Pan-Cancer Analysis of Clinical Relevance via Telomere Maintenance Mechanism
Abstract
:1. Introduction
2. Results
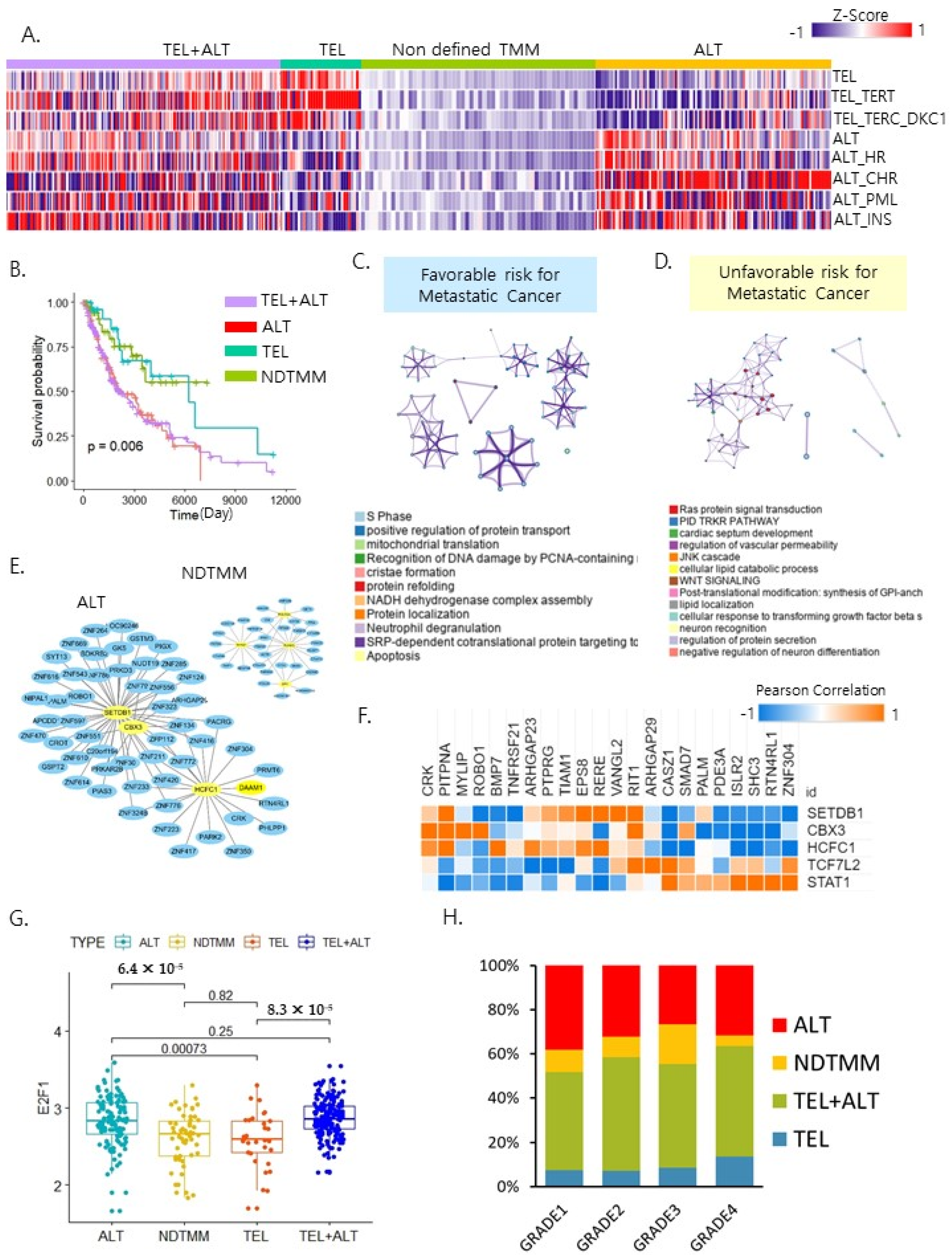
2.1. Telomere Maintenance Mechanism Separated Patient Outcome
2.2. ALT Was Associated with a Poor Prognosis of Metastatic Cancer
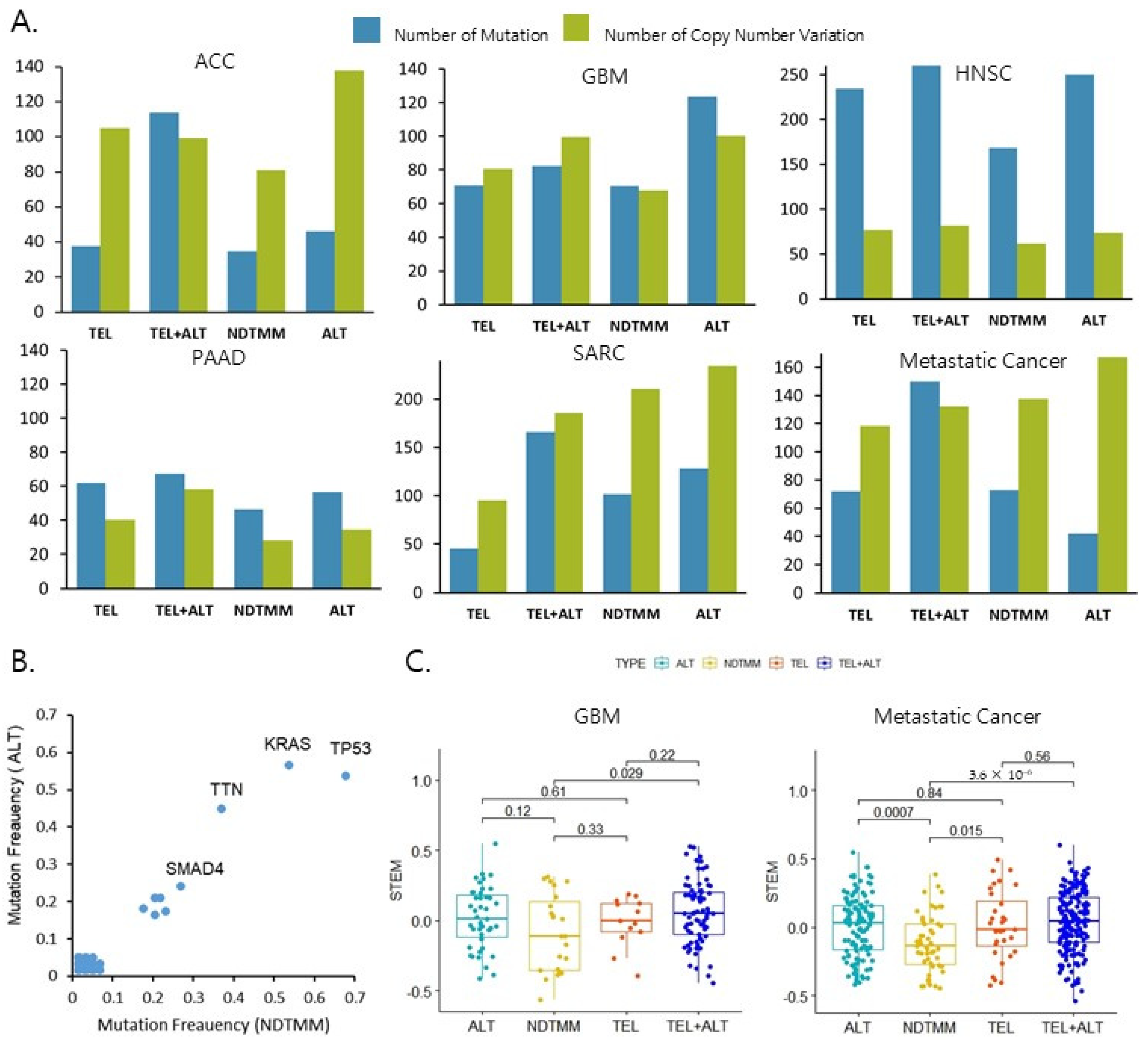
2.3. Molecular Characteristics Based on the Four TMM Types
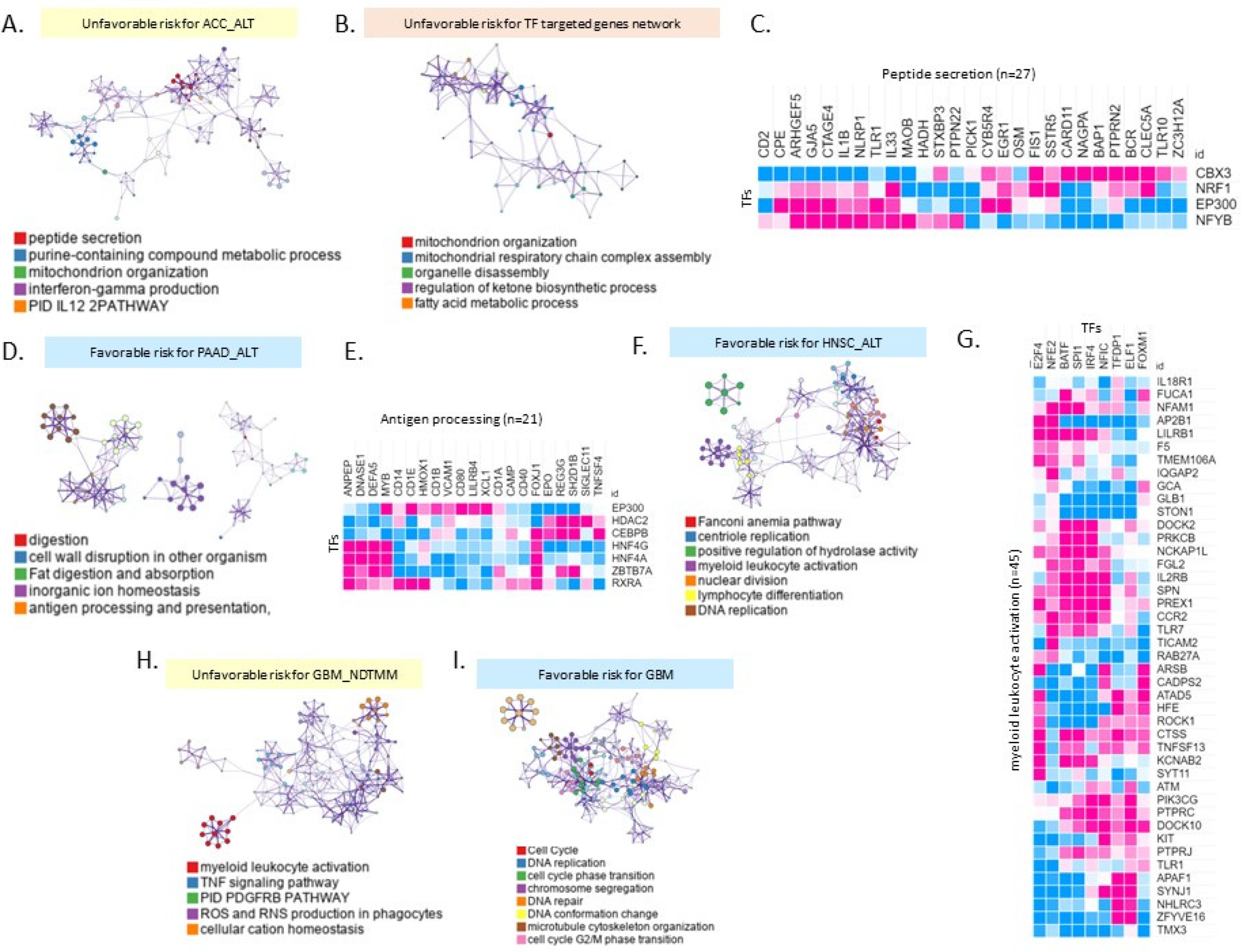
2.4. Different Biological Processes Affected Patient Prognoses of Different TMM Groups
3. Discussion
4. Materials and Methods
4.1. Telomere Maintenance Mechanism Classification
4.2. Differential Expression Gene Analysis in Cancer Types
4.3. Survival Probability Analysis and Gene Ontology and Correlation Analysis
4.4. Transcription Factor Analysis Protein Association Network
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaspar, T.B.; Sá, A.; Lopes, J.M.; Sobrinho-Simões, M.; Soares, P.; Vinagre, J. Telomere Maintenance Mechanisms in Cancer. Genes 2018, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Wang, W.; Li, F.; Songyang, Z.; Feng, X.; Xin, C.; Dai, Z.; Xiong, Y. Pan-cancer analysis identifies telomerase-associated signatures and cancer subtypes. Mol. Cancer 2019, 18, 106. [Google Scholar] [CrossRef] [Green Version]
- Armendáriz-Castillo, I.; López-Cortés, A.; García-Cárdenas, J.; Guevara-Ramírez, P.; Leone, P.; Pérez-Villa, A.; Yumiceba, V.; Zambrano, A.; Guerrero, S.; Paz-Y-Miño, C. TCGA Pan-Cancer Genomic Analysis of Alternative Lengthening of Telomeres (ALT) Related Genes. Genes 2020, 11, 834. [Google Scholar] [CrossRef]
- Subasri, M.; Shooshtari, P.; Watson, A.; Betts, D. Analysis of TERT Isoforms across TCGA, GTEx and CCLE Datasets. Cancers 2021, 13, 1853. [Google Scholar] [CrossRef] [PubMed]
- Claude, E.; Decottignies, A. Telomere maintenance mechanisms in cancer: Telomerase, ALT or lack thereof. Curr. Opin. Genet. Dev. 2020, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Hakin-Smith, V.; Jellinek, D.A.; Levy, D.; Carroll, T.; Teo, M.; Timperley, W.R.; McKay, M.J.; Reddel, R.R.; Royds, J.A. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet 2003, 361, 836–838. [Google Scholar] [CrossRef]
