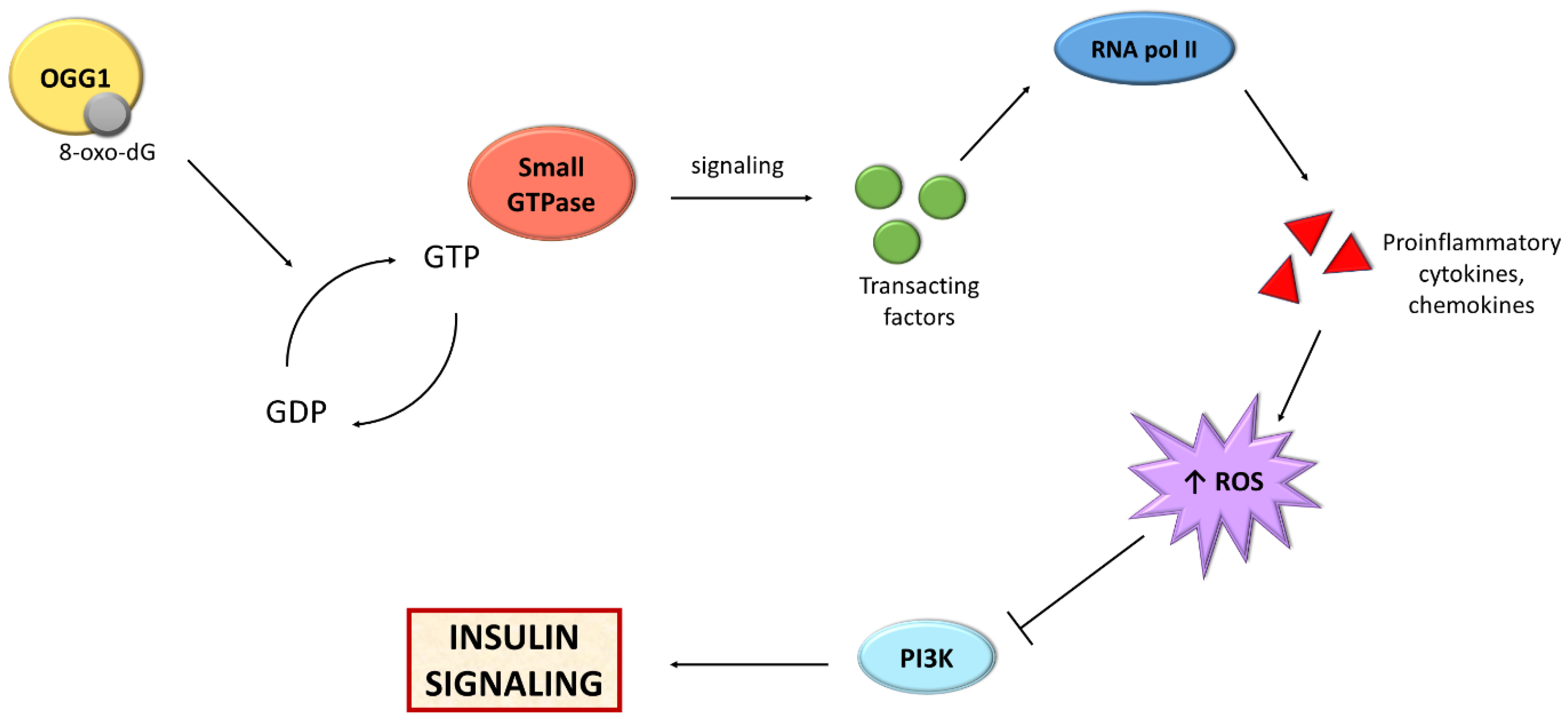
Recent studies highlight the importance of one of the BER mechanism components, i.e., OGG1, in the metabolism of adipose tissue (Figure 3). Findings show that mice with knockout of the OGG1 gene (OGG1−/−) demonstrated an increase in weight and fat content with age [241]. After 10 weeks of high-fat diet feeding, mice were heavier in comparison to WT, and their livers were fatty. These mice demonstrated hyperinsulinemia and impaired glucose tolerance, which results in IR and a prediabetic state. Moreover, these animals showed a higher respiratory exchange ratio, which suggested a prevalence of carbohydrate metabolism over fatty acid oxidation. In addition, the expression of genes associated with hepatic fatty acid oxidation, the amount of glycogen in the liver as well as serum fasting ketone levels were reduced, suggesting a decrease in liver lipid oxidation [241].
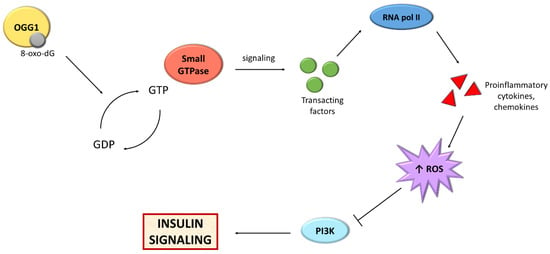
Figure 3.
Scheme presenting the relationship between BER and insulin signaling. Abbreviations: GDP: guanosine diphosphate; GTP: guanosine triphosphate; OGG1: oxoguanine glycosylase; PI3K: phosphoinositide 3-kinase; RNA pol II: RNA polymerase II; ROS; reactive oxygen species.
Furthermore, OGG −/− mice show an increase in skeletal muscle lipid accumulation and dysregulation of genes associated with lipid uptake and mitochondrial fission in muscles. Muscle functionality, i.e., grip strength and treadmill endurance also decreased [242]. The OGG1-deficient mice also demonstrated hyperglycemia, higher mtDNA 8-oxo-dG levels, and elevated liver glycogen concentration due to the inability of suppression of gluconeogenesis in the fed state. The ability of ECT was reduced, which strengthens DNA repair as an important factor in the regulation of metabolism [243].
The OGG1 enzyme exists as nuclear and mitochondrial variants. It has been shown that the lack of a nuclear variant is compensated by the action of the mitochondrial variant. OGG1 −/− mice with mitochondrial-targeted OGG1 show consistent mitochondrial expression of this gene. These animals are protected against obesity, hepatic lipid accumulation, IR, and adipose tissue inflammation [244]. Komakula et al. [245] demonstrated the importance of OGG1 in lipid accumulation and an increase in adipocyte differentiation. The lack of the gene increases fat content and adipogenesis, while overexpression attenuates them. These studies confirm the importance of DNA repair in fat metabolism. Moreover, the presence of the c.1245C>G (substitution of cysteine for serine at codon 326, Ser326Cys; rs1052133) polymorphism in the OGG1 gene increases the risk of cancer, neurodegenerative, cardiovascular, and metabolic diseases, including T2DM and MS [246].