- Sanders, R.P.; Drissi, R.; Billups, C.A.; Daw, N.C.; Valentine, M.B.; Dome, J.S. Telomerase Expression Predicts Unfavorable Outcome in Osteosarcoma. J. Clin. Oncol. 2004, 22, 3790–3797. [Google Scholar] [CrossRef]
- Gagos, S.; Papaioannou, G.; Chiourea, M.; Merk-Loretti, S.; Jefford, C.-E.; Mikou, P.; Irminger-Finger, I.; Liossi, A.; Blouin, J.-L.; Dahoun, S. Unusually stable abnormal karyotype in a highly aggressive melanoma negative for telomerase activity. Mol. Cytogenet. 2008, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.-Y.; Lim, H.-W.; Joung, J.-G.; Park, W.-Y. Pan-Cancer Analysis of Alternative Lengthening of Telomere Activity. Cancers 2020, 12, 2207. [Google Scholar] [CrossRef]
- Sieverling, L.; Hong, C.; Koser, S.D.; Ginsbach, P.; Kleinheinz, K.; Hutter, B.; Braun, D.M.; Cortés-Ciriano, I.; Xi, R.; Kabbe, R.; et al. Genomic footprints of activated telomere maintenance mechanisms in cancer. Nat. Commun. 2020, 11, 733. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.N.; Schiemann, W.P. Means to the ends: The role of telomeres and telomere processing machinery in metastasis. Biochim. Biophys. Acta 2016, 1866, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Nersisyan, L.; Hopp, L.; Loeffler-Wirth, H.; Galle, J.; Loeffler, M.; Arakelyan, A.; Binder, H. Telomere Length Maintenance and Its Transcriptional Regulation in Lynch Syndrome and Sporadic Colorectal Carcinoma. Front. Oncol. 2019, 9, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Royds, J.A.; Al Nadaf, S.; Wiles, A.K.; Chen, Y.-J.; Ahn, A.; Shaw, A.; Bowie, S.; Lam, F.; Baguley, B.C.; Braithwaite, A.W.; et al. The CDKN2A G500 Allele Is More Frequent in GBM Patients with No Defined Telomere Maintenance Mechanism Tumors and Is Associated with Poorer Survival. PLoS ONE 2011, 6, e26737. [Google Scholar] [CrossRef] [PubMed]
- Ehrkamp, A.; Herrmann, C.; Stoll, R.; Heumann, R. Ras and Rheb Signaling in Survival and Cell Death. Cancers 2013, 5, 639–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009, 460, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaphy, C.M.; de Wilde, R.F.; Jiao, Y.; Klein, A.P.; Edil, B.H.; Shi, C.; Bettegowda, C.; Rodriguez, F.J.; Eberhart, C.G.; Hebbar, S.; et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 2011, 333, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.; Shay, J.W. TERT Promoter Mutations Enhance Telomerase Activation by Long-Range Chromatin Interactions. Cancer Discov. 2016, 6, 1212–1214. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, J.R.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Hackett, A.J.; Feldser, D.M.; Greider, C.W. Telomere dysfunction increases mutation rate and genomic instability. Cell 2001, 106, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Riethman, H. Human subtelomeric copy number variations. Cytogenet. Genome Res. 2008, 123, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Hwang, S.S.; Liesa, M.; Gan, B.; Sahin, E.; Jaskelioff, M.; Ding, Z.; Ying, H.; Boutin, A.T.; Zhang, H.; et al. Antitelomerase Therapy Provokes ALT and Mitochondrial Adaptive Mechanisms in Cancer. Cell 2012, 148, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Raghav, L.; Chang, Y.-H.; Hsu, Y.-C.; Li, Y.-C.; Chen, C.-Y.; Yang, T.-Y.; Chen, K.-C.; Hsu, K.-H.; Tseng, J.-S.; Chuang, C.-Y.; et al. Landscape of Mitochondria Genome and Clinical Outcomes in Stage 1 Lung Adenocarcinoma. Cancers 2020, 12, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henson, J.; Neumann, A.A.; Yeager, T.R.; Reddel, R. Alternative lengthening of telomeres in mammalian cells. Oncogene 2002, 21, 598–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Else, T.; Giordano, T.J.; Hammer, G.D. Evaluation of Telomere Length Maintenance Mechanisms in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2008, 93, 1442–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaphy, C.M.; Subhawong, A.P.; Hong, S.