In addition, some studies indicate that mice lacking another glycosylase, NEIL1, show metabolic alterations. Animals fed a high-fat diet showed obesity and glucose intolerance present in MS [247,248]. The findings confirm in general importance of BER pathway in NAFLD (Table 1).

Table 1.
The studies demonstrating the role of DNA repair in the course of lipid and insulin metabolism as well as fatty liver.
3.2.2. DNA Repair in NAFLD
The above studies showed mainly the influence of BER-related proteins on adipose tissue and lipid metabolism, which in turn influence the development of IR. In contrast, the following research provides a general picture of the relationship between NAFLD and alterations in DNA repair pathways, mainly in BER.
Recent studies have examined the relationship between the expression level of genes involved in BER and fatty liver. MCD diet-fed mice were found to demonstrate increased gene expression of thymine-DNA glycosylase and APEX1, enzymes related to BER [252]. In addition, expression is also influenced by pioglitazone, a drug used in T2DM, which can improve insulin sensitivity and the general condition of NASH patients. When administered to animals, pioglitazone reversed high-fat diet-induced steatosis and increased the expression of the OGG1 and MUTYH genes. This suggests that DNA repair may have a significant impact on improving fatty liver [250]. Although fatty liver in animals is observed to co-occur with elevated DNA repair activity, Gao et al. [249] report that MUTYH expression was decreased in animals fed MCD diet. In the case of rats fed a fructose-rich diet rich, a decrease in the expression level of gamma DNA polymerase and a lower mtDNA copy number could be observed [253].
NAFLD is associated not only with BER but also with other DNA repair mechanisms. The liver steatosis appears to be associated with Gadd45α, which encodes the protein involved in cells differentiation, apoptosis, necrosis, and base and nucleotide excision DNA repair. Gadd45α is upregulated in NAFLD patients, probably in response to oxidative and ER stress in hepatocytes. In addition, it plays a protective role against steatohepatitis in Gadd45α−/− mice fed MCD diet. In comparison to a group of WT animals, more severe hepatic inflammation and fibrosis have been observed [254,255].
In patients with obesity and fatty livers, the activity of the nucleotide excision repair, pathway responsible for the repair of lesions caused by UV light, environmental factors, or some DNA adducts, has been significantly decreased [251]. These findings suggest that DNA repair mechanisms may be involved in the pathophysiology of NAFLD.
4. Conclusions
Despite the fact that NAFLD is a common disorder, many of the underlying mechanisms of pathophysiology still remain elusive. Nevertheless, it is clear that the key causative factors of the disease include fat accumulation, hepatic inflammation, oxidative and ER stress, as well as mitochondrial dysfunction. It is a multi-factorial disease that is closely associated with MS, T2DM, and obesity, and the common denominator of these conditions is IR. DNA repair mechanisms are also related to IR and the development of NAFLD; this is particularly the case for BER, which contributes to the course of the disease through OGG1. Nevertheless, further research is needed to better understand this topic.
Author Contributions
Conceptualization, S.Z., P.C., and J.S.; Methodology, S.Z., A.B., and P.C.; Writing—Original Draft Preparation, S.Z., A.B., P.C., and J.S.; Writing—Review and Editing, P.C., J.S.; Visualization, S.Z.; Supervision, J.S., and M.J.; Project Administration, P.C., M.J., and J.S.; Funding Acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by National Science Centre (2019/35/O/NZ5/02502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009, 13, 9–19. [Google Scholar]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, M.; Yamasandhi, P.G. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Indian J. Endocrinol. Metab. 2018, 22, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, K.; Kohjima, M.; Kotoh, K.; Nakashima, M.; Nakamuta, M.; Enjoji, M. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Czarny, P.; Merecz-Sadowska, A.; Majchrzak, K.; Jablkowski, M.; Szemraj, J.; Sliwinski, T.; Karwowski, B. The Influence of Hepatitis C Virus Therapy on the DNA Base Excision Repair System of Peripheral Blood Mononuclear Cells. DNA Cell Biol. 2017, 36, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Jornayvaz, F.R. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol. Cell. Endocrinol. 2015, 418 Pt 1, 55–65. [Google Scholar] [CrossRef]
- Hübscher, S.G. Histological assessment of non-alcoholic fatty liver disease. Histopathology 2006, 49, 450–465. [Google Scholar] [CrossRef]
- Gariani, K.; Philippe, J.; Jornayvaz, F.R. Non-alcoholic fatty liver disease and insulin resistance: From bench to bedside. Diabetes Metab. 2013, 39, 16–26. [Google Scholar] [CrossRef]
- López-Velázquez, J.A.; Silva-Vidal, K.V.; Ponciano-Rodríguez, G.; Chávez-Tapia, N.C.; Arrese, M.; Uribe, M.; Méndez-Sánchez, N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann. Hepatol. 2014, 13, 166–178. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef]
- Schwimmer, J.B. Definitive diagnosis and assessment of risk for nonalcoholic fatty liver disease in children and adolescents. Semin. Liver Dis. 2007, 27, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zheng, L.; Wang, M.; Du, Y.; Jiang, J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990–2017: A population-based observational study. BMJ Open 2020, 10, e036663. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Neuschwander-Tetri, B.A. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterol. Hepatol. 2006, 2, 282–291. [Google Scholar]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Celsa, C.; Giammanco, A.; Spatola, F.; Petta, S. The Relevance of Noninvasive Tools To Assess Fibrosis in Non-Alcoholic Fatty Liver Disease. Curr. Pharm. Des. 2020, 26, 3928–3938. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Mignery, G.A.; Pikaard, C.S.; Park, W.D. Molecular characterization of the patatin multigene family of potato. Gene 1988, 62, 27–44. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Donati, B.; Fares, R.; Lombardi, R.; Mancina, R.M.; Romeo, S.; Valenti, L. PNPLA3 I148M polymorphism and progressive liver disease. World J. Gastroenterol. 2013, 19, 6969–6978. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Patman, G.L.; Leathart, J.B.S.; Piguet, A.-C.; Burt, A.D.; Dufour, J.-F.; Day, C.P.; Daly, A.K.; Reeves, H.L.; Anstee, Q.M. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 2014, 61, 75–81. [Google Scholar] [CrossRef]