-M.; Goggins, M.G.; Montgomery, E.A.; Gabrielson, E.; Netto, G.J.; Epstein, J.I.; Lotan, T.; Westra, W.H.; et al. Prevalence of the Alternative Lengthening of Telomeres Telomere Maintenance Mechanism in Human Cancer Subtypes. Am. J. Pathol. 2011, 179, 1608–1615. [Google Scholar] [CrossRef]
- Dagg, R.A.; Pickett, H.A.; Neumann, A.A.; Napier, C.E.; Henson, J.; Teber, E.T.; Arthur, J.W.; Reynolds, C.P.; Murray, J.; Haber, M.; et al. Extensive Proliferation of Human Cancer Cells with Ever-Shorter Telomeres. Cell Rep. 2017, 19, 2544–2556. [Google Scholar] [CrossRef] [Green Version]
- Henson, J.D.; Hannay, J.A.; McCarthy, S.W.; Royds, J.A.; Yeager, T.R.; Robinson, R.; Wharton, S.B.; Jellinek, D.A.; Arbuckle, S.M.; Yoo, J.; et al. A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin. Cancer Res. 2005, 11, 217–225. [Google Scholar]
- Subhawong, A.P.; Heaphy, C.M.; Argani, P.; Konishi, Y.; Kouprina, N.; Nassar, H.; Vang, R.; Meeker, A.K. The alternative lengthening of telomeres phenotype in breast carcinoma is associated with HER-2 overexpression. Mod. Pathol. 2009, 22, 1423–1431. [Google Scholar] [CrossRef] [Green Version]
- Abedalthagafi, M.; Phillips, J.J.; Kim, G.E.; Mueller, S.; Haas-Kogen, D.A.; Marshall, R.E.; Croul, S.E.; Santi, M.R.; Cheng, J.; Zhou, S.; et al. The alternative lengthening of telomere phenotype is significantly associated with loss of ATRX expression in high-grade pediatric and adult astrocytomas: A multi-institutional study of 214 astrocytomas. Mod. Pathol. 2013, 26, 1425–1432. [Google Scholar] [CrossRef]
- McDonald, K.L.; McDonnell, J.; Muntoni, A.; Henson, J.; Hegi, M.; von Deimling, A.; Wheeler, H.R.; Cook, R.J.; Biggs, M.T.; Little, N.S.; et al. Presence of Alternative Lengthening of Telomeres Mechanism in Patients With Glioblastoma Identifies a Less Aggressive Tumor Type With Longer Survival. J. Neuropathol. Exp. Neurol. 2010, 69, 729–736. [Google Scholar] [CrossRef]