- Pennisi, G.; Celsa, C.; Giammanco, A.; Spatola, F.; Petta, S. The Burden of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: Screening Issue and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 5613. [Google Scholar] [CrossRef]
- Eslam, M.; George, J. Genetic contributions to NAFLD: Leveraging shared genetics to uncover systems biology. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 40–52. [Google Scholar] [CrossRef]
- Simoes, I.C.M.; Karkucinska-wieckowska, A.; Janikiewicz, J.; Szymanska, S.; Pronicki, M.; Dobrzyn, P.; Dabrowski, M.; Dobrzyn, A.; Oliveira, P.J.; Zischka, H.; et al. Western diet causes obesity-induced nonalcoholic fatty liver disease development by differentially compromising the autophagic response. Antioxidants 2020, 9, 995. [Google Scholar] [CrossRef]
- Wu, W.; Tsuchida, H.; Kato, T.; Niwa, H.; Horikawa, Y.; Takeda, J.; Iizuka, K. Fat and carbohydrate in western diet contribute differently to hepatic lipid accumulation. Biochem. Biophys. Res. Commun. 2015, 461, 681–686. [Google Scholar] [CrossRef]
- Feng, R.; Luo, C.; Li, C.; Du, S.; Okekunle, A.P.; Li, Y.; Chen, Y.; Zi, T.; Niu, Y. Free fatty acids profile among lean, overweight and obese non-alcoholic fatty liver disease patients: A case—Control study. Lipids Health Dis. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Diraison, F.; Moulin, P.; Beylot, M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003, 29, 478–485. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos–Roman, M.A.; Browning, J.D.; Parks, E.J. Increased De novo Lipogenesis Is a Distinct Characteristic of Individuals With Nonalcoholic Fatty Liver Disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Hasty, A.H.; Osuga, J.I.; Tamura, Y.; Shionoiri, F.; Iizuka, Y.; Ohashi, K.; Harada, K.; et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem. 1999, 274, 35832–35839. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Foufelle, F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm. Res. 2007, 68, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Prieto, P.; Postic, C. Carbohydrate sensing through the transcription factor ChREBP. Front. Genet. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Pacot, C.; Dugail, I.; Lemarchand, P.; Guichard, C.; le Lièpvre, X.; Berthelier-Lubrano, C.; Spiegelman, B.; Kim, J.B.; Ferré, P.; et al. ADD1/SREBP-1c Is Required in the Activation of Hepatic Lipogenic Gene Expression by Glucose. Mol. Cell. Biol. 1999, 19, 3760–3768. [Google Scholar] [CrossRef]
- Liang, G.; Yang, J.; Horton, J.D.; Hammer, R.E.; Goldstein, J.L.; Brown, M.S. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 2002, 277, 9520–9528. [Google Scholar] [CrossRef] [PubMed]
- Knebel, B.; Haas, J.; Hartwig, S.; Jacob, S.; Köllmer, C.; Nitzgen, U.; Muller-Wieland, D.; Kotzka, J. Liver-specific expression of transcriptionally active srebp-1c is associated with fatty liver and increased visceral fat mass. PLoS ONE 2012, 7, 1–15. [Google Scholar] [CrossRef]
- Kohjima, M.; Enjoji, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2007, 20, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Bruick, R.K.; Liang, G.; Horton, J.D.; Uyeda, K. From The Cover: Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA 2004, 101, 7281–7286. [Google Scholar] [CrossRef]
- Kato, T.; Iizuka, K.; Takao, K.; Horikawa, Y.; Kitamura, T.; Takeda, J. ChREBP-knockout mice show sucrose intolerance and fructose malabsorption. Nutrients 2018, 10, 340. [Google Scholar] [CrossRef]
- Diraison, F.; Dusserre, E.; Vidal, H.; Sothier, M.; Beylot, M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am. J. Physiol. Endocrinol. Metab. 2002, 282, 46–51. [Google Scholar] [CrossRef]
- Forcheron, F.; Cachefo, A.; Thevenon, S.; Pinteur, C.; Beylot, M. Mechanisms of the triglyceride- and cholesterol-lowering effect of fenofibrate in hyperlipidemic type 2 diabetic patients. Diabetes 2002, 51, 3486–3491. [Google Scholar] [CrossRef]
- Dentin, R.; Benhamed, F.; Hainault, I.; Fauveau, V.; Foufelle, F.; Dyck, J.R.B.; Girard, J.; Postic, C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes 2006, 55, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Benhamed, F.; Denechaud, P.D.; Lemoine, M.; Robichon, C.; Moldes, M.; Bertrand-Michel, J.; Ratziu, V.; Serfaty, L.; Housset, C.; Capeau, J.; et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 2012, 122, 2176–2194. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; DeMayo, F.J.; Li, H.; Abu-Elheiga, L.; Gu, Z.; Shaikenov, T.E.; Kordari, P.; Chirala, S.S.; Heird, W.C.; Wakil, S.J. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc. Natl. Acad. Sci. USA 2006, 103, 8552–8557. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A. Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism. Biochem. Soc. Trans. 2002, 30, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Ip, E.; Farrell, G.; Hall, P.; Robertson, G.; Leclercq, I. Administration of the Potent PPARα Agonist, Wy-14,643, Reverses Nutritional Fibrosis and Steatohepatitis in Mice. Hepatology 2004, 39, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central role of PPARα-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Larter, C.Z.; Yeh, M.M.; van Rooyen, D.M.; Brooling, J.; Ghatora, K.; Farrell, G.C. Peroxisome proliferator-activated receptor-α agonist, Wy 14643, improves metabolic indices, steatosis and ballooning in diabetic mice with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2012, 27, 341–350. [Google Scholar] [CrossRef]
- Guerre-Millo, M.; Gervois, P.; Raspé, E.; Madsen, L.; Poulain, P.; Derudas, B.; Herbert, J.M.; Winegar, D.A.; Willson, T.M.; Fruchart, J.C.; et al. Peroxisome proliferator-activated receptor α activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 2000, 275, 16638–16642. [Google Scholar] [CrossRef]
- Tordjman, K.; Bernal-Mizrachi, C.; Zemany, L.; Weng, S.; Feng, C.; Zhang, F.; Leone, T.C.; Coleman, T.; Kelly, D.P.; Semenkovich, C.F. PPARalpha deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J. Clin. Invest. 2001, 107, 1025–1034. [Google Scholar] [CrossRef]
- Vancells Lujan, P.; Viñas Esmel, E.; Sacanella Meseguer, E. Overview of non-alcoholic fatty liver disease (Nafld) and the role of sugary food consumption and other dietary components in its development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef]