- Boardman, L.A.; Johnson, R.A.; Viker, K.B.; Hafner, K.A.; Jenkins, R.B.; Riegert-Johnson, D.L.; Smyrk, T.C.; Litzelman, K.; Seo, S.; Gangnon, R.; et al. Correlation of Chromosomal Instability, Telomere Length and Telomere Maintenance in Microsatellite Stable Rectal Cancer: A Molecular Subclass of Rectal Cancer. PLoS ONE 2013, 8, e80015. [Google Scholar] [CrossRef] [Green Version]
- Dilley, L.R.; Greenberg, R.A. ALTernative Telomere Maintenance and Cancer. Trends Cancer 2015, 1, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Amorim, J.P.; Santos, G.; Vinagre, J.; Soares, P. The Role of ATRX in the Alternative Lengthening of Telomeres (ALT) Phenotype. Genes 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.; Hunger, F.; Pasquinelli, S.; Speel, E.; Perren, A. Loss of DAXX and ATRX Are Associated With Chromosome Instability and Reduced Survival of Patients With Pancreatic Neuroendocrine Tumors. Gastroenterology 2014, 146, 453–460.e5. [Google Scholar] [CrossRef] [PubMed]
- Viceconte, N.; Dheur, M.-S.; Majerova, E.; Pierreux, C.E.; Baurain, J.-F.; van Baren, N.; Decottignies, A. Highly Aggressive Metastatic Melanoma Cells Unable to Maintain Telomere Length. Cell Rep. 2017, 19, 2529–2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, T.; Shay, J.W.; Wright, W.E.; Hiyama, E.; Shimose, S.; Kubo, T.; Sugita, T.; Yasunaga, Y.; Ochi, M. Telomere-Maintenance Mechanisms in Soft-Tissue Malignant Fibrous Histiocytomas. J. Bone Jt. Surg.-Am. 2009, 91, 928–937. [Google Scholar] [CrossRef]
- Liau, J.-Y.; Tsai, J.-H.; Jeng, Y.-M.; Lee, J.-C.; Hsu, H.-H.; Yang, C.-Y. Leiomyosarcoma with Alternative Lengthening of Telomeres Is Associated With Aggressive Histologic Features, Loss of ATRX Expression, and Poor Clinical Outcome. Am. J. Surg. Pathol. 2015, 39, 236–244. [Google Scholar] [CrossRef]