- Hydes, T.; Alam, U.; Cuthbertson, D.J. The Impact of Macronutrient Intake on Non-alcoholic Fatty Liver Disease (NAFLD): Too Much Fat, Too Much Carbohydrate, or Just Too Many Calories? Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Green, C.J.; Hodson, L. The influence of dietary fat on liver fat accumulation. Nutrients 2014, 6, 5018–5033. [Google Scholar] [CrossRef]
- Velázquez, K.T.; Enos, R.T.; Bader, J.E.; Sougiannis, A.T.; Carson, M.S.; Chatzistamou, I.; Carson, J.A.; Nagarkatti, P.S.; Nagarkatti, M.; Murphy, E.A. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019, 11, 619–637. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Tang, W.; Sun, W.; Ma, Y.; Lin, S.; Jing, J.; Jiang, L.; Shi, H.; Song, Z.; et al. Western diet induces severe nonalcoholic steatohepatitis, ductular reaction, and hepatic fibrosis in liver CGI-58 knockout mice. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients 2019, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Sertoglu, E.; Kayadibi, H.; Uyanik, M. A biochemical view: Increase in polyunsaturated fatty acid ω-6/ω-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. J. Diabetes Complications 2015, 29, 157. [Google Scholar] [CrossRef] [PubMed]
- De Castro, G.S.; Calder, P.C. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin. Nutr. 2018, 37, 37–55. [Google Scholar] [CrossRef]
- Khadge, S.; Sharp, J.G.; Thiele, G.M.; McGuire, T.R.; Klassen, L.W.; Duryee, M.J.; Britton, H.C.; Dafferner, A.J.; Beck, J.; Black, P.N.; et al. Dietary omega-3 and omega-6 polyunsaturated fatty acids modulate hepatic pathology. J. Nutr. Biochem. 2018, 52, 92–102. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Li, Z.; Kei Lam, C.W.; Xiao, Y.; Wu, Q.; Zhang, W. Consumption of sugar-sweetened beverages has a dose-dependent effect on the risk of non-alcoholic fatty liver disease: An updated systematic review and dose-response meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 2192. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Edmonds, P.J.; Cheungpasitporn, W. Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: A systematic review and meta-analysis. QJM 2016, 109, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Sevastianova, K.; Santos, A.; Kotronen, A.; Hakkarainen, A.; Makkonen, J.; Silander, K.; Peltonen, M.; Romeo, S.; Lundbom, J.; Lundbom, N.; et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am. J. Clin. Nutr. 2012, 96, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef]
- Bilzer, M.; Roggel, F.; Gerbes, A.L. Role of Kupffer cells in host defense and liver disease. Liver Int. Off. J. Int. Assoc. Study Liver 2006, 26, 1175–1186. [Google Scholar] [CrossRef]
- Wan, J.; Benkdane, M.; Teixeira-Clerc, F.; Bonnafous, S.; Louvet, A.; Lafdil, F.; Pecker, F.; Tran, A.; Gual, P.; Mallat, A.; et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: A protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014, 59, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Invernizzi, P.; Mantovani, A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology 2014, 59, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.-N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W.J. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 2006, 116, 115–124. [Google Scholar] [CrossRef]
- Wasmuth, H.E.; Lammert, F.; Zaldivar, M.M.; Weiskirchen, R.; Hellerbrand, C.; Scholten, D.; Berres, M.-L.; Zimmermann, H.; Streetz, K.L.; Tacke, F.; et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology 2009, 137, 309–319.e3. [Google Scholar] [CrossRef]
- Kitade, H.; Sawamoto, K.; Nagashimada, M.; Inoue, H.; Yamamoto, Y.; Sai, Y.; Takamura, T.; Yamamoto, H.; Miyamoto, K.; Ginsberg, H.N.; et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 2012, 61, 1680–1690. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 2012, 18, 727–735. [Google Scholar] [CrossRef]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.S.; Cortez-Pinto, H.; Solá, S.; Castro, R.E.; Ramalho, R.M.; Baptista, A.; Moura, M.C.; Camilo, M.E.; Rodrigues, C.M.P. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am. J. Gastroenterol. 2004, 99, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Xu, C.-F.; Yu, C.-H.; Chen, W.-X.; Li, Y.-M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, Y.; Pagliassotti, M.J. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006, 147, 943–951. [Google Scholar] [CrossRef]
- Lenna, S.; Trojanowska, M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr. Opin. Rheumatol. 2012, 24, 663–668. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Wagner, M.; Moore, D.D. Endoplasmic reticulum stress and glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 367–373. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2006, 38, 317–332. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef]
- Malhi, H.; Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011, 54, 795–809. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef]
- Du, K.; Herzig, S.; Kulkarni, R.N.; Montminy, M. TRB3: A tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003, 300, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.; Schroeder-Gloeckler, J.M.; Janssen, R.C.; Jiang, H.; Qadri, I.; Maclean, K.N.; Friedman, J.E. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology 2007, 45, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Sreejayan, N.; Dong, F.; Kandadi, M.R.; Yang, X.; Ren, J. Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice. Obesity 2008, 16, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Mirshahi, F.; Cheung, O.; Natarajan, R.; Maher, J.W.; Kellum, J.M.; Sanyal, A.J. Activation and Dysregulation of the Unfolded Protein Response in Nonalcoholic Fatty Liver Disease. Gastroenterology 2008, 134, 568–576. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Human requirement for N-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef]
- Colagar, A.H.; Marzony, E.T. Ascorbic Acid in Human Seminal Plasma: Determination and Its Relationship to Sperm Quality. J. Clin. Biochem. Nutr. 2009, 45, 144–149. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Polimeni, L.; Del Ben, M.; Baratta, F.; Perri, L.; Albanese, F.; Pastori, D.; Violi, F.; Angelico, F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J. Hepatol. 2015, 7, 1325–1336. [Google Scholar] [CrossRef]