- Costa, A.; Daidone, M.G.; Daprai, L.; Villa, R.; Cantù, S.; Pilotti, S.; Mariani, L.; Gronchi, A.; Henson, J.D.; Reddel, R.R.; et al. Telomere Maintenance Mechanisms in Liposarcomas: Association with Histologic Subtypes and Disease Progression. Cancer Res. 2006, 66, 8918–8924. [Google Scholar] [CrossRef] [Green Version]
- Omori, Y.; Nakayama, F.; Li, D.; Kanemitsu, K.; Semba, S.; Ito, A.; Yokozaki, H. Alternative lengthening of telomeres frequently occurs in mismatch repair system-deficient gastric carcinoma. Cancer Sci. 2009, 100, 413–418. [Google Scholar] [CrossRef]
- Wang, N.; Xu, D.; Sofiadis, A.; Höög, A.; Vukojević, V.; Bäckdahl, M.; Zedenius, J.; Larsson, C. Telomerase-dependent and independent telomere maintenance and its clinical implications in medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, E1571–E1579. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.-Y.; Cheong, J.H. Alternative lengthening of telomeres is mechanistically linked to potential therapeutic vulnerability in the stem-like subtype of gastric cancer. Clin. Transl. Med. 2021, 11, e561. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Benhattar, J.; Coindre, J.-M.; Guillou, L. Telomerase activity and hTERT mRNA expression can be heterogeneous and does not correlate with telomere length in soft tissue sarcomas. Int. J. Cancer 2002, 98, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.E.; Varkonyi, R.J.; Schwalm, J.; Cragle, R.; Klein-Szanto, A.; Patchefsky, A.; Cukierman, E.; Von Mehren, M.; Broccoli, D. Multiple Mechanisms of Telomere Maintenance Exist in Liposarcomas. Clin. Cancer Res. 2005, 11, 5347–5355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, R.; Daidone, M.G.; Motta, R.; Venturini, L.; De Marco, C.; Vannelli, A.; Kusamura, S.; Baratti, D.; Deraco, M.; Costa, A.; et al. Multiple Mechanisms of Telomere Maintenance Exist and Differentially Affect Clinical Outcome in Diffuse Malignant Peritoneal Mesothelioma. Clin. Cancer Res. 2008, 14, 