- Hançer, N.J.; Qiu, W.; Cherella, C.; Li, Y.; Copps, K.D.; White, M.F. Insulin and metabolic stress stimulate multisite serine/threonine phosphorylation of insulin receptor substrate 1 and inhibit tyrosine phosphorylation. J. Biol. Chem. 2014, 289, 12467–12484. [Google Scholar] [CrossRef]
- Blanco, C.L.; McGill-Vargas, L.L.; Gastaldelli, A.; Seidner, S.R.; McCurnin, D.C.; Leland, M.M.; Anzueto, D.G.; Johnson, M.C.; Liang, H.; DeFronzo, R.A.; et al. Peripheral insulin resistance and impaired insulin signaling contribute to abnormal glucose metabolism in preterm baboons. Endocrinology 2015, 156, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Nakagawa, Y.; Kojima, I.; Shibata, H. Prolonged insulin stimulation down-regulates GLUT4 through oxidative stress-mediated retromer inhibition by a protein kinase CK2-dependent mechanism in 3T3-L1 adipocytes. J. Biol. Chem. 2014, 289, 133–142. [Google Scholar] [CrossRef]
- Campa, C.C.; Ciraolo, E.; Ghigo, A.; Germena, G.; Hirsch, E. Crossroads of PI3K and Rac pathways. Small GTPases 2015, 6, 71–80. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-H.; Wang, W.-J.; Lin, C.-W.; Pai, P.; Lai, T.-Y.; Tsai, C.-Y.; Kuo, W.-W. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-κB in cardiomyocytes exposed to high glucose. J. Cell. Physiol. 2012, 227, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; de Bari, O.; Bernardo, T.C.; Oliveira, P.J.; Wang, D.Q.-H.; Portincasa, P. Role of mitochondria in nonalcoholic fatty liver disease--from origin to propagation. Clin. Biochem. 2012, 45, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.H.; Swerdlow, R.H.; Khan, E.M.; Iezzoni, J.C.; Hespenheide, E.E.; Parks, J.K.; Parker, W.D.J. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J. Hepatol. 1999, 31, 430–434. [Google Scholar] [CrossRef]
- Eaton, S.; Zaitoun, A.M.; Record, C.O.; Bartlett, K. beta-Oxidation in human alcoholic and non-alcoholic hepatic steatosis. Clin. Sci. 1996, 90, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Robin, M.-A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef]
- Cusi, K. Role of Insulin Resistance and Lipotoxicity in Non-Alcoholic Steatohepatitis. Clin. Liver Dis. 2009, 13, 545–563. [Google Scholar] [CrossRef]
- Silva, M.F.B.; Aires, C.C.P.; Luis, P.B.M.; Ruiter, J.P.N.; IJlst, L.; Duran, M.; Wanders, R.J.A.; Tavares de Almeida, I. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: A review. J. Inherit. Metab. Dis. 2008, 31, 205–216. [Google Scholar] [CrossRef]
- Ding, R.-B.; Bao, J.; Deng, C.-X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Li, M.; Guo, K.; Vanella, L.; Taketani, S.; Adachi, Y.; Ikehara, S. Stem cell transplantation upregulates Sirt1 and antioxidant expression, ameliorating fatty liver in type 2 diabetic mice. Int. J. Biol. Sci. 2015, 11, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, A.A.; Choudhury, M.; Rahman, S.M.; McCurdy, C.E.; Friederich, M.; Van Hove, J.L.K.; Watson, P.A.; Birdsey, N.; Bao, J.; Gius, D.; et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem. J. 2011, 433, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Menzies, K.J.; Ryu, D.; Wegner, C.J.; Wang, X.; Ropelle, E.R.; Moullan, N.; Zhang, H.; Perino, A.; Lemos, V.; et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology 2016, 63, 1190–1204. [Google Scholar] [CrossRef]
- Tan, M.; Mosaoa, R.; Graham, G.T.; Kasprzyk-Pawelec, A.; Gadre, S.; Parasido, E.; Catalina-Rodriguez, O.; Foley, P.; Giaccone, G.; Cheema, A.; et al. Inhibition of the mitochondrial citrate carrier, Slc25a1, reverts steatosis, glucose intolerance, and inflammation in preclinical models of NAFLD/NASH. Cell Death Differ. 2020, 27, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, H. Role of Carnitine in Non-alcoholic Fatty Liver Disease and Other Related Diseases: An Update. Front. Med. 2021, 8, 689042. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yang, L.; McCall, S.; Huang, J.; Yu, X.X.; Pandey, S.K.; Bhanot, S.; Monia, B.P.; Li, Y.-X.; Diehl, A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007, 45, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Kalavalapalli, S.; Williams, C.M.; Nautiyal, M.; Mathew, J.T.; Martinez, J.; Reinhard, M.K.; McDougall, D.J.; Rocca, J.R.; Yost, R.A.; et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E484–E494. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 14205–14218. [Google Scholar] [CrossRef]
- Cheng, Z.; Tseng, Y.; White, M.F. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol. Metab. 2010, 21, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Maassen, J.A.; ’T Hart, L.M.; Van Essen, E.; Heine, R.J.; Nijpels, G.; Jahangir Tafrechi, R.S.; Raap, A.K.; Janssen, G.M.C.; Lemkes, H.H.P.J. Mitochondrial diabetes: Molecular mechanisms and clinical presentation. Diabetes 2004, 53 (Suppl. S1), S103–S109. [Google Scholar] [CrossRef]
- Chalasani, N.; Gorski, J.C.; Asghar, M.S.; Asghar, A.; Foresman, B.; Hall, S.D.; Crabb, D.W. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 2003, 37, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Weltman, M.D.; Farrell, G.C.; Liddle, C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology 1996, 111, 1645–1653. [Google Scholar] [CrossRef]
- Varela, N.M.; Quiñones, L.A.; Orellana, M.; Poniachik, J.; Csendes, A.; Smok, G.; Rodrigo, R.; Cáceres, D.D.; Videla, L.A. Study of cytochrome P450 2E1 and its allele variants in liver injury of nondiabetic, nonalcoholic steatohepatitis obese women. Biol. Res. 2008, 41, 81–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caballero, F.; Fernández, A.; Matías, N.; Martínez, L.; Fucho, R.; Elena, M.; Caballeria, J.; Morales, A.; Fernández-Checa, J.C.; García-Ruiz, C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: Impact on mitochondrial S-adenosyl-L-methionine and glutathione. J. Biol. Chem. 2010, 285, 18528–18536. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Donati, B.; Panera, N.; Vongsakulyanon, A.; Alisi, A.; Dallapiccola, B.; Valenti, L. A 4-polymorphism risk score predicts steatohepatitis in children with nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 632–636. [Google Scholar] [CrossRef]
- Reynolds, K.; He, J. Epidemiology of the metabolic syndrome. Am. J. Med. Sci. 2005, 330, 273–279. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.J.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Reaven, G. Why a cluster is truly a cluster: Insulin resistance and cardiovascular disease. Clin. Chem. 2008, 54, 785–787. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Defronzo, R.A. The insulin resistance syndrome: Physiological considerations. Diabetes Vasc. Dis. Res. 2007, 4, 13–19. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Invest. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Katagiri, H.; Yamada, T.; Oka, Y. Adiposity and cardiovascular disorders: Disturbance of the regulatory system consisting of humoral and neuronal signals. Circ. Res. 2007, 101, 27–39. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Bakris, G.L. Review: Insulin and endothelin: An interplay contributing to hypertension development? J. Clin. Endocrinol. Metab. 2007, 92, 379–385. [Google Scholar] [CrossRef][Green Version]
- Soresi, M.; Giannitrapani, L.; Noto, D.; Terranova, A.; Campagna, M.E.; Cefalù, A.B.; Giammanco, A.; Montalto, G. Effects of steatosis on hepatic hemodynamics in patients with metabolic syndrome. Ultrasound Med. Biol. 2015, 41, 1545–1552. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; McCullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef]
- Kotronen, A.; Westerbacka, J.; Bergholm, R.; Pietiläinen, K.H.; Yki-Järvinen, H. Liver fat in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 3490–3497. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- EASL; EASD; EASO. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.H.; Gwak, G.-Y.; Park, H.N.; Kim, J.E.; Min, Y.W.; Kim, K.M.; Kim, Y.J.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am. J. Gastroenterol. 2012, 107, 561–567. [Google Scholar] [CrossRef]
- Jeong, S.-K.; Kim, Y.-K.; Park, J.-W.; Shin, Y.-J.; Kim, D.-S. Impact of visceral fat on the metabolic syndrome and nonalcoholic fatty liver disease. J. Korean Med. Sci. 2008, 23, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Eguchi, T.; Mizuta, T.; Ide, Y.; Yasutake, T.; Iwakiri, R.; Hisatomi, A.; Ozaki, I.; Yamamoto, K.; Kitajima, Y.; et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J. Gastroenterol. 2006, 41, 462–469. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Savage, D.B.; Bilz, S.; Solomon, G.; Yonemitsu, S.; Cline, G.W.; Befroy, D.; Zemany, L.; Kahn, B.B.; et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 12587–12594. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Magkos, F.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity 2009, 17, 1872–1877. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.; Wildman, R.P. Update on the metabolic syndrome: Hypertension. Curr. Hypertens. Rep. 2009, 11, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Diehl, A.M. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Lipidol. 2008, 19, 295–300. [Google Scholar] [CrossRef]
- Parker, R. The role of adipose tissue in fatty liver diseases. Liver Res. 2018, 2, 35–42. [Google Scholar] [CrossRef]
- Sharma, M.; Mitnala, S.; Vishnubhotla, R.K.; Mukherjee, R.; Reddy, D.N.; Rao, P.N. The Riddle of Nonalcoholic Fatty Liver Disease: Progression From Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis. J. Clin. Exp. Hepatol. 2015, 5, 147–158. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 2008, 52, 885–897. [Google Scholar] [CrossRef]
- Haffner, S.M. Insulin resistance, inflammation, and the prediabetic state. Am. J. Cardiol. 2003, 92, 18J–26J. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Ghosh, A.K.; O’Brien, M.; Mau, T.; Yung, R. Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging 2017, 9, 1971–1982. [Google Scholar] [CrossRef]
- Reyna, S.M.; Ghosh, S.; Tantiwong, P.; Meka, C.S.R.; Eagan, P.; Jenkinson, C.P.; Cersosimo, E.; Defronzo, R.A.; Coletta, D.K.; Sriwijitkamol, A.; et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 2008, 57, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Könner, A.C.; Brüning, J.C. Toll-like receptors: Linking inflammation to metabolism. Trends Endocrinol. Metab. 2011, 22, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Fernández Rodríguez, C.M.; Aller, R.; Gutiérrez García, M.L.; Ampuero, J.; Gómez-Camarero, J.; Martín-Mateos, R.M.; Burgos-Santamaría, D.; Rosales, J.M.; Aspichueta, P.; Buque, X.; et al. Higher levels of serum uric acid influences hepatic damage in patients with non-alcoholic fatty liver disease (NAFLD). Rev. Esp. Enfermedades Dig. Organo Of. la Soc. Esp. Patol. Dig. 2019, 111, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Castaño, G.O.; Scian, R.; Fernández Gianotti, T.; Dopazo, H.; Rohr, C.; Gaj, G.; San Martino, J.; Sevic, I.; Flichman, D.; et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am. J. Clin. Nutr. 2016, 103, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Bacon, B.R.; Farahvash, M.J.; Janney, C.G.; Neuschwander-Tetri, B.A. Nonalcoholic steatohepatitis: An expanded clinical entity. Gastroenterology 1994, 107, 1103–1109. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Bottai, M.; Ekstedt, M.; Kechagias, S.; Hultcrantz, R.; Stål, P. Elevated serum ferritin is associated with increased mortality in non-alcoholic fatty liver disease after 16 years of follow-up. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 1688–1695. [Google Scholar] [CrossRef]
- Yokota, S.; Oda, T.; Fahimi, H.D. The role of 15-lipoxygenase in disruption of the peroxisomal membrane and in programmed degradation of peroxisomes in normal rat liver. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2001, 49, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef]
- Cornejo, P.; Varela, P.; Videla, L.A.; Fernández, V. Chronic iron overload enhances inducible nitric oxide synthase expression in rat liver. Nitric Oxide Biol. Chem. 2005, 13, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Minamiyama, Y.; Takemura, S.; Kodai, S.; Shinkawa, H.; Tsukioka, T.; Ichikawa, H.; Naito, Y.; Yoshikawa, T.; Okada, S. Iron restriction improves type 2 diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1140–E1149. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Maggi, D.; Maurizi, A.R.; Pozzilli, P.; Buzzetti, R. Prevention of type 2 diabetes mellitus: Is it feasible? Diabetes. Metab. Res. Rev. 2014, 30 (Suppl. S1), 4–12. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Mattace Raso, G.; Simeoli, R.; Russo, R.; Iacono, A.; Santoro, A.; Paciello, O.; Ferrante, M.C.; Canani, R.B.; Calignano, A.; Meli, R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS ONE 2013, 8, e68626. [Google Scholar] [CrossRef]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef]
- Subichin, M.; Clanton, J.; Makuszewski, M.; Bohon, A.; Zografakis, J.G.; Dan, A. Liver disease in the morbidly obese: A review of 1000 consecutive patients undergoing weight loss surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2015, 11, 137–141. [Google Scholar] [CrossRef]