4134–4140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vitis, M.; Berardinelli, F.; Sgura, A. Telomere Length Maintenance in Cancer: At the Crossroad between Telomerase and Alternative Lengthening of Telomeres (ALT). Int. J. Mol. Sci. 2018, 19, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojovic, B.; Booth, R.E.; Jin, Y.; Zhou, X.; Crowe, D.L. Alternative lengthening of telomeres in cancer stem cells in vivo. Oncogene 2015, 34, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Durisová, M.; Dedík, L. SURVIVAL--an integrated software package for survival curve estimation and statistical comparison of survival rates of two groups of patients or experimental animals. Methods Find. Exp. Clin. Pharmacol. 1993, 15, 535–540. [Google Scholar]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Janky, R.; Verfaillie, A.; Imrichova, H.; Van de Sande, B.; Standaert, L.; Christiaens, V.; Hulselmans, G.; Herten, K.; Sanchez, M.N.; Potier, D.; et al. iRegulon: From a Gene List to a Gene Regulatory Network Using Large Motif and Track Collections. PLoS Comput. Biol. 2014, 10, e1003731. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.-Y.; Cheong, J.-H. Pan-Cancer Analysis of Clinical Relevance via Telomere Maintenance Mechanism. Int. J. Mol. Sci. 2021, 22, 11101. https://doi.org/10.3390/ijms222011101
Sung J-Y, Cheong J-H. Pan-Cancer Analysis of Clinical Relevance via Telomere Maintenance Mechanism. International Journal of Molecular Sciences. 2021; 22(20):11101. https://doi.org/10.3390/ijms222011101
Chicago/Turabian StyleSung, Ji-Yong, and Jae-Ho Cheong. 2021. "Pan-Cancer Analysis of Clinical Relevance via Telomere Maintenance Mechanism" International Journal of Molecular Sciences 22, no. 20: 11101. https://doi.org/10.3390/ijms222011101
APA StyleSung, J.-Y., & Cheong, J.-H. (2021). Pan-Cancer Analysis of Clinical Relevance via Telomere Maintenance Mechanism. International Journal of Molecular Sciences, 22(20), 11101. https://doi.org/10.3390/ijms222011101