- Van Vliet-Ostaptchouk, J.V.; Nuotio, M.-L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Keach, J.C.; Batts, K.P.; Lindor, K.D. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999, 30, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Moretto, M.; Kupski, C.; Mottin, C.C.; Repetto, G.; Garcia Toneto, M.; Rizzolli, J.; Berleze, D.; de Souza Brito, C.L.; Casagrande, D.; Colossi, F. Hepatic steatosis in patients undergoing bariatric surgery and its relationship to body mass index and co-morbidities. Obes. Surg. 2003, 13, 622–624. [Google Scholar] [CrossRef]
- Andersen, T.; Christoffersen, P.; Gluud, C. The liver in consecutive patients with morbid obesity: A clinical, morphological, and biochemical study. Int. J. Obes. 1984, 8, 107–115. [Google Scholar] [PubMed]
- Lotta, L.A.; Gulati, P.; Day, F.R.; Payne, F.; Ongen, H.; van de Bunt, M.; Gaulton, K.J.; Eicher, J.D.; Sharp, S.J.; Luan, J.; et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 2017, 49, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Das, K.; Mukherjee, P.S.; Ghosh, A.; Ghosh, S.; Mridha, A.R.; Dhibar, T.; Bhattacharya, B.; Bhattacharya, D.; Manna, B.; et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology 2010, 51, 1593–1602. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef]
- Chiyanika, C.; Wong, V.W.-S.; Wong, G.L.-H.; Chan, H.L.-Y.; Hui, S.C.N.; Yeung, D.K.W.; Chu, W.C.W. Implications of Abdominal Adipose Tissue Distribution on Nonalcoholic Fatty Liver Disease and Metabolic Syndrome: A Chinese General Population Study. Clin. Transl. Gastroenterol. 2021, 12, e00300. [Google Scholar] [CrossRef]
- Asrih, M.; Jornayvaz, F.R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J. Endocrinol. 2013, 218, R25–R36. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Lang, C.H.; Dobrescu, C.; Bagby, G.J. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology 1992, 130, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Uysal, K.T.; Becherer, J.D.; Arner, P.; Hotamisligil, G.S. Altered Tumor Necrosis Factor-α (TNF-α) Processing in Adipocytes and Increased Expression of Transmembrane TNF-α in Obesity. Diabetes 2002, 51, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. The adipocyte in insulin resistance: Key molecules and the impact of the thiazolidinediones. Trends Endocrinol. Metab. 2003, 14, 137–145. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Itoh, Y.; Yokomizo, C.; Nishimura, T.; Niimi, T.; Fujii, H.; Okanoue, T.; Yoshikawa, T. Blockade of interleukin-6 signaling enhances hepatic steatosis but improves liver injury in methionine choline-deficient diet-fed mice. Lab. Investig. 2010, 90, 1169–1178. [Google Scholar] [CrossRef]
- Honda, H.; Ikejima, K.; Hirose, M.; Yoshikawa, M.; Lang, T.; Enomoto, N.; Kitamura, T.; Takei, Y.; Sato, N. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology 2002, 36, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Margetic, S.; Gazzola, C.; Pegg, G.G.; Hill, R.A. Leptin: A review of its peripheral actions and interactions. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2002, 26, 1407–1433. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Milic, S.; Turk Wensveen, T.; Grgic, I.; Jakopcic, I.; Stimac, D.; Wensveen, F.; Orlic, L. Nonalcoholic fatty liver disease—A multisystem disease? World J. Gastroenterol. 2016, 22, 9488–9505. [Google Scholar] [CrossRef]
- Dewidar, B.; Kahl, S.; Pafili, K.; Roden, M. Metabolic liver disease in diabetes—From mechanisms to clinical trials. Metabolism. 2020, 111S, 154299. [Google Scholar] [CrossRef]
- Bazick, J.; Donithan, M.; Neuschwander-Tetri, B.A.; Kleiner, D.; Brunt, E.M.; Wilson, L.; Doo, E.; Lavine, J.; Tonascia, J.; Loomba, R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care 2015, 38, 1347–1355. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2015, 47, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Bellentani, S.; Argo, C.K.; Ballestri, S.; Byrne, C.D.; Caldwell, S.H.; Cortez-Pinto, H.; Grieco, A.; Machado, M.V.; Miele, L.; et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2015, 47, 997–1006. [Google Scholar] [CrossRef]
- Dai, W.; Ye, L.; Liu, A.; Wen, S.W.; Deng, J.; Wu, X.; Lai, Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine 2017, 96, e8179. [Google Scholar] [CrossRef] [PubMed]
- Doycheva, I.; Cui, J.; Nguyen, P.; Costa, E.A.; Hooker, J.; Hofflich, H.; Bettencourt, R.; Brouha, S.; Sirlin, C.B.; Loomba, R. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment. Pharmacol. Ther. 2016, 43, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Choi, K.C.; Wong, G.L.-H.; Zhang, Y.; Chan, H.L.-Y.; Luk, A.O.-Y.; Shu, S.S.-T.; Chan, A.W.-H.; Yeung, M.-W.; Chan, J.C.-N.; et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: A prospective cohort study. Gut 2016, 65, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Vilgrain, V.; Angulo, P. Noninvasive evaluation of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J. Nonalcoholic Fatty Liver Disease and Diabetes: An Epidemiological Perspective. Endocrinol. Metab. 2019, 34, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016, 65, 1096–1108. [Google Scholar] [CrossRef]
- Wild, S.H.; Morling, J.R.; McAllister, D.A.; Kerssens, J.; Fischbacher, C.; Parkes, J.; Roderick, P.J.; Sattar, N.; Byrne, C.D. Type 2 diabetes and risk of hospital admission or death for chronic liver diseases. J. Hepatol. 2016, 64, 1358–1364. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef]
- Adams, L.A.; Waters, O.R.; Knuiman, M.W.; Elliott, R.R.; Olynyk, J.K. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: An eleven-year follow-up study. Am. J. Gastroenterol. 2009, 104, 861–867. [Google Scholar] [CrossRef]
- Xia, M.-F.; Bian, H.; Gao, X. NAFLD and Diabetes: Two Sides of the Same Coin? Rationale for Gene-Based Personalized NAFLD Treatment. Front. Pharmacol. 2019, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Fracanzani, A.L.; Valenti, L.; Bugianesi, E.; Andreoletti, M.; Colli, A.; Vanni, E.; Bertelli, C.; Fatta, E.; Bignamini, D.; Marchesini, G.; et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology 2008, 48, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.-F.; Bian, H.; Yan, H.-M.; Lin, H.-D.; Chang, X.-X.; Li, X.-M.; Ma, H.; He, W.-Y.; Zhao, N.-Q.; Xia, P.; et al. Assessment of liver fat content using quantitative ultrasonography to evaluate risks for metabolic diseases. Obesity 2015, 23, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.A.; Maglio, C.; Pirazzi, C.; Burza, M.A.; Adiels, M.; Burch, L.; Donnelly, L.A.; Colhoun, H.; Doney, A.S.; Dillon, J.F.; et al. Paradoxical lower serum triglyceride levels and higher type 2 diabetes mellitus susceptibility in obese individuals with the PNPLA3 148M variant. PLoS ONE 2012, 7, e39362. [Google Scholar] [CrossRef] [PubMed]
- Dubuquoy, C.; Burnol, A.-F.; Moldes, M. PNPLA3, a genetic marker of progressive liver disease, still hiding its metabolic function? Clin. Res. Hepatol. Gastroenterol. 2013, 37, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. an Off. J. Int. Assoc. Study Obes. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, A.; Strycharz, J.; Świderska, E.; Balcerczyk, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Chronic and Transient Hyperglycemia Induces Changes in the Expression Patterns of IL6 and ADIPOQ Genes and Their Associated Epigenetic Modifications in Differentiating Human Visceral Adipocytes. Int. J. Mol. Sci. 2021, 22, 6964. [Google Scholar] [CrossRef]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; An, W.; Song, J.; Zhang, Y.; Zhao, X. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J. Clin. Invest. 2019, 129, 546–555. [Google Scholar] [CrossRef]
- Lomonaco, R.; Bril, F.; Portillo-Sanchez, P.; Ortiz-Lopez, C.; Orsak, B.; Biernacki, D.; Lo, M.; Suman, A.; Weber, M.H.; Cusi, K. Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients With Type 2 Diabetes. Diabetes Care 2016, 39, 632–638. [Google Scholar] [CrossRef]
- Erridge, C. Diet, commensals and the intestine as sources of pathogen-associated molecular patterns in atherosclerosis, type 2 diabetes and non-alcoholic fatty liver disease. Atherosclerosis 2011, 216, 1–6. [Google Scholar] [CrossRef]
- Firneisz, G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: The liver disease of our age? World J. Gastroenterol. 2014, 20, 9072–9089. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.N.; Heilbronn, L.H.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N.L.-M. Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mabalirajan, U.; Ghosh, B. Mitochondrial dysfunction in metabolic syndrome and asthma. J. Allergy 2013, 2013, 340476. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.-T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The maintenance of mitochondrial DNA integrity--critical analysis and update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Wilso, D.M., 3rd. Overview of base excision repair biochemistry. Curr. Mol. Pharmacol. 2012, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef]
- Sampath, H.; Vartanian, V.; Rollins, M.R.; Sakumi, K.; Nakabeppu, Y.; Lloyd, R.S. 8-Oxoguanine DNA glycosylase (OGG1) deficiency increases susceptibility to obesity and metabolic dysfunction. PLoS ONE 2012, 7, e51697. [Google Scholar] [CrossRef]
- Vartanian, V.; Tumova, J.; Dobrzyn, P.; Dobrzyn, A.; Nakabeppu, Y.; Lloyd, R.S.; Sampath, H. 8-oxoguanine DNA glycosylase (OGG1) deficiency elicits coordinated changes in lipid and mitochondrial metabolism in muscle. PLoS ONE 2017, 12, e0181687. [Google Scholar] [CrossRef]
- Scheffler, K.; Rachek, L.; You, P.; Rowe, A.D.; Wang, W.; Kuśnierczyk, A.; Kittelsen, L.; Bjørås, M.; Eide, L. 8-oxoguanine DNA glycosylase (Ogg1) controls hepatic gluconeogenesis. DNA Repair 2018, 61, 56–62. [Google Scholar] [CrossRef]
- Komakula, S.S.B.; Tumova, J.; Kumaraswamy, D.; Burchat, N.; Vartanian, V.; Ye, H.; Dobrzyn, A.; Lloyd, R.S.; Sampath, H. The DNA Repair Protein OGG1 Protects Against Obesity by Altering Mitochondrial Energetics in White Adipose Tissue. Sci. Rep. 2018, 8, 14886. [Google Scholar] [CrossRef]
- Komakula, S.S.B.; Blaze, B.; Ye, H.; Dobrzyn, A.; Sampath, H. A Novel Role for the DNA Repair Enzyme 8-Oxoguanine DNA Glycosylase in Adipogenesis. Int. J. Mol. Sci. 2021, 22, 1152. [Google Scholar] [CrossRef]
- Sampath, H.; Lloyd, R.S. Roles of OGG1 in transcriptional regulation and maintenance of metabolic homeostasis. DNA Repair 2019, 81, 102667. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Batra, A.K.; Vartanian, V.; Carmical, J.R.; Prusak, D.; King, I.B.; Lowell, B.; Earley, L.F.; Wood, T.G.; Marks, D.L.; et al. Variable penetrance of metabolic phenotypes and development of high-fat diet-induced adiposity in NEIL1-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E724–E734. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, V.; Lowell, B.; Minko, I.G.; Wood, T.G.; Ceci, J.D.; George, S.; Ballinger, S.W.; Corless, C.L.; McCullough, A.K.; Lloyd, R.S. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase. Proc. Natl. Acad. Sci. USA 2006, 103, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wei, C.; Chen, L.; Huang, J.; Yang, S.; Diehl, A.M. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1070–G1077. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.-J.; Hsieh, T.-J.; Kuo, K.-K.; Hung, W.-W.; Tsai, K.-B.; Yang, C.-H.; Yu, M.-L.; Shin, S.-J. Pioglitazone retrieves hepatic antioxidant DNA repair in a mice model of high fat diet. BMC Mol. Biol. 2008, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Schults, M.A.; Nagle, P.W.; Rensen, S.S.; Godschalk, R.W.; Munnia, A.; Peluso, M.; Claessen, S.M.; Greve, J.W.; Driessen, A.; Verdam, F.J.; et al. Decreased nucleotide excision repair in steatotic livers associates with myeloperoxidase-immunoreactivity. Mutat. Res. 2012, 736, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Takumi, S.; Okamura, K.; Yanagisawa, H.; Sano, T.; Kobayashi, Y.; Nohara, K. The effect of a methyl-deficient diet on the global DNA methylation and the DNA methylation regulatory pathways. J. Appl. Toxicol. 2015, 35, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Senese, R.; Lasala, P.; Ziello, A.; Mazzoli, A.; Crescenzo, R.; Liverini, G.; Lanni, A.; Goglia, F.; Iossa, S. Fructose-Rich Diet Affects Mitochondrial DNA Damage and Repair in Rats. Nutrients 2017, 9, 323. [Google Scholar] [CrossRef]
- Tanaka, N.; Takahashi, S.; Hu, X.; Lu, Y.; Fujimori, N.; Golla, S.; Fang, Z.-Z.; Aoyama, T.; Krausz, K.W.; Gonzalez, F.J. Growth arrest and DNA damage-inducible 45α protects against nonalcoholic steatohepatitis induced by methionine- and choline-deficient diet. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 3170–3182. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Xia, C.; Li, S.; Zhu, L.; Xu, C. Comparative analysis of the expression profiles of genes related to the Gadd45α signaling pathway in four kinds of liver diseases. Histol. Histopathol. 2020, 35, 949–960. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).